Rhizobium leguminosarum bv. viciae-Mediated Silver Nanoparticles for Controlling Bean Yellow Mosaic Virus (BYMV) Infection in Faba Bean Plants
Abstract
1. Introduction
2. Materials and Methods
2.1. Virus Isolate
2.2. Isolation and Identification of the Rhizobial Isolate
2.3. Characterization of Biosynthesized Silver Nanoparticles
2.4. Greenhouse Experiment for Bionanoparticle Foliar Applications against BYMV Virus
2.4.1. Determination of Total Chlorophyll in Plants
2.4.2. Determination of Total Proteins
2.5. Estimation of Malondialdehyde (MDA) and Hydrogen Peroxide (H2O2)
2.5.1. Malondialdehyde (MDA)
2.5.2. Hydrogen Peroxide (H2O2)
2.6. Estimation of Antioxidant Enzymes
2.6.1. Peroxidase (POX)
2.6.2. Polyphenol Oxidase (PPO)
2.7. Total RNA Extraction and cDNA Synthesis
2.8. Quantitative Real-Time PCR
2.9. GC-MS Analysis
2.10. Data Analysis
3. Results
3.1. Isolation and Characterization of Rhizobium Isolate
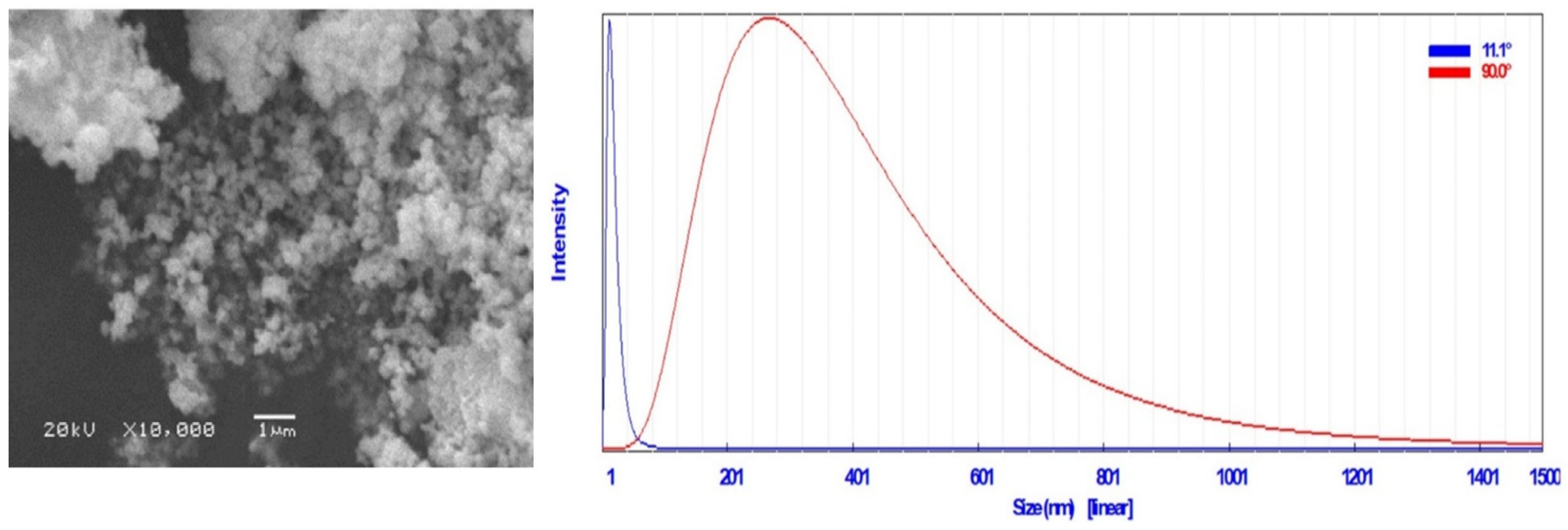
3.2. Visual Inspection, SEM, and DLS Analysis
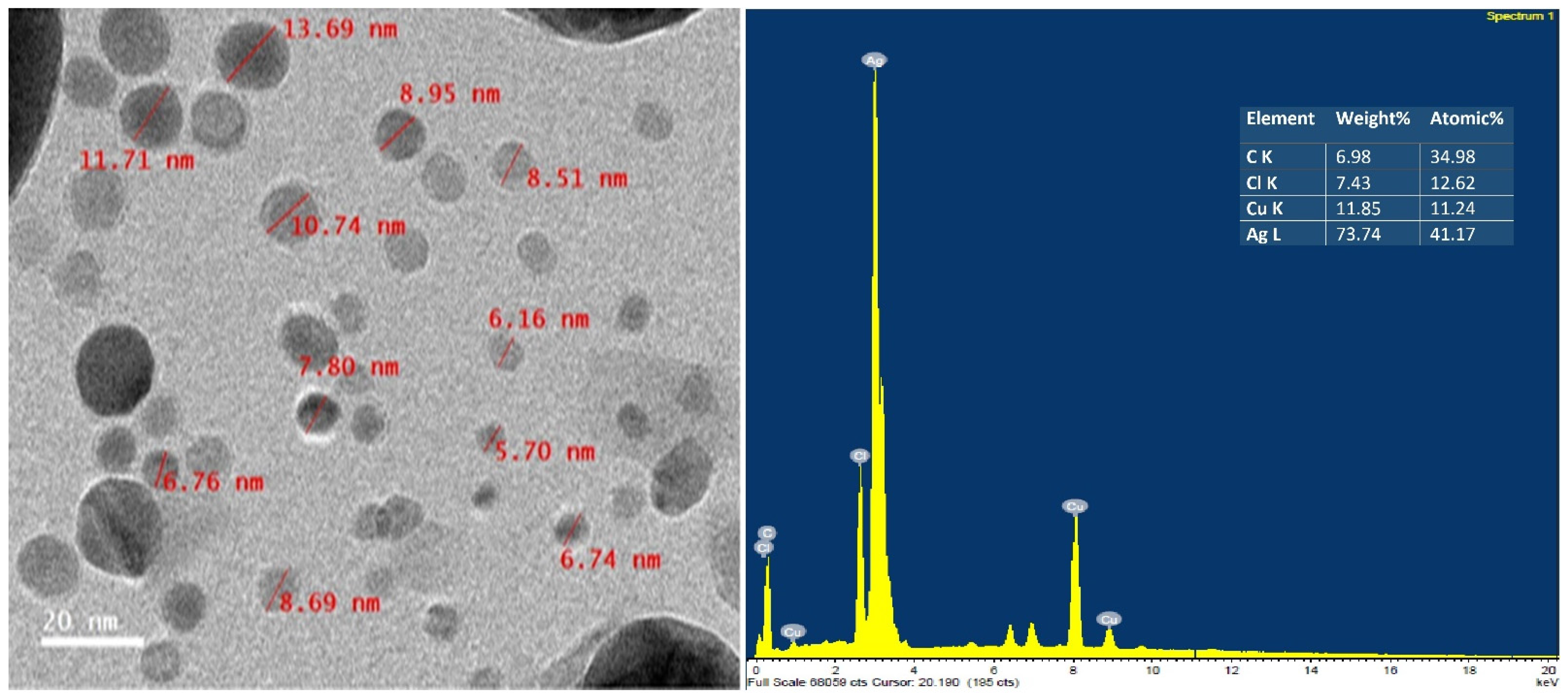
3.3. TEM and EDX Analysis
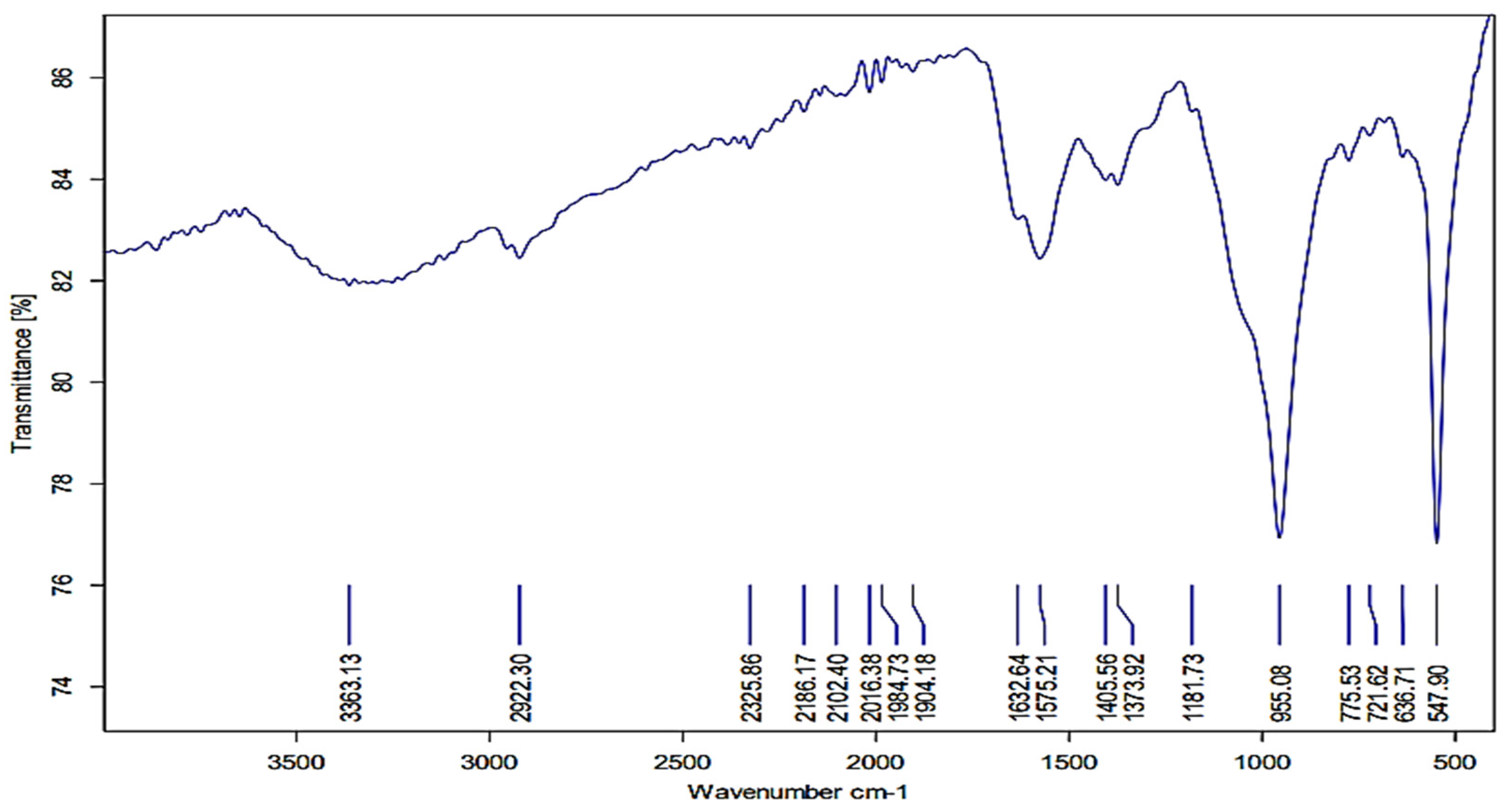
3.4. FTIR Analysis
3.5. Effect of AgNPs on Growth Parameters, Total Chlorophyll Content, and Total Protein
3.6. Effect of AgNPs on Antioxidant Enzymatic Activities (POX and PPO)
3.7. Effect of AgNPs on Oxidative Stress Markers Assay (H2O2 and MDA)
3.8. Effect of AgNPs on BYMV Accumulation Level and Transcriptional Levels of Defense-Related Genes
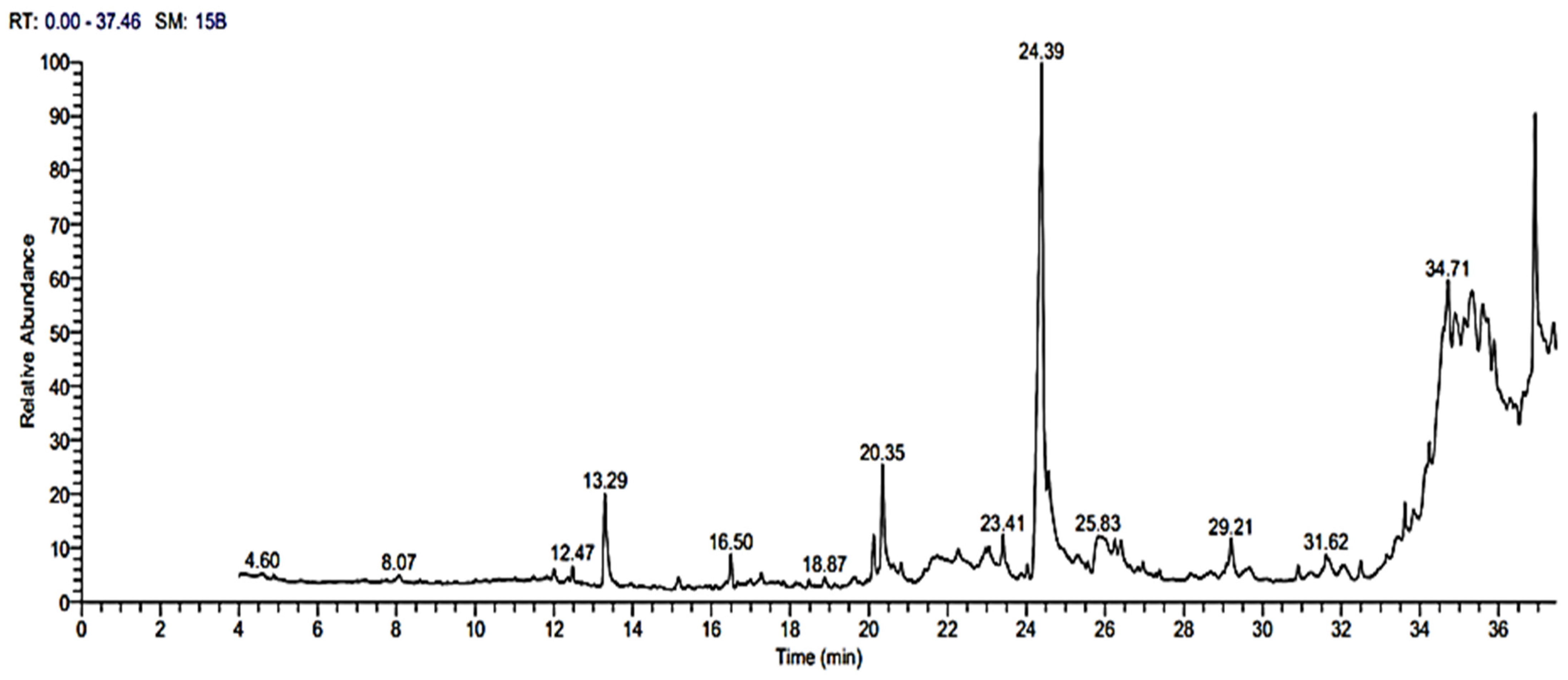
3.9. GC-MS Analysis of Bioactive Metabolites
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Agegnehu, G.; Fessehaite, R. Response of faba bean to phosphate fertilizer and weed control on Nitisols of Ethiopian highlands [Vicia faba L.]. Ital. J. Agron. 2006, 1, 281–290. [Google Scholar] [CrossRef]
- Abdelkhalek, A.; El-Gendi, H.; Al-Askar, A.A.; Maresca, V.; Moawad, H.; Elsharkawy, M.M.; Younes, H.A.; Behiry, S.I. Enhancing systemic resistance in faba bean (Vicia faba L.) to Bean yellow mosaic virus via soil application and foliar spray of nitrogen-fixing Rhizobium leguminosarum bv. viciae strain 33504-Alex1. Front. Plant Sci. 2022, 13, 933498. [Google Scholar] [CrossRef] [PubMed]
- Fath-Allah, M.M. Purification, Serological and Molecular Studies on an Egyptian Isolate of Faba Bean Necrotic yellows Virus (FBNYV) Infecting Faba Bean Plants. Egypt. J. Phytopathol. 2010, 38, 185–199. [Google Scholar] [CrossRef]
- Miteva, E.; Hristova, D.; Nenova, V.; Maneva, S. Arsenic as a factor affecting virus infection in tomato plants: Changes in plant growth, peroxidase activity and chloroplast pigments. Sci. Hortic. 2005, 105, 343–358. [Google Scholar] [CrossRef]
- Omar, S.A.M. The Importance of Faba Bean (Vicia faba L.) Diseases in Egypt. In Mitigating Environmental Stresses for Agricultural Sustainability in Egypt; Springer: Berlin, Germany, 2021; pp. 371–388. [Google Scholar]
