Capacity of Nerium oleander to Phytoremediate Sb-Contaminated Soils Assisted by Organic Acids and Oxygen Nanobubbles
Abstract
:1. Introduction
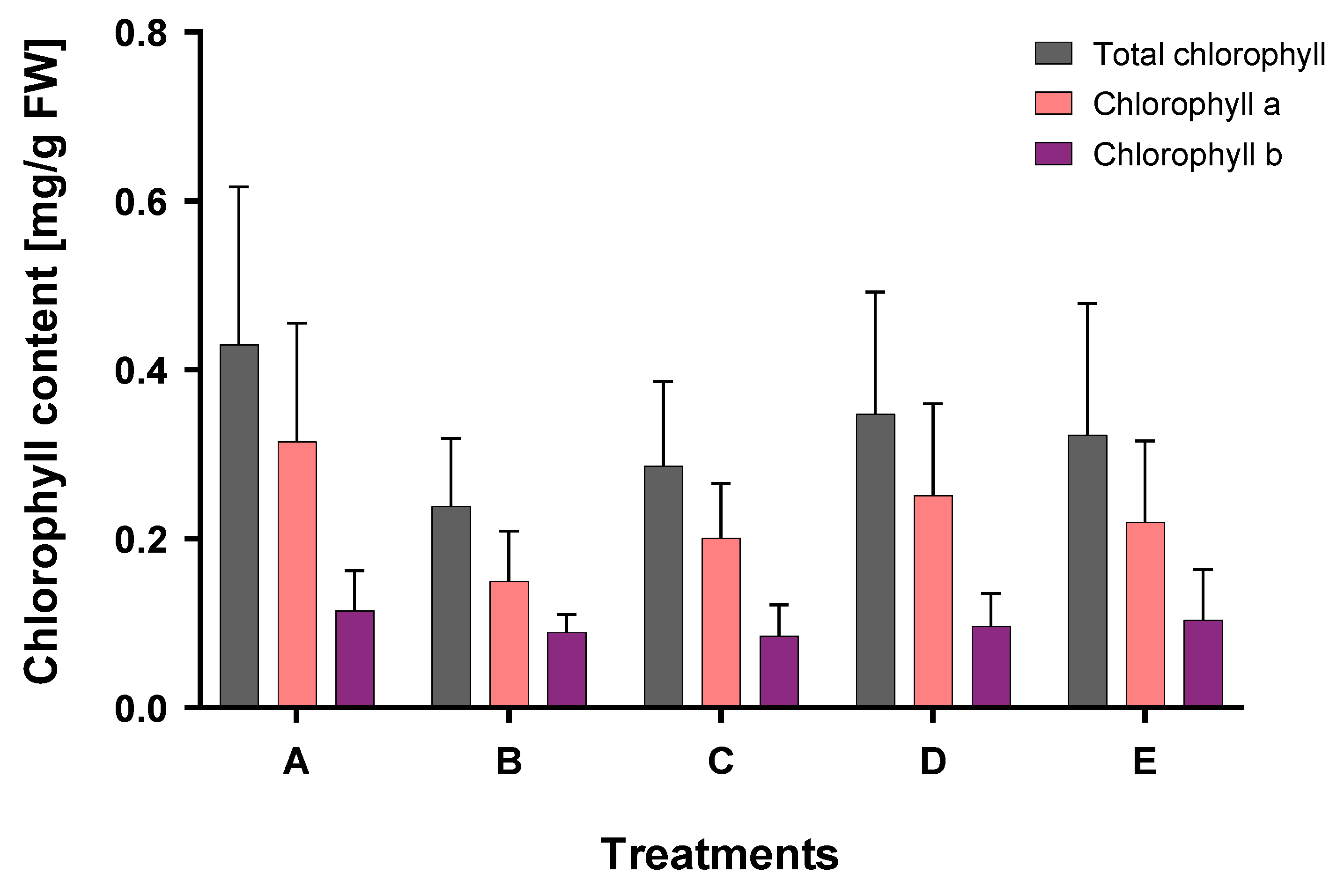
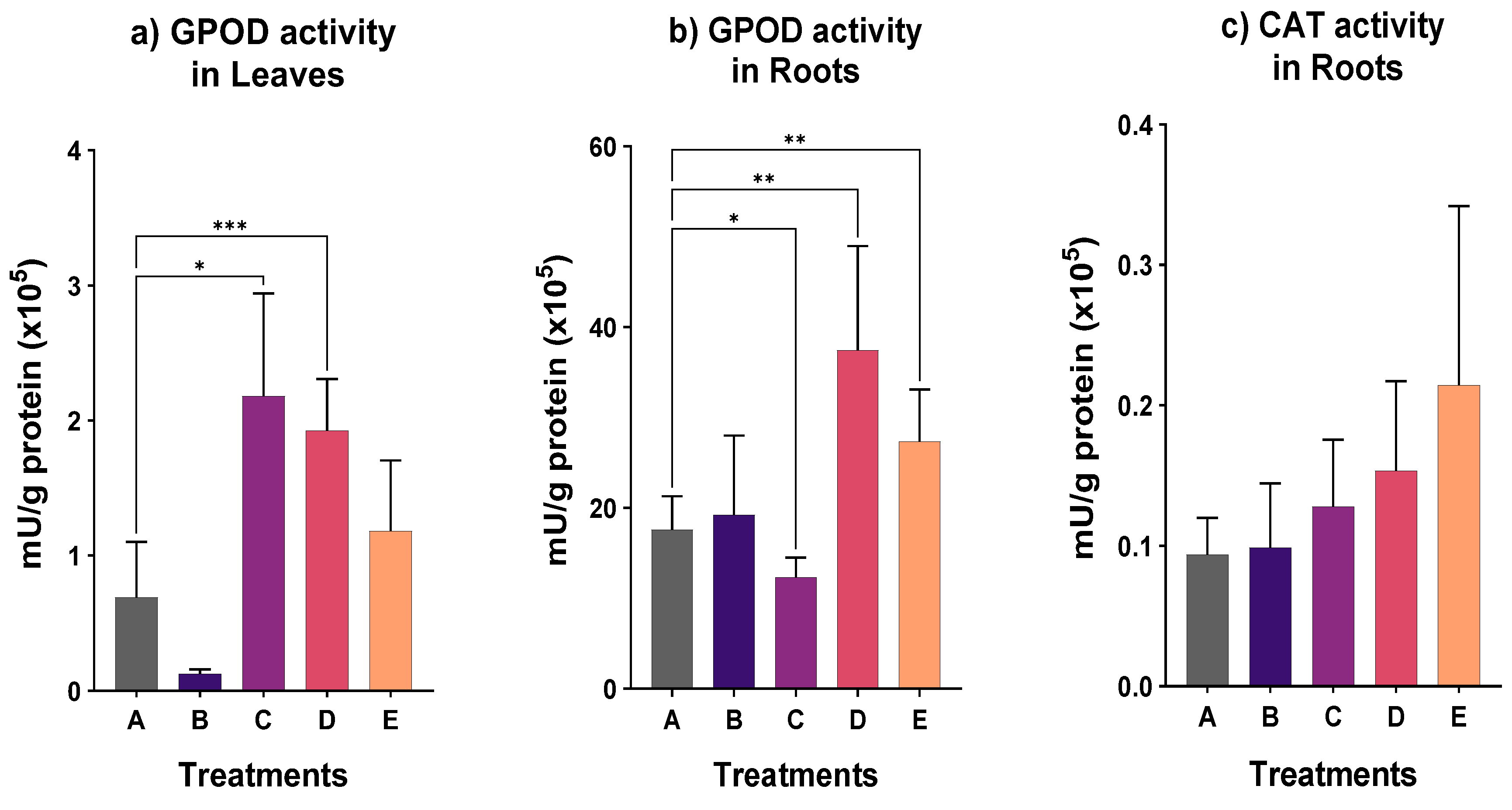
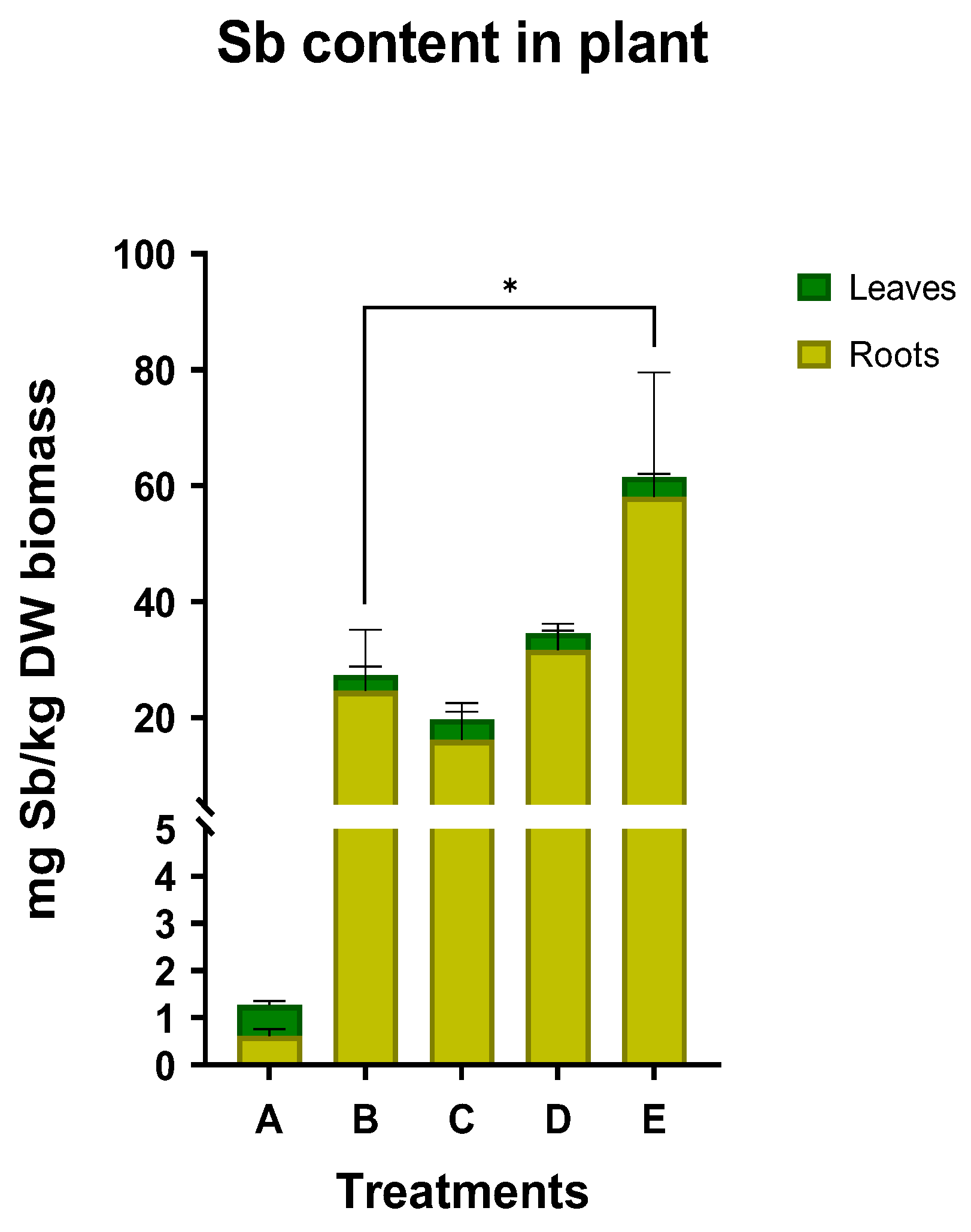
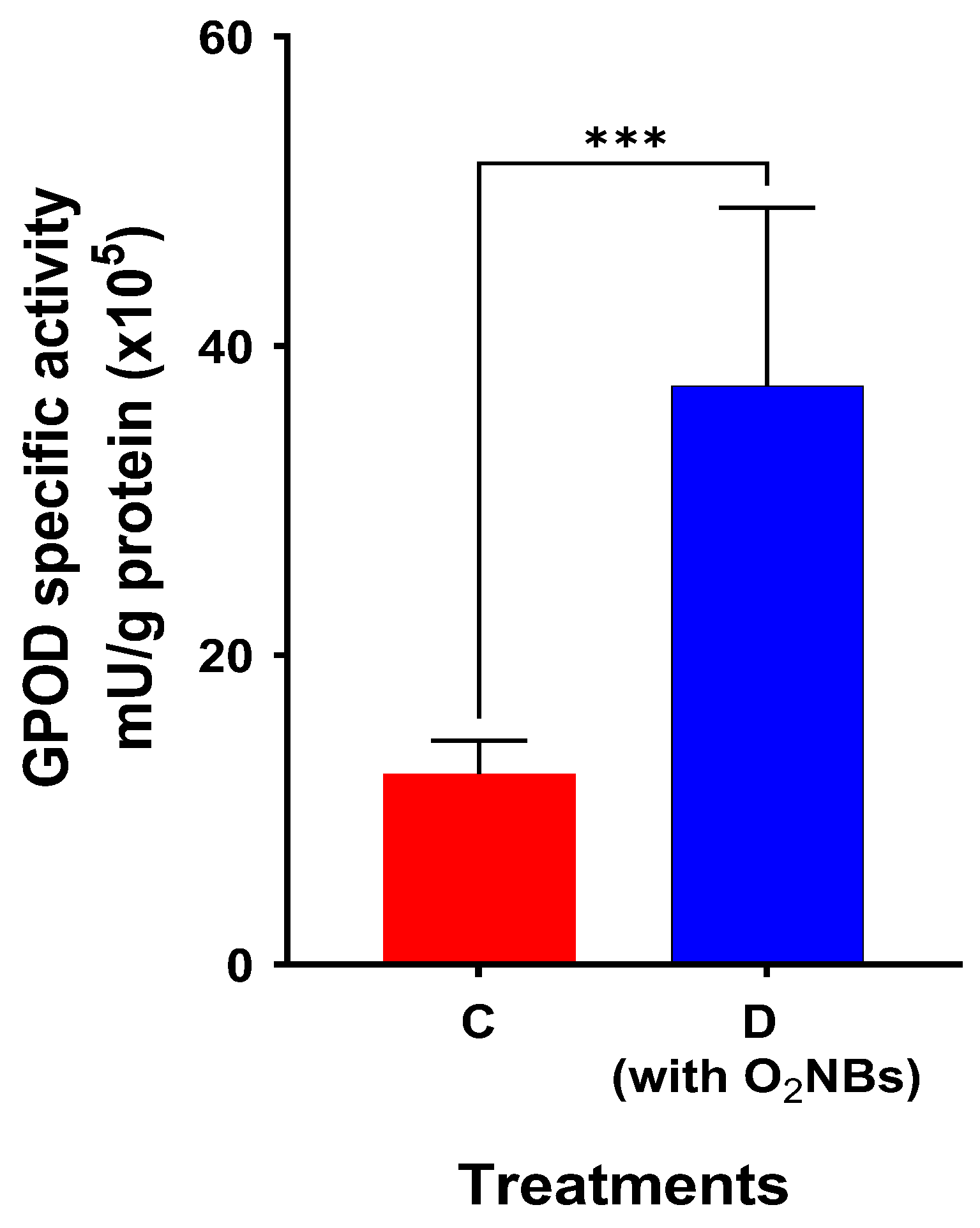
2. Results
3. Discussion
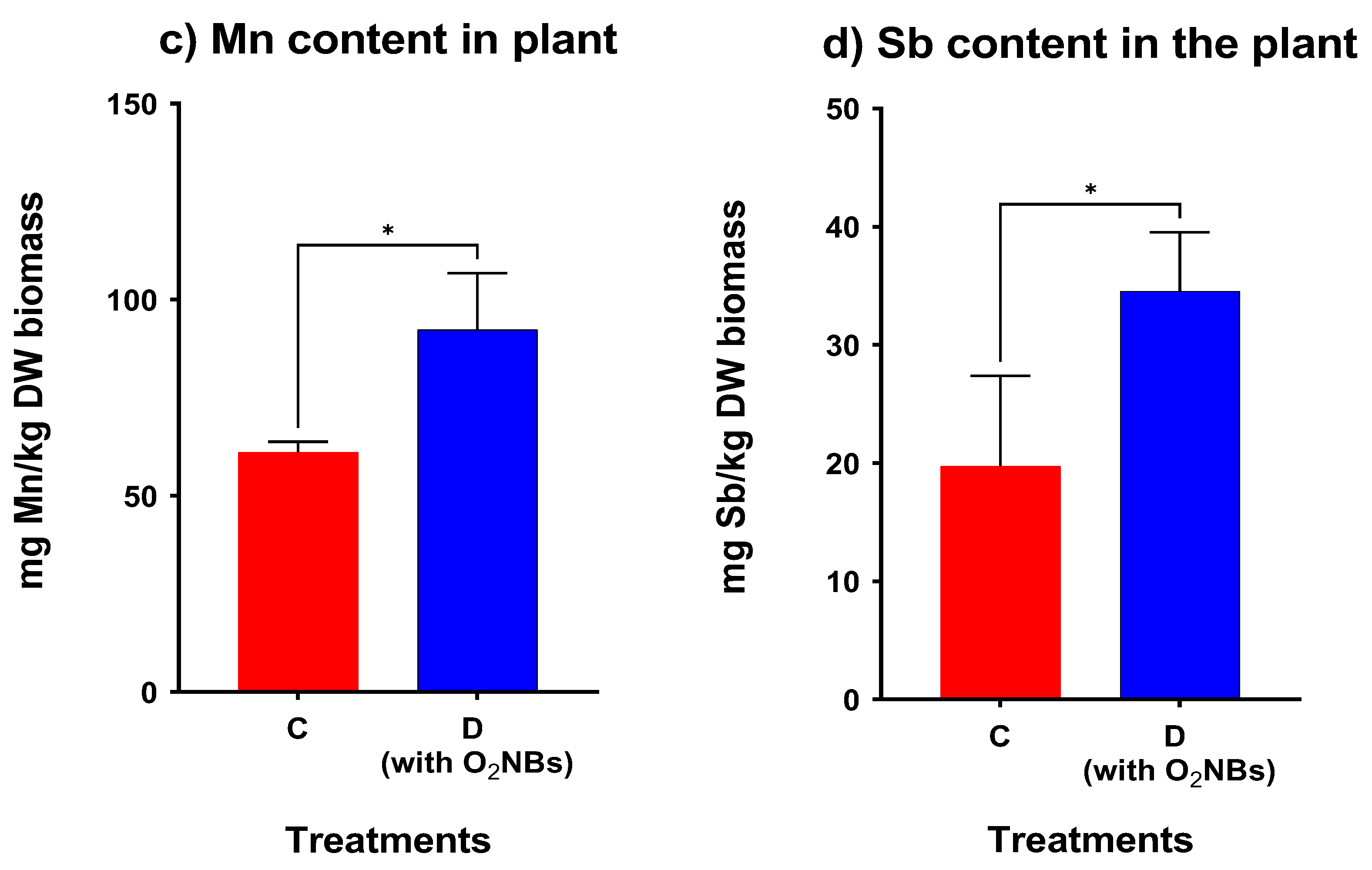
3.1. Sb Uptake by N. oleander
3.2. Effect of Supplementation with Organic Acids
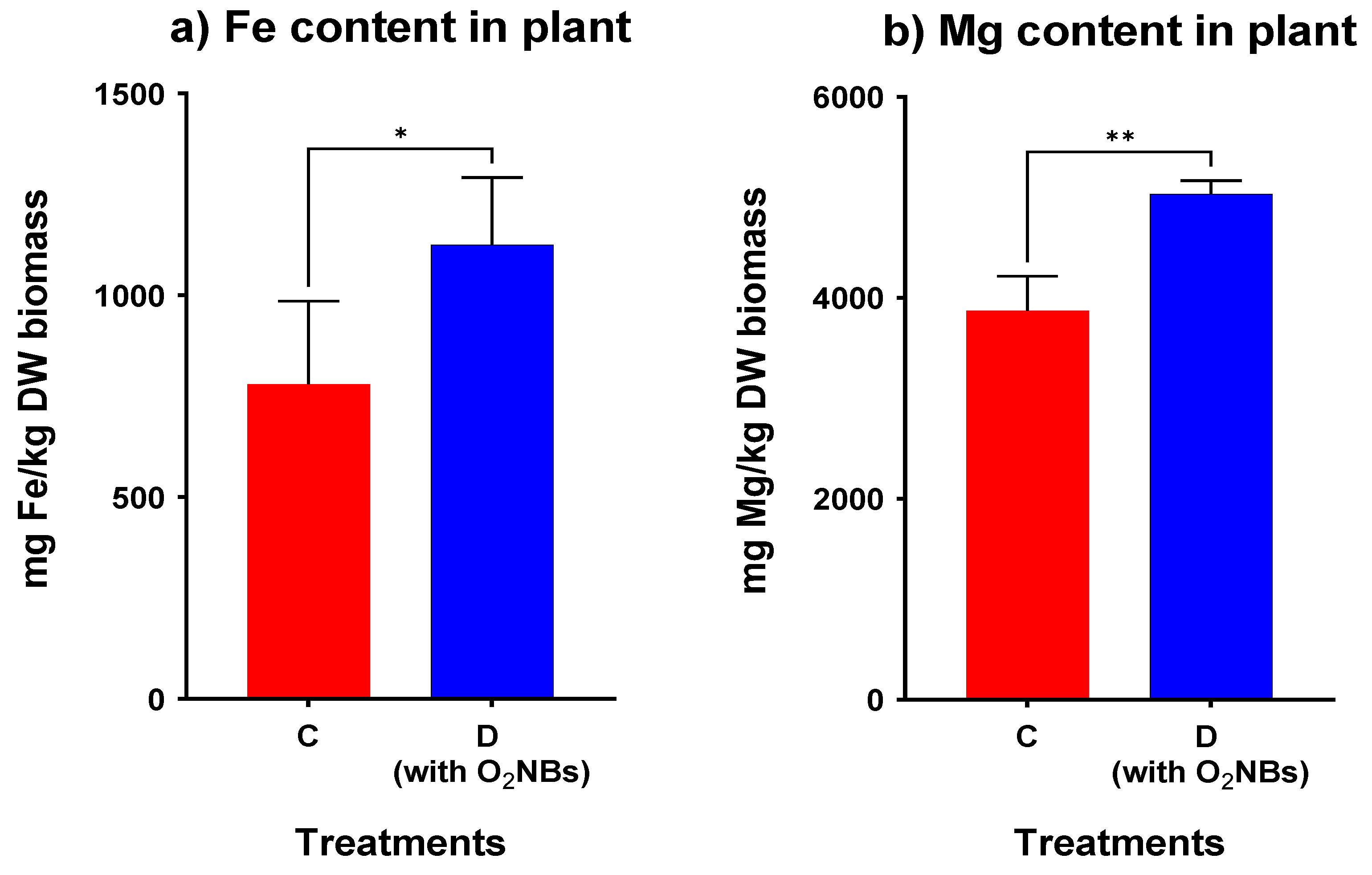
3.3. Effect of Supplementation with Oxygen Nanobubbles
3.4. Phytoremediation Capacity of N. oleander
4. Materials and Methods
4.1. Soil Characterization
4.2. Soil Amendements
4.2.1. Organic Acids
4.2.2. Oxygen Nanobubbles (O2NBs) Production
4.3. Pot Experiment
4.4. Chlorophyll Measurements
4.5. Measurement of Antioxidant Enzymes Activity
4.6. Water Content and Biomass Measurement
4.7. Heavy Metal Analysis
4.8. Bioaccumulation Factor (BC) and Translocation Factor (TF)
4.9. Quality Control and Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hooda, P.S. Trace Elements in Soils; Blackwell Publishing Ltd.: Hoboken, NJ, USA, 2010. [Google Scholar] [CrossRef]
- He, M.; Wang, N.; Long, X.; Zhang, C.; Ma, C.; Zhong, Q.; Wang, A.; Wang, Y.; Pervaiz, A.; Shan, J. Antimony speciation in the environment: Recent advances in understanding the biogeochemical processes and ecological effects. J. Environ. Sci. 2019, 75, 14–39. [Google Scholar] [CrossRef] [PubMed]
- He, M.; Wang, X.; Wu, F.; Fu, Z. Antimony pollution in China. Sci. Total Environ. 2012, 421–422, 41–50. [Google Scholar] [CrossRef]
- Lai, Z.; He, M.; Lin, C.; Ouyang, W.; Liu, X. Interactions of antimony with biomolecules and its effects on human health. Ecotoxicol. Environ. Saf. 2022, 233, 113317. [Google Scholar] [CrossRef] [PubMed]
- Winship, K.A. Toxicity of antimony and its compounds. Adverse Drug React. Acute Poisoning Rev. 1987, 6, 67–90. [Google Scholar] [PubMed]
