Herbivory in Myrtillocactus geometrizans (Cactaceae): Do Parasitoids Provide Indirect Defense or a Direct Advantage?
Abstract
:1. Introduction
2. Results
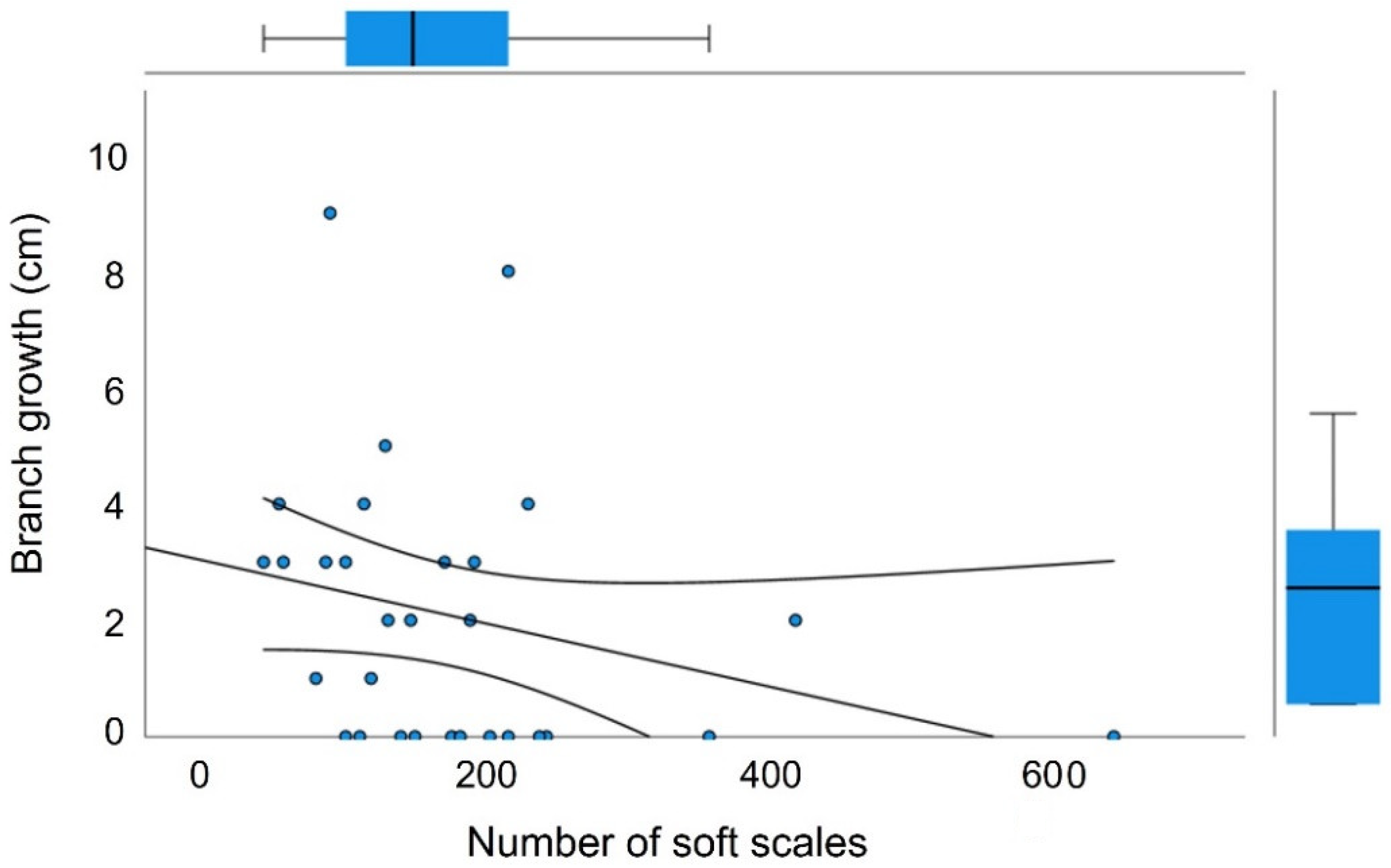
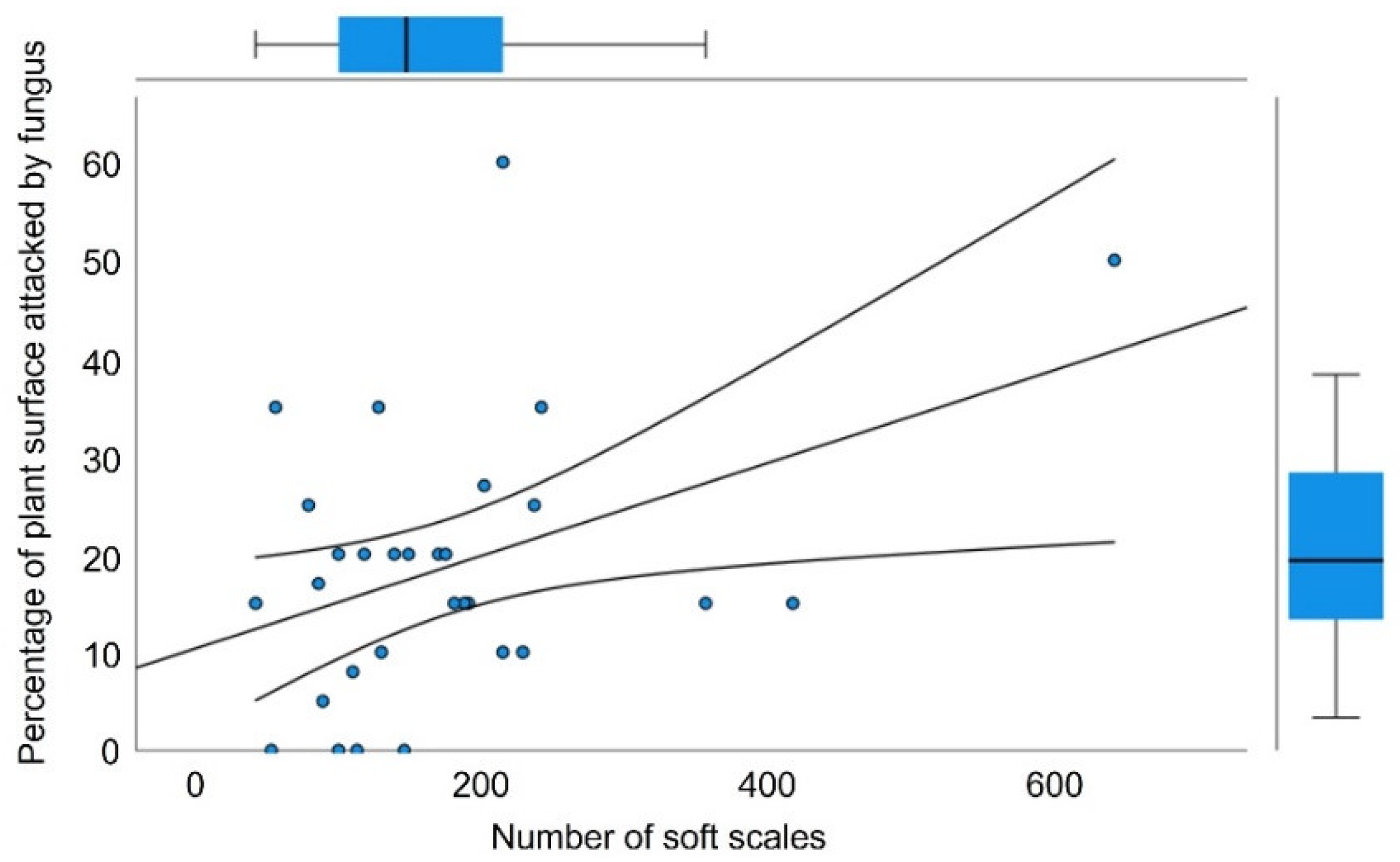
2.1. Effect of the Soft Scale T. martinezae on M. geometrizans
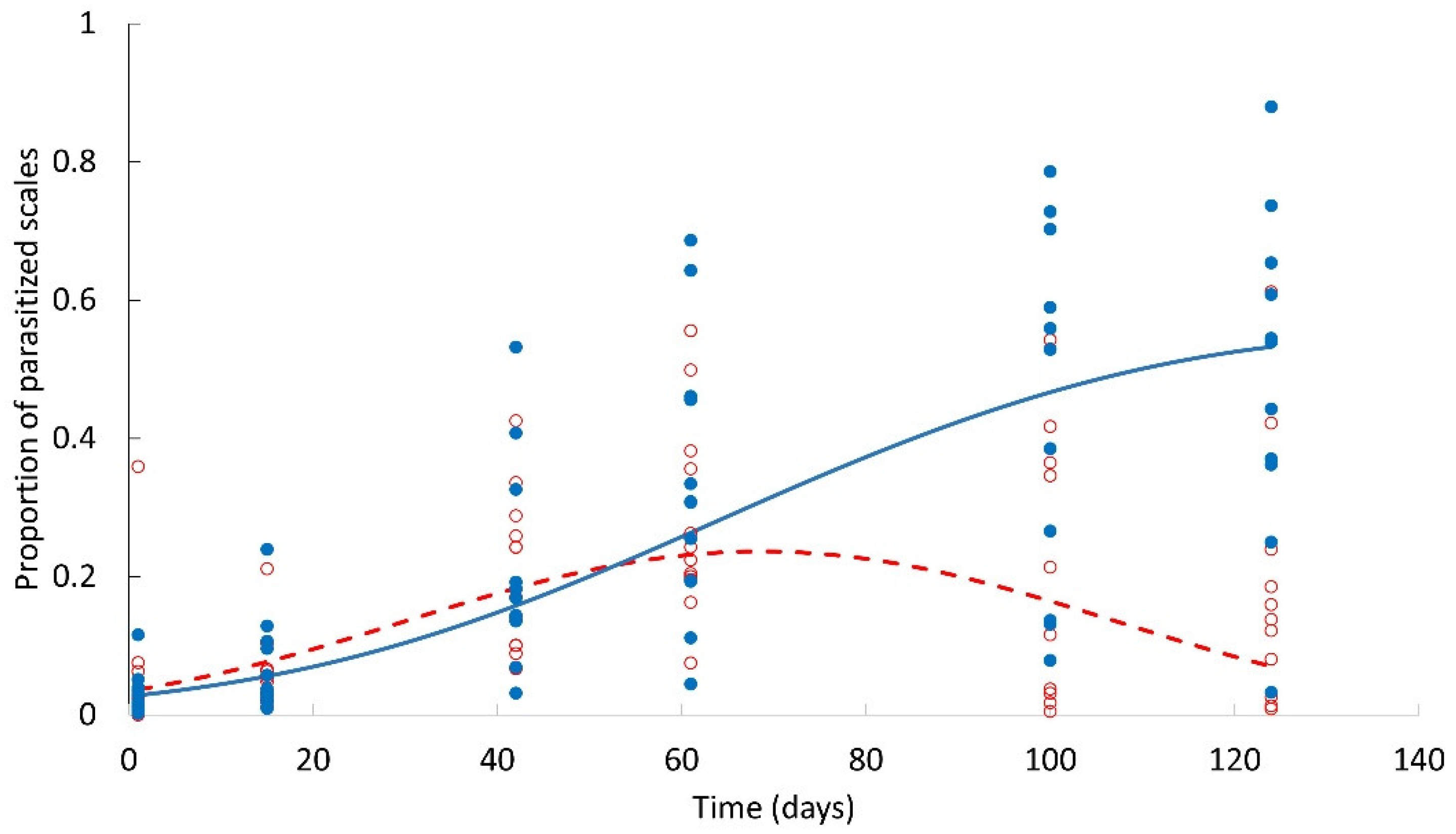
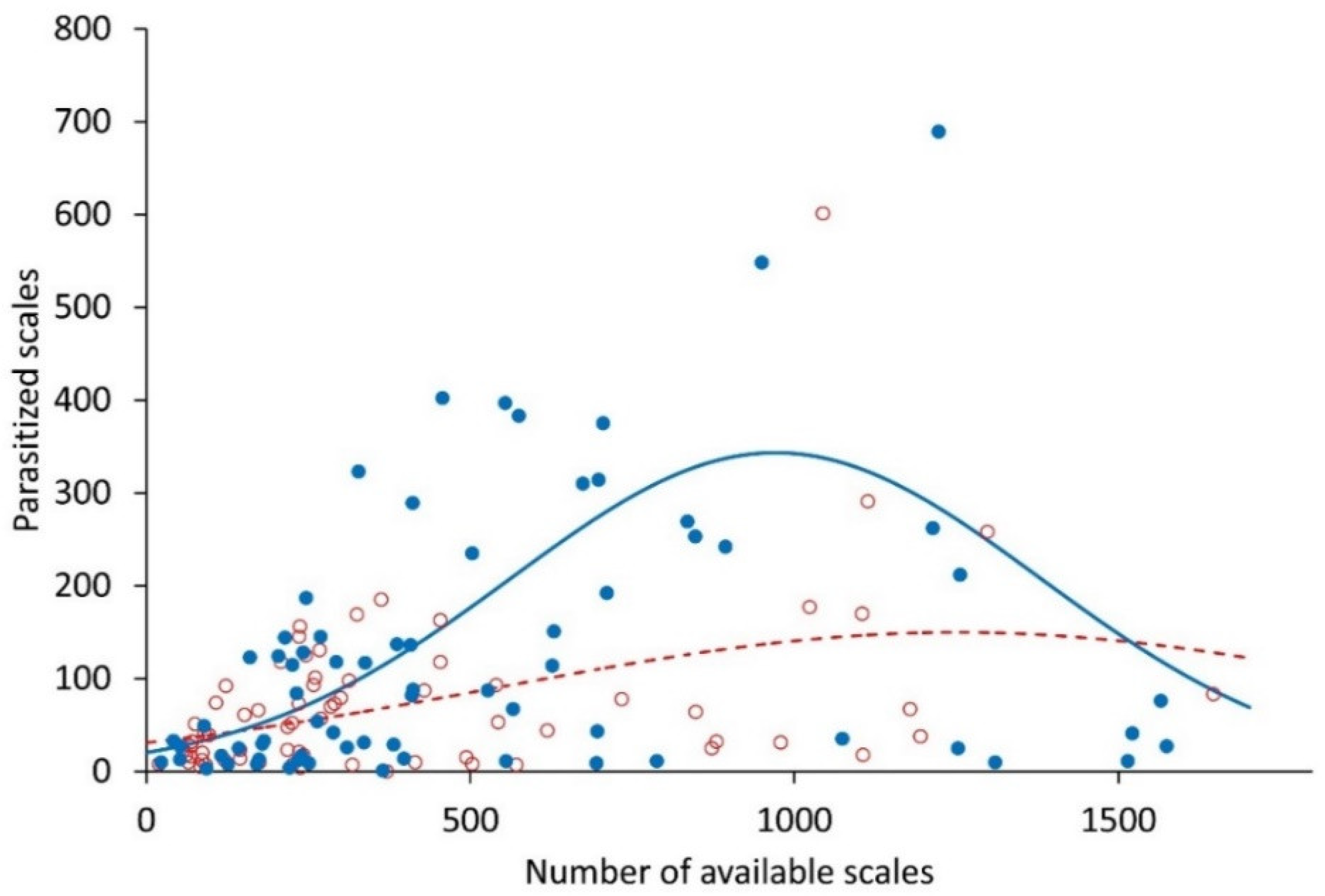
2.2. Infestation Dynamics and Response to Scale Density
3. Discussion
4. Methods
4.1. Study System
4.2. Field Work
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mohamed, A.A.M.; Mohamed, H.H. Tritrophic Interactions and Insect Behavior; Lambert Academic Publishing: Berlin/Heidelberg, Germany, 2013; 133p. [Google Scholar]