- Khalil, S.A.; Erskine, W. Combating disease problems of grain legumes in Egypt. Grain Legum. 2001, 32, 24–26. [Google Scholar]
- Radwan, D.E.M.; Lu, G.; Fayez, K.A.; Mahmoud, S.Y. Protective action of salicylic acid against bean yellow mosaic virus infection in Vicia faba leaves. J. Plant Physiol. 2008, 165, 845–857. [Google Scholar] [CrossRef]
- Piccoli, C.; Cremonese, C.; Koifman, R.J.; Koifman, S.; Freire, C. Pesticide exposure and thyroid function in an agricultural population in Brazil. Environ. Res. 2016, 151, 389–398. [Google Scholar] [CrossRef]
- Bass, C.; Denholm, I.; Williamson, M.S.; Nauen, R. The global status of insect resistance to neonicotinoid insecticides. Pestic. Biochem. Physiol. 2015, 121, 78–87. [Google Scholar] [CrossRef]
- Fox, J.E.; Gulledge, J.; Engelhaupt, E.; Burow, M.E.; McLachlan, J.A. Pesticides reduce symbiotic efficiency of nitrogen-fixing rhizobia and host plants. Proc. Natl. Acad. Sci. USA 2007, 104, 10282–10287. [Google Scholar] [CrossRef]
- Franco-Andreu, L.; Gómez, I.; Parrado, J.; García, C.; Hernández, T.; Tejada, M. Behavior of two pesticides in a soil subjected to severe drought. Effects on soil biology. Appl. Soil Ecol. 2016, 105, 17–24. [Google Scholar] [CrossRef]
- Masry, S.H.D.; Taha, T.H.; Botros, W.A.; Mahfouz, H.; Al-Kahtani, S.N.; Ansari, M.J.; Hafez, E.E. Antimicrobial activity of camphor tree silver nano-particles against foulbrood diseases and finding out new strain of Serratia marcescens via DGGE-PCR, as a secondary infection on honeybee larvae. Saudi J. Biol. Sci. 2021, 28, 2067–2075. [Google Scholar] [CrossRef] [PubMed]
- Abdelkhalek, A.; Al-Askar, A.A. Green Synthesized ZnO Nanoparticles Mediated by Mentha Spicata Extract Induce Plant Systemic Resistance against Tobacco Mosaic Virus. Appl. Sci. 2020, 10, 5054. [Google Scholar] [CrossRef]
- Nassar, A.M. Research Article Effectiveness of Silver Nano-particles of Extracts of Urtica urens (Urticaceae) against Root-knot Nematode Meloidogyne incognita. Asian J. Nematol. 2016, 5, 14–19. [Google Scholar] [CrossRef]
- Alamdari, S.; Sasani Ghamsari, M.; Lee, C.; Han, W.; Park, H.-H.; Tafreshi, M.J.; Afarideh, H.; Ara, M.H.M. Preparation and Characterization of Zinc Oxide Nanoparticles Using Leaf Extract of Sambucus ebulus. Appl. Sci. 2020, 10, 3620. [Google Scholar] [CrossRef]
- Bhuyan, T.; Mishra, K.; Khanuja, M.; Prasad, R.; Varma, A. Biosynthesis of zinc oxide nanoparticles from Azadirachta indica for antibacterial and photocatalytic applications. Mater. Sci. Semicond. Process. 2015, 32, 55–61. [Google Scholar] [CrossRef]
- Volpiano, C.G.; Lisboa, B.B.; Granada, C.E.; José, J.F.B.S.; de Oliveira, A.M.R.; Beneduzi, A.; Perevalova, Y.; Passaglia, L.M.P.; Vargas, L.K. Rhizobia for biological control of plant diseases. In Microbiome in Plant Health and Disease; Springer: Berlin, Germany, 2019; pp. 315–336. [Google Scholar]
