A Green Light to Switch on Genes: Revisiting Trithorax on Plants
Abstract
1. Introduction
2. What Does It Mean to Be a TrxG Member?
2.1. TrxG Histone Methyltransferases
2.2. Histone Demethylases
2.3. Chromatin Remodeler Factors
2.4. COMPASS Proteins
2.5. TrxG Accessory Proteins, the Case of ULTRAPETALA1 and SECRET AGENT
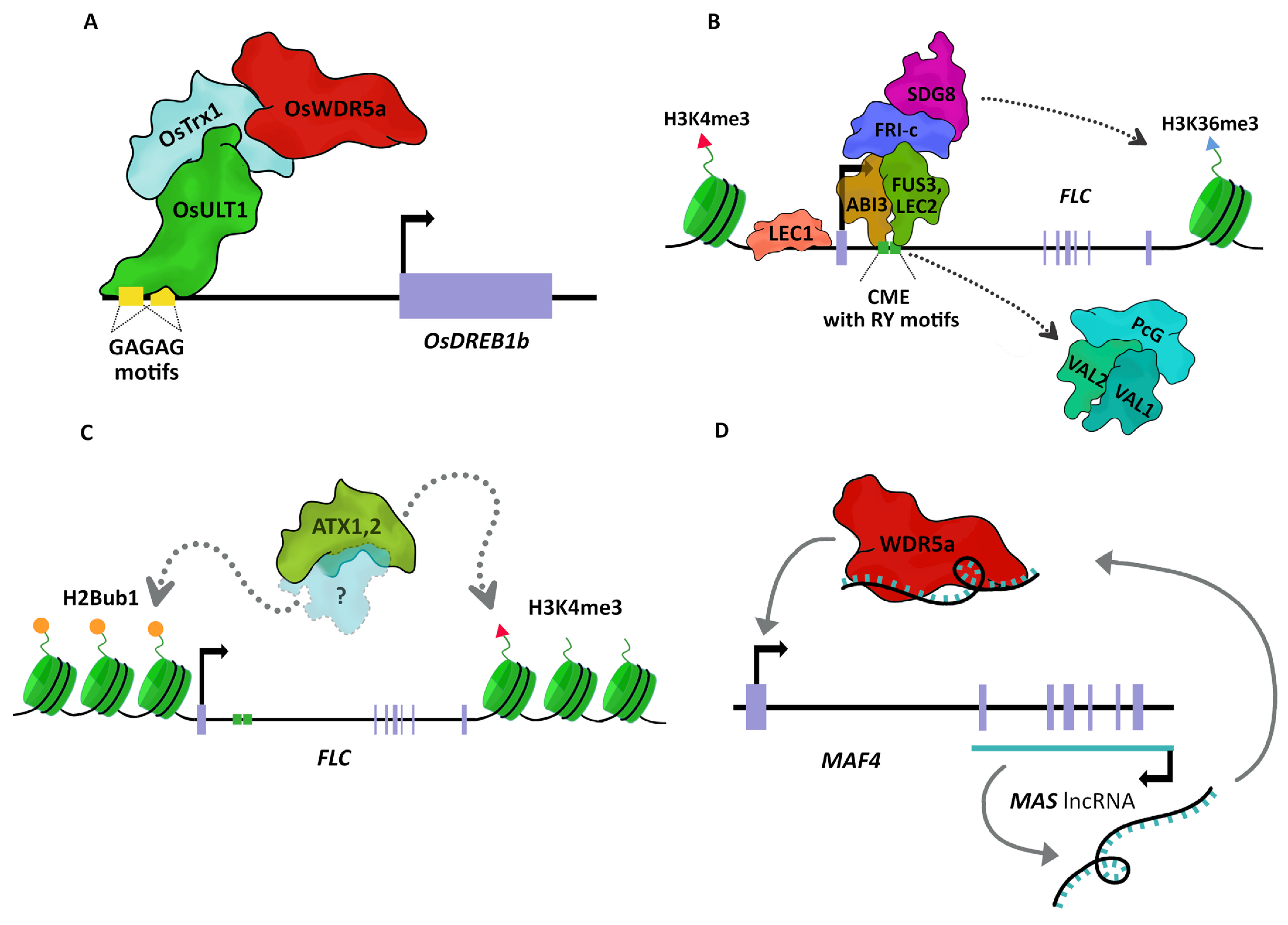
3. A Call for TrxG to Activate Genes
4. How Does TrxG Machinery Activate Transcription?
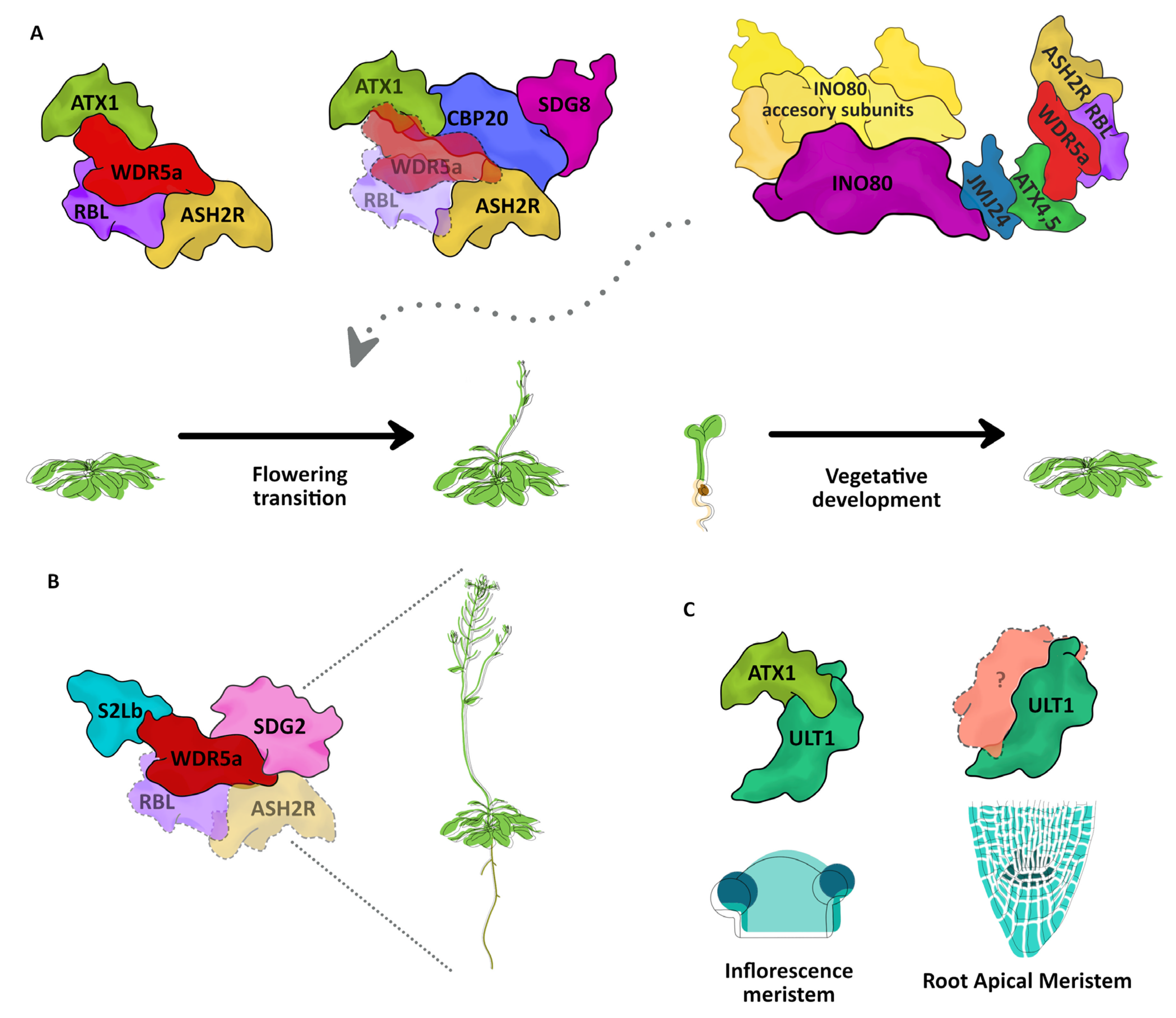
5. Trithorax Teaming Up: Known TrxG Complexes
6. Role of TrxG Proteins during Development
6.1. From Vegetative Growth to Flower Development
6.2. Root Development
7. Life Being Plastic, Is Fantastic! Trithorax and Plant Plasticity
8. Trithorax Breaking the Mold: Atypical Roles in the Regulation
9. Conclusions and Perspectives
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sanchez, M.P.; Aceves-Garcia, P.; Petrone, E.; Steckenborn, S.; Vega-Leon, R.; Alvarez-Buylla, E.R.; Garay-Arroyo, A.; Garcia-Ponce, B. The impact of Polycomb group (PcG) and Trithorax group (TrxG) epigenetic factors in plant plasticity. New Phytol. 2015, 208, 684–694. [Google Scholar] [CrossRef] [PubMed]
- Pigliucci, M.; Murren, C.J.; Schlichting, C.D. Phenotypic plasticity and evolution by genetic assimilation. J. Exp. Biol. 2006, 209, 2362–2367. [Google Scholar] [CrossRef] [PubMed]
- Burton, T.; Ratikainen, I.I.; Einum, S. Environmental change and the rate of phenotypic plasticity. Glob. Chang. Biol. 2022, 28, 5337–5345. [Google Scholar] [CrossRef] [PubMed]
- Holliday, R. Epigenetics: A historical overview. Epigenetics 2006, 1, 76–80. [Google Scholar] [CrossRef] [PubMed]
- Jablonka, E.; Lamb, M.J. The changing concept of epigenetics. Ann. N. Y. Acad. Sci. 2002, 981, 82–96. [Google Scholar] [CrossRef] [PubMed]
- Schlichting, C.D.; Wund, M.A. Phenotypic plasticity and epigenetic marking: An assessment of evidence for genetic accommodation. Evolution 2014, 68, 656–672. [Google Scholar] [CrossRef] [PubMed]
- Duncan, E.J.; Gluckman, P.D.; Dearden, P.K. Epigenetics, plasticity, and evolution: How do we link epigenetic change to phenotype? J. Exp. Zool. B Mol. Dev. Evol. 2014, 322, 208–220. [Google Scholar] [CrossRef]
- Alejo-Vinogradova, M.T.; Ornelas-Ayala, D.; Vega-León, R.; Garay-Arroyo, A.; García-Ponce, B.; R Álvarez-Buylla, E.; Sanchez, M.D.L.P. Unraveling the role of epigenetic regulation in asymmetric cell division during plant development. J. Exp. Bot. 2022, 73, 38–49. [Google Scholar] [CrossRef]
- Zhao, T.; Zhan, Z.; Jiang, D. Histone modifications and their regulatory roles in plant development and environmental memory. J. Genet. Genom. 2019, 46, 467–476. [Google Scholar] [CrossRef]
- Pu, L.; Sung, Z.R. PcG and trxG in plants-friends or foes. Trends Genet. 2015, 31, 252–262. [Google Scholar] [CrossRef]
- Bemer, M.; Grossniklaus, U. Dynamic regulation of Polycomb group activity during plant development. Curr. Opin. Plant Biol. 2012, 15, 523–529. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, J.C. State of the Art: TrxG Factor Regulation of Post-embryonic Plant Development. Front. Plant Sci. 2017, 8, 1925. [Google Scholar] [CrossRef] [PubMed]
- Kohler, C.; Hennig, L. Regulation of cell identity by plant Polycomb and trithorax group proteins. Curr. Opin. Genet. Dev. 2010, 20, 541–547. [Google Scholar] [CrossRef] [PubMed]
- Pien, S.; Grossniklaus, U. Polycomb group and trithorax group proteins in Arabidopsis. Biochim. Biophys. Acta 2007, 1769, 375–382. [Google Scholar] [CrossRef] [PubMed]
- Ingham, P.W. Differential expression of bithorax complex genes in the absence of the extra sex combs and trithorax genes. Nature 1983, 306, 591–593. [Google Scholar] [CrossRef]
- Pien, S.; Fleury, D.; Mylne, J.S.; Crevillen, P.; Inze, D.; Avramova, Z.; Dean, C.; Grossniklaus, U. ARABIDOPSIS TRITHORAX1 dynamically regulates FLOWERING LOCUS C activation via histone 3 lysine 4 trimethylation. Plant Cell 2008, 20, 580–588. [Google Scholar] [CrossRef]
- Saleh, A.; Alvarez-Venegas, R.; Yilmaz, M.; Le, O.; Hou, G.; Sadder, M.; Al-Abdallat, A.; Xia, Y.; Lu, G.; Ladunga, I.; et al. The highly similar Arabidopsis homologs of trithorax ATX1 and ATX2 encode proteins with divergent biochemical functions. Plant Cell 2008, 20, 568–579. [Google Scholar] [CrossRef]
- Chen, L.Q.; Luo, J.H.; Cui, Z.H.; Xue, M.; Wang, L.; Zhang, X.Y.; Pawlowski, W.P.; He, Y. ATX3, ATX4, and ATX5 Encode Putative H3K4 Methyltransferases and Are Critical for Plant Development. Plant Physiol. 2017, 174, 1795–1806. [Google Scholar] [CrossRef]
- Choi, S.C.; Lee, S.; Kim, S.R.; Lee, Y.S.; Liu, C.; Cao, X.; An, G. Trithorax group protein Oryza sativa Trithorax1 controls flowering time in rice via interaction with early heading date3. Plant Physiol. 2014, 164, 1326–1337. [Google Scholar] [CrossRef]
- Jiang, P.; Wang, S.; Ikram, A.U.; Xu, Z.; Jiang, H.; Cheng, B.; Ding, Y. SDG721 and SDG705 are required for rice growth. J. Integr. Plant Biol. 2018, 60, 530–535. [Google Scholar] [CrossRef]