- Price, P.W.; Bouton, C.E.; Gross, P.; McPheron, B.A.; Thompson, J.N.; Weis, A.E. Interactions among three trophic levels: Influence of plants on interactions between insect herbivores and natural enemies. Annu. Rev. Ecol. Syst. 1980, 11, 41–65. [Google Scholar] [CrossRef] [Green Version]
- Callejas-Chavero, A.; Castaño-Meneses, G.; Razo-Gonzalez, M.; Perez-Velazquez, D.; Palacios-Vargas, J.G.; Flores-Martinez, A. Soil Microarthropods and Their Relationship to Higher Trophic Levels in the Pedregal de San Angel Ecological Reserve, Mexico. J. Insect Sci. 2015, 15, 59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Triapitsyn, S.V.; Aguirre, M.B.; Logarzo, G.A.; Hight, S.D. Hyperparasitoids (Hymenoptera: Encyrtidae and Signiphoridae) of Hypogeococcus spp. mealybugs (Hemiptera: Pseudococcidae) in Argentina and Paraguay. Acta Zoológica 2020, 64, 148–165. [Google Scholar] [CrossRef]
- Van Dam, N.M.; Heil, M. Multitrophic interactions below and above ground: En route to the next level. J. Ecol. 2011, 99, 77–88. [Google Scholar] [CrossRef]
- Meiners, T. Chemical ecology and evolution of plant–insect interactions: A multitrophic perspective. Curr. Opin. Insect Sci. 2015, 8, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Moncada, O.A. Competencia entre Toumeyella martinezae (Coccidae) y Opuntiaspis philococcus (Diaspididae) asociadas a Myrtillocactus geometrizans (Cactaceae) en presencia y ausencia de la hormiga Liometopum apiculatum (Formicidae). Master’s Thesis, Escuela Nacional Ciencias Biológicas, Mexico City, Mexico, 2019; 52p. Available online: http://tesis.ipn.mx/handle/123456789/28349 (accessed on 20 August 2022).
