Sterols and Sphingolipids as New Players in Cell Wall Building and Apical Growth of Nicotiana tabacum L. Pollen Tubes
Abstract
1. Introduction
2. Results
2.1. Squalestatin and Myriocin Alter Lipid Profile and Pollen Tube Length
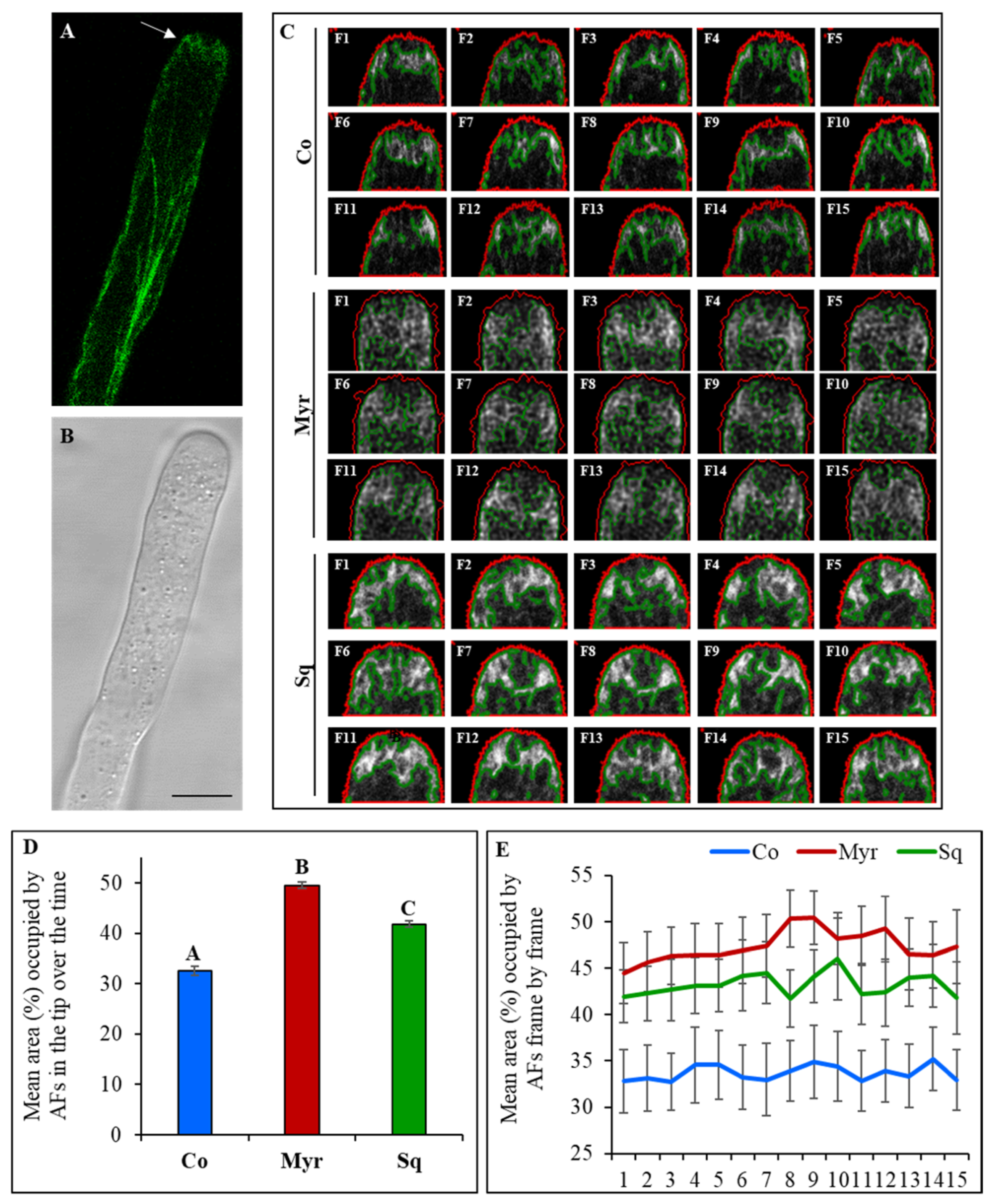
2.2. Squalestatin and Myriocin Alter the Morphology and Dynamics of the Actin Fringe
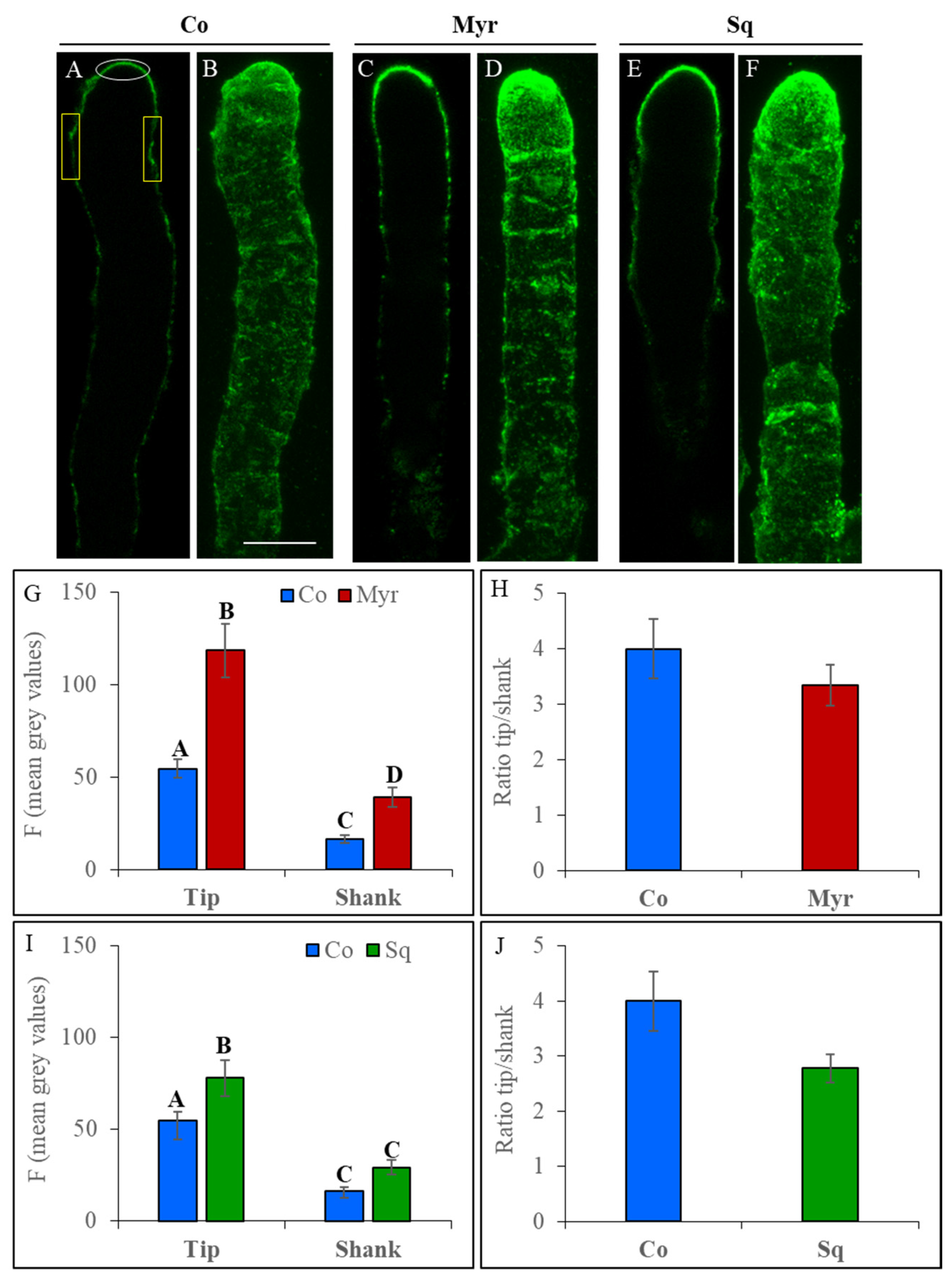
2.3. Sterols and Sphingolipids Regulate Cell Wall Deposition
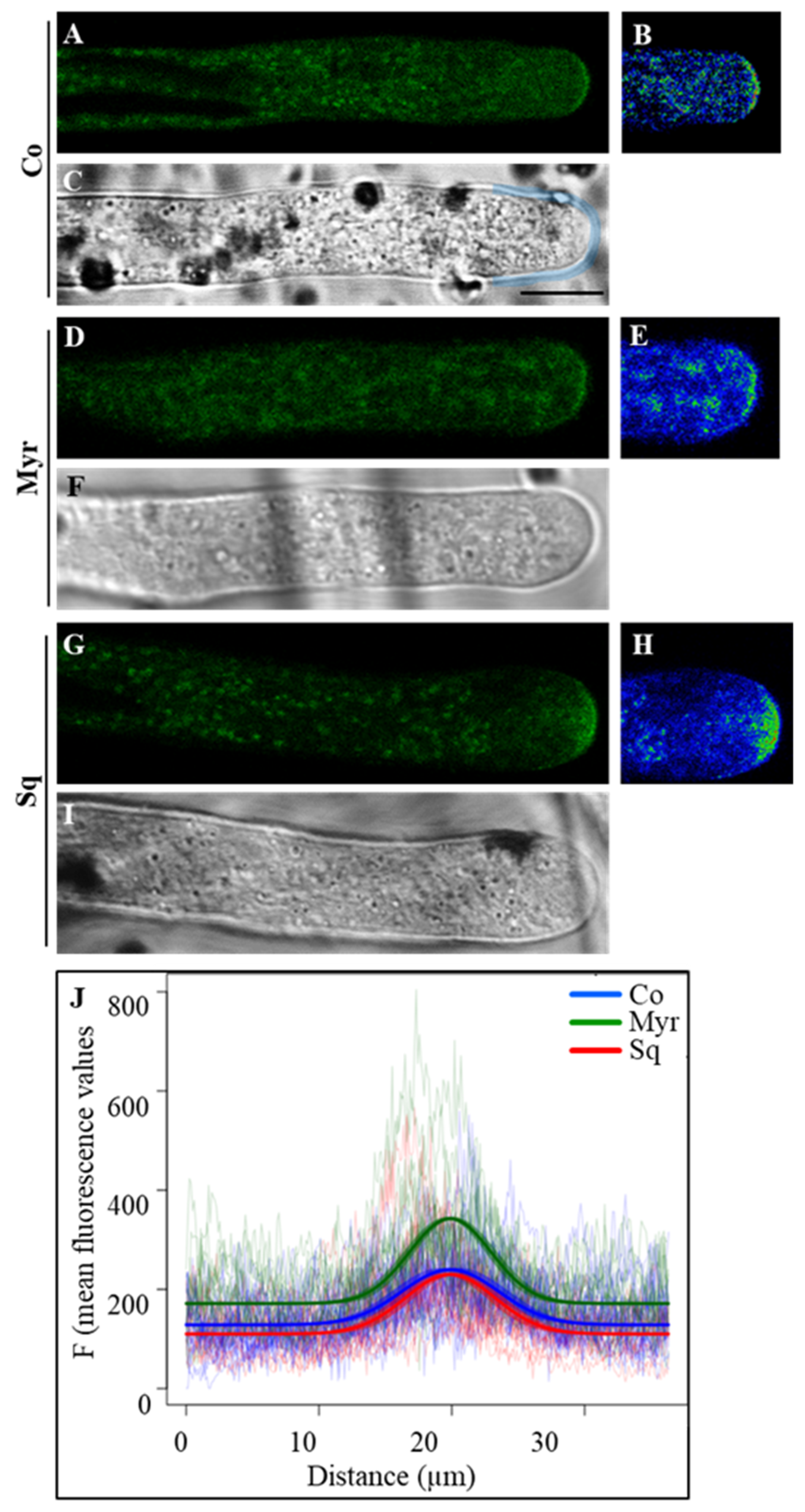
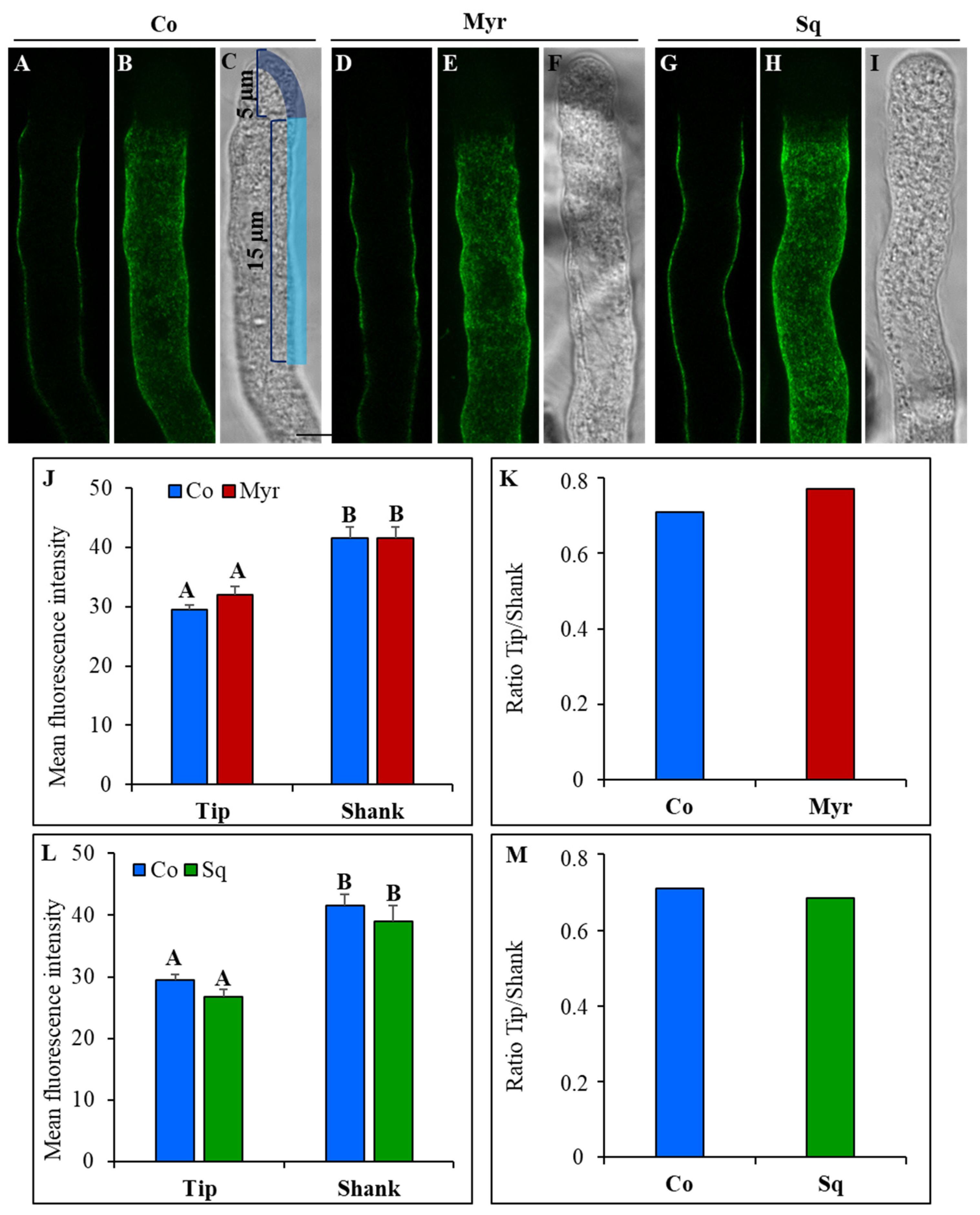
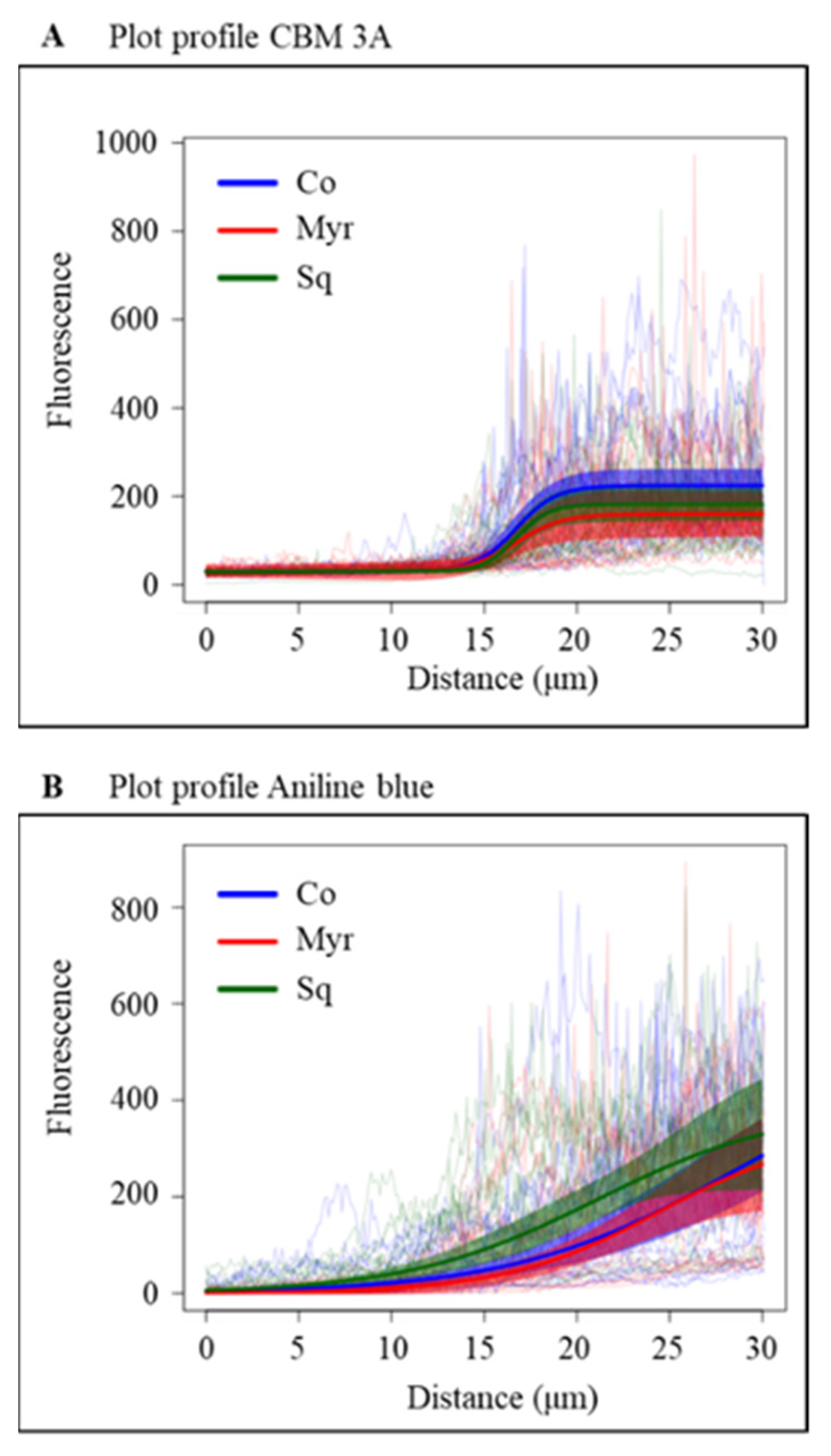
2.3.1. Pectin Deposition
2.3.2. Cellulose and Callose Deposition
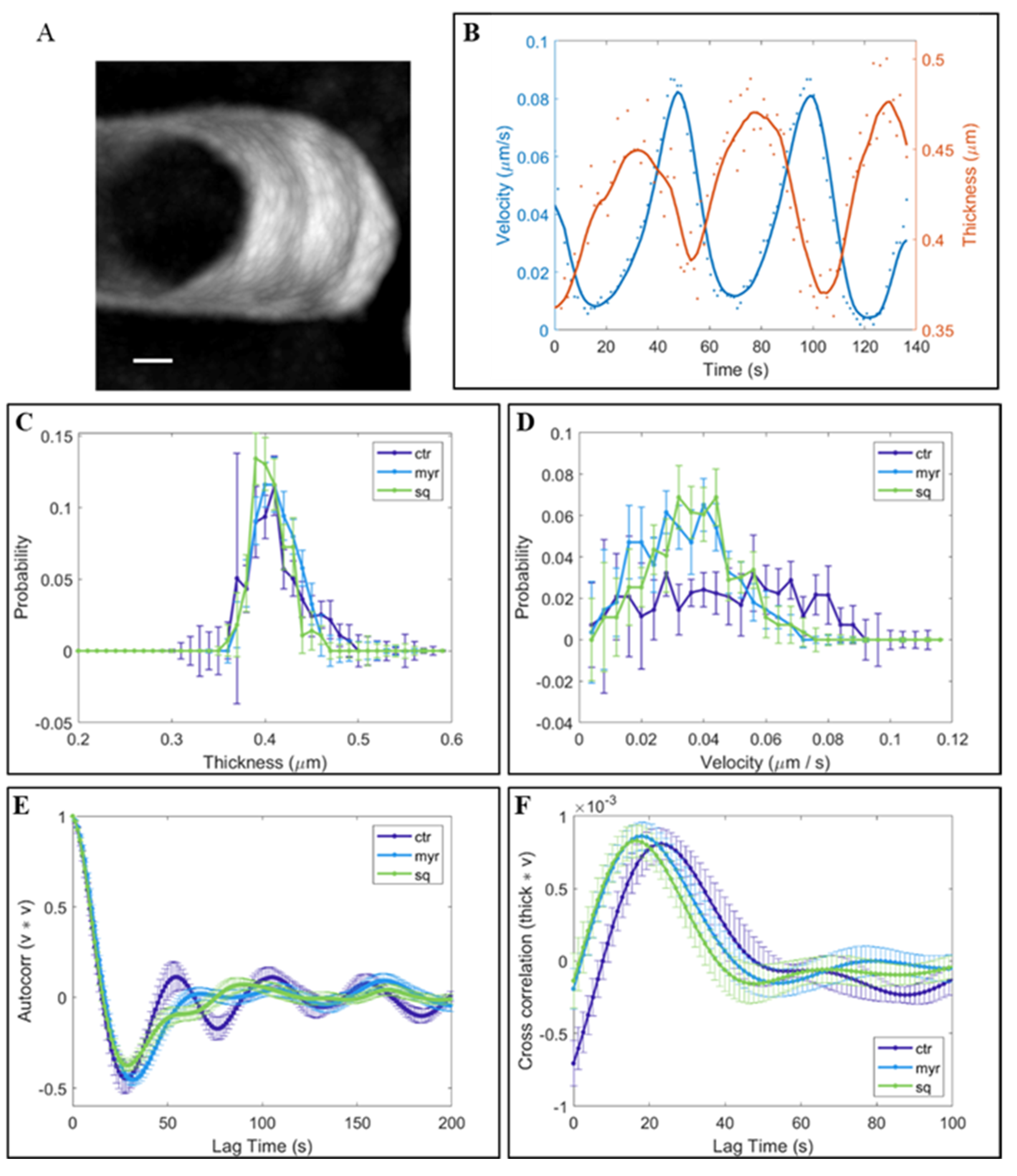
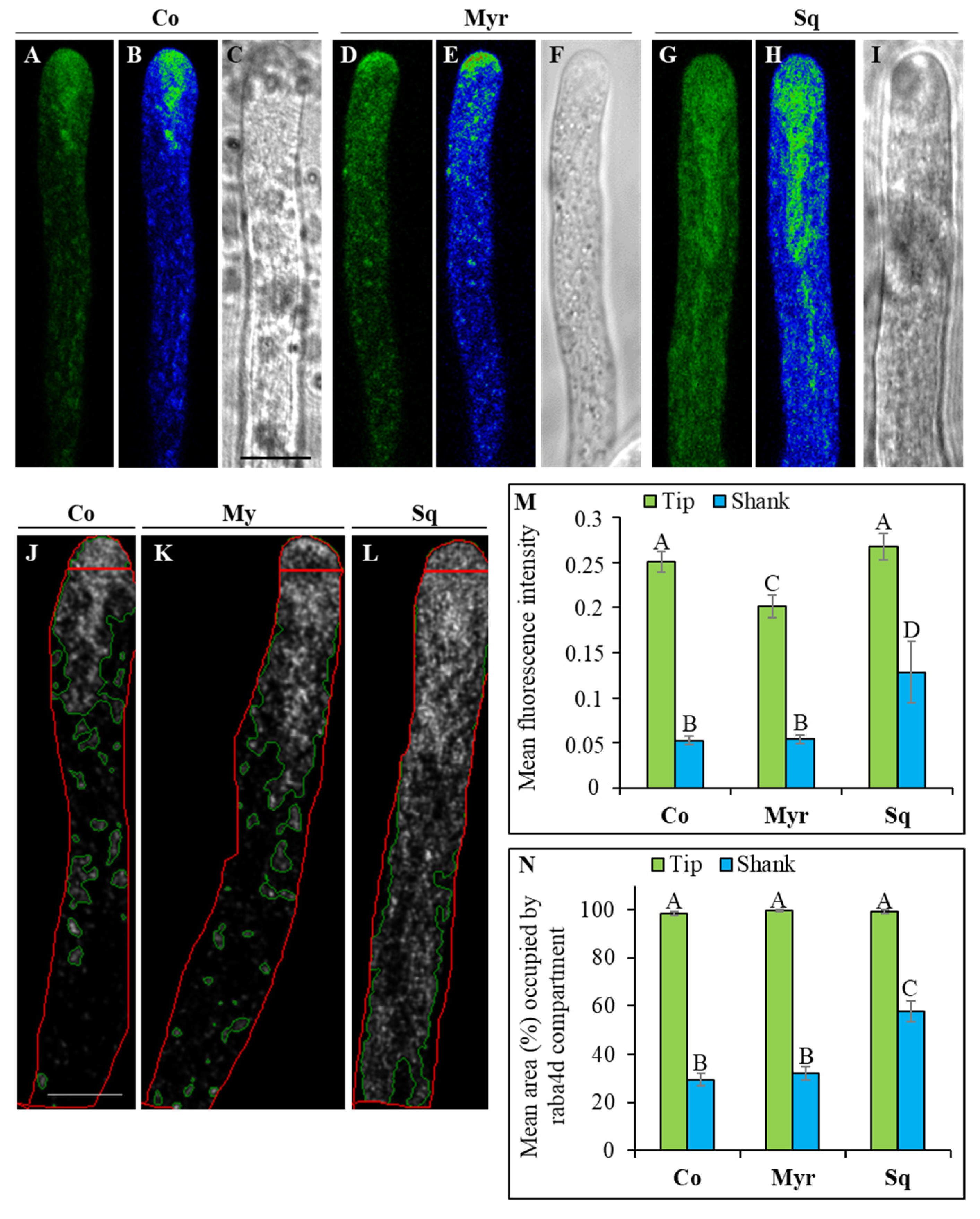
2.4. Investigating the Morphology and Dynamics of the Clear Zone
3. Discussion
3.1. Sterols and Sphingolipids Play a Role in Modulating Actin Filament Organization and Dynamics in the Apical Region and in Defining Pollen Tube Pulsed Growth
3.2. Pollen Tube Cell Wall Building Requires Integrity of Sterol/Sphingolipid or Ganization in Membranes
3.3. The Morphology and Dynamics of the Clear Zone Depend on Stability of the Sterol/Sphingolipid Ratio
4. Conclusions
5. Materials and Methods
5.1. Probes and Drugs
5.2. Germination Assay and Pollen Tube Measurement
5.3. Pollen Tube Microsomes
5.4. Lipid Analysis
5.5. SDS-PAGE, Western Blot
5.6. Immunofluorescence Analysis
5.7. GFP-CBM3A and Aniline Blue Staining
5.8. Filipin Staining
5.9. Transient Gene Expression
5.10. Tube Growth Data Analysis
5.11. Statistical Analysis of Fluorescence and Profile Variation of NtPPME1, GFP-CBM3A and Aniline Blue
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Heslop-Harrison, J. Pollen germination and pollen tube growth. Int. Rev. Cytol. 1987, 107, 1–78. [Google Scholar] [CrossRef]
- Steer, M.W.; Steer, J.M. Pollen tube tip growth. New Phytol. 1989, 111, 323–358. [Google Scholar] [CrossRef] [PubMed]
- Holdaway-Clarke, T.L.; Hepler, P.K. Control of pollen tube growth: Role of ion gradients and fluxes. New Phytol. 2003, 159, 539–563. [Google Scholar] [CrossRef] [PubMed]
- Chebli, Y.; Geitmann, A. Mechanical principles governing pollen tube growth. Funct. Plant Sci. Biotechnol. 2007, 1, 232–245. [Google Scholar]
- Krichevsky, A.; Kozlovsky, S.V.; Tian, G.W.; Chen, M.H.; Zaltsman, A.; Citovsky, V. How pollen tubes grow. Develop. Biol. 2007, 303, 405–420. [Google Scholar] [CrossRef]
- Cheung, A.Y.; Wu, H.M. Structural and signalling networks for the polar cell growth machinery in pollen tubes. Ann. Rev. Plant Biol. 2008, 59, 547–572. [Google Scholar] [CrossRef]
- Michard, E.; Simon, A.A.; Tavares, B.; Wudick, M.M.; Feijó, J.A. Signaling with ions: The keystone for apical cell growth and morphogenesis in pollen tubes. Plant. Physiol. 2017, 173, 91–111. [Google Scholar] [CrossRef]
- Guo, J.; Yang, Z. Exocytosis and endocytosis: Coordinating and fine-tuning the polar tip growth domain in pollen tubes. J. Exp. Bot. 2020, 71, 2428–2438. [Google Scholar] [CrossRef]
- Heslop-Harrison, J.; Heslop-Harrison, Y. The actin cytoskeleton in unfixed pollen tubes following microwave-accelerated DMSO permeabilization and TRITC-phalloidin staining. Sex. Plant Reprod. 1991, 4, 6–11. [Google Scholar] [CrossRef]
- Vidali, L.; McKenna, S.T.; Hepler, P.K. Actin polymerization is essential for pollen tube growth. Mol. Biol. Cell 2001, 12, 2534–2545. [Google Scholar] [CrossRef]
- Cárdenas, L.; Lovy-Wheeler, A.; Wilsen, K.L.; Hepler, P.K. Actin polymerization promotes the reversal of streaming in the apex of pollen tubes. Cell Motil. Cytoskelet. 2005, 61, 112–127. [Google Scholar] [CrossRef] [PubMed]
- Onelli, E.; Moscatelli, A. Endocytic pathways and recycling in growing pollen tubes. Plants 2013, 2, 211–229. [Google Scholar] [CrossRef] [PubMed]
- Hazan, I.; Liu, H. Hyphal tip-associated localization of cdc42 is F-actin dependent in Candida albicans. Eukar. Cell 2002, 1, 856–864. [Google Scholar] [CrossRef] [PubMed]
