Plastic Covers and Potassium Pre-Harvest Sprays and Their Influence on Antioxidant Properties, Phenolic Profile, and Organic Acids Composition of Sweet Cherry Fruits Cultivated in Southern Chile
Abstract
1. Introduction
2. Results
2.1. Fruit Technological Quality at Harvest
2.2. Total Antioxidant Activity and Total Phenolic Content
2.3. Phenolic Compound Composition in Fruits
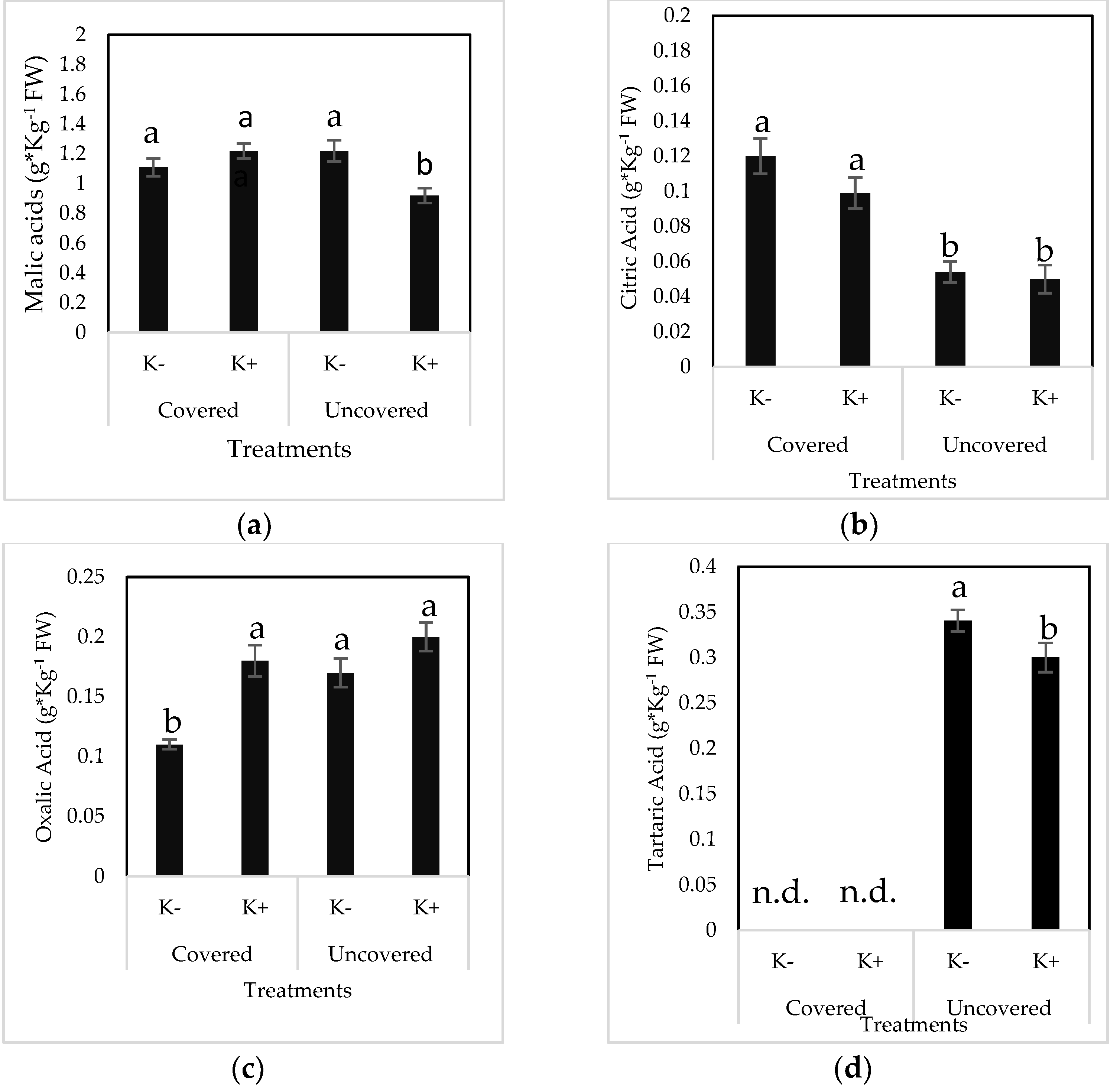
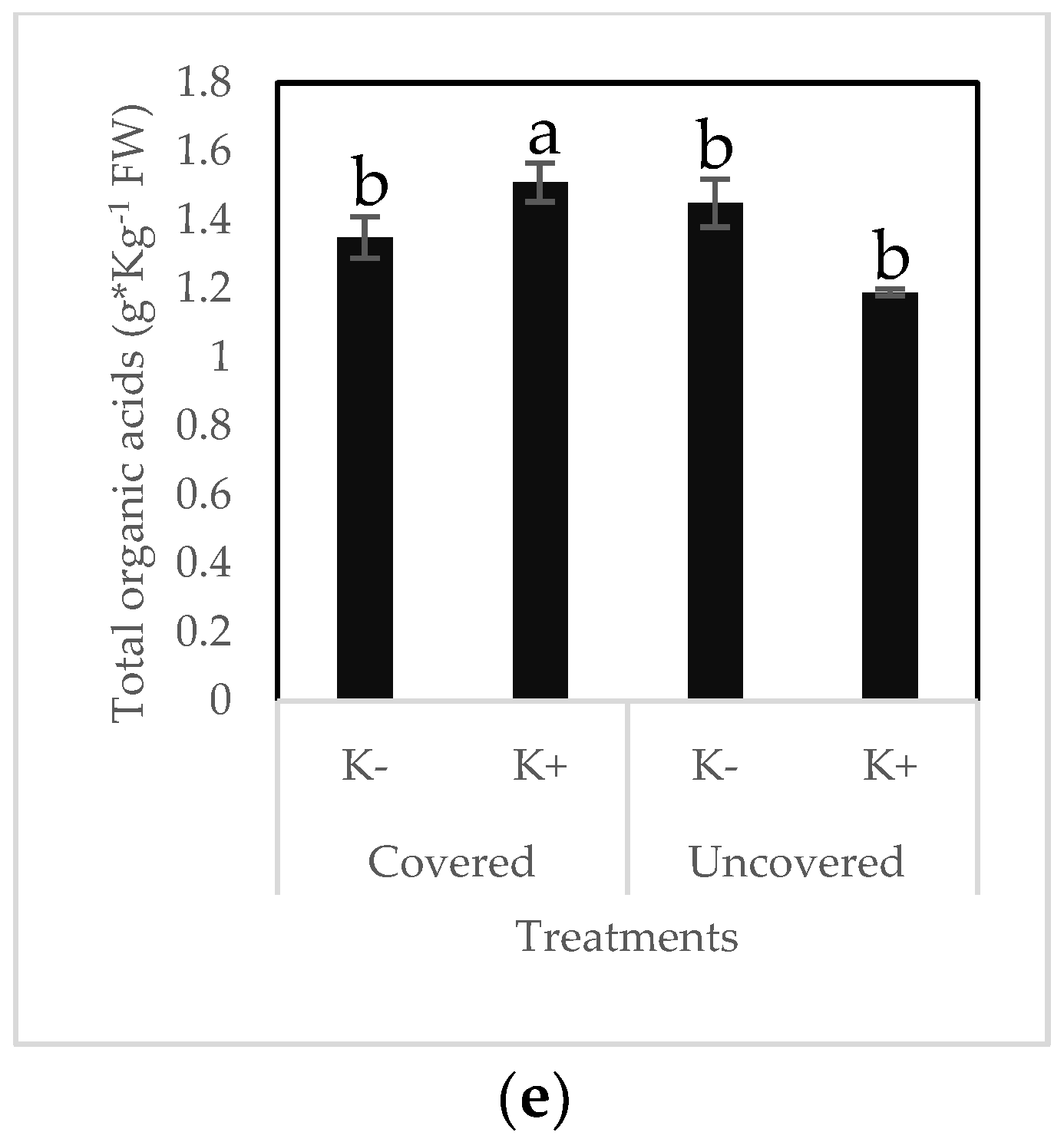
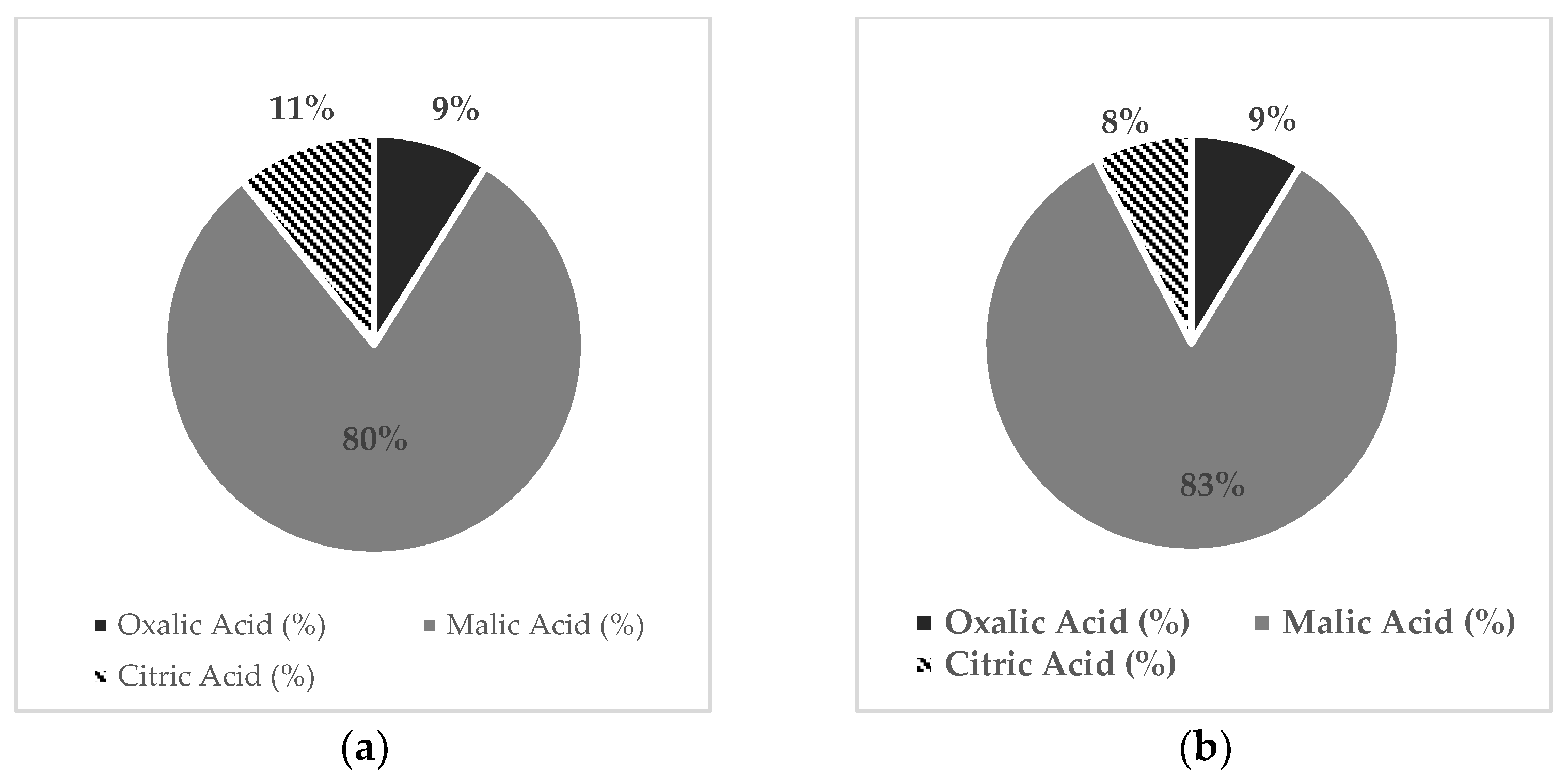
2.4. Organic Acid Composition in Fruits
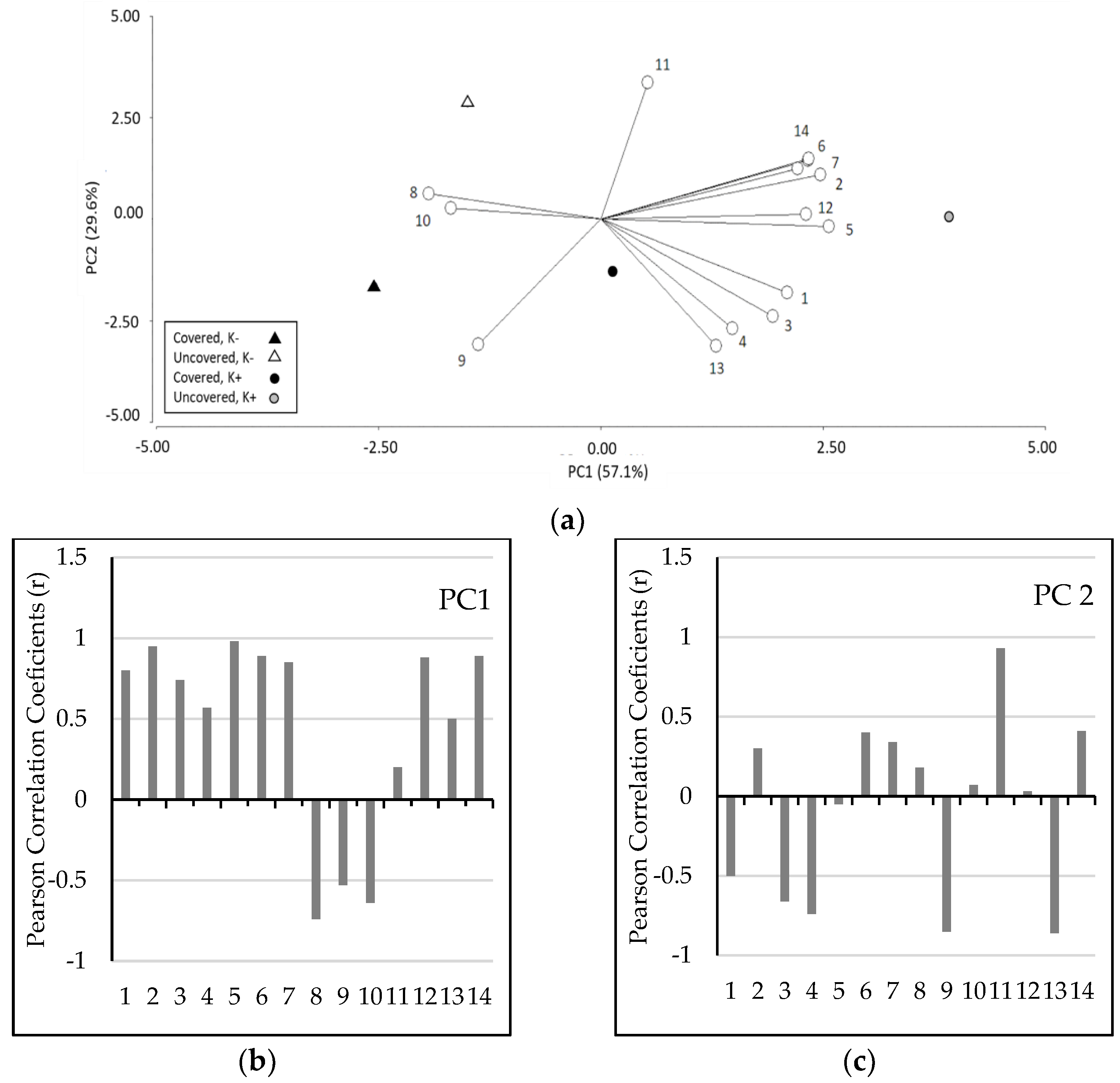
2.5. PCA Analysis
3. Discussion
4. Materials and Methods
4.1. Plant Material, Treatments, and Experimental Design
4.2. Treatments
4.3. Experimental Design
4.4. Evaluation of Fruit Quality at Harvest
4.5. Antioxidant Activity Determinations in Fruits
4.6. Analysis of Phenolic Composition in Fruits
4.7. Measuring Total Phenolic Compounds in Fruits
4.8. Analysis of Low Molecular Weight Organic Acid Composition in Fruits
4.9. Statistical Data Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAOSTAT. Agriculture Data. Available online: https://www.fao.org/faostat/en/#data/QCL (accessed on 11 October 2022).