- Aguirre, M.B.; Bruzzone, O.A.; Triapitsyn, S.V.; Diaz-Soltero, H.; Hight, S.D.; Logarzo, G.A. Influence of competition and intraguild predation between two candidate biocontrol parasitoids on their potential impact against Harrisia cactus mealybug, Hypogeococcus sp. (Hemiptera: Pseudococcidae). Sci. Rep. 2021, 11, 13377. [Google Scholar] [CrossRef] [PubMed]
- Fineblum, W.L.; Rausher, M.D. Trade off between resistance and tolerance to herbivore damage in a morning glory. Nature 1995, 377, 517–520. [Google Scholar] [CrossRef]
- Hagenbucher, S.; Olson, D.M.; Ruberson, J.R.; Wäckers, F.L.; Romeis, J. Resistance Mechanisms Against Arthropod Herbivores in Cotton and Their Interactions with Natural Enemies. Crit. Rev. Plant Sci. 2013, 32, 458–482. [Google Scholar] [CrossRef]
- Xu, H.; Veyrat, N.; Degen, T.; Turlings TC, J. Exceptional use of sex pheromones in parasitoids of the genus Cotesia: Males are strongly attracted to virgin females but are no longer attracted to or even repelled by mated females. Insects 2014, 5, 499–512. [Google Scholar] [CrossRef] [Green Version]
- De Moraes, C.M.; Lewis, W.J.; Tumlinson, J.H. Plant-Parasitoid Interactions in Tritrophic Systems. In Tritrophic Interactions and Insect Behavior; Mohamed, A.A.M., Mohamed, H.H., Eds.; Lambert Academic Publishing: Berlin/Heidelberg, Germany, 2013; pp. 8–17. [Google Scholar]
- Mumm, R.; Dicke, M. Variation in natural plant products and the attraction of bodyguards involved in indirect plant defense. Can. J. Zool. 2010, 88, 628–667. [Google Scholar] [CrossRef]
- Pearse, I.S.; LoPresti, E.; Schaeffer, R.N.; Wetzel, W.C.; Mooney, K.A.; Ali, J.G.; Ode, P.J.; Eubanks, M.D.; Bronstein, J.L.; Weber, M.G. Generalising indirect defense and resistance of plants. Ecol. Lett. 2020, 23, 1137–1152. [Google Scholar] [CrossRef] [PubMed]
- Rostás, M.; Ton, J.; Mauch-Mani, B. Fungal Infection Reduces Herbivore-Induced Plant Volatiles of Maize but does not Affect Naïve Parasitoids. J. Chem. Ecol. 2006, 32, 1897–1909. [Google Scholar] [CrossRef] [PubMed]
- McCormick, A.C.; Unsicker, S.B.; Gershenzon, J. The specificity of herbivore-induced plant volatiles in attracting herbivore enemies. Trends Plant Sci. 2012, 17, 303–310. [Google Scholar] [CrossRef] [PubMed]
- Turlings, T.C.J.; Tumlinson, J.H.; Lewis, W.J. Exploitation of herbivore-induced plant odors by host-seeking parasitic wasps. Science 1990, 250, 1251–1253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Acevedo, F.E.; Rivera-Vega, L.J.; Chung, S.H.; Ray, S.; Felton, G.W. Cues from chewing insects—The intersection of DAMPs, HAMPs, MAMPs and effectors. Curr. Opin. Plant Biol. 2015, 26, 80–86. [Google Scholar] [CrossRef]
- Huffaker, A.; Pearce, G.; Veyrat, N.; Erb, M.; Turlings, T.C.; Sartor, R.; Shen, Z.; Briggs, S.P.; Vaughan, M.M.; Alborn, H.T.; et al. Plant elicitor peptides are conserved signals regulating direct and indirect antiherbivore defense. Proc. Natl. Acad. Sci. USA 2013, 110, 5707–5712. [Google Scholar] [CrossRef] [Green Version]
- Rostás, M.; Wölfling, M. Caterpillar footprints as host location kairomones for Cotesia marginiventris: Persistence and chemical nature. J. Chem. Ecol. 2009, 35, 20–27. [Google Scholar] [CrossRef]
- Lo Giudice, D.; Riedel, M.; Rostás, M. Host Sex Discrimination by an egg parasitoid on Brassica leaves. J. Chem. Ecol. 2011, 37, 622–628. [Google Scholar] [CrossRef]
- Meiners, T.; Peri, E. Chemical Ecology of Insect Parasitoids: Essential Elements for Developing Effective Biological Control Programmes. In Chemical Ecology of Insect Parasitoids, 1st ed.; Eric Wajnberg, E., Colazza, S., Eds.; Wiley Blackwell John Wiley & Sons, Ltd.: Oxford, UK, 2013; pp. 191–224. [Google Scholar] [CrossRef]
- Furlong, M.J.; Ang, G.C.K.; Silva, R.; Zalucki, M.P. Bringing Ecology Back: How Can the Chemistry of Indirect Plant Defenses Against Herbivory Be Manipulated to Improve Pest Management? Front. Plant Sci. 2018, 9, 1436. [Google Scholar] [CrossRef]
- Woodard, A.M.; Ervin, G.N.; Marsico, T.D. Host plant defense signaling in response to a coevolved herbivore combats introduced herbivore attack. Ecol. Evol. 2012, 2, 1056–1064. [Google Scholar] [CrossRef]