- Mañes, S.; Ana Lacalle, R.; Gómez-Moutón, C.; Martínez, A.C. From rafts to crafts: Membrane asymmetry in moving cells. Trends Immunol. 2003, 24, 320–326. [Google Scholar] [CrossRef] [PubMed]
- Martin, S.W.; Konopka, J.B. Lipid raft polarization contributes to hyphal growth in Candida albicans. Eukar. Cell 2004, 3, 675–684. [Google Scholar] [CrossRef]
- Moscatelli, A.; Gagliardi, A.; Maneta-Peyret, L.; Bini, L.; Stroppa, N.; Onelli, E.; Scali, M.; Idilli, A.I.; Moreau, P. Characterization of detergent-insoluble membranes in pollen tubes of Nicotiana tabacum (L.). Biol. Open 2015, 4, 378–399. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Fratini, M.; Krishnamoorthy, P.; Stenzel, I.; Riechmann, M.; Matzner, M.; Bacia, K.; Heilmann, M.; Heilmann, I. Plasma membrane nano-organization specifies phosphoinositide effects on Rho-GTPases and actin dynamics in tobacco pollen tubes. Plant Cell 2021, 33, 642–670, Erratum in: Plant Cell 2021, 33, 3176. [Google Scholar] [CrossRef] [PubMed]
- Ovecka, M.; Berson, T.; Beck, M.; Derksen, J.; Samaj, J.; Baluska, F.; Lichtscheidl, I.K. Structural sterols are involved in both the initiation and tip growth of root hairs in Arabidopsis thaliana. Plant Cell 2010, 22, 2999–3019. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Zhang, X.; Qu, Y.; Li, R.; Baluška, F.; Wan, Y. Mapping of membrane lipid order in root apex zones of Arabidopsis thaliana. Front. Plant Sci. 2015, 6, 1151. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Li, R.; Lu, C.; Baluška, F.; Wan, Y. Di-4-ANEPPDHQ, a fluorescent probe for the visualisation of membrane microdomains in living Arabidopsis thaliana cells. Plant Physiol. Biochem. 2015, 87, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Simons, K.; van Meer, G. Lipid sorting in epithelial cells. Biochemistry 1988, 27, 6197–6202. [Google Scholar] [CrossRef] [PubMed]
- Rosenberger, C.M.; Brumell, J.H.; Finlay, B.B. Microbial pathogenesis: Lipid rafts as pathogen portals. Curr. Biol. 2000, 10, R823–R825. [Google Scholar] [CrossRef] [PubMed]
- Hoessli, D.C.; Ilangumaran, S.; Soltermann, A.; Robinson, P.J.; Borisch, B.; Din, N. Signaling through sphingolipid microdomains of the plasma membrane: The concept of signaling platform. Glycoconj. J. 2000, 17, 191–197. [Google Scholar] [CrossRef] [PubMed]
- London, E. Insights into lipid raft structure and formation from experiments in model membranes. Curr. Opin. Struct. Biol. 2002, 12, 480–486. [Google Scholar] [CrossRef]
- Salun, C.; James, D.J.; Chamberlain, L.H. Lipid rafts and the regulation of exocytosis. Traffic 2004, 5, 255–264. [Google Scholar] [CrossRef]
- Rajendran, L.; Simons, K. Lipid rafts and membrane dynamics. J. Cell Sci. 2005, 118, 1099–1102. [Google Scholar] [CrossRef]
- Simons, K.; Sampaio, J.L. Membrane organization and lipid rafts. Cold Spring Harb. Perspect. Biol. 2011, 3, a004697. [Google Scholar] [CrossRef]
- Mongrand, S.; Morel, J.; Laroche, J.; Claverol, S.; Carde, J.P.; Hartman, M.A.; Bonneu, M.; Simon Plas, F.; Lessire, R.; Bessoule, J.J. Lipid rafts in higher plant cells: Purification and characterization of Triton X-100-insoluble microdomains from tobacco plasma membrane. J. Biol. Chem. 2004, 279, 36277–36286. [Google Scholar] [CrossRef]
- Cacas, J.L.; Furt, F.; Le Guedard, M.; Schmitter, J.M.; Bure’, C.; Gerbeau-Pissot, P.; Moreau, P.; Bessoule, J.J.; Simon-Plas, F.; Mongrand, S. Lipids of plant membrane rafts. Prog. Lipid Res. 2012, 51, 272–299. [Google Scholar] [CrossRef]
- Mamode Cassim, A.; Gouguet, P.; Gronnier, J.; Laurent, N.; Germain, V.; Grison, M.; Boutté, Y.; Gerbeau-Pissot, P.; Simon-Plas, F.; Mongrand, S. Plant lipids: Key players of plasma membrane organization and function. Prog. Lipid Res. 2019, 73, 1–27. [Google Scholar] [CrossRef]
- Furt, F.; Konig, S.; Bessoule, J.J.; Sargueil, F.; Zallot, R.; Stanislas, T.; Noirot, E.; Lherminier, J.; Simon-Plas, F.; Heilmann, I.; et al. Polyphosphoinositides are enriched in plant membrane rafts and form microdomains in the plasma membrane. Plant Physiol. 2010, 152, 2173–2187. [Google Scholar] [CrossRef] [PubMed]