- PRO CHILE. Estadísticas de Exportación. Available online: https://www.asoex.cl/estadisticas-de-exportacion.html (accessed on 11 October 2022).
- ODEPA-CIREN. Catastro Frutícola Región de O’Higgins. 2021. Available online: https://www.ciren.cl/proyectos/catastros/catastro-fruticola/ (accessed on 25 October 2022).
- Murakami, Y.; Hernández, R. The Impacts of China on Economic Growth: Evidence for Brazil, Chile, and Peru. J. Post Keynes. Econ. 2018, 41, 430–454. [Google Scholar] [CrossRef]
- Blanke, M.; Yuri, A. Chile: Record Exports of Fruit Grown by the Andes. Erwerbsobstbau 2020, 62, 175–180. [Google Scholar] [CrossRef]
- Santibáñez, Q.F. Atlas Agroclimático de Chile. Estado Actual y Tendencias del Clima. Tomo IV: Regiones del Biobío y de la Araucanía, 1st ed.; Facultad de Ciencias Agronómicas, Universidad de Chile, FIA: Santiago, Chile, 2017; p. 74. [Google Scholar]
- Roco, L.; Engler, A.; Bravo-Ureta, B.; Jara-Rojas, R. Farm Level Adaptation Decisions to Face Climatic Change and Variability: Evidence from Central Chile. Environ. Sci. Pol. 2014, 44, 86–96. [Google Scholar] [CrossRef]
