A New Fluorescence Detection Method for Tryptophan- and Tyrosine-Derived Allelopathic Compounds in Barley and Lupin
Abstract
:1. Introduction
2. Results
2.1. Method Development
2.2. Selectivity, Accuracy, Matrix Effect and Precision
2.3. Calibration Curves and Sensitivity
2.4. Carryover
2.5. Stability
2.6. Applications
2.6.1. Screening the Barley Pangenome
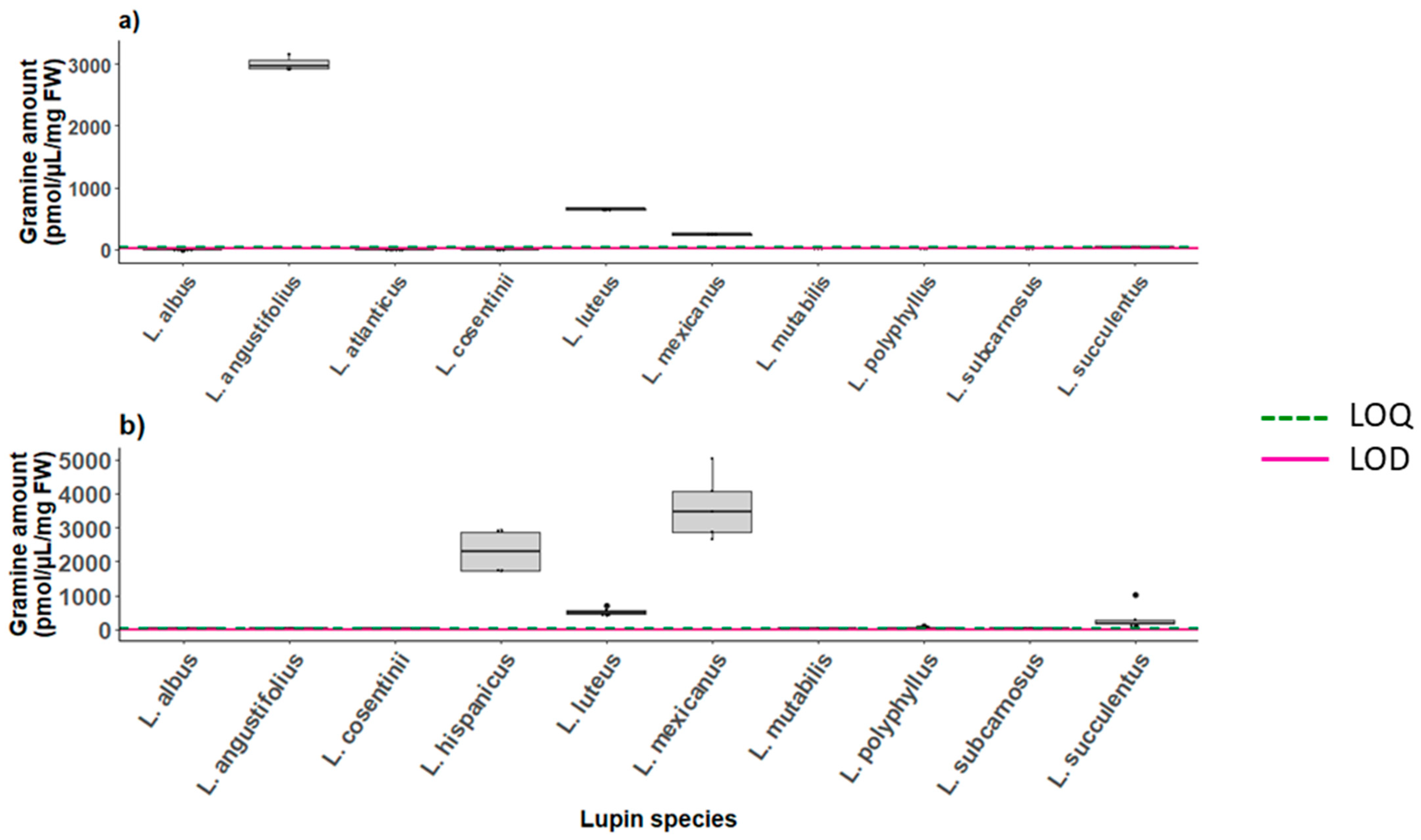
2.6.2. Gramine in Lupins
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Plant Growth Conditions
4.3. Sample Preparation
4.3.1. Barley Sample Preparation
4.3.2. Lupin Sample Preparation
4.4. Chemicals and Reagents
4.5. Instrumentation and Conditions
4.6. Standards and Calibration Curves
4.7. Method Validation
LOQ = 10σ/S’,
4.8. Figures
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Glossary
| AMI | 3-aminomethylindole |
| DAG | days after germination |
| ELISA | enzyme-linked immunosorbent assay |
| FID | flame ionization detector |
| GM-MS | gas chromatography mass spectrometry |
| GLC-MS | gas liquid chromatography mass spectrometry |
| HPLC | high-performance liquid chromatography |
| LC-MS | high-performance liquid chromatography mass spectrometry |
| LOD | limit of detection |
| LOQ | limit of quantification |
| MAMI | N-methyl-3-aminomethylindole |
| MS | mass spectrometry |
| PDA | photodiode array detector |
| QTL | quantitative trait locus |
| RSD | relative standard deviation |
| SD | standard deviation |
| UPLC | ultra performance liquid chromatography |
References
- FAO. Food Outlook—Biannual Report on Global Food Markets; FAO: Rome, Italy, 2022. [Google Scholar] [CrossRef]
- Aktar, M.W.; Sengupta, D.; Chowdhury, A. Impact of pesticides use in agriculture: Their benefits and hazards. Interdiscip. Toxicol. 2009, 2, 1–12. [Google Scholar] [CrossRef]
- Wu, H.; Pratley, J.; Lemerle, D.; Haig, T. Crop cultivars with allelopathic capability. Weed Res. 2002, 39, 171–180. [Google Scholar] [CrossRef]
- Jabran, K.; Mahajan, G.; Sardana, V.; Chauhan, B.S. Allelopathy for weed control in agricultural systems. Crop Prot. 2015, 72, 57–65. [Google Scholar] [CrossRef]
- Corcuera, L.J. Biochemical basis for the resistance of barley to aphids. Phytochemistry 1993, 33, 741–747. [Google Scholar] [CrossRef]
- Matsuo, H.; Taniguchi, K.; Hiramoto, T.; Yamada, T.; Ichinose, Y.; Toyoda, K.; Takeda, K.; Shiraishi, T. Gramine Increase Associated with Rapid and Transient Systemic Resistance in Barley Seedlings Induced by Mechanical and Biological Stresses. Plant Cell Physiol. 2001, 42, 1103–1111. [Google Scholar] [CrossRef] [PubMed]