- Somasegaran, P.; Hoben, H.J. Methods in Legume-Rhizobium Technology; University of Hawaii NifTAL Project and MIRCEN: Paia, HI, USA, 1985. [Google Scholar]
- Laguerre, G.; Nour, S.M.; Macheret, V.; Sanjuan, J.; Drouin, P.; Amarger, N. Classification of rhizobia based on nodC and nifH gene analysis reveals a close phylogenetic relationship among Phaseolus vulgaris symbiontsThe GenBank accession numbers for the sequences reported in this paper are AF217261 through AF217272 for nodC and A. Microbiology 2001, 147, 981–993. [Google Scholar] [CrossRef]
- Heflish, A.A.; Hanfy, A.E.; Ansari, M.J.; Dessoky, E.S.; Attia, A.O.; Elshaer, M.M.; Gaber, M.K.; Kordy, A.; Doma, A.S.; Abdelkhalek, A.; et al. Green biosynthesized silver nanoparticles using Acalypha wilkesiana extract control root-knot nematode. J. King Saud Univ. Sci. 2021, 33, 101516. [Google Scholar] [CrossRef]
- Singh, V.; Shrivastava, A.; Wahi, N. Biosynthesis of silver nanoparticles by plants crude extracts and their characterization using UV, XRD, TEM and EDX. Afr. J. Biotechnol. 2015, 14, 2554–2567. [Google Scholar]
- Strain, H.H.; Svec, W.A. Extraction, separation, estimation, and isolation of the chlorophylls. In The Chlorophylls; Elsevier: Amsterdam, The Netherlands, 1966; pp. 21–66. [Google Scholar]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Heath, R.L.; Packer, L. Photoperoxidation in isolated chloroplasts: II. Role of electron transfer. Arch. Biochem. Biophys. 1968, 125, 850–857. [Google Scholar] [CrossRef]
- Velikova, V.; Yordanov, I.; Edreva, A. Oxidative stress and some antioxidant systems in acid rain-treated bean plants: Protective role of exogenous polyamines. Plant Sci. 2000, 151, 59–66. [Google Scholar] [CrossRef]
- Beauchamp, C.; Fridovich, I. Superoxide dismutase: Improved assays and an assay applicable to acrylamide gels. Anal. Biochem. 1971, 44, 276–287. [Google Scholar] [CrossRef] [PubMed]
- Kato, M.; Shimizu, S. Chlorophyll metabolism in higher plants. VII. Chlorophyll degradation in senescing tobacco leaves; phenolic-dependent peroxidative degradation. Can. J. Bot. 1987, 65, 729–735. [Google Scholar] [CrossRef]
- Kumar, K.B.; PA, K. Peroxidase & Polyphenol Oxidase in Excised Ragi (Eleusine Coracana Cv Pr 202) Leaves during Senescence. Indian J. Exp. Biol. 1982, 20, 412–416. [Google Scholar]
- Hafez, E.E.; Abdelkhalek, A.A.; Abd El-Wahab, A.S.E.-D.; Galal, F.H. Altered gene expression: Induction/suppression in leek elicited by Iris Yellow Spot Virus infection (IYSV) Egyptian isolate. Biotechnol. Biotechnol. Equip. 2013, 27, 4061–4068. [Google Scholar] [CrossRef]
- Abdelkhalek, A.; Al-Askar, A.A.; Hafez, E. Differential induction and suppression of the potato innate immune system in response to Alfalfa mosaic virus infection. Physiol. Mol. Plant Pathol. 2020, 110, 101485. [Google Scholar] [CrossRef]