- Ischebeck, T.; Stenzel, I.; Hempel, F.; Jin, X.; Mosblech, A.; Heilmann, I. Phosphatidylinositol-4,5-bisphosphate influences Nt-Rac5-mediated cell expansion in pollen tubes of Nicotiana tabacum. Plant J. 2011, 65, 453–468. [Google Scholar] [CrossRef]
- Heilmann, I.; Ischebeck, T. Male functions and malfunctions: The impact of phosphoinositides on pollen development and pollen tube growth. Plant Reprod. 2016, 29, 3–20. [Google Scholar] [CrossRef] [PubMed]
- Borner, G.H.; Sherrier, D.J.; Weimar, T.; Michaelson, L.V.; Hawkins, N.D.; Macaskill, A.; Napier, J.A.; Beale, M.H.; Lilley, K.S.; Dupree, P. Analysis of detergent-resistant membranes in Arabidopsis. Evidence for plasma membrane lipid rafts. Plant Physiol. 2005, 137, 104–116. [Google Scholar] [CrossRef] [PubMed]
- Lefebvre, B.; Furt, F.; Hartmann, M.A.; Michaelson, L.V.; Carde, J.P.; Sargueil-Boiron, F.; Rossignol, M.; Napier, J.A.; Cullimore, J.; Bessoule, J.J.; et al. Characterization of lipid rafts from Medicago truncatula root plasma membranes: A proteomic study reveals the presence of a raft-associated redox system. Plant Physiol. 2007, 144, 402–418. [Google Scholar] [CrossRef] [PubMed]
- McKenna, J.F.; Tolmie, A.F.; Runions, J. Across the great divide: The plant cell surface continuum. Curr. Opin. Plant Biol. 2014, 22, 132–140. [Google Scholar] [CrossRef]
- Szymanski, D.B.; Cosgrove, D.J. Dynamic coordination of cytoskeletal and cell wall systems during plant cell morphogenesis. Curr. Biol. 2009, 19, R800–R811. [Google Scholar] [CrossRef]
- Szymanski, W.G.; Zauber, H.; Erban, A.; Gorka, M.; Wu, X.N.; Schulze, W.X. Cytoskeletal components define protein location to membrane microdomains. Mol. Cell Proteom. 2015, 14, 2493–2509. [Google Scholar] [CrossRef]
- Lv, X.; Jing, Y.; Xiao, J.; Zhang, Y.; Zhu, Y.; Julian, R.; Lin, J. Membrane microdomains and the cytoskeleton constrain AtHIR1 dynamics and facilitate the formation of an AtHIR1-associated immune complex. Plant J. 2017, 90, 3–16. [Google Scholar] [CrossRef]
- Yu, M.; Cui, Y.; Zhang, X.; Li, R.; Lin, J. Organization and dynamics of functional plant membrane microdomains. Cell. Mol. Life Sci. 2020, 77, 275–287. [Google Scholar] [CrossRef]
- Martiniere, A.; Lavagi, I.; Nageswaran, G.; Rolfe, D.J.; Maneta-Peyret, L.; Luu, D.T.; Botchway, S.W.; Webb, S.E.; Mongrand, S.; Maurel, C.; et al. Cell wall constrains lateral diffusion of plant plasma-membrane proteins. Proc. Natl. Acad. Sci. USA 2012, 109, 12805–12810. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.Q.; Chen, F.; Linskens, H.F.; Cresti, M. Distribution of unesterified and esterified pectins in cell wall of pollen tubes. Sex. Plant Reprod. 1994, 7, 145–152. [Google Scholar] [CrossRef]
- Taylor, L.P.; Hepler, P.K. Pollen germination and tube growth. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1997, 48, 461–491. [Google Scholar] [CrossRef] [PubMed]
- Hepler, P.K.; Vidali, L.; Cheung, A.Y. Polarized cell growth in higher plants. Annu. Rev. Cell Dev. Biol. 2001, 17, 159–187. [Google Scholar] [CrossRef] [PubMed]
- Vogler, H.; Santos-Fernandez, G.; Mecchia, M.A.; Grossniklaus, U. To preserve or to destroy, that is the question: The role of the cell wall integrity pathway in pollen tube growth. Curr. Opin. Plant Biol. 2019, 52, 131–139. [Google Scholar] [CrossRef]
- Lord, E. Adhesion and cell movement during pollination: Cherchez la femme. Trends Plant Sci. 2000, 5, 368–373. [Google Scholar] [CrossRef]
- Li, Y.Q.; Moscatelli, A.; Cai, G.; Cresti, M. Functional interaction among cytoskeleton, membranes and cell wall in the pollen tube of flowering plants. Int. Rev. Cytol. 1997, 176, 133–199. [Google Scholar] [CrossRef]
- Derksen, J.; Janssen, G.J.; Wolters-Arts, M.; Lichtscheidl, I.; Adlassnig, W.; Ovecka, M.; Doris, F.; Steer, M. Wall architecture with high porosity is established at the tip and maintained in growing pollen tubes of Nicotiana tabacum. Plant J. 2011, 68, 495–506. [Google Scholar] [CrossRef]
- Hepler, P.K.; Bosch, M. Pectin methylesterases and pectin dynamics in pollen tubes. Plant Cell 2005, 17, 3219–3226. [Google Scholar] [CrossRef]