- Lu, A.; Wang, T.; Hui, H.; Wei, X.; Cui, W.; Zhou, C.; Li, H.; Wang, Z.; Guo, J.; Ma, D.; et al. Natural Products for Drug Discovery: Discovery of Gramines as Novel Agents against a Plant Virus. J. Agric. Food Chem. 2019, 67, 2148–2156. [Google Scholar] [CrossRef]
- Ishiai, S.; Kondo, H.; Hattori, T.; Mikami, M.; Aoki, Y.; Enoki, S.; Suzuki, S. Hordenine is responsible for plant defense response through jasmonate-dependent defense pathway. Physiol. Mol. Plant Pathol. 2016, 96, 94–100. [Google Scholar] [CrossRef]
- Smith, T.A. Tryptamine and related compounds in plants. Phytochemistry 1977, 16, 171–175. [Google Scholar] [CrossRef]
- Williams, M.; Barnes, R.F.; Cassady, J.M. Characterization of Alkaloids in Palatable and Unpalatable Clones of Phalaris arundinacea L1. Crop Sci. 1971, 11, 213–217. [Google Scholar] [CrossRef]
- Hong, Y.; Hu, H.-Y.; Sakoda, A.; Sagehashi, M. Isolation and characterization of antialgal allelochemicals from Arundo donax L. Allelopath. J. 2009, 25, 357–368. [Google Scholar]
- Duke, J.A. Handbook of Phytochemical Constituent Grass, Herbs and Other Economic Plants: Herbal Reference Library; Routledge: London, UK, 2017. [Google Scholar]
- Massicot, J.; Marion, L. Biogenesis of Alkaloids: XVIII. The Formation of Hordenine from Phenylalanine in Barley. Can. J. Chem. 1957, 35, 1–4. [Google Scholar] [CrossRef]
- Argandoña, V.H.; Zuñiga, G.E.; Corcuera, L.J. Distribution of gramine and hydroxamic acids in barley and wheat leaves. Phytochemistry 1987, 26, 1917–1918. [Google Scholar] [CrossRef]
- Ghosal, S.; Mukherjee, B. Indole-3-alkylamine Bases of Desmodium pulchellum. J. Org. Chem. 1966, 31, 2284–2288. [Google Scholar] [CrossRef]
- Leete, E. Biosynthesis and metabolism of gramine in Lupinus hartwegii. Phytochemistry 1975, 14, 471–474. [Google Scholar] [CrossRef]
- Wink, M.; Meißner, C.; Witte, L. Patterns of quinolizidine alkaloids in 56 species of the genus Lupinus. Phytochemistry 1995, 38, 139–153. [Google Scholar] [CrossRef]
- Pachter, I.J.; Zacharias, D.E.; Ribeiro, O. Indole Alkaloids of Acer saccharinum (the Silver Maple), Dictyoloma incanescens, Piptadenia colubrina, and Mimosa hostilis. J. Org. Chem. 1959, 24, 1285–1287. [Google Scholar] [CrossRef]
- Baik, B.-K.; Ullrich, S.E. Barley for food: Characteristics, improvement, and renewed interest. J. Cereal Sci. 2008, 48, 233–242. [Google Scholar] [CrossRef]
- Larsson, K.A.E.; Zetterlund, I.; Delp, G.; Jonsson, L.M.V. N-Methyltransferase involved in gramine biosynthesis in barley: Cloning and characterization. Phytochemistry 2006, 67, 2002–2008. [Google Scholar] [CrossRef]