- Behiry, S.I.; Ashmawy, N.A.; Abdelkhalek, A.A.; Younes, H.A.; Khaled, A.E.; Hafez, E.E. Compatible- and incompatible-type interactions related to defense genes in potato elucidation by Pectobacterium carotovorum. J. Plant Dis. Prot. 2018, 125, 197–204. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Abdelkhalek, A.; Behiry, S.I.; Al-Askar, A.A. Bacillus velezensis PEA1 Inhibits Fusarium oxysporum Growth and Induces Systemic Resistance to Cucumber Mosaic Virus. Agronomy 2020, 10, 1312. [Google Scholar] [CrossRef]
- Abd El-Rahim, W.M.; Moawad, H.; Azeiz, A.Z.A.; Sadowsky, M.J. Biodegradation of azo dyes by bacterial or fungal consortium and identification of the biodegradation products. Egypt. J. Aquat. Res. 2021, 47, 269–276. [Google Scholar] [CrossRef]
- Abdelkhalek, A.; Hafez, E. Plant Viral Diseases in Egypt and Their Control. In Cottage Industry of Biocontrol Agents and Their Applications; Springer: Berlin, Germany, 2020; pp. 403–421. [Google Scholar]
- Moawad, H.; Abd el-Rahim, W.M.; Abd el-Aleem, D.; Abo Sedera, S.A. Persistence of two Rhizobium etli inoculant strains in clay and silty loam soils. J. Basic Microbiol. 2005, 45, 438–446. [Google Scholar] [CrossRef] [PubMed]
- Moawad, H.; Abd El-Rahim, W.M.; Abd El-Haleem, D. Performance of phaseolus bean rhizobia in soils from the major production sites in the Nile Delta. C. R. Biol. 2004, 327, 445–453. [Google Scholar] [CrossRef] [PubMed]
- Kirubha, R.; Alagumuthu, G. Morinda tinctoria fruit assisted biosynthesis of silver nanoparticles. Asian J. Pharm. Clin. Res. 2013, 6, 60–64. [Google Scholar]
- Kumar, C.G.; Mamidyala, S.K. Extracellular synthesis of silver nanoparticles using culture supernatant of Pseudomonas aeruginosa. Colloids Surf. B Biointerfaces 2011, 84, 462–466. [Google Scholar] [CrossRef]
- Kulikov, K.G.; Koshlan, T.V. Measurement of sizes of colloid particles using dynamic light scattering. Tech. Phys. 2015, 60, 1758–1764. [Google Scholar] [CrossRef]
- Elbeshehy, E.K.F.; Almaghrabi, O.A.; Mahmoud, W.M.A.; Elazzazy, A.M. Effect of biosynthesized silver nanoparticles on physiological parameters of Vicia faba infected by bean yellow mosaic virus. J. Pure Appl. Microbiol. 2014, 8, 803–812. [Google Scholar]
- Kathiresan, K.; Alikunhi, N.M.; Pathmanaban, S.; Nabikhan, A.; Kandasamy, S. Analysis of antimicrobial silver nanoparticles synthesized by coastal strains of Escherichia coli and Aspergillus niger. Can. J. Microbiol. 2010, 56, 1050–1059. [Google Scholar] [CrossRef]
- Datta, A.; Patra, C.; Bharadwaj, H.; Kaur, S.; Dimri, N.; Khajuria, R. Green synthesis of zinc oxide nanoparticles using parthenium hysterophorus leaf extract and evaluation of their antibacterial properties. J. Biotechnol. Biomater. 2017, 7, 271–276. [Google Scholar] [CrossRef]
- Chanda, S. Silver nanoparticles (medicinal plants mediated): A new generation of antimicrobials to combat microbial pathogens-a review. Microb. Pathog. Strateg. Combat. Sci. Technol. Educ. 2013, 4, 1314–1323. [Google Scholar]
- Mittal, A.K.; Chisti, Y.; Banerjee, U.C. Synthesis of metallic nanoparticles using plant extracts. Biotechnol. Adv. 2013, 31, 346–356. [Google Scholar] [CrossRef]