- Maurer, M.M.; Baker, M.A. Volatile profiling of cacti: A preliminary assessment of the taxonomic and evolutionary significance of volatile compounds in Cylindropuntia, Grusonia, Consolea, Opuntia, Quiabentia, and Tacinga. J. Plant Res. 2021, 134, 1095–1103. [Google Scholar] [CrossRef] [PubMed]
- Robert, C.A.M.; Frank, D.L.; Leach, K.A.; Turlings, T.C.J.; Hibbard, B.E.; Erb, M. Direct and indirect plant defenses are not suppressed by endosymbionts of a specialist root herbivore. J. Chem. Ecol. 2013, 39, 507–515. [Google Scholar] [CrossRef] [PubMed]
- Desumont, G.A.; Xu, H.; Turlings, T.C.J. Powdery mildew suppresses herbivore = induced plant volatiles and interferes with parasitoid attraction in Brassica rapa. Plant Cell Environ. 2016, 39, 1920–1927. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, Q.; Yang, T.; Jiang, J. Herbivore-induced rice grain volatiles affect attraction behavior of herbivore enemies. Interciencia 2016, 41, 319–324. [Google Scholar] [CrossRef]
- Castellanos Rojas, J.L. Evaluación de las Funciones Ecosistémicas de los Enemigos Naturales Mediadas por Volátiles de Plantas Inducidos por la Herbivoría en Cultivos de Café de la Provincia del Sumapaz. Bachelor’s Thesis, Escuela de Ciencias Agrícolas Pecuarios y del Medio Ambiente, Universidad Nacional Abierta y a Distancia, Bogotá, Colombia, 2019. Available online: https://repository.unad.edu.co/handle/10596/31830 (accessed on 25 August 2022).
- Musengi, K. The Biological Control of Cacti (Cactaceae: Opuntioideae) in South Africa: Basis of Host Selection In The ‘Stricta’Biotype of Dactylopius opuntiae (Cockerell)(Hemiptera: Dactylopiidae). Ph.D. Thesis, University of the Witwatersrand, Johannesburg, South Africa, 2018. Available online: https://core.ac.uk/display/188776458?utm_source=pdf&utm_medium=banner&utm_campaign=pdf-decoration-v1 (accessed on 18 October 2022).
- Varone, L.; Mengoni, G.C.; Faltlhauser, A.C.; Guala, M.E.; Wolaver, D.; Srivastava, M.; Hight, S.D. Effect of rearing Cactoblastis cactorum on an artificial diet on the behaviour of Apanteles opuntiarum. J. Appl. Entomol. 2020, 144, 278–286. [Google Scholar] [CrossRef]
- Vet, L.E.M.; Lewis, W.J.; Cardé, R.T. Parasitoid Foraging and Learning. In Chemical Ecology of Insects; Springer Science & Bussines Media: Boston, MA, USA, 1995; pp. 65–101. [Google Scholar] [CrossRef]
- Vet, L.E.M.; Hemerik, L.; Visser, M.E.; Wäckers, F.L. Patch-Use Strategies of Insect Parasitoids. In The Behavioural Ecology of Parasites; CABI Publishing: Wallingford, UK, 2002; pp. 39–64. [Google Scholar]
- Zito, P.; Sajeva, M.; Bruno, M.; Rosselli, S.; Maggio, A.; Senatore, F. Essential oils composition of two Sicilian cultivars of Opuntia ficus-indica (L.) Mill. (Cactaceae) fruits (prickly pear). Nat. Prod. Res. 2012, 27, 1305–1314. [Google Scholar] [CrossRef]
- Aguirre, M.B.; Diaz-Soltero, H.; Claps, L.E.; Bottero, A.S.; Triapitsyn, S.; Hasson, E.; Logarzo, G.A. Studies on the biology of Hypogeococcus pungens (sensu stricto) (Hemiptera: Pseudococcidae) in Argentina to aid the identification of the mealybug pest of cactaceae in Puerto Rico. J. Insect Sci. 2016, 16, 58. [Google Scholar] [CrossRef] [Green Version]
- Céspedes, C.L.; Salazar, J.R.; Martínez, M.; Aranda, E. Insect growth regulatory effects of some extracts and sterols from Myrtillocactus geometrizans (Cactaceae) against Spodoptera frugiperda and Tenebrio molitor. Phytochemistry 2005, 66, 2481–2493. [Google Scholar] [CrossRef]
- Callejas-Chavero, A.; Martínez-Hernández, D.G.; Flores-Martínez, A.; Moncada-Orellana, A.; Díaz-Quiñones, Y.; Fabián Vargas-Mendoza, C. Herbivory in Cacti: Fitness Effects of Two Herbivores, One Tending-Ant on Myrtillocactus geometrizans (Cactaceae). In Evolutionary Ecology of Plant-Herbivore Interaction; Chapter 6; Núñez-Farfán, J., L-Valverde, P., Eds.; Springer: Berlin/Heidelberg, Germany, 2021; pp. 109–134. [Google Scholar] [CrossRef]
- Godfray, H.C.J. Parasitoids: Behavioral and Evolutionary Ecology; Princeton University Press: Princeton, NJ, USA, 1994; Volume 67, 465p. [Google Scholar]
- Bernal, J.S. Biología, Ecología y Etología de Parasitoides. In Teoría y Aplicación del Control Biológico; Rodríguez-del-Bosque, L.A., Arredondo-Bernal, H.C., Eds.; Sociedad Mexicana de Control Biológico: Mexico City, Mexico, 2007; pp. 61–74. [Google Scholar]