- Zhang, X.; Xie, N.; Guo, Y.; Niu, D.; Sun, H.; Yang, Y. Insights into adsorptive removal of antimony contaminants: Functional materials, evaluation and prospective. J. Hazard. Mater. 2021, 418, 126345. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Liu, Y.; Shen, C.; Li, F.; Wang, C.-C.; Huang, M.; Yang, B.; Wang, Z.; Yang, J.; Sand, W. One-step Sb(III) decontamination using a bifunctional photoelectrochemical filter. J. Hazard. Mater. 2019, 389, 121840. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Lou, Z.; Yang, K.; Wang, Z.; Zhou, C.; Li, Y.; Cao, Z.; Xu, X. Coagulation removal of Sb(V) from textile wastewater matrix with enhanced strategy: Comparison study and mechanism analysis. Chemosphere 2019, 237, 124494. [Google Scholar] [CrossRef] [PubMed]
- Long, X.; Wang, X.; Guo, X.; He, M. A review of removal technology for antimony in aqueous solution. J. Environ. Sci. 2020, 90, 189–204. [Google Scholar] [CrossRef]
- Filella, M.; Belzile, N.; Chen, Y.W. Antimony in the environment: A review focused on natural waters I. Occurrence. Earth-Sci. Rev. 2002, 57, 125–176. [Google Scholar] [CrossRef]
- Shahid, M.; Khalid, S.; Dumat, C.; Pierart, A.; Niazi, N.K. Biogeochemistry of antimony in soil-plant system: Ecotoxicology and human health. Appl. Geochem. 2019, 106, 45–59. [Google Scholar] [CrossRef]
- Grob, M.; Wilcke, W.; Mestrot, A. Release and biomethylation of antimony in shooting range soils upon flooding. Soil Syst. 2018, 2, 34. [Google Scholar] [CrossRef] [Green Version]
- Okkenhaug, G.; Zhu, Y.; Luo, L.; Lei, M.; Li, X.; Mulder, J. Distribution, speciation and availability of antimony (Sb) in soils and terrestrial plants from an active Sb mining area. Environ. Pollut. 2011, 159, 2427–2434. [Google Scholar] [CrossRef] [PubMed]
- Barker, A.J.; Mayhew, L.; Douglas, T.; Ilgen, A.; Trainor, T.P. Lead and antimony speciation associated with the weathering of bullets in a historic shooting range in Alaska. Chem. Geol. 2020, 553, 119797. [Google Scholar] [CrossRef]