- Raj, S.; Singh, H.; Trivedi, R.; Soni, V. Biogenic synthesis of AgNPs employing Terminalia arjuna leaf extract and its efficacy towards catalytic degradation of organic dyes. Sci. Rep. 2020, 10, 9616. [Google Scholar] [CrossRef] [PubMed]
- Babu Nagati, V.; Koyyati, R.; Donda, M.R.; Alwala, J.; Kundle, K.R.; Padigya, P.R.M. Green synthesis and characterization of silver nanoparticles from Cajanus cajan leaf extract and its antibacterial activity. Int. J. Nanomater. Biostruct. 2012, 2, 39–43. [Google Scholar]
- Jagtap, U.B.; Bapat, V.A. Green synthesis of silver nanoparticles using Artocarpus heterophyllus Lam. seed extract and its antibacterial activity. Ind. Crops Prod. 2013, 46, 132–137. [Google Scholar] [CrossRef]
- Suriyakala, G.; Sathiyaraj, S.; Babujanarthanam, R.; Alarjani, K.M.; Hussein, D.S.; Rasheed, R.A.; Kanimozhi, K. Green synthesis of gold nanoparticles using Jatropha integerrima Jacq. flower extract and their antibacterial activity. J. King Saud Univ. 2022, 34, 101830. [Google Scholar] [CrossRef]
- Sharma, K.; Agrawal, S.S.; Gupta, M. Development and validation of UV spectrophotometric method for the estimation of curcumin in bulk drug and pharmaceutical dosage forms. Int. J. Drug Dev. Res. 2012, 4, 375–380. [Google Scholar]
- Nandiyanto, A.B.D.; Oktiani, R.; Ragadhita, R. How to read and interpret FTIR spectroscope of organic material. Indones. J. Sci. Technol. 2019, 4, 97–118. [Google Scholar] [CrossRef]
- Benakashani, F.; Allafchian, A.R.; Jalali, S.A.H. Biosynthesis of silver nanoparticles using Capparis spinosa L. leaf extract and their antibacterial activity. Karbala Int. J. Mod. Sci. 2016, 2, 251–258. [Google Scholar] [CrossRef]
- Erjaee, H.; Rajaian, H.; Nazifi, S. Synthesis and characterization of novel silver nanoparticles using Chamaemelum nobile extract for antibacterial application. Adv. Nat. Sci. Nanosci. Nanotechnol. 2017, 8, 25004. [Google Scholar] [CrossRef]
- Yang, X.X.; Li, C.M.; Huang, C.Z. Curcumin modified silver nanoparticles for highly efficient inhibition of respiratory syncytial virus infection. Nanoscale 2016, 8, 3040–3048. [Google Scholar] [CrossRef]
- Cai, L.; Cai, L.; Jia, H.; Liu, C.; Wang, D.; Sun, X. Foliar exposure of Fe3O4 nanoparticles on Nicotiana benthamiana: Evidence for nanoparticles uptake, plant growth promoter and defense response elicitor against plant virus. J. Hazard. Mater. 2020, 393, 122415. [Google Scholar] [CrossRef]
- Hemida, S.K. Effect of bean yellow mosaic virus on physiological parameters ofVicia faba and Phaseolus vulgaris. Int. J. Agric. Biol. 2005, 7, 154–157. [Google Scholar]
- Rostamizadeh, E.; Iranbakhsh, A.; Majd, A.; Arbabian, S.; Mehregan, I. Green synthesis of Fe2O3 nanoparticles using fruit extract of Cornus mas L. and its growth-promoting roles in Barley. J. Nanostruct. Chem. 2020, 10, 125–130. [Google Scholar] [CrossRef]