- Lee, K.; Park, O.S.; Seo, P.J. Arabidopsis ATXR2 deposits H3K36me3 at the promoters of LBD genes to facilitate cellular dedifferentiation. Sci. Signal. 2017, 10, eaan0316. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Yu, Y.; Law, J.A.; Zhang, X. SET DOMAIN GROUP2 is the major histone H3 lysine [corrected] 4 trimethyltransferase in Arabidopsis. Proc. Natl. Acad. Sci. USA 2010, 107, 18557–18562. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Yu, Y.; Dong, A.; Shen, W.H. SET DOMAIN GROUP701 encodes a H3K4-methytransferase and regulates multiple key processes of rice plant development. New Phytol. 2017, 215, 609–623. [Google Scholar] [CrossRef] [PubMed]
- Tamada, Y.; Yun, J.Y.; Woo, S.C.; Amasino, R.M. ARABIDOPSIS TRITHORAX-RELATED7 is required for methylation of lysine 4 of histone H3 and for transcriptional activation of FLOWERING LOCUS C. Plant Cell 2009, 21, 3257–3269. [Google Scholar] [CrossRef]
- Berr, A.; Xu, L.; Gao, J.; Cognat, V.; Steinmetz, A.; Dong, A.; Shen, W.H. SET DOMAIN GROUP25 encodes a histone methyltransferase and is involved in FLOWERING LOCUS C activation and repression of flowering. Plant Physiol. 2009, 151, 1476–1485. [Google Scholar] [CrossRef]
- Li, Y.; Mukherjee, I.; Thum, K.E.; Tanurdzic, M.; Katari, M.S.; Obertello, M.; Edwards, M.B.; McCombie, W.R.; Martienssen, R.A.; Coruzzi, G.M. The histone methyltransferase SDG8 mediates the epigenetic modification of light and carbon responsive genes in plants. Genome Biol. 2015, 16, 79. [Google Scholar] [CrossRef]
- Sui, P.; Jin, J.; Ye, S.; Mu, C.; Gao, J.; Feng, H.; Shen, W.H.; Yu, Y.; Dong, A. H3K36 methylation is critical for brassinosteroid-regulated plant growth and development in rice. Plant J. Cell Mol. Biol. 2012, 70, 340–347. [Google Scholar] [CrossRef]
- Sun, C.; Fang, J.; Zhao, T.; Xu, B.; Zhang, F.; Liu, L.; Tang, J.; Zhang, G.; Deng, X.; Chen, F.; et al. The histone methyltransferase SDG724 mediates H3K36me2/3 deposition at MADS50 and RFT1 and promotes flowering in rice. Plant Cell 2012, 24, 3235–3247. [Google Scholar] [CrossRef]
- Berr, A.; Shafiq, S.; Pinon, V.; Dong, A.; Shen, W.H. The trxG family histone methyltransferase SET DOMAIN GROUP 26 promotes flowering via a distinctive genetic pathway. Plant J. Cell Mol. Biol. 2015, 81, 316–328. [Google Scholar] [CrossRef]
- Liu, B.; Wei, G.; Shi, J.; Jin, J.; Shen, T.; Ni, T.; Shen, W.H.; Yu, Y.; Dong, A. SET DOMAIN GROUP 708, a histone H3 lysine 36-specific methyltransferase, controls flowering time in rice (Oryza sativa). New Phytol. 2016, 210, 577–588. [Google Scholar] [CrossRef]
- Cartagena, J.A.; Matsunaga, S.; Seki, M.; Kurihara, D.; Yokoyama, M.; Shinozaki, K.; Fujimoto, S.; Azumi, Y.; Uchiyama, S.; Fukui, K. The Arabidopsis SDG4 contributes to the regulation of pollen tube growth by methylation of histone H3 lysines 4 and 36 in mature pollen. Dev. Biol. 2008, 315, 355–368. [Google Scholar] [CrossRef] [PubMed]
- Jiang, D.; Gu, X.; He, Y. Establishment of the winter-annual growth habit via FRIGIDA-mediated histone methylation at FLOWERING LOCUS C in Arabidopsis. Plant Cell 2009, 21, 1733–1746. [Google Scholar] [CrossRef] [PubMed]
- Jiang, P.; Wang, S.; Jiang, H.; Cheng, B.; Wu, K.; Ding, Y. The COMPASS-Like Complex Promotes Flowering and Panicle Branching in Rice. Plant Physiol. 2018, 176, 2761–2771. [Google Scholar] [CrossRef] [PubMed]
- Jiang, D.; Kong, N.C.; Gu, X.; Li, Z.; He, Y. Arabidopsis COMPASS-like complexes mediate histone H3 lysine-4 trimethylation to control floral transition and plant development. PLoS Genet. 2011, 7, e1001330. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; Wang, J.; Chen, X.; Sha, H.; Liu, X.; Han, Y.; Qiu, G.; Zhang, F.; Fang, J. OsASHL1 and OsASHL2, two members of the COMPASS-like complex, control floral transition and plant development in rice. J. Genet. Genom. 2022, 49, 870–880. [Google Scholar] [CrossRef] [PubMed]
- Fiorucci, A.S.; Bourbousse, C.; Concia, L.; Rougee, M.; Deton-Cabanillas, A.F.; Zabulon, G.; Layat, E.; Latrasse, D.; Kim, S.K.; Chaumont, N.; et al. Arabidopsis S2Lb links AtCOMPASS-like and SDG2 activity in H3K4me3 independently from histone H2B monoubiquitination. Genome Biol. 2019, 20, 100. [Google Scholar] [CrossRef] [PubMed]
- Farrona, S.; Hurtado, L.; Bowman, J.L.; Reyes, J.C. The Arabidopsis thaliana SNF2 homolog AtBRM controls shoot development and flowering. Development 2004, 131, 4965–4975. [Google Scholar] [CrossRef]
- Kwon, C.S.; Chen, C.; Wagner, D. WUSCHEL is a primary target for transcriptional regulation by SPLAYED in dynamic control of stem cell fate in Arabidopsis. Genes Dev. 2005, 19, 992–1003. [Google Scholar] [CrossRef]
- Shang, J.Y.; Lu, Y.J.; Cai, X.W.; Su, Y.N.; Feng, C.; Li, L.; Chen, S.; He, X.J. COMPASS functions as a module of the INO80 chromatin remodeling complex to mediate histone H3K4 methylation in Arabidopsis. Plant Cell 2021, 33, 3250–3271. [Google Scholar] [CrossRef]