- Red Agrícola Nuevas Variedades de Cerezo: Tempranas y Tardías, para Escapar del Vendaval Productivo. Available online: https://www.redagricola.com/cl/nuevas-variedades-de-cerezo-tempranas-y-tardias-para-escapar-del-vendaval-productivo/ (accessed on 11 October 2022).
- Stojanović, M.; Milatović, D.; Kulina, M.; Džanović, Z.A. Susceptibility of Sweet Cherry Cultivars to Rain Induced Fruit Cracking in the Region of Sarajevo. Agro-Knowl. J. 2013, 14, 179–184. [Google Scholar] [CrossRef][Green Version]
- Blanco, V.; Zoffoli, J.P.; Ayala, M. High Tunnel Cultivation of Sweet Cherry (Prunus avium L.): Physiological and Production Variables. Sci. Hortic. 2019, 251, 108–117. [Google Scholar] [CrossRef]
- Zoffoli, J.P.; Ayala, M. Año Nuevo Chino, Mejoramiento Genético y la Búsqueda de la Cereza Perfecta. Available online: http://www.uc.cl/noticias/ano-nuevo-chino-mejoramiento-genetico-y-la-busqueda-de-la-cereza-perfecta/ (accessed on 25 October 2022).
- Roversi, A.; Ughini, V. Influence of Weather Conditions of the Flowering Period on Sweet Cherry Fruit Set. Available online: http://www.actahort.org/books/410/410_69.htm (accessed on 13 October 2022).