- Al-Huqail, A.A.; Hatata, M.M.; Al-Huqail, A.A.; Ibrahim, M.M. Preparation, characterization of silver phyto nanoparticles and their impact on growth potential of Lupinus termis L. seedlings. Saudi J. Biol. Sci. 2018, 25, 313–319. [Google Scholar] [CrossRef] [PubMed]
- El Gamal, A.; Tohamy, M.R.; Abou-Zaid, M.I.; Atia, M.M.; El Sayed, T.; Farroh, K. Silver Nanoparticles as A Viricidal Agent to Inhibit Plant-Infecting Viruses and Disrupt their Acquisition and Transmission by their Aphid Vector. Arch. Virol. 2022, 167, 85–97. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, M.; Kazemi, H. Changes in peroxidase and polyphenol oxidase activities in susceptible and resistant wheat heads inoculated with Fusarium graminearum and induced resistance. Plant Sci. 2002, 162, 491–498. [Google Scholar] [CrossRef]
- Mondal, S.; Phadke, R.R.; Badigannavar, A.M. Genetic variability for total phenolics, flavonoids and antioxidant activity of testaless seeds of a peanut recombinant inbred line population and identification of their controlling QTLs. Euphytica 2015, 204, 311–321. [Google Scholar] [CrossRef]
- Anthony, K.K.; George, D.S.; Baldev Singh, H.K.; Fung, S.M.; Santhirasegaram, V.; Razali, Z.; Somasundram, C. Reactive oxygen species activity and antioxidant properties of Fusarium infected bananas. J. Phytopathol. 2017, 165, 213–222. [Google Scholar] [CrossRef]
- Dempsey, D.A.; Vlot, A.C.; Wildermuth, M.C.; Klessig, D.F. Salicylic Acid Biosynthesis and Metabolism. Arab. B. 2011, 9, e0156. [Google Scholar] [CrossRef]
- Abo-Zaid, G.; Abdelkhalek, A.; Matar, S.; Darwish, M.; Abdel-Gayed, M. Application of Bio-Friendly Formulations of Chitinase-Producing Streptomyces cellulosae Actino 48 for Controlling Peanut Soil-Borne Diseases Caused by Sclerotium rolfsii. J. Fungi 2021, 7, 167. [Google Scholar] [CrossRef]
- Abdelkhalek, A. Expression of tomato pathogenesis related genes in response to Tobacco mosaic virus. JAPS J. Anim. Plant Sci. 2019, 29, 1596–1602. [Google Scholar]
- ElMorsi, A.; Abdelkhalek, A.; Alshehaby, O.; Hafez, E.E. Pathogenesis-related genes as tools for discovering the response of onion defence system against iris yellow spot virus infection. Botany 2015, 93, 735–744. [Google Scholar] [CrossRef]
- Akyol, H.; Riciputi, Y.; Capanoglu, E.; Caboni, M.; Verardo, V. Phenolic compounds in the potato and its byproducts: An overview. Int. J. Mol. Sci. 2016, 17, 835. [Google Scholar] [CrossRef] [PubMed]
- Leiss, K.A.; Maltese, F.; Choi, Y.H.; Verpoorte, R.; Klinkhamer, P.G.L. Identification of chlorogenic acid as a resistance factor for thrips in chrysanthemum. Plant Physiol. 2009, 150, 1567–1575. [Google Scholar] [CrossRef] [PubMed]
- Niggeweg, R.; Michael, A.J.; Martin, C. Engineering plants with increased levels of the antioxidant chlorogenic acid. Nat. Biotechnol. 2004, 22, 746. [Google Scholar] [CrossRef] [PubMed]