- Australia New Zealand Food Authority. Lupin Alkaloids in Food a Toxicological Review and Risk Assessment; Technical Report Series Number 3; Australia New Zealand Food: Canberra, Australia, 2001. [Google Scholar]
- Hickisch, A.; Beer, R.; Vogel, R.F.; Toelstede, S. Influence of lupin-based milk alternative heat treatment and exopolysaccharide-producing lactic acid bacteria on the physical characteristics of lupin-based yogurt alternatives. Food Res. Int. 2016, 84, 180–188. [Google Scholar] [CrossRef]
- Plancquaet, P. The lupin, an up-to-date look at what is known about it world-wide. In Proceedings of the Report of the 3 International Lupin Congress, La Rochelle, France, 4–8 June 1984. [Google Scholar]
- Tian, L.; Peel, G.J.; Lei, Z.; Aziz, N.; Dai, X.; He, J.; Watson, B.; Zhao, P.X.; Sumner, L.W.; Dixon, R.A. Transcript and proteomic analysis of developing white lupin (Lupinus albus L.) roots. BMC Plant Biol. 2009, 9, 1. [Google Scholar] [CrossRef]
- Rebolledo-Leiva, R.; Almeida-García, F.; Pereira-Lorenzo, S.; Ruíz-Nogueira, B.; Moreira, M.T.; González-García, S. Determining the environmental and economic implications of lupin cultivation in wheat-based organic rotation systems in Galicia, Spain. Sci. Total Environ. 2022, 845, 157342. [Google Scholar] [CrossRef] [PubMed]
- Gower, B.G.; Leete, E. Biosynthesis of Gramine: The Immediate Precursors of the Alkaloid. J. Am. Chem. Soc. 1963, 85, 3683–3685. [Google Scholar] [CrossRef]
- Ishikawa, E.; Kanai, S.; Sue, M. Detection of a novel intramolecular rearrangement during gramine biosynthesis in barley using stable isotope-labeled tryptophan. Biochem. Biophys. Rep. 2023, 34, 101439. [Google Scholar] [CrossRef] [PubMed]
- Larsson, K.A.E.; Saheed, S.A.; Gradin, T.; Delp, G.; Karpinska, B.; Botha, C.E.J.; Jonsson, L.M.V. Differential regulation of 3-aminomethylindole/N-methyl-3-aminomethylindole N-methyltransferase and gramine in barley by both biotic and abiotic stress conditions. Plant Physiol. Biochem. 2011, 49, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Dieter, G.; Lehmann, H.; Schütte, H.R. Zur Biosynthese des Gramins: Biosynthesis of Gramine. Biochem. Physiol. Pflanz 1974, 166, 281–287. [Google Scholar] [CrossRef]
- Leete, E.; Marion, L. The biogenesis of alkaloids: Vii. The formation of hordenine and n-methyltyramine from tyrosine in barley. Can. J. Chem. 1953, 31, 126–128. [Google Scholar] [CrossRef]
- Meyer, E. Separation of two distinct S-adenosylmethionine dependent N-methyltransferases involved in hordenine biosynthesis in Hordeum vulgare. Plant Cell Rep. 1982, 1, 236–239. [Google Scholar] [CrossRef]
- Schenck, C.A.; Maeda, H.A. Tyrosine biosynthesis, metabolism, and catabolism in plants. Phytochemistry 2018, 149, 82–102. [Google Scholar] [CrossRef]