- Aichinger, E.; Villar, C.B.; Di Mambro, R.; Sabatini, S.; Kohler, C. The CHD3 chromatin remodeler PICKLE and polycomb group proteins antagonistically regulate meristem activity in the Arabidopsis root. Plant Cell 2011, 23, 1047–1060. [Google Scholar] [CrossRef]
- Chen, X.; Liu, X.; Zhao, Y.; Zhou, D.X. Histone H3K4me3 and H3K27me3 regulatory genes control stable transmission of an epimutation in rice. Sci. Rep. 2015, 5, 13251. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Howard, M.; Dean, C. Physical coupling of activation and derepression activities to maintain an active transcriptional state at FLC. Proc. Natl. Acad. Sci. USA 2016, 113, 9369–9374. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Chen, X.; Zhong, X.; Zhao, Y.; Liu, X.; Zhou, S.; Cheng, S.; Zhou, D.X. Jumonji C domain protein JMJ705-mediated removal of histone H3 lysine 27 trimethylation is involved in defense-related gene activation in rice. Plant Cell 2013, 25, 4725–4736. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Zhou, D.X. Rice jmjC domain-containing gene JMJ706 encodes H3K9 demethylase required for floral organ development. Proc. Natl. Acad. Sci. USA 2008, 105, 13679–13684. [Google Scholar] [CrossRef] [PubMed]
- Roy, D.; Chakrabarty, J.; Mallik, R.; Chaudhuri, S. Rice Trithorax factor ULTRAPETALA 1 (OsULT1) specifically binds to “GAGAG” sequence motif present in Polycomb response elements. Biochim. Biophys. Acta Gene Regul. Mech. 2019, 1862, 582–597. [Google Scholar] [CrossRef] [PubMed]
- Ng, D.W.; Wang, T.; Chandrasekharan, M.B.; Aramayo, R.; Kertbundit, S.; Hall, T.C. Plant SET domain-containing proteins: Structure, function and regulation. Biochim. Biophys. Acta 2007, 1769, 316–329. [Google Scholar] [CrossRef]
- Zhou, H.; Liu, Y.; Liang, Y.; Zhou, D.; Li, S.; Lin, S.; Dong, H.; Huang, L. The function of histone lysine methylation related SET domain group proteins in plants. Protein Sci. 2020, 29, 1120–1137. [Google Scholar] [CrossRef]
- Lee, J.; Yun, J.Y.; Zhao, W.; Shen, W.H.; Amasino, R.M. A methyltransferase required for proper timing of the vernalization response in Arabidopsis. Proc. Natl. Acad. Sci. USA 2015, 112, 2269–2274. [Google Scholar] [CrossRef]
- Zhai, H.; Zhang, X.; You, Y.; Lin, L.; Zhou, W.; Li, C. SEUSS integrates transcriptional and epigenetic control of root stem cell organizer specification. EMBO J. 2020, 39, e105047. [Google Scholar] [CrossRef]
- Oya, S.; Takahashi, M.; Takashima, K.; Kakutani, T.; Inagaki, S. Transcription-coupled and epigenome-encoded mechanisms direct H3K4 methylation. Nat. Commun. 2022, 13, 4521. [Google Scholar] [CrossRef]
- Zhang, X.; Bernatavichute, Y.V.; Cokus, S.; Pellegrini, M.; Jacobsen, S.E. Genome-wide analysis of mono-, di- and trimethylation of histone H3 lysine 4 in Arabidopsis thaliana. Genome Biol. 2009, 10, R62. [Google Scholar] [CrossRef] [PubMed]
- Sequeira-Mendes, J.; Araguez, I.; Peiro, R.; Mendez-Giraldez, R.; Zhang, X.; Jacobsen, S.E.; Bastolla, U.; Gutierrez, C. The Functional Topography of the Arabidopsis Genome Is Organized in a Reduced Number of Linear Motifs of Chromatin States. Plant Cell 2014, 26, 2351–2366. [Google Scholar] [CrossRef] [PubMed]
- Yan, W.; Chen, D.; Smaczniak, C.; Engelhorn, J.; Liu, H.; Yang, W.; Graf, A.; Carles, C.C.; Zhou, D.X.; Kaufmann, K. Dynamic and spatial restriction of Polycomb activity by plant histone demethylases. Nat. Plants 2018, 4, 681–689. [Google Scholar] [CrossRef] [PubMed]
- Crevillen, P.; Yang, H.; Cui, X.; Greeff, C.; Trick, M.; Qiu, Q.; Cao, X.; Dean, C. Epigenetic reprogramming that prevents transgenerational inheritance of the vernalized state. Nature 2014, 515, 587–590. [Google Scholar] [CrossRef]
- Han, S.K.; Wu, M.F.; Cui, S.; Wagner, D. Roles and activities of chromatin remodeling ATPases in plants. Plant J. Cell Mol. Biol. 2015, 83, 62–77. [Google Scholar] [CrossRef]
- Ho, K.K.; Zhang, H.; Golden, B.L.; Ogas, J. PICKLE is a CHD subfamily II ATP-dependent chromatin remodeling factor. Biochim. Biophys. Acta 2013, 1829, 199–210. [Google Scholar] [CrossRef]
- Jing, Y.; Guo, Q.; Lin, R. The Chromatin-Remodeling Factor PICKLE Antagonizes Polycomb Repression of FT to Promote Flowering. Plant Physiol. 2019, 181, 656–668. [Google Scholar] [CrossRef]
- Wu, M.F.; Sang, Y.; Bezhani, S.; Yamaguchi, N.; Han, S.K.; Li, Z.; Su, Y.; Slewinski, T.L.; Wagner, D. SWI2/SNF2 chromatin remodeling ATPases overcome polycomb repression and control floral organ identity with the LEAFY and SEPALLATA3 transcription factors. Proc. Natl. Acad. Sci. USA 2012, 109, 3576–3581. [Google Scholar] [CrossRef]
- Xue, M.; Zhang, H.; Zhao, F.; Zhao, T.; Li, H.; Jiang, D. The INO80 chromatin remodeling complex promotes thermomorphogenesis by connecting H2A.Z eviction and active transcription in Arabidopsis. Mol. Plant 2021, 14, 1799–1813. [Google Scholar] [CrossRef]