- Hepler, P.K.; Rounds, C.M.; Winship, L.J. Control of cell wall extensibility during pollen tube growth. Mol. Plant 2013, 6, 998–1017. [Google Scholar] [CrossRef]
- Wang, H.; Zhuang, X.H.; Cai, Y.; Cheung, A.Y.; Jiang, L.W. Apical F-actin-regulated exocytic targeting of NtPPME1 is essential for construction and rigidity of the pollen tube cell wall. Plant J. 2013, 76, 367–379. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhuang, X.H.; Wang, X.F.; Law, A.H.Y.; Zhao, T.; Du, S.W.; Loy, M.M.; Jiang, L. A distinct pathway for polar exocytosis in plant cell wall formation. Plant Physiol. 2016, 172, 1003–1018. [Google Scholar] [CrossRef] [PubMed]
- Fayant, P.; Girlanda, O.; Chebli, Y.; Aubin, C.-E.; Villemure, I.; Geitmann, A. Finite element model of polar growth in pollen tubes. Plant Cell 2010, 22, 2579–2593. [Google Scholar] [CrossRef]
- Aouar, L.; Chebli, Y.; Geitmann, A. Morphogenesis of complex plant cell shapes: The mechanical role of crystalline cellulose in growing pollen tubes. Sex Plant Reprod. 2010, 23, 15–27. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Wang, L.; Chen, C.; Xiong, G.; Tan, X.Y.; Yang, K.Z.; Wang, Z.C.; Zhou, Y.; Ye, D.; Chen, L.Q. Arabidopsis CSLD1 and CSLD4 are required for cellulose deposition and normal growth of pollen tubes. J. Exp. Bot. 2011, 62, 5161–5177. [Google Scholar] [CrossRef] [PubMed]
- Bessueille, L.; Sindt, N.; Guichardent, M.; Uichardant, M.; Djerbis, S.; Teeri, T.T.; Bulone, V. Plasma membrane microdomains from hybrid aspen cells are involved in cell wall polysaccharide biosynthesis. Biochem. J. 2009, 420, 93–103. [Google Scholar] [CrossRef] [PubMed]
- Alexandersson, E.; Saalbach, G.; Larsson, C.; Kjellbom, P. Arabidopsis plasma membrane proteomics identifies components of transport, signal transduction and membrane trafficking. Plant Cell Physiol. 2004, 45, 1543–1556. [Google Scholar] [CrossRef]
- Turner, A.; Bacic, A.; Harris, P.J.; Read, S.M. Membrane fractionation and enrichment of callose synthase from pollen tubes of Nicotiana alata Link et Otto. Planta 1998, 205, 380–388. [Google Scholar] [CrossRef]
- Brownfield, L.; Ford, K.; Doblin, M.S.; Newbigin, E.; Read, S.; Bacic, A. Proteomic and biochemical evidence links the callose synthase in Nicotiana alata pollen tubes to the product of the NaGSL1 gene. Plant J. 2007, 52, 147–156. [Google Scholar] [CrossRef]
- Morel, J.S.; Claverol, S.; Mongrand, F.; Furt, J.; Fromentin, J.J.; Bessoule, J.P.; Blein, F.; Simon-Plas, F. Proteomics of plant detergent-resistant membranes. Mol. Cell. Prot. 2006, 5, 1396–1411. [Google Scholar] [CrossRef]
- Picton, J.M.; Steer, M.W. A model for the mechanism of tip extension in pollen tubes. J. Theor. Biol. 1982, 98, 15–20. [Google Scholar] [CrossRef]
- Derksen, J.; Rutten, T.; van Amstel, T.; de Win, A.; Doris, F.; Steer, M. Regulation of pollen tube growth. Acta Bot. Neer. 1995, 4, 103–119. [Google Scholar] [CrossRef]
- Bove, J.; Vaillancourt, B.; Kroeger, J.; Hepler, P.K.; Wiseman, P.W.; Geitmann, A. Magnitude and direction of vesicle dynamics in growing pollen tubes using spatiotemporal image correlation spectroscopy and fluorescence recovery after photobleaching. Plant Physiol. 2008, 147, 1646–1658. [Google Scholar] [CrossRef]
- Moscatelli, A.; Ciampolini, F.; Rodighiero, S.; Onelli, E.; Cresti, M.; Santo, N.; Idilli, A.I. Distinct endocytic pathways identified in tobacco pollen tubes using charged nanogold. J. Cell Sci. 2007, 120, 3804–3819. [Google Scholar] [CrossRef]
- Moscatelli, A.; Idilli, A.I. Pollen tube growth: A delicate equilibrium between secretory and endocytic pathways. J. Integr. Plant Biol. 2009, 51, 727–739. [Google Scholar] [CrossRef] [PubMed]
- Kost, B.; Mathur, J.; Chua, N.-H. Cytoskeleton in plant development. Curr. Opin. Plant Biol. 1999, 2, 462–470. [Google Scholar] [CrossRef]
- Pechlivanis, M.; Kuhlmann, J. Hydrophobic modifications of Ras proteins by isoprenoid groups and fatty acids—More than just membrane anchoring. BBA 2006, 1764, 1914–1931. [Google Scholar] [CrossRef]