- Kirkwood, S.; Marion, L. The Biogenesis of Alkaloids. I. The Isolation of N-Methyltyramine from Barley. J. Am. Chem. Soc. 1950, 72, 2522–2524. [Google Scholar] [CrossRef]
- Lebecque, S.; Crowet, J.-M.; Lins, L.; Delory, B.M.; du Jardin, P.; Fauconnier, M.-L.; Deleu, M. Interaction between the barley allelochemical compounds gramine and hordenine and artificial lipid bilayers mimicking the plant plasma membrane. Sci. Rep. 2018, 8, 9784. [Google Scholar] [CrossRef]
- Simons, A.B.; Marten, G.C. Relationship of Indole Alkaloids to Palatability of Phalaris arundinacea L. Agron. J. 1971, 63, 915–919. [Google Scholar] [CrossRef]
- Goelz, M.F.B.; Rothenbacher, H.; Wiggins, J.P.; Kendall, W.A.; Hershberger, T.V. Some hematological and histopathological effects of the alkaloids gramine and hordenine on meadow voles (Microtus pennsylvanicus). Toxicology 1980, 18, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Bourke, C.; Carrigan, M.; Dixon, R. Experimental evidence that tryptamine alkaloids do not cause Phalaris aquatica sudden death syndrome in sheep. Aust. Vet. J. 1988, 65, 218–220. [Google Scholar] [CrossRef] [PubMed]
- Boschin, G.; Tesio, E.; Arnoldi, A. A field case of pig poisoning by accidental feed contamination by alkaloid-rich lupin seeds. J. Appl. Anim. Res. 2022, 50, 725–731. [Google Scholar] [CrossRef]
- Luque Marquez, R.; Gutierrez-Rave, M.; Miranda, F.I. Acute poisoning by lupine seed debittering water. Vet. Hum. Toxicol. 1991, 33, 265–267. [Google Scholar]
- Westcott, N.D.; Hinks, C.F.; Olfert, O. Dietary effects of secondary plant compounds on nymphs of Melanoplus sanguinipes (Orthoptera: Acrididae). Ann. Entomol. Soc. Am. USA 1992, 85, 304–309. [Google Scholar] [CrossRef]
- Corcuera, L.J. Effects of indole alkaloids from gramineae on aphids. Phytochemistry 1984, 23, 539–541. [Google Scholar] [CrossRef]
- Griffiths, M.R.; Strobel, B.W.; Hama, J.R.; Cedergreen, N. Toxicity and risk of plant-produced alkaloids to Daphnia magna. Environ. Sci. Eur. 2021, 33, 10. [Google Scholar] [CrossRef]
- Sepulveda, B.A.; Corcuera, L.J. Effect of gramine on the susceptibility of barley leaves to Pseudomonas syringae. Phytochemistry 1990, 29, 465–467. [Google Scholar] [CrossRef]
- Mary, M.A.; Rashel, R.H.; Patiño, R. Growth inhibition of the harmful alga Prymnesium parvum by plant-derived products and identification of ellipticine as highly potent allelochemical. J. Appl. Phycol. 2021, 33, 3853–3860. [Google Scholar] [CrossRef]
- Wippich, C.; Wink, M. Biological properties of alkaloids. Influence of quinolizidine alkaloids and gramine on the germination and development of powdery mildew, Erysiphe graminis f.sp.hordei. Experientia 1985, 41, 1477–1479. [Google Scholar] [CrossRef]