- Hockmann, K.; Tandy, S.; Lenz, M.; Reiser, R.; Conesa, H.M.; Keller, M.; Studer, B.; Schulin, R. Antimony retention and release from drained and waterlogged shooting range soil under field conditions. Chemosphere 2015, 134, 536–543. [Google Scholar] [CrossRef]
- Wang, L.; Ji, B.; Hu, Y.; Liu, R.; Sun, W. A review on in situ phytoremediation of mine tailings. Chemosphere 2017, 184, 594–600. [Google Scholar] [CrossRef]
- Huang, Y.; Chen, Z.; Liu, W. Influence of iron plaque and cultivars on antimony uptake by and translocation in rice (Oryza sativa L.) seedlings exposed to Sb(III) or Sb(V). Plant Soil 2012, 352, 41–49. [Google Scholar] [CrossRef]
- Ji, Y.; Sarret, G.; Schulin, R.; Tandy, S. Fate and chemical speciation of antimony (Sb) during uptake, translocation and storage by rye grass using XANES spectroscopy. Environ. Pollut. 2017, 231, 1322–1329. [Google Scholar] [CrossRef]
- Ren, J.H.; Ma, L.; Sun, H.; Cai, F.; Luo, J. Antimony uptake, translocation and speciation in rice plants exposed to antimonite and antimonate. Sci. Total Environ. 2014, 475, 83–89. [Google Scholar] [CrossRef]
- Shtangeeva, I.; Steinnes, E.; Lierhagen, S. Uptake of different forms of antimony by wheat and rye seedlings. Environ. Sci. Pollut. Res. 2012, 19, 502–509. [Google Scholar] [CrossRef]
- Xi, L.; Shen, Y.; Zhao, X.; Zhou, M.; Mi, Y.; Li, X.; Chen, H.; Wei, Y.; Su, H.; Hou, H. Effects of arbuscular mycorrhizal fungi on frond antimony enrichment, morphology, and proteomics in Pteris cretica var. nervosa during antimony phytoremediation. Sci. Total Environ. 2022, 804, 149904. [Google Scholar] [CrossRef]
- Zand, A.D.; Heir, A.V. Phytoremediation: Data on effects of titanium dioxide nanoparticles on phytoremediation of antimony polluted soil. Data Br. 2020, 31, 105959. [Google Scholar] [CrossRef] [PubMed]