- Li, C.; Chen, C.; Gao, L.; Yang, S.; Nguyen, V.; Shi, X.; Siminovitch, K.; Kohalmi, S.E.; Huang, S.; Wu, K.; et al. The Arabidopsis SWI2/SNF2 chromatin Remodeler BRAHMA regulates polycomb function during vegetative development and directly activates the flowering repressor gene SVP. PLoS Genet. 2015, 11, e1004944. [Google Scholar] [CrossRef]
- Xu, Y.; Guo, C.; Zhou, B.; Li, C.; Wang, H.; Zheng, B.; Ding, H.; Zhu, Z.; Peragine, A.; Cui, Y.; et al. Regulation of Vegetative Phase Change by SWI2/SNF2 Chromatin Remodeling ATPase BRAHMA. Plant Physiol. 2016, 172, 2416–2428. [Google Scholar] [CrossRef] [PubMed]
- Archacki, R.; Yatusevich, R.; Buszewicz, D.; Krzyczmonik, K.; Patryn, J.; Iwanicka-Nowicka, R.; Biecek, P.; Wilczynski, B.; Koblowska, M.; Jerzmanowski, A.; et al. Arabidopsis SWI/SNF chromatin remodeling complex binds both promoters and terminators to regulate gene expression. Nucleic Acids Res. 2017, 45, 3116–3129. [Google Scholar] [CrossRef] [PubMed]
- Shu, J.; Chen, C.; Li, C.; Thapa, R.K.; Song, J.; Xie, X.; Nguyen, V.; Bian, S.; Liu, J.; Kohalmi, S.E.; et al. Genome-wide occupancy of Arabidopsis SWI/SNF chromatin remodeler SPLAYED provides insights into its interplay with its close homolog BRAHMA and Polycomb proteins. Plant J. Cell Mol. Biol. 2021, 106, 200–213. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Bishop, B.; Ringenberg, W.; Muir, W.M.; Ogas, J. The CHD3 remodeler PICKLE associates with genes enriched for trimethylation of histone H3 lysine 27. Plant Physiol. 2012, 159, 418–432. [Google Scholar] [CrossRef]
- Schuettengruber, B.; Bourbon, H.M.; Di Croce, L.; Cavalli, G. Genome Regulation by Polycomb and Trithorax: 70 Years and Counting. Cell 2017, 171, 34–57. [Google Scholar] [CrossRef]
- Dou, Y.; Milne, T.A.; Ruthenburg, A.J.; Lee, S.; Lee, J.W.; Verdine, G.L.; Allis, C.D.; Roeder, R.G. Regulation of MLL1 H3K4 methyltransferase activity by its core components. Nat. Struct. Mol. Biol. 2006, 13, 713–719. [Google Scholar] [CrossRef]
- Schneider, J.; Wood, A.; Lee, J.S.; Schuster, R.; Dueker, J.; Maguire, C.; Swanson, S.K.; Florens, L.; Washburn, M.P.; Shilatifard, A. Molecular regulation of histone H3 trimethylation by COMPASS and the regulation of gene expression. Mol. Cell 2005, 19, 849–856. [Google Scholar] [CrossRef]
- Li, Z.; Jiang, D.; Fu, X.; Luo, X.; Liu, R.; He, Y. Coupling of histone methylation and RNA processing by the nuclear mRNA cap-binding complex. Nat. Plants 2016, 2, 16015. [Google Scholar] [CrossRef]
- Soares, L.M.; Buratowski, S. Yeast Swd2 is essential because of antagonism between Set1 histone methyltransferase complex and APT (associated with Pta1) termination factor. J. Biol. Chem. 2012, 287, 15219–15231. [Google Scholar] [CrossRef]
- Ding, Y.; Ndamukong, I.; Xu, Z.; Lapko, H.; Fromm, M.; Avramova, Z. ATX1-generated H3K4me3 is required for efficient elongation of transcription, not initiation, at ATX1-regulated genes. PLoS Genet. 2012, 8, e1003111. [Google Scholar] [CrossRef]
- Ornelas-Ayala, D.; Vega-Leon, R.; Petrone-Mendoza, E.; Garay-Arroyo, A.; Garcia-Ponce, B.; Alvarez-Buylla, E.R.; Sanchez, M.P. ULTRAPETALA1 maintains Arabidopsis root stem cell niche independently of ARABIDOPSIS TRITHORAX1. New Phytol. 2020, 225, 1261–1272. [Google Scholar] [CrossRef] [PubMed]
- Carles, C.C.; Fletcher, J.C. The SAND domain protein ULTRAPETALA1 acts as a trithorax group factor to regulate cell fate in plants. Genes Dev. 2009, 23, 2723–2728. [Google Scholar] [CrossRef] [PubMed]
- Ornelas-Ayala, D.; Garay-Arroyo, A.; Garcia-Ponce, B.; R Álvarez-Buylla, E.; Sanchez, M.D.L.P. The Epigenetic Faces of ULTRAPETALA1. Front. Plant Sci. 2021, 12, 637244. [Google Scholar] [CrossRef]
- Xing, L.; Liu, Y.; Xu, S.; Xiao, J.; Wang, B.; Deng, H.; Lu, Z.; Xu, Y.; Chong, K. Arabidopsis O-GlcNAc transferase SEC activates histone methyltransferase ATX1 to regulate flowering. EMBO J. 2018, 37, e98115. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.L.; Chalkley, R.J.; Maynard, J.C.; Wang, W.; Ni, W.; Jiang, X.; Shin, K.; Cheng, L.; Savage, D.; Huhmer, A.F.; et al. Proteomic analysis reveals O-GlcNAc modification on proteins with key regulatory functions in Arabidopsis. Proc. Natl. Acad. Sci. USA 2017, 114, E1536–E1543. [Google Scholar] [CrossRef]
- Deplus, R.; Delatte, B.; Schwinn, M.K.; Defrance, M.; Mendez, J.; Murphy, N.; Dawson, M.A.; Volkmar, M.; Putmans, P.; Calonne, E.; et al. TET2 and TET3 regulate GlcNAcylation and H3K4 methylation through OGT and SET1/COMPASS. EMBO J. 2013, 32, 645–655. [Google Scholar] [CrossRef]
- Schuettengruber, B.; Martinez, A.M.; Iovino, N.; Cavalli, G. Trithorax group proteins: Switching genes on and keeping them active. Nat. Rev. Mol. Cell Biol. 2011, 12, 799–814. [Google Scholar] [CrossRef]
- Bredesen, B.A.; Rehmsmeier, M. DNA sequence models of genome-wide Drosophila melanogaster Polycomb binding sites improve generalization to independent Polycomb Response Elements. Nucleic Acids Res. 2019, 47, 7781–7797. [Google Scholar] [CrossRef]