- Quero-Garcia, J.; Lezzoni, A.; Paulawska, J.; Lang, G. Cherries: Botany, Production and Uses; McCann, E., Ed.; Cabi: Wallingford, UK, 2017; Volume 4. [Google Scholar]
- Kviklys, D.; Viškelis, J.; Liaudanskas, M.; Janulis, V.; Laužikė, K.; Samuolienė, G.; Uselis, N.; Lanauskas, J. Apple Fruit Growth and Quality Depend on the Position in Tree Canopy. Plants 2022, 11, 196. [Google Scholar] [CrossRef]
- Palmer, J.W.; Harker, F.R.; Tustin, D.S.; Johnston, J. Fruit Dry Matter Concentration: A New Quality Metric for Apples. J. Sci. Food. Agric. 2010, 90, 2586–2594. [Google Scholar] [CrossRef]
- Lester, G.E.; Jifon, J.L.; Makus, D.J. Supplemental Foliar Potassium Applications with or without a Surfactant Can Enhance Netted Muskmelon Quality. HortScience 2006, 41, 741–744. [Google Scholar] [CrossRef]
- Lester, G.E.; Jifon, J.L.; Rogers, G. Supplemental Foliar Potassium Applications during Muskmelon Fruit Development Can Improve Fruit Quality, Ascorbic Acid, and Beta-Carotene Contents. HorstScience 2005, 130, 649–653. [Google Scholar] [CrossRef]
- Kanai, S.; Ohkura, K.; Adu-Gyamfi, J.J.; Mohapatra, P.K.; Nguyen, N.T.; Saneoka, H.; Fujita, K. Depression of Sink Activity Precedes the Inhibition of Biomass Production in Tomato Plants Subjected to Potassium Deficiency Stress. J. Exp. Bot. 2007, 58, 2917–2928. [Google Scholar] [CrossRef]
- Yener, H.; Altuntaş, Ö. Effects of Potassium Fertilization on Leaf Nutrient Content and Quality Attributes of Sweet Cherry Fruits (Prunus avium L.). J. Plant Nutr. 2021, 44, 946–957. [Google Scholar] [CrossRef]
- Bustamante, M.; Muñoz, A.; Romero, I.; Osorio, P.; Mánquez, S.; Arriola, R.; Reyes-Díaz, M.; Ribera-Fonseca, A. Impact of Potassium Pre-Harvest Applications on Fruit Quality and Condition of Sweet Cherry (Prunus avium L.) Cultivated under Plastic Covers in Southern Chile Orchards. Plants 2021, 10, 2778. [Google Scholar] [CrossRef] [PubMed]
- Crisosto, C.H.; Crisosto, G.M.; Metheney, P. Consumer Acceptance of ‘Brooks’ and ‘Bing’ Cherries Is Mainly Dependent on Fruit SSC and Visual Skin Color. Postharvest Biol. Technol. 2003, 28, 159–167. [Google Scholar] [CrossRef]
- Mahmood, T.; Anwar, F.; Abbas, M.; Boyce, M.C.; Saari, N. Compositional Variation in Sugars and Organic Acids at Different Maturity Stages in Selected Small Fruits from Pakistan. Int. J. Mol. Sci. 2012, 13, 1380–1392. [Google Scholar] [CrossRef] [PubMed]
- Serrano, M.; Guillén, F.; Martínez-Romero, D.; Castillo, S.; Valero, D. Chemical Constituents and Antioxidant Activity of Sweet Cherry at Different Ripening Stages. J. Agric. Food Chem. 2005, 53, 2741–2745. [Google Scholar] [CrossRef]
- Usenik, V.; Fabčič, J.; Štampar, F. Sugars, Organic Acids, Phenolic Composition and Antioxidant Activity of Sweet Cherry (Prunus avium L.). J. Agric. Food Chem. 2008, 107, 185–192. [Google Scholar] [CrossRef]
- Winkler, A.; Ossenbrink, M.; Knoche, M. Malic acid promotes cracking of sweet cherry fruit. HorstScience 2015, 140, 280–287. [Google Scholar] [CrossRef]
- Faienza, M.F.; Corbo, F.; Carocci, A.; Catalano, A.; Clodoveo, M.L.; Grano, M.; Wang, D.Q.-H.; D’Amato, G.; Muraglia, M.; Franchini, C.; et al. Novel Insights in Health-Promoting Properties of Sweet Cherries. J. Funct. Foods 2020, 69, 103945. [Google Scholar] [CrossRef] [PubMed]
- Correia, S.; Schouten, R.; Silva, A.P.; Gonçalves, B. Factors Affecting Quality and Health Promoting Compounds during Growth and Postharvest Life of Sweet Cherry (Prunus avium L.). Fronts. Plant. Sci. 2017, 8, 2166. [Google Scholar] [CrossRef]
- Gao, L.; Mazza, G. Characterization, Quantitation, and Distribution of Anthocyanins and Colorless Phenolics in Sweet Cherries. Available online: https://pubs.acs.org/doi/pdf/10.1021/jf00050a015 (accessed on 13 October 2022).
- Gonçalves, B.; Landbo, A.-K.; Knudsen, D.; Silva, A.P.; Moutinho-Pereira, J.; Rosa, E.; Meyer, A.S. Effect of Ripeness and Postharvest Storage on the Phenolic Profiles of Cherries (Prunus avium L.). J. Agric. Food Chem. 2004, 52, 523–530. [Google Scholar] [CrossRef] [PubMed]
- Hu, T.; Subbiah, V.; Wu, H.; Amrit, B.K.; Rauf, A.; Alhumaydhi, F.A.; Suleria, H.A.R. Determination and Characterization of Phenolic Compounds from Australia-Grown Sweet Cherries (Prunus avium L.) and Their Potential Antioxidant Properties. ACS Omega 2021, 6, 34687–34699. [Google Scholar] [CrossRef] [PubMed]
- Serra, A.T.; Duarte, R.O.; Bronze, M.R.; Duarte, C.M.M. Identification of Bioactive Response in Traditional Cherries from Portugal. Food Chem. 2011, 125, 318–325. [Google Scholar] [CrossRef]
- Kelebek, H.; Selli, S. Evaluation of Chemical Constituents and Antioxidant Activity of Sweet Cherry (Prunus Avium L.) Cultivars. Int. J. Food Sci. Technol. 2011, 46, 2530–2537. [Google Scholar] [CrossRef]
- Solhjoo, S.; Gharaghani, A.; Fallahi, E. Calcium and Potassium Foliar Sprays Affect Fruit Skin Color, Quality Attributes, and Mineral Nutrient Concentrations of ‘Red Delicious’ Apples. Int. J. Fruit Sci. 2017, 17, 358–373. [Google Scholar] [CrossRef]
- Chockchaisawasdee, S.; Golding, J.B.; Vuong, Q.V.; Papoutsis, K.; Stathopoulos, C.E. Sweet Cherry: Composition, Postharvest Preservation, Processing and Trends for Its Future Use. Trends Food Sci. Technol. 2016, 55, 72–83. [Google Scholar] [CrossRef]
- Moure, A.; Cruz, J.M.; Franco, D.; Domínguez, J.M.; Sineiro, J.; Domínguez, H.; José Núñez, M.; Parajó, J.C. Natural Antioxidants from Residual Sources. Food. Chem. 2001, 72, 145–171. [Google Scholar] [CrossRef]
- Rios, J.C.; Robledo, F.; Schreiber, L.; Zeisler, V.; Lang, E.; Carrasco, B.; Silva, H. Association between the Concentration of N-Alkanes and Tolerance to Cracking in Commercial Varieties of Sweet Cherry Fruits. Sci. Hort. 2015, 197, 57–65. [Google Scholar] [CrossRef]
- Blanco, V.; Zoffoli, J.P.; Ayala, M. Influence of High Tunnel Microclimate on Fruit Quality and Calcium Concentration in ‘Santina’ Sweet Cherries in a Mediterranean Climate. Agronomy 2021, 11, 1186. [Google Scholar] [CrossRef]
- Dong, S.; Cheng, L.; Scagel, C.; Fuchigami, L. Timing of Urea Application Affects Leaf and Root N Uptake in Young Fuji/M.9 Apple Trees. J. Hort. Sci. Biotechnol. 2005, 80, 116–120. [Google Scholar] [CrossRef]
- Al-Obeed, R.S.; Ahmed, M.A.-A.; Kassem, H.A.; Al-Saif, A.M. Improvement of “Kinnow” Mandarin Fruit Productivity and Quality by Urea, Boron and Zinc Foliar Spray. J. Plant Nutr. 2018, 41, 609–618. [Google Scholar] [CrossRef]
- Marschner, H. Marschner’s Mineral Nutrition of Higher Plants—Google Libros. Available online: https://books.google.cl/books?hl=es&lr=&id=yqKV3USG41cC&oi=fnd&pg=PP1&dq=Marchner,+2011&ots=Vc8IZ6wZCk&sig=PzikM8ntYtiJ_Fk6K0lT7Vg7AeQ#v=onepage&q=Marchner%2C%202011&f=false (accessed on 13 October 2022).