- Zou, Y.; Aggarwal, M.; Zheng, W.G.; Wu, H.M.; Cheung, A.Y. Receptor-like kinases as surface regulators for RAC/ROP-mediated pollen tube growth and interaction with the pistil. AoB Plants 2011, 2011, plr017. [Google Scholar] [CrossRef]
- Ohtani, Y.; Irie, T.; Uekama, K.; Fukunaga, K.; Pitha, J. Differential effects of alpha-, beta- and gamma-cyclodextrins on human erythrocytes. Eur. J. Biochem. 1989, 186, 17–22. [Google Scholar] [CrossRef]
- Roche, Y.; Gerbeau-Pissot, P.; Buhot, B.; Thomas, D.; Bonneau, L.; Gresti, J.; Mongrand, S.; Perrier-Cornet, J.M.; Simon-Plas, F. Depletion of phytosterols from the plant plasma membrane provides evidence for disruption of lipid rafts. FASEB J. 2008, 22, 3980–3991. [Google Scholar] [CrossRef]
- Bosch, M.; Cheung, A.Y.; Hepler, P.K. Pectin methylesterase, a regulator of pollen tube growth. Plant Physiol. 2005, 138, 1334–1346. [Google Scholar] [CrossRef] [PubMed]
- Cardenas, L.; Lovy-Wheeler, A.; Kunkel, J.G.; Hepler, P.K. Pollen tube oscillatory and intracellular calcium levels are reversibly modulated by actin polymerization. Plant Physiol. 2008, 146, 1611–1621. [Google Scholar] [CrossRef] [PubMed]
- McKenna, S.T.; Kunkel, J.G.; Bosch, M.; Rounds, C.M.; Vidali, L.; Winship, L.J.; Hepler, P.K. Exocytosis precedes and predicts the increase in growth in oscillating pollen tubes. Plant Cell 2009, 21, 3026–3040. [Google Scholar] [CrossRef]
- Szumlanski, A.L.; Nielsen, E. The Rab GTPase RabA4d regulates pollen tube tip growth in Arabidopsis thaliana. Plant Cell 2009, 21, 526–544. [Google Scholar] [CrossRef] [PubMed]
- Idilli, A.I.; Morandini, P.; Onelli, E.; Rodighiero, S.; Caccianiga, M.; Moscatelli, A. Microtubule depolymerization affects endocytosis and exocytosis in the tip and influences endosome movement in tobacco pollen tubes. Mol. Plant 2013, 6, 1109–1130. [Google Scholar] [CrossRef]
- Hou, S.; Shi, J.; Hao, L.; Wang, Z.; Liao, Y.; Gu, H.; Dong, J.; Dresselhaus, T.; Zhong, S.; Qu, L.J. VPS18-regulated vesicle trafficking controls the secretion of pectin and its modifying enzyme during pollen tube growth in Arabidopsis. Plant Cell 2021, 33, 3042–3056. [Google Scholar] [CrossRef]
- Kong, J.N.; Hardina, K.; Dinkinsa, M.; Wanga, G.; Hea, Q.; Mujadzica, T.; Zhua, G.; Bielawski, J.; Spassieva, S.; Bieberich, E. Regulation of Chlamydomonas flagella and ependymal cell motile cilia by ceramide-mediated translocation of GSK3. Mol. Biol. Cell 2015, 26, 4451–4465. [Google Scholar] [CrossRef]
- Villette, C.; Berna, A.; Compagnon, V.; Schaller, H. Plant sterol diversity in pollen from angiosperms. Lipids 2015, 50, 749–760. [Google Scholar] [CrossRef]
- Rotsch, A.H.; Kopka, J.; Feussner, I.; Ischebeck, T. Central metabolite and sterol profiling divides tobacco male gametophyte development and pollen tube growth into eight metabolic phases. Plant J. 2017, 92, 129–146. [Google Scholar] [CrossRef]
- Grosjean, K.; Mongrand, S.; Beney, L.; Simon-Plas, F.; Gerbeau-Pissot, P. Differential effect of plant lipids on membrane organization: Specificities of phytosphingolipids and phytosterols. J. Biol. Chem. 2015, 290, 5810–5825. [Google Scholar] [CrossRef]
- Cacas, J.L.; Buré, C.; Grosjean, K.; Gerbeau-Pissot, P.; Lherminier, J.; Rombouts, Y.; Maes, E.; Bossard, C.; Gronnier, J.; Furt, F.; et al. Revisiting plant plasma membrane lipids in tobacco: A focus on sphingolipids. Plant Physiol. 2016, 170, 367–384. [Google Scholar] [CrossRef] [PubMed]
- Qu, X.; Zhang, R.; Zhang, M.; Diao, M.; Xue, Y.; Huang, S. Organizational innovation of apical actin filaments drives rapid pollen tube growth and turning. Mol. Plant 2017, 10, 930–947. [Google Scholar] [CrossRef] [PubMed]
- Vidali, L.; Rounds, C.M.; Hepler, P.K.; Bezanilla, M. Lifeact-mEGFP reveals a dynamic apical f-actin network in tip growing plant cells. PLoS ONE 2009, 4, e5744. [Google Scholar] [CrossRef]
- Rounds, C.M.; Hepler, P.K.; Winship, L.J. The apical actin fringe contributes to localized cell wall deposition and polarized growth in the Lily pollen tube. Plant Physiol. 2014, 166, 139–151. [Google Scholar] [CrossRef]