- Deng, W.; Buzas, D.M.; Ying, H.; Robertson, M.; Taylor, J.; Peacock, W.J.; Dennis, E.S.; Helliwell, C. Arabidopsis Polycomb Repressive Complex 2 binding sites contain putative GAGA factor binding motifs within coding regions of genes. BMC Genom. 2013, 14, 593. [Google Scholar] [CrossRef]
- Kassis, J.A.; Brown, J.L. Polycomb group response elements in Drosophila and vertebrates. Adv. Genet. 2013, 81, 83–118. [Google Scholar] [CrossRef]
- Farkas, G.; Gausz, J.; Galloni, M.; Reuter, G.; Gyurkovics, H.; Karch, F. The Trithorax-like gene encodes the Drosophila GAGA factor. Nature 1994, 371, 806–808. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Jin, R.; Yu, X.; Shen, M.; Wagner, J.D.; Pai, A.; Song, C.; Zhuang, M.; Klasfeld, S.; He, C.; et al. Cis and trans determinants of epigenetic silencing by Polycomb repressive complex 2 in Arabidopsis. Nat. Genet. 2017, 49, 1546–1552. [Google Scholar] [CrossRef] [PubMed]
- Baulcombe, D.C.; Dean, C. Epigenetic regulation in plant responses to the environment. Cold Spring Harb. Perspect. Biol. 2014, 6, a019471. [Google Scholar] [CrossRef] [PubMed]
- Yuan, W.; Luo, X.; Li, Z.; Yang, W.; Wang, Y.; Liu, R.; Du, J.; He, Y. A cis cold memory element and a trans epigenome reader mediate Polycomb silencing of FLC by vernalization in Arabidopsis. Nat. Genet. 2016, 48, 1527–1534. [Google Scholar] [CrossRef] [PubMed]
- Questa, J.I.; Song, J.; Geraldo, N.; An, H.; Dean, C. Arabidopsis transcriptional repressor VAL1 triggers Polycomb silencing at FLC during vernalization. Science 2016, 353, 485–488. [Google Scholar] [CrossRef]
- Xu, G.; Tao, Z.; He, Y. Embryonic reactivation of FLOWERING LOCUS C by ABSCISIC ACID-INSENSITIVE 3 establishes the vernalization requirement in each Arabidopsis generation. Plant Cell 2022, 34, 2205–2221. [Google Scholar] [CrossRef]
- Tao, Z.; Shen, L.; Gu, X.; Wang, Y.; Yu, H.; He, Y. Embryonic epigenetic reprogramming by a pioneer transcription factor in plants. Nature 2017, 551, 124–128. [Google Scholar] [CrossRef]
- Tao, Z.; Hu, H.; Luo, X.; Jia, B.; Du, J.; He, Y. Embryonic resetting of the parental vernalized state by two B3 domain transcription factors in Arabidopsis. Nat. Plants 2019, 5, 424–435. [Google Scholar] [CrossRef]
- Roudier, F.; Ahmed, I.; Berard, C.; Sarazin, A.; Mary-Huard, T.; Cortijo, S.; Bouyer, D.; Caillieux, E.; Duvernois-Berthet, E.; Al-Shikhley, L.; et al. Integrative epigenomic mapping defines four main chromatin states in Arabidopsis. EMBO J. 2011, 30, 1928–1938. [Google Scholar] [CrossRef]
- Sridhar, V.V.; Kapoor, A.; Zhang, K.; Zhu, J.; Zhou, T.; Hasegawa, P.M.; Bressan, R.A.; Zhu, J.K. Control of DNA methylation and heterochromatic silencing by histone H2B deubiquitination. Nature 2007, 447, 735–738. [Google Scholar] [CrossRef]
- Cao, Y.; Dai, Y.; Cui, S.; Ma, L. Histone H2B monoubiquitination in the chromatin of FLOWERING LOCUS C regulates flowering time in Arabidopsis. Plant Cell 2008, 20, 2586–2602. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.S.; Shukla, A.; Schneider, J.; Swanson, S.K.; Washburn, M.P.; Florens, L.; Bhaumik, S.R.; Shilatifard, A. Histone crosstalk between H2B monoubiquitination and H3 methylation mediated by COMPASS. Cell 2007, 131, 1084–1096. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Luo, X.; Sun, F.; Hu, J.; Zha, X.; Su, W.; Yang, J. Overexpressing lncRNA LAIR increases grain yield and regulates neighbouring gene cluster expression in rice. Nat. Commun. 2018, 9, 3516. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Li, J.; Lian, B.; Gu, H.; Li, Y.; Qi, Y. Global identification of Arabidopsis lncRNAs reveals the regulation of MAF4 by a natural antisense RNA. Nat. Commun. 2018, 9, 5056. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.C.; Yang, Y.W.; Liu, B.; Sanyal, A.; Corces-Zimmerman, R.; Chen, Y.; Lajoie, B.R.; Protacio, A.; Flynn, R.A.; Gupta, R.A.; et al. A long noncoding RNA maintains active chromatin to coordinate homeotic gene expression. Nature 2011, 472, 120–124. [Google Scholar] [CrossRef]
- Gomez, J.A.; Wapinski, O.L.; Yang, Y.W.; Bureau, J.F.; Gopinath, S.; Monack, D.M.; Chang, H.Y.; Brahic, M.; Kirkegaard, K. The NeST long ncRNA controls microbial susceptibility and epigenetic activation of the interferon-gamma locus. Cell 2013, 152, 743–754. [Google Scholar] [CrossRef]
- Luo, S.; Lu, J.Y.; Liu, L.; Yin, Y.; Chen, C.; Han, X.; Wu, B.; Xu, R.; Liu, W.; Yan, P.; et al. Divergent lncRNAs Regulate Gene Expression and Lineage Differentiation in Pluripotent Cells. Cell Stem Cell 2016, 18, 637–652. [Google Scholar] [CrossRef]
- Heo, J.B.; Lee, Y.S.; Sung, S. Epigenetic regulation by long noncoding RNAs in plants. Chromosome Res. 2013, 21, 685–693. [Google Scholar] [CrossRef]
- Kim, D.H.; Sung, S. Vernalization-Triggered Intragenic Chromatin Loop Formation by Long Noncoding RNAs. Dev. Cell 2017, 40, 302–312.e4. [Google Scholar] [CrossRef]