- Grandi, M.; Lugli, S.; Piccinini, L.; Correale, R.; Costa, G.; Etiopi, C.; Monari, W. Effectiveness of New Rain-Protection Systems on Cracking, Ripening Date and Fruit Quality of Sweet Cherry Cultivars. Acta Hortic. 2017, 1161, 213–220. [Google Scholar] [CrossRef]
- Hodges, D.M.; Lester, G.E.; Munro, K.; Toivonen, P. Oxidative Stress: Importance for Postharvest Quality. HortScience 2004, 39, 924–929. [Google Scholar] [CrossRef]
- McCune, L.M.; Kubota, C.; Stendell-Hollis, N.R.; Thomson, C.A. Cherries and Health: A Review. Crit. Rev. Food. Sci. Nutr. 2010, 51, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Cao, G.; Prior, R.L. Total Antioxidant Capacity of Fruits. J. Agric. Food Chem. 1996, 44, 701–705. [Google Scholar] [CrossRef]
- Kim, D.-O.; Jeong, S.W.; Lee, C.Y. Antioxidant Capacity of Phenolic Phytochemicals from Various Cultivars of Plums. Food Chem. 2003, 81, 321–326. [Google Scholar] [CrossRef]
- Pande, G.; Akoh, C.C. Organic Acids, Antioxidant Capacity, Phenolic Content and Lipid Characterisation of Georgia-Grown Underutilized Fruit Crops. Food Chem. 2010, 120, 1067–1075. [Google Scholar] [CrossRef]
- González-Gómez, D.; Lozano, M.; Fernández-León, M.F.; Bernalte, M.J.; Ayuso, M.C.; Rodríguez, A.B. Sweet Cherry Phytochemicals: Identification and Characterization by HPLC-DAD/ESI-MS in Six Sweet-Cherry Cultivars Grown in Valle Del Jerte (Spain). Int. J. Food Comp. Anal. 2010, 23, 533–539. [Google Scholar] [CrossRef]
- Pissard, A.; Lateur, M.; Baeten, V.; Magein, H.; Dupont, P.; Tabart, J.; Pincemail, J.; Kevers, C. Determination of Total Phenolic Compound Content and Antioxidant Activity in Cherry Species and Cultivars. J. Berry Res. 2016, 6, 81–91. [Google Scholar] [CrossRef]
- Martini, S.; Conte, A.; Tagliazucchi, D. Phenolic Compounds Profile and Antioxidant Properties of Six Sweet Cherry (Prunus avium L.) Cultivars. Int. Food. Res. 2017, 97, 15–26. [Google Scholar] [CrossRef]
- Acero, N.; Gradillas, A.; Beltran, M.; García, A.; Muñoz Mingarro, D. Comparison of Phenolic Compounds Profile and Antioxidant Properties of Different Sweet Cherry (Prunus avium L.) Varieties. Food Chem. 2019, 279, 260–271. [Google Scholar] [CrossRef]
- Gonçalves, A.C.; Bento, C.; Jesus, F.; Alves, G.; Silva, L.R. Chapter 2—Sweet Cherry Phenolic Compounds: Identification, Characterization, and Health Benefits. In Studies in Natural Products Chemistry; Atta-ur-Rahman, Ed.; Elsevier: Amsterdam, The Netherlands, 2018; Volume 59, pp. 31–78. [Google Scholar]
- Hayaloglu, A.A.; Demir, N. Phenolic Compounds, Volatiles, and Sensory Characteristics of Twelve Sweet Cherry (Prunus avium L.) Cultivars Grown in Turkey. J. Food Sci. 2016, 81, C7–C18. [Google Scholar] [CrossRef] [PubMed]
- Ferretti, G.; Bacchetti, T.; Belleggia, A.; Neri, D. Cherry Antioxidants: From Farm to Table. Molecules 2010, 15, 6993–7005. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.-O.; Heo, H.J.; Kim, Y.J.; Yang, H.S.; Lee, C.Y. Sweet and Sour Cherry Phenolics and Their Protective Effects on Neuronal Cells. J. Agric. Food Chem. 2005, 53, 9921–9927. [Google Scholar] [CrossRef] [PubMed]
- Ballistreri, G.; Continella, A.; Gentile, A.; Amenta, M.; Fabroni, S.; Rapisarda, P. Fruit Quality and Bioactive Compounds Relevant to Human Health of Sweet Cherry (Prunus avium L.) Cultivars Grown in Italy. Food Chem. 2013, 140, 630–638. [Google Scholar] [CrossRef]
- Ribera, A.E.; Reyes-Diaz, M.; Alberdi, M.; Zuñiga, G.E.; Mora, M.L. Antioxidant Compounds in Skin and Pulp of Fruits Change among Genotypes and Maturity Stages in Highbush Blueberry (Vaccinium corymbosum L.) Grown in Southern Chile. J. Soil Sci. Plant Nutr. 2010, 10, 509–536. [Google Scholar] [CrossRef]
- Schmitz-Eiberger, M.A.; Blanke, M.M. Bioactive Components in Forced Sweet Cherry Fruit (Prunus avium L.), Antioxidative Capacity and Allergenic Potential as Dependent on Cultivation under Cover. LWT–Int. J. Food Sci. Technol. 2012, 46, 388–392. [Google Scholar] [CrossRef]
- Lang, G.A. Growing Sweet Cherries under Plastic Covers and Tunnels: Physiological Aspects and Practical Considerations. Acta Hortic. 2014, 1020, 303–312. [Google Scholar] [CrossRef]
- Bharti, A.K.; Khurana, J.P. Mutants of Arabidopsis as Tools to Understand the Regulation of Phenylpropanoid Pathway and UVB Protection Mechanisms. J. Photochem. Photobiol. 1997, 65, 765–776. [Google Scholar] [CrossRef]
- Nguyen, P.M.; Kwee, E.M.; Niemeyer, E.D. Potassium Rate Alters the Antioxidant Capacity and Phenolic Concentration of Basil (Ocimum basilicum L.) Leaves. Food Chem. 2010, 123, 1235–1241. [Google Scholar] [CrossRef]