- Ehmann, A. The van URK-Salkowski reagent—A sensitive and specific chromogenic reagent for silica gel thin-layer chromatographic detection and identification of indole derivatives. J. Chromatogr. A 1977, 132, 267–276. [Google Scholar] [CrossRef] [PubMed]
- Clemensen, A.K.; Provenza, F.D.; Lee, S.T.; Gardner, D.R.; Rottinghaus, G.E.; Villalba, J.J. Plant Secondary Metabolites in Alfalfa, Birdsfoot Trefoil, Reed Canarygrass, and Tall Fescue Unaffected by Two Different Nitrogen Sources. Crop Sci. 2017, 57, 964–970. [Google Scholar] [CrossRef]
- Anderton, N.; Cockrum, P.A.; Colegate, S.M.; Edgar, J.A.; Flower, K. Assessment of potential for toxicity of Phalaris spp. via alkaloid content determination: P. coerulescens, a case example. Phytochem. Anal. 1999, 10, 113–118. [Google Scholar] [CrossRef]
- Popp, D.; Harms, H.; Sträuber, H. The alkaloid gramine in the anaerobic digestion process—Inhibition and adaptation of the methanogenic community. Appl. Microbiol. Biotechnol. 2016, 100, 7311–7322. [Google Scholar] [CrossRef]
- Tietje, C.; Brouder, A. (Eds.) International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use. In Handbook of Transnational Economic Governance Regimes; Brill Nijhoff: Boston, MA, USA, 2010; pp. 1041–1053. [Google Scholar]
- Snyder, L.R.; Kirkland, J.J.; Dolan, J. The Column. In Introduction to Modern Liquid Chromatography; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2009; pp. 199–252. [Google Scholar]
- Snyder, L.R.; Stadalius, M.A. High-Performance Liquid Chromatography Separations of Large Molecules: A General Model. In High-Performance Liquid Chromatography; Horváth, C., Ed.; Academic Press: Cambridge, MA, USA, 1986; pp. 195–312. [Google Scholar]
- Muir, A.D.; Westcott, N.D.; Hinks, C.F. New quantitative high-performance liquid chromatography method for analysis of gramine in cereal leaf tissue. J. Agric. Food Chem. 1992, 40, 1836–1838. [Google Scholar] [CrossRef]
- Hoult, A.H.C.; Lovett, J.V. Biologically Active Secondary Metabolites of Barley. III. A Method for Identification and Quantification of Hordenine and Gramine in Barley by High-Performance Liquid Chromatography. J. Chem. Ecol. 1993, 19, 2245–2254. [Google Scholar] [CrossRef]
- Khedr, T.; Juhasz, A.; Singh, K.B.; Foley, R.; Nye-Wood, M.G.; Colgrave, M.L. Development and Validation of a Rapid and Sensitive Lc-Ms/Ms Approach for Alkaloids Testing in Different Lupin Species. 2023. Available online: https://papers.ssrn.com/sol3/papers.cfm?abstract_id=4331110 (accessed on 24 April 2023).