- Chang, M.; Li, Z.; Huang, S. Monomeric G-actin is uniformly distributed in pollen tubes and is rapidly redistributed via cytoplasmic streaming during pollen tube growth. Plant J. 2017, 92, 509–519. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Huang, S. Control of the actin cytoskeleton within apical and subapical regions of pollen tubes. Front. Cell Develop. Biol. 2020, 8, 614821. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.Y.; Shi, D.Q.; Zhang, W.J.; Tang, Z.S.; Liu, J.; Yang, W.C. The Arabidopsis alkaline ceramidase TOD1 is a key turgor pressure regulator in plant cells. Nat. Commun. 2015, 6, 6030. [Google Scholar] [CrossRef]
- Zonia, L.; Müller, M.; Munnik, T. Hydrodynamics and cell volume oscillations in the pollen tube apical region are integral components of the biomechanics of Nicotiana tabacum pollen tube growth. Cell Biochem. Biophys. 2006, 46, 209–232. [Google Scholar] [CrossRef]
- Winship, L.J.; Obermeyer, G.; Geitmann, A.; Hepler, P.K. Under pressure, cell walls set the pace. Trends Plant Sci. 2010, 15, 363–369. [Google Scholar] [CrossRef]
- Winship, L.J.; Obermeyer, G.; Geitmann, A.; Hepler, P.K. Pollen tube sand the physical world. Trends Plant Sci. 2011, 16, 353–355. [Google Scholar] [CrossRef]
- Liu, J.; Hussey, P.J. Dissecting the regulation of pollen tube growth by modeling the interplay of hydrodynamics, cell wall and ion dynamics. Front. Plant Sci. 2014, 5, 392. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chebli, Y.; Kaneda, M.; Zerzour, R.; Geitmann, A. The cell wall of the Arabidopsis pollen tube-spatial distribution, recycling and network formation of polysaccharides. Plant Physiol. 2012, 160, 1940–1955. [Google Scholar] [CrossRef] [PubMed]
- Dehors, J.; Mareck, A.; Kiefer-Meyer, M.-C.; Menu-Bouaouiche, L.; Lehner, A.; Mollet, J.-C. Evolution of cell wall polymers in tip-growing land plant gametophytes: Composition, distribution, functional aspects and their remodeling. Front. Plant Sci. 2019, 10, 441. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.; Hlavacka, A.; Matoh, T.; Volkmann, D.; Menzel, D.; Goldbach, H.E.; Baluska, F. Short-term boron deprivation inhibits endocytosis of cell wall pectins in meristematic cells of maize and wheat root apices. Plant Physiol. 2002, 130, 415–421. [Google Scholar] [CrossRef] [PubMed]
- Baluska, F.; Hlavacka, A.; Samaj, J.; Palme, K.; Robinson, D.G.; Matoh, T.; McCurdy, D.W.; Menzel, D.; Volkmann, D. F-actin-dependent endocytosis of cell wall pectins in meristematic root cells. Insights from brefeldin A-induced compartments. Plant Physiol. 2002, 130, 422–431. [Google Scholar] [CrossRef]
- Baluska, F.; Liners, F.; Hlava, A.; Schlicht, M.; Van Cutsem, P.; Mccurdy, D.W.; Menzel, D. Cell wall pectins and xyloglucans are internalized into dividing root cells and accumulate within cell plates during cytokinesis. Protoplasma 2005, 225, 141–155. [Google Scholar] [CrossRef]
- Rong, D.; Luo, N.; Mollet, J.C.; Liu, X.; Yang, Z. Salicylic acid regulates pollen tip growth through an NPR3/NPR4-independent pathway. Mol. Plant 2016, 9, 1478–1491. [Google Scholar] [CrossRef]
- Kaneda, M.; van Oostende-Triplet, C.; Chebli, Y.; Testerink, C.; Bednarek, S.Y.; Geitmann, A. Plant AP180 N-terminal homolog proteins are involved in clathrin-dependent endocytosis during pollen tube growth in Arabidopsis thaliana. Plant Cell Physiol. 2019, 60, 1316–1330. [Google Scholar] [CrossRef]
- Zhao, Y.; Yan, A.; Feijó, J.A.; Furutani, M.; Takenawa, T.; Hwang, I.; Fu, Y.; Yang, Z. Phosphoinositides regulate clathrin-dependent endocytosis at the tip of pollen tubes in Arabidopsis and tobacco. Plant Cell 2010, 22, 4031–4044. [Google Scholar] [CrossRef]
- Giovane, A.; Balestrieri, C.; Quagliuolo, L.; Castaldo, D.; Servillo, L. A glycoprotein inhibitor of pectin methylesterase in kiwi fruit purification by affinity-chromatography and evidence of a ripening related precursor. Eur. J. Biochem. 1995, 233, 926–929. [Google Scholar] [CrossRef]