- Gaaliche, B.; Ladhari, A.; Zarrelli, A.; Ben Mimoun, M. Impact of Foliar Potassium Fertilization on Biochemical Composition and Antioxidant Activity of Fig (Ficus carica L.). Sci. Hort. 2019, 253, 111–119. [Google Scholar] [CrossRef]
- Zareei, E.; Javadi, T.; Aryal, R. Biochemical Composition and Antioxidant Activity Affected by Spraying Potassium Sulfate in Black Grape (Vitis vinifera L. Cv. Rasha). J. Sci. Food Agric. 2018, 98, 5632–5638. [Google Scholar] [CrossRef] [PubMed]
- Araujo, L.; Bispo, W.S.; Rios, V.S.; Fernandes, S.A.; Rodrigues, F.A. Induction of the phenylpropanoid pathway by acibenzolar-s-methyl and potassium phosphite increases mango resistance to Ceratocystis fimbriata infection. Plant Dis. 2015, 99, 447–459. [Google Scholar] [CrossRef]
- Wu, L.; Li, P.; Jia, H.; Phillip, F.O.; Bao, X.; Zhao, F.; Zhao, B.; Feng, J.; Yu, K. The Effect of Foliar Application of K2SO4 or KH2PO4 on Skin Color of the ‘Kyoho’Grape. Agronomy 2021, 11, 2361. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, X.; Zhong, F.; Tian, R.; Zhang, K.; Zhang, X.; Li, T. Comparative Study of Phenolic Compounds and Antioxidant Activity in Different Species of Cherries. J. Food Sci. 2011, 76, C633–C638. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Jiang, N.; Wang, Y.; Jiang, D.; Feng, X. Characterization of Phenolic Compounds from Early and Late Ripening Sweet Cherries and Their Antioxidant and Antifungal Activities. J. Agric. Food Chem. 2017, 65, 5413–5420. [Google Scholar] [CrossRef] [PubMed]
- Picariello, G.; De Vito, V.; Ferranti, P.; Paolucci, M.; Volpe, M.G. Species- and Cultivar-Dependent Traits of Prunus avium and Prunus cerasus Polyphenols. J. Food Compos. Anal. 2016, 45, 50–57. [Google Scholar] [CrossRef]
- Pacifico, S.; Di Maro, A.; Petriccione, M.; Galasso, S.; Piccolella, S.; Di Giuseppe, A.M.A.; Scortichini, M.; Monaco, P. Chemical Composition, Nutritional Value and Antioxidant Properties of Autochthonous Prunus avium Cultivars from Campania Region. Food Res. Int. 2014, 64, 188–199. [Google Scholar] [CrossRef] [PubMed]
- Ağlar, E.; Saraçoğlu, O. Role of the Foliar Fertilization Treatments on Quality Attributes of Sweet Cherry Fruits (Prunus avium L.). Akad. Ziraat Derg. 2018, 7, 131–136. [Google Scholar] [CrossRef]
- Facteau, T.J.; Chestnut, N.E.; Rowe, K.E. Tree, Fruit Size and Yield of ‘Bing’ Sweet Cherry as Influenced by Rootstock, Replant Area, and Training System. Sci. Hort. 1996, 67, 13–26. [Google Scholar] [CrossRef]
- Jakobek, L.; Šeruga, M.; Voća, S.; Šindrak, Z.; Dobričević, N. Flavonol and Phenolic Acid Composition of Sweet Cherries (Cv. Lapins) Produced on Six Different Vegetative Rootstocks. Sci. Hort. 2009, 123, 23–28. [Google Scholar] [CrossRef]
- Mozetič, B.; Trebše, P.; Hribar, J. Determination and Quantitation of Anthocyanins and Hydroxycinnamic Acids in Different Cultivars of Sweet Cherries (Prunus avium L.) from Nova Gorica Region (Slovenia). Food Technol. Biotechnol. 2002, 40, 207–212. [Google Scholar]
- Mozetič, B.; Trebše, P.; Simčič, M.; Hribar, J. Changes of Anthocyanins and Hydroxycinnamic Acids Affecting the Skin Colour during Maturation of Sweet Cherries (Prunus avium L.). LWT Int. J. Food Sci. Technol. 2004, 37, 123–128. [Google Scholar] [CrossRef]
- Serradilla, M.J.; Lozano, M.; Bernalte, M.J.; Ayuso, M.C.; López-Corrales, M.; González-Gómez, D. Physicochemical and Bioactive Properties Evolution during Ripening of ‘Ambrunés’ Sweet Cherry Cultivar. LWT Int. J. Food Sci. Technol. 2011, 44, 199–205. [Google Scholar] [CrossRef]
- Chaovanalikit, A.; Wrolstad, R.E. Total Anthocyanins and Total Phenolics of Fresh and Processed Cherries and Their Antioxidant Properties. J. Food Sci. 2004, 69, FCT67–FCT72. [Google Scholar] [CrossRef]
- Esti, M.; Cinquanta, L.; Sinesio, F.; Moneta, E.; Di Matteo, M. Physicochemical and Sensory Fruit Characteristics of Two Sweet Cherry Cultivars after Cool Storage. Food Chem. 2002, 76, 399–405. [Google Scholar] [CrossRef]
- Delgado, R.; González, M.R.; Martín, P. Interaction Effects of Nitrogen and Potassium Fertilization on Anthocyanin Composition and Chromatic Features of Tempranillo Grapes. OENO One 2006, 40, 141. [Google Scholar] [CrossRef]
- Tehranifar, A.; Tabar, S.M. Foliar Application of Potassium and Boron during Pomegranate (Punica granatum) Fruit Development Can Improve Fruit Quality. Hortic. Environ. Biotechnol. 2009, 50, 191–196. [Google Scholar]
- Nava, G.; Dechen, A.R.; Nachtigall, G.R. Nitrogen and Potassium Fertilization Affect Apple Fruit Quality in Southern Brazil. Commun. Soil Sci. Plant Anal. 2007, 39, 96–107. [Google Scholar] [CrossRef]
- Revell, J.M. Sensory Profile & Consumer Acceptability of Sweet Cherries. Available online: http://eprints.nottingham.ac.uk/10931/ (accessed on 13 October 2022).