- Liu, Z.W.; Zhao, N.; Su, Y.N.; Chen, S.S.; He, X.J. Exogenously overexpressed intronic long noncoding RNAs activate host gene expression by affecting histone modification in Arabidopsis. Sci. Rep. 2020, 10, 3094. [Google Scholar] [CrossRef]
- Khalil, A.M.; Guttman, M.; Huarte, M.; Garber, M.; Raj, A.; Rivea Morales, D.; Thomas, K.; Presser, A.; Bernstein, B.E.; van Oudenaarden, A.; et al. Many human large intergenic noncoding RNAs associate with chromatin-modifying complexes and affect gene expression. Proc. Natl. Acad. Sci. USA 2009, 106, 11667–11672. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.W.; Flynn, R.A.; Chen, Y.; Qu, K.; Wan, B.; Wang, K.C.; Lei, M.; Chang, H.Y. Essential role of lncRNA binding for WDR5 maintenance of active chromatin and embryonic stem cell pluripotency. Elife 2014, 3, e02046. [Google Scholar] [CrossRef]
- Fromm, M.; Avramova, Z. ATX1/AtCOMPASS and the H3K4me3 marks: How do they activate Arabidopsis genes? Curr. Opin. Plant Biol. 2014, 21, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Cao, L.; Rong, L.; An, Z.; Zhou, W.; Ma, J.; Shen, W.H.; Zhu, Y.; Dong, A. The chromatin-remodeling factor AtINO80 plays crucial roles in genome stability maintenance and in plant development. Plant J. Cell Mol. Biol. 2015, 82, 655–668. [Google Scholar] [CrossRef] [PubMed]
- Berr, A.; McCallum, E.J.; Menard, R.; Meyer, D.; Fuchs, J.; Dong, A.; Shen, W.H. Arabidopsis SET DOMAIN GROUP2 is required for H3K4 trimethylation and is crucial for both sporophyte and gametophyte development. Plant Cell 2010, 22, 3232–3248. [Google Scholar] [CrossRef]
- Li, F.; Cheng, C.; Cui, F.; de Oliveira, M.V.; Yu, X.; Meng, X.; Intorne, A.C.; Babilonia, K.; Li, M.; Li, B.; et al. Modulation of RNA polymerase II phosphorylation downstream of pathogen perception orchestrates plant immunity. Cell Host Microbe 2014, 16, 748–758. [Google Scholar] [CrossRef] [PubMed]
- Jiang, P.; Wang, S.; Zheng, H.; Li, H.; Zhang, F.; Su, Y.; Xu, Z.; Lin, H.; Qian, Q.; Ding, Y. SIP1 participates in regulation of flowering time in rice by recruiting OsTrx1 to Ehd1. New Phytol. 2018, 219, 422–435. [Google Scholar] [CrossRef]
- Doi, K.; Izawa, T.; Fuse, T.; Yamanouchi, U.; Kubo, T.; Shimatani, Z.; Yano, M.; Yoshimura, A. Ehd1, a B-type response regulator in rice, confers short-day promotion of flowering and controls FT-like gene expression independently of Hd1. Genes Dev. 2004, 18, 926–936. [Google Scholar] [CrossRef]
- Alvarez-Venegas, R.; Pien, S.; Sadder, M.; Witmer, X.; Grossniklaus, U.; Avramova, Z. ATX-1, an Arabidopsis homolog of trithorax, activates flower homeotic genes. Curr. Biol. 2003, 13, 627–637. [Google Scholar] [CrossRef]
- Thorstensen, T.; Grini, P.E.; Mercy, I.S.; Alm, V.; Erdal, S.; Aasland, R.; Aalen, R.B. The Arabidopsis SET-domain protein ASHR3 is involved in stamen development and interacts with the bHLH transcription factor ABORTED MICROSPORES (AMS). Plant Mol. Biol. 2008, 66, 47–59. [Google Scholar] [CrossRef]
- Wu, M.F.; Yamaguchi, N.; Xiao, J.; Bargmann, B.; Estelle, M.; Sang, Y.; Wagner, D. Auxin-regulated chromatin switch directs acquisition of flower primordium founder fate. Elife 2015, 4, e09269. [Google Scholar] [CrossRef] [PubMed]
- Jing, Y.; Guo, Q.; Zha, P.; Lin, R. The chromatin-remodelling factor PICKLE interacts with CONSTANS to promote flowering in Arabidopsis. Plant Cell Env. 2019, 42, 2495–2507. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, J.C. The ULTRAPETALA gene controls shoot and floral meristem size in Arabidopsis. Development 2001, 128, 1323–1333. [Google Scholar] [CrossRef] [PubMed]
- Napsucialy-Mendivil, S.; Alvarez-Venegas, R.; Shishkova, S.; Dubrovsky, J.G. Arabidopsis homolog of trithorax1 (ATX1) is required for cell production, patterning, and morphogenesis in root development. J. Exp. Bot. 2014, 65, 6373–6384. [Google Scholar] [CrossRef]
- Yao, X.; Feng, H.; Yu, Y.; Dong, A.; Shen, W.H. SDG2-mediated H3K4 methylation is required for proper Arabidopsis root growth and development. PLoS ONE 2013, 8, e56537. [Google Scholar] [CrossRef]
- Xu, L.; Zhao, Z.; Dong, A.; Soubigou-Taconnat, L.; Renou, J.P.; Steinmetz, A.; Shen, W.H. Di- and tri- but not monomethylation on histone H3 lysine 36 marks active transcription of genes involved in flowering time regulation and other processes in Arabidopsis thaliana. Mol. Cell Biol. 2008, 28, 1348–1360. [Google Scholar] [CrossRef]
- Yang, S.; Li, C.; Zhao, L.; Gao, S.; Lu, J.; Zhao, M.; Chen, C.Y.; Liu, X.; Luo, M.; Cui, Y.; et al. The Arabidopsis SWI2/SNF2 Chromatin Remodeling ATPase BRAHMA Targets Directly to PINs and Is Required for Root Stem Cell Niche Maintenance. Plant Cell 2015, 27, 1670–1680. [Google Scholar] [CrossRef]
- Wagner, D.; Meyerowitz, E.M. SPLAYED, a novel SWI/SNF ATPase homolog, controls reproductive development in Arabidopsis. Curr. Biol. 2002, 12, 85–94. [Google Scholar] [CrossRef]