- Kumar, D.; Al Hassan, M.; Naranjo, M.A.; Agrawal, V.; Boscaiu, M.; Vicente, O. Effects of salinity and drought on growth, ionic relations, compatible solutes and activation of antioxidant systems in oleander (Nerium oleander L.). PLoS ONE 2017, 12, e0185017. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, N.; El Afandi, G. Phytoremediation uptake model of heavy metals (Pb, Cd and Zn) in soil using Nerium oleander. Heliyon 2020, 6, e04445. [Google Scholar] [CrossRef] [PubMed]
- Al-Shayeb, S. Comparison study of Phoenix dactylifera L. and Nerium oleander L. as biomonitors for lead and other elements. Asian J. Chem. 2002, 14, 597. [Google Scholar]
- Qiao, D.; Han, Y.; Zhao, Y. Organic acids in conjunction with various oilseed sunflower cultivars promote Cd phytoextraction through regulating micro-environment in root zone. Ind. Crops Prod. 2022, 183, 114932. [Google Scholar] [CrossRef]
- Wang, S.T.; Dong, Q.; Wang, Z.L. Differential effects of citric acid on cadmium uptake and accumulation between tall fescue and Kentucky bluegrass. Ecotoxicol. Environ. Saf. 2017, 145, 200–206. [Google Scholar] [CrossRef]
- Qiao, D.; Lu, H.; Zhang, X. Change in phytoextraction of Cd by rapeseed (Brassica napus L.) with application rate of organic acids and the impact of Cd migration from bulk soil to the rhizosphere. Environ. Pollut. 2020, 267, 115452. [Google Scholar] [CrossRef]
- Liu, Y.; Zhou, Y.; Wang, T.; Pan, J.; Zhou, B.; Muhammad, T.; Zhou, C.; Li, Y. Micro-nano bubble water oxygation: Synergistically improving irrigation water use efficiency, crop yield and quality. J. Clean. Prod. 2019, 222, 835–843. [Google Scholar] [CrossRef]
- Wu, Y.; Lyu, T.; Yue, B.; Tonoli, E.; Verderio, E.A.M.; Ma, Y.; Pan, G. Enhancement of Tomato Plant Growth and Productivity in Organic Farming by Agri-Nanotechnology Using Nanobubble Oxygation. J. Agric. Food Chem. 2019, 67, 10823–10831. [Google Scholar] [CrossRef] [Green Version]
- Seridou, P.; Kalogerakis, N. Disinfection applications of ozone micro- And nanobubbles. Environ. Sci. Nano 2021, 8, 3493–3510. [Google Scholar] [CrossRef]