- Villavicencio, J.D.; Zoffoli, J.P.; Plotto, A.; Contreras, C. Aroma Compounds Are Responsible for an Herbaceous Off-Flavor in the Sweet Cherry (Prunus avium L.) Cv. Regina during Fruit Development. Agronomy 2021, 11, 2020. [Google Scholar] [CrossRef]
- Girard, B.; Kopp, T.G. Physicochemical Characteristics of Selected Sweet Cherry Cultivars. J. Agric. Food Chem. 1998, 46, 471–476. [Google Scholar] [CrossRef]
- Nagy, P.T.; Thurzó, S.; Szabó, Z.; Nyéki, J.; Silva, A.P.; Gonçalves, B. Influence of Foliar Fertilization on Mineral Composition, Sugar and Organic Acid Content of Sweet Cherry. Acta Hortic. 2010, 868, 353–358. [Google Scholar] [CrossRef]
- Defilippi, B.; Manríquez, D. Evaluación de sistemas de medición de firmeza para uva de mesa y cerezas utilizados en la industria frutícola. Rev. Frutícola 2011, 2, 26–32. [Google Scholar]
- Parada, J.; Valenzuela, T.; Gómez, F.; Tereucán, G.; García, S.; Cornejo, P.; Winterhalter, P.; Ruiz, A. Effect of Fertilization and Arbuscular Mycorrhizal Fungal Inoculation on Antioxidant Profiles and Activities in Fragaria Ananassa Fruit. J. Sci. Food Agric. 2019, 99, 1397–1404. [Google Scholar] [CrossRef] [PubMed]
- Maldonado, P.D.; Rivero-Cruz, I.; Mata, R.; Pedraza-Chaverrí, J. Antioxidant activity of A-type proanthocyanidins from Geranium niveum (Geraniaceae). J. Agric. Food Chem. 2005, 53, 1996–2001. [Google Scholar] [CrossRef] [PubMed]
- Millaleo, R.; Alvear, M.; Aguilera, P.; González-Villagra, J.; de la Luz Mora, M.; Alberdi, M.; Reyes-Díaz, M. Mn Toxicity Differentially Affects Physiological and Biochemical Features in Highbush Blueberry (Vaccinium corymbosum L.) Cultivars. J. Soil Sci. Plant Nutr. 2020, 20, 795–805. [Google Scholar] [CrossRef]

| Quality Parameter | Canopy Zone | Treatments | |||
|---|---|---|---|---|---|
| Uncovered Trees | Covered Trees | ||||
| K− | K+ | K− | K+ | ||
| Fruit weight (g) | Lower | 9.5 ± 0.2 Ba | 9.5 ± 1.0 Ba | 9.9 ± 0.2 Ab | 10.3 ± 0.2 Aa |
| Upper | 9.9 ± 0.2 Ba | 9.8 ± 0.2 Ba | 10.4 ± 0.2 Aa | 10.3 ± 0.2 Aa | |
| Significance | n.s. | n.s. | * | n.s. | |
| Fruit size (mm) | Lower | 26.6 ± 0.3 Ba | 27.3 ± 0.2 Aa | 27.1 ± 0.3 Ba | 27.7 ± 0.3 Aa |
| Upper | 26.8 ± 0.3 Aa | 27.3 ± 0.2 Aa | 27.1 ± 0.2 Aa | 27.3 ± 0.3 Aa | |
| Significance | n.s. | n.s. | n.s. | n.s. | |
| Firmness (g mm−1) | Lower | 341.3 ± 10.1 Ba | 373.3 ± 6.9 Aa | 293.6 ± 6.8 Ca | 315.2 ± 7.1 Ca |
| Upper | 323.4 ± 10.3 Ba | 369.2 ± 8.3 Aa | 296.7 ± 7.8 Ca | 311.6 ± 9.7 BCa | |
| Significance | n.s. | n.s. | n.s. | n.s. | |
| SSC (Brix) | Lower | 17.5 ± 0.9 Ba | 19.1 ± 0.5 Ab | 15.9 ± 0.7 Cb | 18.1 ± 0.5 Bb |
| Upper | 18.5 ± 0.9 Bb | 19.9 ± 0.3 Aa | 17.9 ± 0.5 Ba | 20.2 ± 0.5 Aa | |
| Significance | ** | * | *** | *** | |
| TA (% of malic acid) | Lower | 1.1 ± 0.0 Aa | 1.0 ± 0.0 Aa | 0.9 ± 0.0 Ba | 0.9 ± 0.0 Bb |
| Upper | 1.1 ± 0.0 Aa | 0.9 ± 0.0 BCa | 0.9 ± 0.0 Ca | 1.0 ± 0.0 ABa | |
| Significance | n.s. | n.s. | n.s. | * | |
| Maturity index | Lower | 16.7 ± 0.9 Ba | 18.8 ± 0.6 Aa | 18.4 ± 1.0 Ba | 20.5 ± 0.9 Aa |
| Upper | 16.9 ± 0.6 Ba | 21.0 ± 1.0 Aa | 19.9 ± 0.6 Aa | 19.4 ± 0.3 Aa | |
| Significance | n.s. | n.s. | n.s. | n.s. | |
| Quality Parameters | Canopy Zone | Potassium | Cover | Potassium × Cover |
|---|---|---|---|---|
| Fruit weight | Lower | n.s. | ** | n.s. |
| Upper | n.s. | ** | n.s. | |
| Fruit size | Lower | ** | n.s. | n.s. |
| Upper | n.s. | n.s. | n.s. | |
| Firmness | Lower | ** | *** | n.s. |
| Upper | *** | *** | n.s. | |
| SCC | Lower | *** | *** | n.s. |
| Upper | *** | n.s. | n.s. | |
| TA | Lower | n.s. | *** | n.s. |
| Upper | n.s. | n.s. | *** | |
| Maturity index | Lower | * | n.s. | n.s. |
| Upper | * | n.s. | ** |
| Compounds | Canopy Zone | Treatments | |||
|---|---|---|---|---|---|
| Uncovered Trees | Covered Trees | ||||
| K− | K+ | K− | K+ | ||
| Antioxidant activity (using DPPH) (µmol TE g−1 FW) | Lower | 1.6 ± 0.0 Aa | 1.5 ± 0.0 Ab | 1.5 ± 0.1 Aa | 1.6 ± 0.1 Aa |
| Upper | 1.5 ± 0.1 Ba | 1.8 ± 0.0 Aa | 1.5 ± 0.1 Ba | 1.6 ± 0.1 Aa | |
| Significance | n.s. | *** | n.s. | n.s. | |
| Antioxidant activity (using ABTS) (µmol TE g−1 FW) | Lower | 2.4 ± 0.1 Aa | 2.3 ± 0.1 Aa | 2.3 ± 0.1 Aa | 2.4 ± 0.1 Aa |
| Upper | 2.4 ± 0.1 Ba | 3.0 ± 0.1 Aa | 2.3 ± 0.1 Ba | 2.3 ± 0.1 Ba | |
| Significance | n.s. | n.s. | n.s. | n.s. | |
| Total phenolic content (µg GAE g−1 FW) | Lower | 2.5 ± 0.1 Ba | 2.9 ± 0.2 Aa | 2.8 ± 0.4 Ba | 3.4 ± 0.4 Aa |
| Upper | 2.5 ± 0.3 Aa | 3.3 ± 0.4 Aa | 2.8 ± 0.2 Aa | 2.9 ± 0.2 Aa | |
| Significance | n.s. | n.s. | n.s. | n.s. | |
| Zone | Significance | |||
|---|---|---|---|---|
| Compounds | Potassium | Cover | Potassium × Cover | |
| Antioxidant activity (using DPPH) | Lower | n.s. | n.s. | n.s. |
| Upper | * | n.s. | n.s. | |
| Antioxidant activity (using ABTS) | Lower | n.s. | n.s. | n.s. |
| Upper | ** | *** | * | |
| Total phenolic content | Lower | * | * | n.s. |
| Upper | n.s. | n.s. | n.s. | |