- Ma, J.; Wang, S.; Huang, X.; Geng, P.; Wen, C.; Zhou, Y.; Yu, L.; Wang, X. Validated UPLC–MS/MS method for determination of hordenine in rat plasma and its application to pharmacokinetic study. J. Pharm. Biomed. Anal. 2015, 111, 131–137. [Google Scholar] [CrossRef]
- Snyder, L.R.; Kirkland, J.J.; Dolan, J. Qualitative and Quantitative Analysis. In Introduction to Modern Liquid Chromatography; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2009; pp. 499–530. [Google Scholar]
- Joshi, S.; Upadhyay, K.S.; Rathore, A. Ion exchange chromatography hyphenated with fluorescence detector as a sensitive alternative to UV detector: Applications in biopharmaceutical analysis. J. Chromatogr. B 2022, 1212, 123511. [Google Scholar] [CrossRef]
- Lawrence, J.F. Advantages and limitations of HPLC in environmental analysis. Chromatographia 1987, 24, 45–50. [Google Scholar] [CrossRef]
- Lin, L.-L.; Lien, C.-Y.; Cheng, Y.-C.; Ku, K.-L. An effective sample preparation approach for screening the anticancer compound piceatannol using HPLC coupled with UV and fluorescence detection. J. Chromatogr. B 2007, 853, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Główka, F.K.; Karaźniewicz-Łada, M. Determination of roxithromycin in human plasma by HPLC with fluorescence and UV absorbance detection: Application to a pharmacokinetic study. J. Chromatogr. B 2007, 852, 669–673. [Google Scholar] [CrossRef] [PubMed]
- Mulvena, D.P.; Slaytor, M. Separation of tryptophan derivatives in Phalaris aquatica by thin-layer chromatography. J. Chromatogr. A 1982, 245, 155–157. [Google Scholar] [CrossRef]
- Ganzera, M.; Krüger, A.; Wink, M. Determination of quinolizidine alkaloids in different Lupinus species by NACE using UV and MS detection. J. Pharm. Biomed. Anal. 2010, 53, 1231–1235. [Google Scholar] [CrossRef]
- Lee, H.-W.; Hwang, I.-M.; Lee, H.M.; Yang, J.-S.; Park, E.J.; Choi, J.W.; Seo, H.Y.; Kim, S.H. Validation and Determination of Quinolizidine Alkaloids (QAs) in Lupin Products by Gas Chromatography with Flame Ionization Detection (GC-FID). Anal. Lett. 2020, 53, 606–613. [Google Scholar] [CrossRef]
- Eugelio, F.; Palmieri, S.; Fanti, F.; Messuri, L.; Pepe, A.; Compagnone, D.; Sergi, M. Development of an HPLC-MS/MS Method for the Determination of Alkaloids in Lupins. Molecules 2023, 28, 1531. [Google Scholar] [CrossRef]
- Holme, I.B.; Dionisio, G.; Brinch-Pedersen, H.; Wendt, T.; Madsen, C.K.; Vincze, E.; Holm, P.B. Cisgenic barley with improved phytase activity. Plant Biotechnol. J. 2012, 10, 237–247. [Google Scholar] [CrossRef]
- Harwood, W.A.; Bartlett, J.G.; Alves, S.C.; Perry, M.; Smedley, M.A.; Leyland, N.; Snape, J.W. Barley transformation using Agrobacterium-mediated techniques. Methods Mol. Biol. 2009, 478, 137–147. [Google Scholar]
- Kumlehn, J.; Serazetdinova, L.; Hensel, G.; Becker, D.; Loerz, H. Genetic transformation of barley (Hordeum vulgare L.) via infection of androgenetic pollen cultures with Agrobacterium tumefaciens. Plant Biotechnol. J. 2006, 4, 251–261. [Google Scholar] [CrossRef]
- Maver, M.; Miras-Moreno, B.; Lucini, L.; Trevisan, M.; Pii, Y.; Cesco, S.; Mimmo, T. New insights in the allelopathic traits of different barley genotypes: Middle Eastern and Tibetan wild-relative accessions vs. cultivated modern barley. PLoS ONE 2020, 15, e0231976. [Google Scholar] [CrossRef]
- Hanson, A.D.; Traynor, P.L.; Ditz, K.M.; Reicosky, D.A. Gramine in Barley Forage—Effects of Genotype and Environment. Crop Sci. 1981, 21, 726–730. [Google Scholar] [CrossRef]