- Zhang, F.; Sun, L.; Yang, H.; Gui, X.; Schönherr, H.; Kappl, M.; Cao, Y.; Xing, Y. Recent advances for understanding the role of nanobubbles in particles flotation. Adv. Colloid Interface Sci. 2021, 291, 102403. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Zhang, K.; Cen, C.; Wu, X.; Mao, R.; Zheng, Y. Role of bulk nanobubbles in removing organic pollutants in wastewater treatment. AMB Express 2021, 11, 96. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, A.K.A.; Shi, X.; Hua, L.; Manzueta, L.; Qing, W.; Marhaba, T.; Zhang, W. Influences of Air, Oxygen, Nitrogen, and Carbon Dioxide Nanobubbles on Seed Germination and Plant Growth. J. Agric. Food Chem. 2018, 66, 5117–5124. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Oshita, S.; Kawabata, S.; Thuyet, D.Q. Nanobubble Water’s Promotion Effect of Barley (Hordeum vulgare L.) Sprouts Supported by RNA-Seq Analysis. Langmuir 2017, 33, 12478–12486. [Google Scholar] [CrossRef]
- Liu, S.; Oshita, S.; Kawabata, S.; Makino, Y.; Yoshimoto, T. Identification of ROS Produced by Nanobubbles and Their Positive and Negative Effects on Vegetable Seed Germination. Langmuir 2016, 32, 11295–11302. [Google Scholar] [CrossRef]
- Liu, S.; Oshita, S.; Makino, Y.; Wang, Q.; Kawagoe, Y.; Uchida, T. Oxidative Capacity of Nanobubbles and Its Effect on Seed Germination. ACS Sustain. Chem. Eng. 2016, 4, 1347–1353. [Google Scholar] [CrossRef]
- Ebina, K.; Shi, K.; Hirao, M.; Hashimoto, J.; Kawato, Y.; Kaneshiro, S.; Morimoto, T.; Koizumi, K.; Yoshikawa, H. Oxygen and Air Nanobubble Water Solution Promote the Growth of Plants, Fishes, and Mice. PLoS ONE 2013, 8, 2–8. [Google Scholar] [CrossRef] [Green Version]
- Pan, X.; Zhang, D.; Chen, X. Antimony Accumulation, Growth Performance, Antioxidant Defense System and Photosynthesis of Zea mays in Response to Antimony Pollution in Soil. Water Air Soil Pollut. 2012, 215, 517–5231. [Google Scholar] [CrossRef]
- Frohne, T.; Rinklebe, J.; Diaz-Bone, R.A. Contamination of Floodplain Soils along the Wupper River, Germany, with As, Co, Cu, Ni, Sb, and Zn and the Impact of Pre-definite Redox Variations on the Mobility of These Elements. Soil Sediment Contam. 2014, 23, 779–799. [Google Scholar] [CrossRef]