- Kang, H.; Zhang, C.; An, Z.; Shen, W.H.; Zhu, Y. AtINO80 and AtARP5 physically interact and play common as well as distinct roles in regulating plant growth and development. New Phytol. 2019, 223, 336–353. [Google Scholar] [CrossRef]
- Wang, X.; Gao, J.; Gao, S.; Li, Z.; Kuai, B.; Ren, G. REF6 promotes lateral root formation through de-repression of PIN1/3/7 genes. J. Integr. Plant Biol. 2019, 61, 383–387. [Google Scholar] [CrossRef]
- Quint, M.; Delker, C.; Franklin, K.A.; Wigge, P.A.; Halliday, K.J.; van Zanten, M. Molecular and genetic control of plant thermomorphogenesis. Nat. Plants 2016, 2, 15190. [Google Scholar] [CrossRef] [PubMed]
- Casal, J.J.; Balasubramanian, S. Thermomorphogenesis. Annu. Rev. Plant Biol. 2019, 70, 321–346. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.T.; Zhang, L.L.; Han, J.J.; Zhou, M.; Liu, J.X. Histone H3K4 methyltransferases SDG25 and ATX1 maintain heat-stress gene expression during recovery in Arabidopsis. Plant J. Cell Mol. Biol. 2021, 105, 1326–1338. [Google Scholar] [CrossRef]
- Zhang, Y.; Tian, Y.Y.; Wang, L.F.; Li, Y.H.; Li, T.T.; Liu, W.C. WDR5a functions in cadmium-inhibited root meristem growth by regulating nitric oxide accumulation in Arabidopsis. Planta 2020, 252, 78. [Google Scholar] [CrossRef]
- Liu, W.C.; Zheng, S.Q.; Yu, Z.D.; Gao, X.; Shen, R.; Lu, Y.T. WD40-REPEAT 5a represses root meristem growth by suppressing auxin synthesis through changes of nitric oxide accumulation in Arabidopsis. Plant J. Cell Mol. Biol. 2018, 93, 883–893. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.C.; Li, Y.H.; Yuan, H.M.; Zhang, B.L.; Zhai, S.; Lu, Y.T. WD40-REPEAT 5a functions in drought stress tolerance by regulating nitric oxide accumulation in Arabidopsis. Plant Cell Env. 2017, 40, 543–552. [Google Scholar] [CrossRef]
- van Dijk, K.; Ding, Y.; Malkaram, S.; Riethoven, J.J.; Liu, R.; Yang, J.; Laczko, P.; Chen, H.; Xia, Y.; Ladunga, I.; et al. Dynamic changes in genome-wide histone H3 lysine 4 methylation patterns in response to dehydration stress in Arabidopsis thaliana. BMC Plant Biol. 2010, 10, 238. [Google Scholar] [CrossRef]
- Ding, Y.; Avramova, Z.; Fromm, M. The Arabidopsis trithorax-like factor ATX1 functions in dehydration stress responses via ABA-dependent and ABA-independent pathways. Plant J. Cell Mol. Biol. 2011, 66, 735–744. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, A.; Yin, H.; Meng, Q.; Yu, X.; Huang, S.; Wang, J.; Ahmad, R.; Liu, B.; Xu, Z.Y. Trithorax-group proteins ARABIDOPSIS TRITHORAX4 (ATX4) and ATX5 function in abscisic acid and dehydration stress responses. New Phytol. 2018, 217, 1582–1597. [Google Scholar] [CrossRef]
- Miura, K.; Renhu, N.; Suzaki, T. The PHD finger of Arabidopsis SIZ1 recognizes trimethylated histone H3K4 mediating SIZ1 function and abiotic stress response. Commun. Biol. 2020, 3, 23. [Google Scholar] [CrossRef]
- Tyler, L.; Miller, M.J.; Fletcher, J.C. The Trithorax Group Factor ULTRAPETALA1 Regulates Developmental as Well as Biotic and Abiotic Stress Response Genes in Arabidopsis. G3 2019, 9, 4029–4043. [Google Scholar] [CrossRef] [PubMed]
- Wittstock, U.; Burow, M. Glucosinolate breakdown in Arabidopsis: Mechanism, regulation and biological significance. Arab. Book 2010, 8, e0134. [Google Scholar] [CrossRef] [PubMed]
- De-La-Pena, C.; Rangel-Cano, A.; Alvarez-Venegas, R. Regulation of disease-responsive genes mediated by epigenetic factors: Interaction of Arabidopsis-Pseudomonas. Mol. Plant Pathol. 2012, 13, 388–398. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Menard, R.; Li, Y.; Coruzzi, G.M.; Heitz, T.; Shen, W.H.; Berr, A. Arabidopsis SDG8 Potentiates the Sustainable Transcriptional Induction of the Pathogenesis-Related Genes PR1 and PR2 During Plant Defense Response. Front. Plant Sci. 2020, 11, 277. [Google Scholar] [CrossRef]
- Avramova, Z. Transcriptional ‘memory’ of a stress: Transient chromatin and memory (epigenetic) marks at stress-response genes. Plant J. Cell Mol. Biol. 2015, 83, 149–159. [Google Scholar] [CrossRef]
- Cazzonelli, C.I.; Nisar, N.; Roberts, A.C.; Murray, K.D.; Borevitz, J.O.; Pogson, B.J. A chromatin modifying enzyme, SDG8, is involved in morphological, gene expression, and epigenetic responses to mechanical stimulation. Front. Plant Sci. 2014, 5, 533. [Google Scholar] [CrossRef]
- Ding, Y.; Fromm, M.; Avramova, Z. Multiple exposures to drought ‘train’ transcriptional responses in Arabidopsis. Nat. Commun. 2012, 3, 740. [Google Scholar] [CrossRef]
- Farrona, S.; Hurtado, L.; March-Diaz, R.; Schmitz, R.J.; Florencio, F.J.; Turck, F.; Amasino, R.M.; Reyes, J.C. Brahma is required for proper expression of the floral repressor FLC in Arabidopsis. PLoS ONE 2011, 6, e17997. [Google Scholar] [CrossRef]
- Otvos, K.; Miskolczi, P.; Marhavy, P.; Cruz-Ramirez, A.; Benkova, E.; Robert, S.; Bako, L. Pickle Recruits Retinoblastoma Related 1 to Control Lateral Root Formation in Arabidopsis. Int. J. Mol. Sci. 2021, 22, 3862. [Google Scholar] [CrossRef]