- Bosch, M.; Hepler, P.K. Silencing of the tobacco pollen pectin methylesterase NtPPME1results in retarded in vivo pollen tube growth. Planta 2006, 223, 736–745. [Google Scholar] [CrossRef]
- Micheli, F. Pectin methylesterases: Cell wall enzymes with important roles in plant physiology. Trends Plant Sci. 2001, 6, 414–419. [Google Scholar] [CrossRef] [PubMed]
- Sénéchal, F.; Wattier, C.; Rustérucci, C.; Pelloux, J. Homogalacturonan-modifying enzymes: Structure, expression, and roles in plants. J. Exp. Bot. 2014, 65, 5125–5160. [Google Scholar] [CrossRef]
- Ferguson, C.; Teeri, T.T.; Siika-aho, M.; Read, S.M.; Bacic, A. Localization of cellulose and callose in pollen tubes and grains of Nicotiana tabacum. Planta 1998, 206, 452–460. [Google Scholar] [CrossRef]
- Geitmann, A.; Steer, M. The architecture and properties of the pollen tube cell wall. In The Pollen Tube; Plant Cell Monographs; Malho’, R., Ed.; Springer Verlag: Berlin/Heidelberg, Germany, 2006; Volume 3, pp. 177–200. [Google Scholar]
- Crowell, E.F.; Bischoff, V.; Desprez, T.; Rolland, A.; Stierhof, Y.D.; Schumacher, K.; Gonneau, M.; Hofte, H.; Vernhettes, S. Pausing of Golgi bodies on microtubules regulates secretion of cellulose synthase complexes in Arabidopsis. Plant Cell 2009, 21, 1141–1154. [Google Scholar] [CrossRef] [PubMed]
- Schrick, K.; Fujioka, S.; Takatsuto, S.; Stierhof, Y.D.; Stransky, H.; Yoshida, S.; Jürgens, G. A link between sterol biosynthesis, the cell wall, and cellulose in Arabidopsis. Plant J. 2004, 38, 227–243. [Google Scholar] [CrossRef] [PubMed]
- Paredez, A.R.; Somerville, C.R.; Ehrhardt, D.W. Visualization of cellulose synthase demonstrates functional association with microtubules. Science 2006, 312, 1491–1495. [Google Scholar] [CrossRef]
- Gutierrez, R.; Lindeboom, J.J.; Paredez, A.R.; Emons, A.M.; Ehrhardt, D.W. Arabidopsis cortical microtubules position cellulose synthase delivery to the plasma membrane and interact with cellulose synthase trafficking compartments. Nat. Cell Biol. 2009, 11, 797–806. [Google Scholar] [CrossRef] [PubMed]
- Schrick, K.; DeBolt, S.; Bulone, V. Deciphering the molecular functions of sterols in cellulose biosynthesis. Front. Plant Sci. 2012, 3, 4. [Google Scholar] [CrossRef]
- Brownfield, L.; Wilson, S.; Newbigin, E.; Bacic, A.; Read, S. Molecular control of the glucan synthase-like protein NaGSL1 and callose synthesis during growth of Nicotiana alata pollen tubes. Biochem. J. 2008, 414, 43–52. [Google Scholar] [CrossRef]
- Hong, Z.; Zhang, Z.; Olson, J.M.; Verma, D.P.S. A novel UDPglucose transferase is part of the callose synthase complex and interacts with phragmoplastin at the forming cell plate. Plant Cell 2001, 13, 769–779. [Google Scholar] [CrossRef]
- Adhikari, P.B.; Liu, X.; Kasahara, R.D. Mechanics of pollen tube elongation: A perspective. Front. Plant Sci. 2020, 11, 589712. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Sun, Y.; Zhu, X.; Yang, L.; Chen, X.; Miao, Y. Membrane microdomains modulate formin condensation for actin remodeling in Arabidopsis innate immune responses. Plant Cell 2021, 34, 374–394. [Google Scholar] [CrossRef] [PubMed]
- Sorek, N.; Segev, O.; Gutman, O.; Bar, E.; Richter, S.; Poraty, L.; Hirsch, J.A.; Henis, Y.I.; Lewinsohn, E.; Jürgens, G.; et al. An S-acylation switch of conserved G domain cysteines is required for polarity signaling by ROP GTPases. Curr. Biol. 2010, 20, 914–920, Erratum in: Curr Biol. 2010, 20, 1326; Curr Biol. 2015, 25, 2875–2877. [Google Scholar] [CrossRef] [PubMed]
- Platre, M.P.; Bayle, V.; Armengot, L.; Bareille, J.; Marquès-Bueno, M.D.M.; Creff, A.; Maneta-Peyret, L.; Fiche, J.B.; Nollmann, M.; Miège, C.; et al. Developmental control of plant Rho GTPase nano-organization by the lipid phosphatidylserine. Science 2019, 364, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Hepler, P.K.; Winship, L.J. The pollen tube clear zone: Clues to the mechanism of polarized growth. J. Integr. Plant Biol. 2015, 57, 79–92. [Google Scholar] [CrossRef] [PubMed]