- Strømseng, A.E.; Ljønes, M.; Bakka, L.; Mariussen, E. Episodic discharge of lead, copper and antimony from a Norwegian small arm shooting range. J. Environ. Monit. 2009, 11, 1259–1267. [Google Scholar] [CrossRef]
- Adeleke, R.; Nwangburuka, C.; Oboirien, B. Origins, roles and fate of organic acids in soils: A review. S. Afr. J. Bot. 2017, 108, 393–406. [Google Scholar] [CrossRef]
- Wu, L.H.; Luo, Y.; Christie, P.; Wong, M.H. Effects of EDTA and low molecular weight organic acids on soil solution properties of a heavy metal polluted soil. Chemosphere 2003, 50, 819–822. [Google Scholar] [CrossRef] [PubMed]
- Onireti, O.O.; Lin, C.; Qin, J. Combined effects of low-molecular-weight organic acids on mobilization of arsenic and lead from multi-contaminated soils. Chemospher 2017, 170, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Sun, C.; Zhu, P.; Liu, F.E.I. Effects of antimony stress on photosynthesis and growth of acorus calamus. Front. Plant Sci. 2018, 9, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aksoy, A.; Öztürk, M.A. Nerium oleander L. as a biomonitor of lead and other heavy metal pollution in Mediterranean environments. Sci. Total Environ. 1997, 205, 145–150. [Google Scholar] [CrossRef]
- Kaya, G.; Okumus, N.; Yamant, M. Lead, cadmium and copper concentrations in leaves of Nerium oleander L. and Robinia pseudoacacia L. as biomonitors of atmospheric pollution. Fresenius Environ. Bull. 2010, 19, 669–675. [Google Scholar]
- Mingorance, M.D.; Valdés, B.; Oliva, S.R. Strategies of heavy metal uptake by plants growing under industrial emissions. Environ. Int. 2007, 33, 514–520. [Google Scholar] [CrossRef]
- Vázquez, S.; Martín, A.; García, M.; Español, C.; Navarro, E. Metal uptake of Nerium oleander from aerial and underground organs and its use as a biomonitoring tool for airborne metallic pollution in cities. Environ. Sci. Pollut. Res. 2016, 23, 7582–7594. [Google Scholar] [CrossRef]