- Pietrantuono, A.L.; Fernández-Arhex, V.; Jofré, N.; Corley, J.C. Food and Host Searching Decisions Made by Ibalia leucospoides (Hymenoptera: Ibaliidae), a Parasitoid of Sirex noctilio (Hymenoptera:Siricidae). J. Insect Behav. 2011, 25, 320–327. [Google Scholar] [CrossRef]
- Badii, M.H.; Hernández-Ortiz, E.; Flores, A.E.; Landeros, J. Prey stage preference and functional response of Euseius hibisci to Tetranychus urticae (Acari: Phytoseiidae, Tetranychidae). Exp. Appl. Acarol. 2004, 34, 263–273. [Google Scholar] [CrossRef]
- Badii, M.H.; Abreu, J.L. Control biológico una forma sustentable de control de plagas (Biological control a sustainable way of pest control). Daena Int. J. Good Conscienc. 2006, 1, 82–89. [Google Scholar]
- D’Alessandro, M.; Brunner, V.; von Mérey, G.; Turlings, T.C.J. Strong Attraction of the Parasitoid Cotesia marginiventris Towards Minor Volatile Compounds of Maize. J. Chem. Ecol. 2009, 35, 999–1008. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, J.; Wang, L.; Zhu, J.; Zhang, S.; Nandi, O.I.; Kang, L. Plants Attract Parasitic Wasps to Defend Themselves against Insect Pests by Releasing Hexenol. PLoS ONE 2007, 2, e852. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- War, A.R.; Paulraj, M.G.; Ahmad, T.; Buhroo, A.A.; Hussain, B.; Ignacimuthu, S.; Sharma, H.C. Mechanisms of plant defense against insect herbivores. Plant Signal. Behav. 2012, 7, 1306–1320. [Google Scholar] [CrossRef] [Green Version]
- Kaplan, I.; Carrillo, J.; Garvey, M.; Ode, P.J. Indirect plant–parasitoid interactions mediated by changes in herbivore physiology. Curr. Opin. Insect Sci. 2016, 14, 112–119. [Google Scholar] [CrossRef]
- Martínez-Hernández, D.G. Efecto de las Interacciones que Establecen los Herbívoros Toumeyella martinezi y Opuntiaspis philococcus Sobre la Calidad y Reproducción de Myrtillocactus geometrizans. Master’s Thesis, Escuela Nacional Ciencias Biológicas, Mexico City, Mexico, 2017; 103p. Available online: http://tesis.ipn.mx/handle/123456789/28371 (accessed on 9 October 2020).
- González-Villa, H. Efecto del parasitoidismo y mutualismo sobre la demografía de Toumeyella martinezae (Hemíptera: Coccidae) asociada a Myrtillocactus geometrizans (Cactaceae) en un matorral xerófilo de Huichapan, Hidalgo. Master´s Thesis, Escuela Nacional Ciencias Biológicas, Mexico City, Mexico, 2021; 61p. [Google Scholar]
- Martínez-Hernández, D.G. Efecto de Liometopum apiculatum (Hymenoptera: Formicidae) Sobre la Tasa de Parasitoidismo de Toumeyella martinezi (Hemiptera: Coccidae) Asociados a Myrtillocactus geometrizans (Cactaceae) en un Matorral Xerófilo de Huichapan, Hidalgo. Bachelor’s Thesis, Escuela Nacional Ciencias Biológicas, Mexico City, Mexico, 2015; 109p. [Google Scholar]
- Del Val, E.; Dirzo, R. Mirmecofilia: Las plantas con ejército propio. Interciencia 2004, 29, 673–679. [Google Scholar]
- González-Teuber, M.; Kaltenpoth, M.; Boland, W. Mutualistic ants as an indirect defence against leaf pathogens. New Phytol. 2014, 202, 640–650. [Google Scholar] [CrossRef] [Green Version]
- Ohm, J.R.; Miller, T.E. Balancing anti-herbivore benefits and anti-pollinator costs of defensive mutualists. Ecology 2014, 95, 2924–2935. [Google Scholar] [CrossRef] [Green Version]
- Maron, J.L.; Crone, E. Herbivory: Effects on plant abundance, distribution and population growth. Proc. R. Soc. B Biol. Sci. 2006, 273, 2575–2584. [Google Scholar] [CrossRef]
- Miller, G.; Schlauch, K.; Tam, R.; Cortes, D.; Torres, M.A.; Shulaev, V.; Mittler, R. The Plant NADPH Oxidase RBOHD Mediates Rapid Systemic Signaling in Response to Diverse Stimuli. Sci. Signal. 2009, 2, ra45. [Google Scholar] [CrossRef] [Green Version]
- Bashan, Y.; Toledo, G.; Holguin, G. Flat top decay syndrome of the giant cardon cactus (Pachycereus pringlei): Description and distribution in Baja California Sur, Mexico. Can. J. Bot. 1995, 73, 683–692. [Google Scholar] [CrossRef] [Green Version]
- Palafox-Luna, J.A.; Rodríguez-Leyva, E.; Lomeli-Flores, J.R.; Vigueras-Guzmán, A.L.; Vanegas-Rico, J.M. Ciclo de vida y fecundidad de Dactylopius opuntiae (Hemiptera: Dactylopiidae) en Opuntia ficus-indica (Caryophyllales: Cactaceae). Agrociencia 2018, 52, 103–114. Available online: https://www.scielo.org.mx/scielo.php?pid=S1405-31952018000100103&script=sci_arttext (accessed on 30 August 2020).