- Kuwabara, A.; Gruissem, W. Arabidopsis RETINOBLASTOMA-RELATED and Polycomb group proteins: Cooperation during plant cell differentiation and development. J. Exp. Bot. 2014, 65, 2667–2676. [Google Scholar] [CrossRef]
- Xu, F.; Kuo, T.; Rosli, Y.; Liu, M.S.; Wu, L.; Chen, L.O.; Fletcher, J.C.; Sung, Z.R.; Pu, L. Trithorax Group Proteins Act Together with a Polycomb Group Protein to Maintain Chromatin Integrity for Epigenetic Silencing during Seed Germination in Arabidopsis. Mol. Plant. 2018, 11, 659–677. [Google Scholar] [CrossRef] [PubMed]
- Barski, A.; Cuddapah, S.; Cui, K.; Roh, T.Y.; Schones, D.E.; Wang, Z.; Wei, G.; Chepelev, I.; Zhao, K. High-resolution profiling of histone methylations in the human genome. Cell 2007, 129, 823–837. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, K.; Yin, L.; Yu, Y.; Qi, J.; Shen, W.H.; Zhu, J.; Zhang, Y.; Dong, A. H3K4me2 functions as a repressive epigenetic mark in plants. Epigenetics Chromatin 2019, 12, 40. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, D.; Xu, W.; Kong, L.; Ye, X.; Zhuang, Q.; Fan, D.; Luo, K. Histone methyltransferase ATX1 dynamically regulates fiber secondary cell wall biosynthesis in Arabidopsis inflorescence stem. Nucleic Acids Res. 2021, 49, 190–205. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.; Romero-Campero, F.J.; de Los Reyes, P.; Yan, P.; Yang, J.; Tian, G.; Yang, X.; Mo, X.; Zhao, S.; Calonje, M.; et al. H2AK121ub in Arabidopsis associates with a less accessible chromatin state at transcriptional regulation hotspots. Nat. Commun. 2021, 12, 315. [Google Scholar] [CrossRef]
- Wang, Y.; Fan, Y.; Fan, D.; Zhang, Y.; Zhou, X.; Zhang, R.; Wang, Y.; Sun, Y.; Zhang, W.; He, Y.; et al. The Arabidopsis DREAM complex antagonizes WDR5A to modulate histone H3K4me2/3 deposition for a subset of genome repression. Proc. Natl. Acad. Sci. USA 2022, 119, e2206075119. [Google Scholar] [CrossRef]
- Sadasivam, S.; DeCaprio, J.A. The DREAM complex: Master coordinator of cell cycle-dependent gene expression. Nat. Rev. Cancer 2013, 13, 585–595. [Google Scholar] [CrossRef]

| Classification | Plants | Flies | Mammals | Function | Domain | |
|---|---|---|---|---|---|---|
| Arabidopsis | Rice | |||||
| Histone methyltransferases (HMTs) | ATX1/SDG27 [16] ATX2/SDG30 [17] ATX3/SDG14 [18] ATX4/SDG16 ATX5/SDG29 [18] | OsTrx1 /SDG723 [19] OSDG721 [20] OSDG705 [20] | Trx Trr | MLL1/2 MLL 3/4 | H3K4 HMT H3K4 HMT H3K4 HMT | SET SET SET |
| ATXR2/SDG36 [21] ATXR3/SDG2 [22] | OSDG701 [23] | H3K4/36, HMT H3K4 HMT | SET SET | |||
ATXR7/SDG25 [24,25] | Set1 | SET1 A/B | H3K36/4, HMT | SET | ||
| ASHH2/SDG8/EFS [26] | OsSDG724 and OsSDG725 [27,28] | Ash1 | ASH1L | H3K36 HMT | SET | |
| ASHH1/SDG26 [29] | OsSDG708 [30] | H3K36/4, HMT | SET | |||
| ASHR3/SDG4 [31] | H3K36/4, HMT | SET | ||||
| COMPASS Core | WDR5a [32] | OsWDR5a [33] | Wds | WDR5 | Histone binding | WD40 |
| ASH2R/TRO [34] | OsASHL1 OsASHL2 OsASHL3 [35] | Ash2 | ASH2L | DNA binding | Zinc Finger | |
| RBL [34] | OsRBL [35] | Rbbp5 | RBBP5 | Histone binding | WD40 | |
| S2Lb [36] | - | WDR82 | Histone binding | WD40 | ||
| Chromatin Remodelers | SWI/SNF: BRM [37] | BAP | BRM/BRG1 | ATP-dependent chromatin remodeler | Bromodomain | |
| SYD [38] | ATP-dependent chromatin remodeler | Bromodomain | ||||
| INO80 [39] | INO80 | INO80 | ATP-dependent chromatin remodeler | DBINO | ||
| CHD3: PKL [40] | OsCHR729 [41] | CHD3 | CHD3 | ATP-dependent chromatin remodeler | CHD | |
| Histone Demethylases | ELF6 REF6 JMJ13 [42] | OsJMJ705 [43] | Utx | JmjC proteins | H3K27 demethylase | jmjC |
| JMJ24 JMJ26 JMJ28 [39] | OsJMJ706 [44] | H3K9 demethylase | jmjC | |||
| Co-activators | ULT1/2 | OsULT1 [45] | - | - | ATX1 coactivator | SAND |
| SEC | Esc (Polycomb gene) | OGT | ATX1 coactivator | O-linked N-acetylglucosamine transferase | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ornelas-Ayala, D.; Cortés-Quiñones, C.; Olvera-Herrera, J.; García-Ponce, B.; Garay-Arroyo, A.; Álvarez-Buylla, E.R.; Sanchez, M.d.l.P. A Green Light to Switch on Genes: Revisiting Trithorax on Plants. Plants 2023, 12, 75. https://doi.org/10.3390/plants12010075
Ornelas-Ayala D, Cortés-Quiñones C, Olvera-Herrera J, García-Ponce B, Garay-Arroyo A, Álvarez-Buylla ER, Sanchez MdlP. A Green Light to Switch on Genes: Revisiting Trithorax on Plants. Plants. 2023; 12(1):75. https://doi.org/10.3390/plants12010075
Chicago/Turabian StyleOrnelas-Ayala, Diego, Carlos Cortés-Quiñones, José Olvera-Herrera, Berenice García-Ponce, Adriana Garay-Arroyo, Elena R. Álvarez-Buylla, and Maria de la Paz Sanchez. 2023. "A Green Light to Switch on Genes: Revisiting Trithorax on Plants" Plants 12, no. 1: 75. https://doi.org/10.3390/plants12010075
APA StyleOrnelas-Ayala, D., Cortés-Quiñones, C., Olvera-Herrera, J., García-Ponce, B., Garay-Arroyo, A., Álvarez-Buylla, E. R., & Sanchez, M. d. l. P. (2023). A Green Light to Switch on Genes: Revisiting Trithorax on Plants. Plants, 12(1), 75. https://doi.org/10.3390/plants12010075