- Abdelkhalek, A.; Al-Askar, A.A.; Behiry, S.I. Bacillus licheniformis strain POT1 mediated polyphenol biosynthetic pathways genes activation and systemic resistance in potato plants against Alfalfa mosaic virus. Sci. Rep. 2020, 10, 16120. [Google Scholar] [CrossRef] [PubMed]
- Desbois, A.P.; Smith, V.J. Antibacterial free fatty acids: Activities, mechanisms of action and biotechnological potential. Appl. Microbiol. Biotechnol. 2010, 85, 1629–1642. [Google Scholar] [CrossRef]
- Guedes, A.C.; Amaro, H.M.; Malcata, F.X. Microalgae as sources of high added-value compounds—A brief review of recent work. Biotechnol. Prog. 2011, 27, 597–613. [Google Scholar] [CrossRef]
- El-Gendi, H.; Al-Askar, A.A.; Király, L.; Samy, M.A.; Moawad, H.; Abdelkhalek, A. Foliar Applications of Bacillus subtilis HA1 Culture Filtrate Enhance Tomato Growth and Induce Systemic Resistance against Tobacco mosaic virus Infection. Horticulturae 2022, 8, 301. [Google Scholar] [CrossRef]
- Ahsan, T.; Chen, J.; Zhao, X.; Irfan, M.; Wu, Y. Extraction and identification of bioactive compounds (eicosane and dibutyl phthalate) produced by Streptomyces strain KX852460 for the biological control of Rhizoctonia solani AG-3 strain KX852461 to control target spot disease in tobacco leaf. AMB Express 2017, 7, 1–9. [Google Scholar] [CrossRef]
- Octarya, Z.; Novianty, R.; Suraya, N. Saryono Antimicrobial activity and GC-MS analysis of bioactive constituents of Aspergillus fumigatus 269 isolated from Sungai Pinang hot spring, Riau, Indonesia. Biodiversitas 2021, 22, 1839–1845. [Google Scholar] [CrossRef]
- Naeim, H.; El-Hawiet, A.; Abdel Rahman, R.A.; Hussein, A.; El Demellawy, M.A.; Embaby, A.M. Antibacterial activity of Centaurea pumilio L. Root and aerial part extracts against some multidrug resistant bacteria. BMC Complement. Med. Ther. 2020, 20, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Kachroo, A.; Kachroo, P. Fatty acid-derived signals in plant defense. Annu. Rev. Phytopathol. 2009, 47, 153–176. [Google Scholar] [CrossRef] [PubMed]
- Cohen, Y.; Gisi, U.; Mosinger, E. Systemic resistance of potato plants against Phytophthora infestans induced by unsaturated fatty acids. Physiol. Mol. Plant Pathol. 1991, 38, 255–263. [Google Scholar] [CrossRef]
- Zhao, L.; Chen, Y.; Wu, K.; Yan, H.; Hao, X.; Wu, Y. Application of fatty acids as antiviral agents against tobacco mosaic virus. Pestic. Biochem. Physiol. 2017, 139, 87–91. [Google Scholar] [CrossRef] [PubMed]



| Gene | Abbreviation | Sequence |
|---|---|---|
| 16S ribosomal RNA | 16S rRNA | For: AGAGTTTGATCCTGGCTCAG Rev: AAGGAGGTGATCCAGCC |
| Pathogenesis-related protein-1 | PR-1 | For: TTCTTCCCTCGAAAGCTCAA Rev: CGCTACCCCAGGCTAAGTTT |
| Hydroxycinnamoyl-CoA quinate hydroxycinnamoyltransferase | HQT | For: CCCAATGGCTGGAAGATTAGCTA Rev:CATGAATCACTTTCAGCCTCAACAA |
| Bean yellow mosaic virus coat protein | BYMV-CP | For:GGTTTGGCYAGRTATGCTTTTG Rev: GAGAATTTAAAGACGGATA |
| 18s ribosomal RNA. | 18S rRNA | For: CATCAGCTCGCGTTGACTAC Rev: GATCCTTCCGCAGGTTCAC |