| Phenolic Compounds (mg × 100 g of FW) | Canopy Zone | Treatments | |||
|---|---|---|---|---|---|
| Uncovered Trees | Covered Trees | ||||
| K− | K+ | K− | K+ | ||
| Hydroxycinnamic acid 1 * | Lower | 34.6 ± 4.5 Aba | 26.8 ± 3.6 BCb | 21.7 ± 5.2 Ca | 39.4 ± 1.9 Aa |
| Upper | 27.5 ± 3.5 Ba | 43.2 ± 1.3 Aa | 29.1 ± 2.4 Ba | 28.8 ± 5.6 Bb | |
| Hydroxycinnamic acid 2 * | Lower | 91.9 ± 20.1 Aa | 85.6 ± 13.2 Aa | 92.8 ± 26.0 Aa | 123.7 ± 2.2 Aa |
| Upper | 56.9 ± 5.4 Ba | 104.1 ± 9.9 Aa | 93.6 ± 11.8 Aa | 52.5 ± 9.7 Bb | |
| Quinic acid derivative | Lower | 5.1 ± 0.3 Bb | 5.8 ± 0.2 Ab | 5.4 ± 0.26 Ba | 5.4 ± 0.1 Aa |
| Upper | 5.8 ± 0.1 Ba | 6.4 ± 0.1 Aa | 4.9 ± 0.1 Ca | 5.4 ± 0.3 Ba | |
| Total hydroxycinnamic acids | Lower | 120.0 ± 24.6 Aa | 118.2 ± 16.7 Aa | 131.38 ± 31.0 Aa | 167.6 ± 2.3 Aa |
| Upper | 90.18 ± 9 Ba | 153.78 ± 10.7 Aa | 127.73 ± 14.29 Aa | 86.83 ± 15.1 Bb | |
| Quercetin-rutinoside | Lower | 7.3 ± 0.7 Ab | 8.7 ± 0.4 Ab | 8.5 ± 1.2 Aa | 10.0 ± 0.9 Aa |
| Upper | 11.1 ± 1.4 Ba | 15.8 ± 1.1 Aa | 9.4 ± 0.9 Ba | 9.7 ± 0.3 Ba | |
| Flavonol 2 * | Lower | 3.1 ± 0.2 Bb | 3.9 ± 0.4 Aa | 2.7 ± 0.1 Bb | 4.0 ± 0.4 Aa |
| Upper | 4.9 ± 0.3 Aa | 5.4 ± 0.6 Aa | 4.2 ± 0.4 Ba | 3.7 ± 0.3 Ba | |
| Total Flavonols | Lower | 10.5 ± 0.8 B | 12.7 ± 0.8 Ab | 11.12 ± 1.2 Bb | 14.1 ± 1.3 Aa |
| Upper | 18.27 ± 2.48 A | 21.19 ± 1.6 Aa | 13.7 ± 1.3 Ba | 13.4 ± 0.58 Ba | |
| Cyanidin-3-rutinoside | Lower | 74.0 ± 5.7 Ba | 55.9 ± 5.2 BCa | 39.9 ± 13.5 Cb | 109.1 ± 18.5 Aa |
| Upper | 67.5 ± 5.5 Ca | 204.5 ± 13.6 Ab | 103.8 ± 15.7 Ba | 125.8 ± 9.5 Ba | |
| Peonidin-3-rutinoside | Lower | 9.2 ± 0.8 Aa | 5.1 ± 0.1 Bb | 3.7 ± 0.9 Bb | 9.4 ± 1.7 Aa |
| Upper | 6.1 ± 0.7 Ba | 8.9 ± 0.5 Aa | 6.9 ± 0.7 Ba | 10.9 ± 1.3 Aa | |
| Total Anthocyanins | Lower | 83.3 ± 6.4 Ba | 61.1 ± 5.3 BCb | 43.7 ± 14.2 Cb | 131.6 ± 17.5 Aa |
| Upper | 73.7 ± 6.2 Ca | 213.7 ± 13.9 Aa | 110.9 ± 16.3 Ba | 136.9 ± 10.7 Ba | |
| Phenolic Compounds | Zone | Significance | ||
|---|---|---|---|---|
| Significance | Cover | Potassium × Cover | ||
| Cyanidin-3-rutinoside | Lower | * | n.s. | *** |
| Upper | *** | n.s. | *** | |
| Peonidin-3-rutinoside | Lower | n.s. | n.s. | *** |
| Upper | *** | n.s. | n.s. | |
| Total Anthocyanins | Lower | * | n.s. | *** |
| Upper | *** | n.s. | *** | |
| Quercetin-rutinoside | Lower | n.s. | n.s. | n.s. |
| Upper | * | *** | * | |
| Flavo(a)nol 2 * | Lower | *** | n.s. | n.s. |
| Upper | n.s. | ** | n.s. | |
| Total Flavo(a)nols | Lower | ** | n.s. | n.s. |
| Upper | n.s. | *** | n.s. | |
| Hydroxycinnamic acid 1 * | Lower | n.s. | n.s. | ** |
| Upper | * | n.s. | * | |
| Hydroxycinnamic acid 2 * | Lower | n.s. | n.s. | n.s. |
| Upper | n.s. | n.s. | *** | |
| Quinic acid derivative | Lower | * | n.s. | n.s. |
| Upper | *** | *** | n.s. | |
| Total hydroxycinnamic acids | Lower | n.s. | n.s. | n.s. |
| Upper | n.s. | n.s. | *** | |
| Organic Acid (g × Kg−1 FW) | Treatment | |||
|---|---|---|---|---|
| K- | K+ | |||
| Lower | Upper | Lower | Upper | |
| Malic acid | 0.99 ± 0.08 c | 1.21 ± 0.014 ab | 1.2 ± 0.015 ab | 1.27 ± 0.12 a |
| Oxalic acid | 0.11 ± 0.007 c | 0.12 ± 0.007 c | 0.16 ± 0.004 b | 0.20 ± 0.027 a |
| Citric acid | 0.136 ± 0.032 a | 0.11 ± 0.019 ab | 0.12 ± 0.002 ab | 0.085 ± 0.012 b |
| Total OA | 1.24 ± 0.11 c | 1.4 ± 0.016 b | 145.5 ± 15.6 b | 156.3 ± 14.9 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Palacios-Peralta, C.; Ruiz, A.; Ercoli, S.; Reyes-Díaz, M.; Bustamante, M.; Muñoz, A.; Osorio, P.; Ribera-Fonseca, A. Plastic Covers and Potassium Pre-Harvest Sprays and Their Influence on Antioxidant Properties, Phenolic Profile, and Organic Acids Composition of Sweet Cherry Fruits Cultivated in Southern Chile. Plants 2023, 12, 50. https://doi.org/10.3390/plants12010050
Palacios-Peralta C, Ruiz A, Ercoli S, Reyes-Díaz M, Bustamante M, Muñoz A, Osorio P, Ribera-Fonseca A. Plastic Covers and Potassium Pre-Harvest Sprays and Their Influence on Antioxidant Properties, Phenolic Profile, and Organic Acids Composition of Sweet Cherry Fruits Cultivated in Southern Chile. Plants. 2023; 12(1):50. https://doi.org/10.3390/plants12010050
Chicago/Turabian StylePalacios-Peralta, Cristóbal, Antonieta Ruiz, Stefano Ercoli, Marjorie Reyes-Díaz, Marco Bustamante, Ariel Muñoz, Pamela Osorio, and Alejandra Ribera-Fonseca. 2023. "Plastic Covers and Potassium Pre-Harvest Sprays and Their Influence on Antioxidant Properties, Phenolic Profile, and Organic Acids Composition of Sweet Cherry Fruits Cultivated in Southern Chile" Plants 12, no. 1: 50. https://doi.org/10.3390/plants12010050
APA StylePalacios-Peralta, C., Ruiz, A., Ercoli, S., Reyes-Díaz, M., Bustamante, M., Muñoz, A., Osorio, P., & Ribera-Fonseca, A. (2023). Plastic Covers and Potassium Pre-Harvest Sprays and Their Influence on Antioxidant Properties, Phenolic Profile, and Organic Acids Composition of Sweet Cherry Fruits Cultivated in Southern Chile. Plants, 12(1), 50. https://doi.org/10.3390/plants12010050