- Helling, D.; Possart, A.; Cottier, S.; Klahre, U.; Kost, B. Pollen tube tip growth depends on plasma membrane polarization mediated by tobacco PLC3 activity and endocytic membrane recycling. Plant Cell 2006, 18, 3519–3534. [Google Scholar] [CrossRef]
- Preuss, M.L.; Serna, J.; Falbel, T.G.; Bednarek, S.Y.; Nielsen, E. The Arabidopsis Rab GTPase RabA4b localizes to the tips of growing root hair cells. Plant Cell 2004, 16, 1589–1603. [Google Scholar] [CrossRef]
- Laloi, M.; Perret, A.M.; Chatre, L.; Melser, S.; Cantrel, C.; Vaultier, M.N.; Zachowski, A.; Bathany, K.; Schmitter, J.M.; Vallet, M.; et al. Insights into the role of specific lipids in the formation and delivery of lipidmicrodomains to the plasmamembrane of plant cells. Plant Physiol. 2007, 143, 461–472. [Google Scholar] [CrossRef]
- Melser, S.; Batailler, B.; Peypelut, M.; Poujol, C.; Bellec, Y.; Wattelet-Boyer, V.; Maneta-Peyret, L.; Faure, J.D.; Moreau, P. Glucosylceramide biosynthesis is involved in Golgi morphology and protein secretion in plant cells. Traffic 2010, 11, 479–490. [Google Scholar] [CrossRef]
- Wattelet-Boyer, V.; Brocard, L.; Jonsson, K.; Esnay, N.; Joubès, J.; Domergue, F.; Mongrand, S.; Raikhel, N.; Bhalerao, R.P.; Moreau, P.; et al. Enrichment of hydroxylated C24- and C26-acyl-chain sphingolipids mediates PIN2 apical sorting at trans-Golgi network subdomains. Nat. Commun. 2016, 7, 12788. [Google Scholar] [CrossRef] [PubMed]
- Ito, Y.; Esnay, N.; Platre, M.P.; Noack, L.C.; Menzel, W.; Claverol, S.; Moreau, P.; Jaillais, Y.; Boutté, Y. Sphingolipids mediate polar sorting of PIN2 through phosphoinositide consumption at the trans-Golgi Network. Nat. Commun. 2021, 12, 4267. [Google Scholar] [CrossRef] [PubMed]
- Colombani, A.; Djerbi, S.; Bessueille, L.; Blomqvist, K.; Ohlsson, A.; Berglund, T. In vitro synthesis of (1-3)-b-D-glucan (callose) and cellulose by detergent extracts of membranes from cell suspension cultures of hybrid aspen. Cellulose 2004, 11, 313–327. [Google Scholar] [CrossRef]
- Brewbaker, J.L.; Kwack, B.H. The essential role of calcium ions in pollen germination and pollen tube growth. Am. J. Bot. 1963, 50, 859–865. [Google Scholar] [CrossRef]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef]
- Towbin, H.; Staehelin, T.; Gordon, J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets. Procedure and some applications. Proc. Natl. Acad. Sci. USA 1979, 76, 4350–4354. [Google Scholar] [CrossRef]
- Kost, B.; Spielhofer, P.; Chua, N.H. A GFP-mouse talin fusion protein labels plant actin filaments in vivo and visualizes the actin cytoskeleton in growing pollen tubes. Plant J. 1998, 16, 393–401. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stroppa, N.; Onelli, E.; Moreau, P.; Maneta-Peyret, L.; Berno, V.; Cammarota, E.; Ambrosini, R.; Caccianiga, M.; Scali, M.; Moscatelli, A. Sterols and Sphingolipids as New Players in Cell Wall Building and Apical Growth of Nicotiana tabacum L. Pollen Tubes. Plants 2023, 12, 8. https://doi.org/10.3390/plants12010008
Stroppa N, Onelli E, Moreau P, Maneta-Peyret L, Berno V, Cammarota E, Ambrosini R, Caccianiga M, Scali M, Moscatelli A. Sterols and Sphingolipids as New Players in Cell Wall Building and Apical Growth of Nicotiana tabacum L. Pollen Tubes. Plants. 2023; 12(1):8. https://doi.org/10.3390/plants12010008
Chicago/Turabian StyleStroppa, Nadia, Elisabetta Onelli, Patrick Moreau, Lilly Maneta-Peyret, Valeria Berno, Eugenia Cammarota, Roberto Ambrosini, Marco Caccianiga, Monica Scali, and Alessandra Moscatelli. 2023. "Sterols and Sphingolipids as New Players in Cell Wall Building and Apical Growth of Nicotiana tabacum L. Pollen Tubes" Plants 12, no. 1: 8. https://doi.org/10.3390/plants12010008
APA StyleStroppa, N., Onelli, E., Moreau, P., Maneta-Peyret, L., Berno, V., Cammarota, E., Ambrosini, R., Caccianiga, M., Scali, M., & Moscatelli, A. (2023). Sterols and Sphingolipids as New Players in Cell Wall Building and Apical Growth of Nicotiana tabacum L. Pollen Tubes. Plants, 12(1), 8. https://doi.org/10.3390/plants12010008