| Beta-actin | β-actin | For: TGGCATACAAAGACAGGACAGCCT Rev: ACTCAATCCCAAGGCCAACAGAGA |
| Treatments * | Fresh Weight | Dry Weight | Chlorophyll | Total Protein |
|---|---|---|---|---|
| T1 | 41.07 a ± 3.50 | 7.07 ab ± 0.78 | 32.79 b ± 0.18 | 13.24 a ± 0.31 |
| T2 | 31.77 b ± 1.99 | 5.26 b ± 0.98 | 23.42 c ± 0.21 | 13.84 a ± 0.27 |
| T3 | 40.70 a ± 1.31 | 8.57 a ± 0.58 | 45.76 a ± 0.23 | 10.37 b ± 0.15 |
| Treatments * | Antioxidant Enzymes | Oxidative Stress Markers | ||
|---|---|---|---|---|
| POX | PPO | H2O2 | MDA | |
| T1 | 0.88 ± 0.18 b | 0.13 ± 0.01 b | 6.39 ± 0.10 b | 235.60 ± 0.10 b |
| T2 | 1.78 ± 0.15 a | 0.27 ± 0.01 a | 7.56 ± 0.04 a | 383.98 ± 0.16 a |
| T3 | 1.12 ± 0.18 b | 0.15 ± 0.01 b | 4.52 ± 0.07 c | 147.14 ± 0.10 b |
| No | Retention Time | Compound | Area % | Molecular Formula | Molecular Weight |
|---|---|---|---|---|---|
| 1 | 12.47 | Tetradecane, 2,6,10-trimethyl- | 0.57 | C17H36 | 240 |
| 2 | 13.29 | 15-methyltricyclo [6.5.2(13,1 4).0(7,15)] pentadeca-1,3,5,7,9,1 1,13-heptene | 4.13 | C16H14 | 206 |
| 3 | 16.50 | Heptadecane, 2,6,10,15-tetramethyl- | 1.27 | C21H44 | 296 |
| 4 | 20.35 | 7,9-Di-tert-butyl-1-oxaspiro(4,5)dec a-6,9-diene-2,8-dione | 4.19 | C17H24O3 | 276 |
| 5 | 24.39 | Oleic Acid | 20.23 | C18H34O2 | 282 |
| 6 | 25.82 | 1-Hexadecanol, 2-methyl- | 4.48 | C17H36O | 256 |
| 7 | 29.20 | 1,2-benzenedicarboxylic acid | 1.77 | C24H38O4 | 390 |
| 8 | 31.62 | 2,2,3,3,4,4 hexadeutero octadecanal | 0.58 | C18H30D6O | 274 |
| 9 | 34.72 | 4H-1-Benzopyran-4 one, 2-(3,4-dimethoxyphenyl)- 3, 5-dihydroxy-7- methoxy- | 5.66 | C18H16O7 | 344 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdelkhalek, A.; Yassin, Y.; Abdel-Megeed, A.; Abd-Elsalam, K.A.; Moawad, H.; Behiry, S.I. Rhizobium leguminosarum bv. viciae-Mediated Silver Nanoparticles for Controlling Bean Yellow Mosaic Virus (BYMV) Infection in Faba Bean Plants. Plants 2023, 12, 45. https://doi.org/10.3390/plants12010045
Abdelkhalek A, Yassin Y, Abdel-Megeed A, Abd-Elsalam KA, Moawad H, Behiry SI. Rhizobium leguminosarum bv. viciae-Mediated Silver Nanoparticles for Controlling Bean Yellow Mosaic Virus (BYMV) Infection in Faba Bean Plants. Plants. 2023; 12(1):45. https://doi.org/10.3390/plants12010045
Chicago/Turabian StyleAbdelkhalek, Ahmed, Yara Yassin, Ahmed Abdel-Megeed, Kamel A. Abd-Elsalam, Hassan Moawad, and Said I. Behiry. 2023. "Rhizobium leguminosarum bv. viciae-Mediated Silver Nanoparticles for Controlling Bean Yellow Mosaic Virus (BYMV) Infection in Faba Bean Plants" Plants 12, no. 1: 45. https://doi.org/10.3390/plants12010045
APA StyleAbdelkhalek, A., Yassin, Y., Abdel-Megeed, A., Abd-Elsalam, K. A., Moawad, H., & Behiry, S. I. (2023). Rhizobium leguminosarum bv. viciae-Mediated Silver Nanoparticles for Controlling Bean Yellow Mosaic Virus (BYMV) Infection in Faba Bean Plants. Plants, 12(1), 45. https://doi.org/10.3390/plants12010045