- Redmond, C.M.; Auga, J.; Gewa, B.; Segar, S.T.; Miller, S.E.; Molem, K.; Weiblen, G.D.; Butteril, P.T.; Maiyah, G.; Hood, A.S.C.; et al. High specialization and limited structural change in plant-herbivore networks along a successional chronosequence in tropical montane forest. Ecography 2019, 42, 162–172. [Google Scholar] [CrossRef] [Green Version]
- Bravo-Avilez, D.; Zavala-Hurtado, J.A.; Rendón-Aguilar, B. Damage in Cactaceae, their geographic distribution and new evidence. Bot. Sci. 2019, 97, 551–567. [Google Scholar] [CrossRef] [Green Version]
- Vranjic, J.A. Effects on Host Plant. In World Crop Pests; Ben-Dov, Y., Hodgson, C.J., Eds.; Elsevier: Amsterdam, The Netherlands, 1997; Volume 7, pp. 323–336. [Google Scholar]
- Rosenthal, J.P.; Kotanen, P.M. Terrestrial plant tolerance to herbivory. Trends Ecol. Evol. 1994, 9, 145–148. [Google Scholar] [CrossRef]
- Coley, P.D.; Barone, J.A. Herbivory and plant defenses in tropical forests. Annu. Rev. Ecol. Syst 1996, 27, 305–335. [Google Scholar] [CrossRef]
- Roy, B.A.; Gusewell, S.; Harte, J. Response of plant pathogens and herbivores to a warming experiment. Ecology 2004, 85, 2570–2581. [Google Scholar] [CrossRef] [Green Version]
- Hawkes, C.V.; Sullivan, J.J. The impact of herbivory on plants in different resource conditions: A meta-analysis. Ecology 2001, 82, 2045–2058. [Google Scholar] [CrossRef]
- Arias, S.; Gama, S.; Guzmán, L. Flora del Valle de Tehuacán-Cuicatlán. Fascículo 14. Cactaceae; Universidad Nacional Autónoma de México: Mexico City, Mexico, 1997. [Google Scholar]
- Hernández-López, D.; Vaillant, F.; Reynoso-Camacho, R.; Guzman-Maldonado, S.H. Myrtillocactus (Cactaceae): Botanical, agronomic, physicochemical and chemical characteristics of fruits. Fruits 2008, 63, 269–276. [Google Scholar] [CrossRef] [Green Version]
- Myartseva, S.N.; Ruíz-Cancino, E.; Coronado-Blanco, J.M.; Lomelí-Flores, J.R.; Hernández-De La Cruz, R.C. Parasitoids (Hymenoptera: Chalcidoidea) of Toumeyella scales (Hemiptera: Coccidae) in the New World, with description of a new species from Mexico. Fla. Entomol. 2016, 99, 781–784. [Google Scholar] [CrossRef]
- Stephenson, N.; Perroy, R.; Eiben, J.; Klasner, F. High resolution habitat suitability modelling for an endemic restricted-range Hawaiian insect (Nysius wekiuicola, Hemiptera: Lygaeidae). J. Insect Conserv. 2017, 21, 87–96. [Google Scholar] [CrossRef]
- Whitfield, J.B. Phylogeny and evolution of host-parasitoid interactions in Hymenoptera. Annu. Rev. Entomol. 1998, 43, 129–151. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Kumar, A.; Tripathi, C.P.M. Functional Response of Campoletis chloridae Uchida (Hymenoptera: Ichneumonidae), a Parasitoid of Heliothis armigera (Hübner) (Lepidoptera: Noctuidae) in an Enclosed Experimental System. Biol. Agric. Hortic. 1994, 10, 287–295. [Google Scholar] [CrossRef]
- Bruce, T.J.A.; Wadhams, L.J.; Woodcock, C.M. Insect host location: A volatile situation. Trends Plant Sci. 2005, 10, 269–274. [Google Scholar] [CrossRef]
- Randlkofer, B.; Obermaier, E.; Hilker, M.; Meiners, T. Vegetation complexity—The influence of plant species diversity and plant structures on plant chemical complexity and arthropods. Basic Appl. Ecol. 2010, 11, 383–395. [Google Scholar] [CrossRef]
- Wäschke, N.; Meiners, T.; Rostás, M. Foraging Strategies of Parasitoids in Complex Chemical Environments. In Chemical Ecology of Insect Parasitoids, 1st ed.; John Wiley & Sons: Hoboken, NJ, USA, 2013. [Google Scholar]
- Wajnberg, E. Time allocation strategies in insect parasitoids: From ultimate predictions to proximate behavioral mechanisms. Behav. Ecol. Sociobiol. 2006, 60, 589–611. [Google Scholar] [CrossRef]
- Bezemer, M.; Harvey, T.; Kamp, J.; Wagenaar, A.; Gols, R.; Kostenko, R.; Soler, R. Behaviour of male and female parasitoids in the field: Influence of patch size, host density, and habitat complexity. Ecol. Entomol. 2010, 35, 341–351. [Google Scholar] [CrossRef]
- Heimpel, G.E.; Casas, J. Parasitoid Foraging and Oviposition Behavior in the Field. In Behavioral Ecology of Insect Parasitoids: From Theoretical Approaches to Field Applications; Blackwell Publishing Ltd.: Hoboken, NJ, USA, 2008; pp. 52–70. [Google Scholar] [CrossRef]
- Waage, J.K. Aggregation in field parasitoid populations: Foraging time allocation by a population of Diadegma (Hymenoptera, Ichneumonidae). Ecol. Entomol. 1983, 8, 447–453. [Google Scholar] [CrossRef]
- Connor, E.F.; Cargain, M.J. Density-related foraging behaviour in Closterocerus tricinctus, a parasitoid of the leaf-mining moth, Cameraria hamadryadella. Ecol. Entomol. 1994, 19, 327–334. [Google Scholar] [CrossRef]
- Weisser, W.W.; Houston, A.I. Host Discrimination in Parasitic Wasps: When is it Advantageous? Funct. Ecol. 1993, 7, 27–39. [Google Scholar] [CrossRef]
- Miller, T.E.X. Does having multiple partners weaken the benefits of facultative mutualism? A test with cacti and cactus-tending ants. Oikos 2007, 116, 500–512. [Google Scholar] [CrossRef] [Green Version]
- Donald, M.L.; Miller, T.E.X. Does ant–plant mutualism have spillover effects on the non-partner ant community? Ecol. Evol. 2021, 12, e8524. [Google Scholar] [CrossRef] [PubMed]
- Savage, M.A.; Rudgers, A.J. Non-additive benefit or cost? Disentangling the indirect effects that occur when plants bearing extrafloral nectaries and honeydew-producing insects share exotic ant mutualists. Ann. Bot. 2013, 111, 1295–1307. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nelson, S.A.; Mooney, K.A. The Evolution and Ecology of Interactions Between Ants and Honeydew-Producing Hemipteran Insects. Annu. Rev. Ecol. Evol. Syst. 2022, 53, 379–402. [Google Scholar] [CrossRef]
- Styrsky, J.D.; Eubanks, M.D. Ecological consequences of interactions between ants and honeydew-producing insects. Proc. R. Soc. B Biol. Sci. 2007, 274, 151–164. [Google Scholar] [CrossRef] [Green Version]
- Nakabayashi, Y.; Mochioka, Y.; Tokuda, M.; Ohshima, I. Mutualistic ants and parasitoid communities associated with a facultative myrmecophilous lycaenid, Arhopala japonica, and the effects of ant attendance on the avoidance of parasitism. Entomol. Sci. 2020, 23, 233–244. [Google Scholar] [CrossRef]
- Kaneko, S. Aphid-attending ants increase the number of emerging adults of the aphid’s primary parasitoid and hyperparasitoids by repelling intraguild predators. Entomol. Sci. 2002, 5, 131–146. Available online: https://cir.nii.ac.jp/crid/1521136280240663040 (accessed on 16 October 2022).