- Doganlar, Z.B.; Doganlar, O.; Erdogan, S.; Onal, Y. Heavy metal pollution and physiological changes in the leaves of some shrub, palm and tree species in urban areas of Adana, Turkey. Chem. Speciat. Bioavailab. 2012, 24, 65–78. [Google Scholar] [CrossRef] [Green Version]
- Rai, N.; Sharma, N.; Panchal, A. Heavy metal accumulation by selected plant species along the national highway: A case study of Udaipur, Rajasthan, India. Int. J. Environ. Anal. Chem. 2019, 99, 1078–1089. [Google Scholar] [CrossRef]
- Monaci, F.; Trigueros, D.; Mingorance, M.; Rossini-Oliva, S. Phytostabilization potential of Erica australis L. and Nerium oleander L.: A comparative study in the Riotinto mining area (SW Spain). Environ. Geochem. Health 2020, 42, 2345–2360. [Google Scholar] [CrossRef] [PubMed]
- Parra, A.; Zornoza, R.; Conesa, E.; Gómez-López, M.; Faz, A. Evaluation of the suitability of three Mediterranean shrub species for phytostabilization of pyritic mine soils. Catena 2016, 136, 59–65. [Google Scholar] [CrossRef]
- Elloumi, N.; Belhaj, D.; Mseddi, S.; Zouari, M.; Abdallah, F.B.; Woodwar, S.; Kallel, M. Response of Nerium oleander to phosphogypsum amendment and its potential use for phytoremediation. Ecol. Eng. 2017, 99, 164–171. [Google Scholar] [CrossRef]
- Chen, L.; Larson, S.L.; Ballard, J.H.; Ma, Y.; Zhang, Q.; Li, J.; Wu, L.; Arslan, Z.; Han, F.X. Laboratory spiking process of soil with various uranium and other heavy metals. MethodsX 2019, 6, 734–739. [Google Scholar] [CrossRef] [PubMed]
- Reeuwijk, L. Procedures for Soil Analysis. In International Soil Reference and Information Centre (ISRIC) Technical Paper; ISRIC: Wangeningen, The Netherlands, 1993; p. 119. [Google Scholar]
- Gangwar, D.P.; Baskar, M. Texture Determination of Soil by Hydrometer Method for Forensic Purpose. Cent. Forensic Sci. Lab. 2019. [Google Scholar] [CrossRef]