- Kapranas, A.; Tena, A. Encyrtid Parasitoids of Soft Scale Insects: Biology, Behavior, and Their Use in Biological Control. Annu. Rev. Entomol. 2015, 60, 195–211. [Google Scholar] [CrossRef]
- Ramos, A.S.d.J.C.; Lemos, R.N.S.d.; Costa, V.A.; Peronti, A.L.B.G.; Silva, E.A.d.; Mondego, J.M.; Moreira, A.A. Hymenopteran Parasitoids Associated with Scale Insects (Hemiptera: Coccoidea) in Tropical Fruit Trees in the Eastern Amazon. Brazil. Fla. Entomol. 2018, 101, 273–278. [Google Scholar] [CrossRef] [Green Version]
- Quesada, C.R.; Scharf, M.E.; Sadof, C.S. Excretion of non-metabolized insecticides in honeydew of striped pine scale. Chemosphere 2020, 249, 126167. [Google Scholar] [CrossRef]
- Ben-Dov, Y.; Hogson, C.J. (Eds.) World Crop Pests. Soft Scale Insects. Their Biology, Natural Enemies, and Control; Elsevier: Amsterdam, The Netherlands, 1997; Volume 7, pp. 3–452. [Google Scholar]
- Malumphy, C.P. Morphology and Anatomy of Honeydew Eliminating Organs. In World Crop Pests. Soft Scale Insects. Their Biology, Natural Enemies and Control; Ben-Dov, Y., Hogson, C.J., Eds.; Elsevier: Amsterdam, The Netherlands, 1997; Volume 7, pp. 269–274. [Google Scholar] [CrossRef]
- Ide, T.; Suzuki, N.; Katayama, N. The use of honeydew in foraging for aphids by larvae of the ladybird beetle, Coccinella septempunctata L. (Coleoptera: Coccinellidae). Ecol. Entomol. 2007, 32, 455–460. [Google Scholar] [CrossRef]
- Dhami, M.K.; Gardner-Gee, R.; Van Houtte, J. Species-Specific Chemical Signatures in Scale Insect Honeydew. J. Chem. Ecol. 2011, 37, 1231–1241. [Google Scholar] [CrossRef] [PubMed]
- Golan, K.; Najda, A. Differences in the sugar composition of the honeydew of polyphagous brown soft scales Coccus hesperidum (Hemiptera: Sternorrhyncha: Coccoidea) feeding on various host plants. Eur. J. Entomol. 2011, 108, 705–709. [Google Scholar] [CrossRef]
- Islas-Estrada, S.A. Ciclo de vida de Opuntiaspis philococcus (Hemiptera: Diaspididae) e identificación de sus enemigos naturales, asociados a Myrtillocactus geometrizans (Cactaceae) en Hidalgo. Bachelor’s Thesis, Escuela Nacional Ciencias Biológicas, Mexico City, Mexico, 2021. [Google Scholar]
- IBM Corp. IBM SPSS Statistics for Windows, Version 25.0.; IBM Corp.: Armonk, NY, USA, 2017. [Google Scholar]
- Crawley, M. Generalized Linear Models. In The R Book; John Wiley & Sons: Hoboken, NJ, USA, 2012; pp. 557–578. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Callejas-Chavero, A.; Martínez-Hernández, D.G.; Vargas-Mendoza, C.F.; Flores-Martínez, A. Herbivory in Myrtillocactus geometrizans (Cactaceae): Do Parasitoids Provide Indirect Defense or a Direct Advantage? Plants 2023, 12, 47. https://doi.org/10.3390/plants12010047
Callejas-Chavero A, Martínez-Hernández DG, Vargas-Mendoza CF, Flores-Martínez A. Herbivory in Myrtillocactus geometrizans (Cactaceae): Do Parasitoids Provide Indirect Defense or a Direct Advantage? Plants. 2023; 12(1):47. https://doi.org/10.3390/plants12010047
Chicago/Turabian StyleCallejas-Chavero, Alicia, Diana Guadalupe Martínez-Hernández, Carlos Fabian Vargas-Mendoza, and Arturo Flores-Martínez. 2023. "Herbivory in Myrtillocactus geometrizans (Cactaceae): Do Parasitoids Provide Indirect Defense or a Direct Advantage?" Plants 12, no. 1: 47. https://doi.org/10.3390/plants12010047