- Harborne, J. Chlorophylls in Phytochemical methods. Chapman Hall 1984, 214–221. [Google Scholar]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Erdelsky, K.; Fric, E. Practical and Analytical Methods in Plant Physiology. SPN: Bratislava, Slovakia, 1979. [Google Scholar]
- Aebi, H. Catalase in Vitro. In Method Enzyme 105; Academic Press: Cambridge, MA, USA, 1984; pp. 121–130. [Google Scholar]




| pH | Treatment | ||||
|---|---|---|---|---|---|
| A (Control) | B Sb 50 ppm | C Sb 50 ppm OAs 7 mmol/kg | D Sb 50ppm OAs 7 mmol/kg with O2NBs | E Sb 50 ppm OAs 70 mmol/kg | |
| Before | 7.42 | 7.42 | 6.61 | 6.61 | 6.04 |
| After | 7.17 | 7.51 | 7.65 | 7.61 | 7.23 |
| Factors | Treatment | |||
|---|---|---|---|---|
| B | C | D | E | |
| BCF | 0.78 | 0.51 | 0.90 | 1.72 * |
| TF | 0.07 | 0.23 **** | 0.09 | 0.06 |
| Soil Property | Value |
|---|---|
| Sand (%) | 72.53 |
| Clay (%) | 21.87 |
| Slit (%) | 5.6 |
| Texture | sand clay loam |
| Organic matter (%) | 1.83 |
| TKN (g/kg soil) | 0.76 |
| pH | 7.42 |
| Sb concentration (ppm) | 48.8 ± 1.3 |
| Experimental Treatment (Code Name) | Sb Concentration [ppm] | Organic Acids Concentration [mmol/kg] | O2NBs |
|---|---|---|---|
| A (control) | 1.17 ppm (background level) | 0 | - |
| B | 50 | 0 | - |
| C | 50 | 7 | - |
| D | 50 | 7 | + |
| E | 50 | 70 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seridou, P.; Monogyiou, S.; Syranidou, E.; Kalogerakis, N. Capacity of Nerium oleander to Phytoremediate Sb-Contaminated Soils Assisted by Organic Acids and Oxygen Nanobubbles. Plants 2023, 12, 91. https://doi.org/10.3390/plants12010091
Seridou P, Monogyiou S, Syranidou E, Kalogerakis N. Capacity of Nerium oleander to Phytoremediate Sb-Contaminated Soils Assisted by Organic Acids and Oxygen Nanobubbles. Plants. 2023; 12(1):91. https://doi.org/10.3390/plants12010091
Chicago/Turabian StyleSeridou, Petroula, Sofia Monogyiou, Evdokia Syranidou, and Nicolas Kalogerakis. 2023. "Capacity of Nerium oleander to Phytoremediate Sb-Contaminated Soils Assisted by Organic Acids and Oxygen Nanobubbles" Plants 12, no. 1: 91. https://doi.org/10.3390/plants12010091