- Khan, M.K.; Karnpanit, W.; Nasar-Abbas, S.M.; Huma, Z.; Jayasena, V. Phytochemical composition and bioactivities of lupin: A review. Int. J. Food Sci. Technol. 2015, 50, 2004–2012. [Google Scholar] [CrossRef]
- Osorio, C.E.; Till, B.J. A Bitter-Sweet Story: Unraveling the Genes Involved in Quinolizidine Alkaloid Synthesis in Lupinus albus. Front Plant Sci. 2022, 12, 3436. [Google Scholar] [CrossRef] [PubMed]
- Radwanski, E.R.; Last, R.L. Tryptophan biosynthesis and metabolism: Biochemical and molecular genetics. Plant Cell. 1995, 7, 921–934. [Google Scholar] [PubMed]
- Dai, F.; Chen, Z.-H.; Wang, X.; Li, Z.; Jin, G.; Wu, D.; Cai, S.; Wang, N.; Wu, F.; Nevo, E.; et al. Transcriptome profiling reveals mosaic genomic origins of modern cultivated barley. Proc. Natl. Acad. Sci. USA 2014, 111, 13403–13408. [Google Scholar] [CrossRef]
- Moore, R.; Williams, J.; Chia, J. Factors Affecting Concentrations of Dimethylated Indolealkylamines in Phalaris tuberosa L. Aust. J. Biol. Sci. 1967, 20, 1131. [Google Scholar] [CrossRef]
- Soreng, R.J.; Peterson, P.M.; Romaschenko, K.; Davidse, G.; Zuloaga, F.O.; Judziewicz, E.J.; Filgueiras, T.S.; Davis, J.I.; Morrone, O. A worldwide phylogenetic classification of the Poaceae (Gramineae). J. Syst. Evol. 2015, 53, 117–137. [Google Scholar] [CrossRef]
- Kokubo, Y.; Nishizaka, M.; Ube, N.; Yabuta, Y.; Tebayashi, S.; Ueno, K.; Taketa, S.; Ishihara, A. Distribution of the tryptophan pathway-derived defensive secondary metabolites gramine and benzoxazinones in Poaceae. Biosci. Biotechnol. Biochem. 2017, 81, 431–440. [Google Scholar] [CrossRef]
- Prasad, V.; Strömberg, C.A.E.; Leaché, A.D.; Samant, B.; Patnaik, R.; Tang, L.; Mohabey, D.M.; Ge, S.; Sahni, A. Late Cretaceous origin of the rice tribe provides evidence for early diversification in Poaceae. Nat. Commun. 2011, 2, 480. [Google Scholar] [CrossRef]
- Chaw, S.-M.; Chang, C.-C.; Chen, H.-L.; Li, W.-H. Dating the monocot-dicot divergence and the origin of core eudicots using whole chloroplast genomes. J. Mol. Evol. 2004, 58, 424–441. [Google Scholar]
- EFSA Panel on Contaminants in the Food Chain (CONTAM); Schrenk, D.; Bodin, L.; Chipman, J.K.; del Mazo, J.; Grasl-Kraupp, B.; Hogstrand, C.; Hoogenboom, L.; Leblanc, J.-C.; Nebbia, C.S. Scientific opinion on the risks for animal and human health related to the presence of quinolizidine alkaloids in feed and food, in particular in lupins and lupin-derived products. EFSA J. 2019, 17, e05860. [Google Scholar] [PubMed]
- Schütz, V.; Frindte, K.; Cui, J.; Zhang, P.; Hacquard, S.; Schulze-Lefert, P.; Knief, C.; Schulz, M.; Dörmann, P. Differential Impact of Plant Secondary Metabolites on the Soil Microbiota. Front. Microbiol. 2021, 12, 666010. [Google Scholar] [CrossRef] [PubMed]
- Ridsdill-Smith, T.; Edwards, O.; Wang, S.; Ghisalberti, E. Aphid responses to plant defensive compounds in lupin. In Aphids in New Millennium; INRA Editions: Paris, France, 2004. [Google Scholar]
- Wink, M.; Heinen, H.J.; Vogt, H.; Schiebel, H.M. Cellular localization of quinolizidine alkaloids by laser desorption mass spectrometry (LAMMA 1000). Plant Cell Rep. 1984, 3, 230–233. [Google Scholar] [CrossRef]
- Bunsupa, S.; Yamazaki, M.; Saito, K. Lysine-derived Alkaloids: Overview and Update on Biosynthesis and Medicinal Applications with Emphasis on Quinolizidine Alkaloids. Mini Rev. Med. Chem. 2017, 17, 1002–1012. [Google Scholar] [CrossRef] [PubMed]
- Jayakodi, M.; Padmarasu, S.; Haberer, G.; Bonthala, V.S.; Gundlach, H.; Monat, C.; Lux, T.; Kamal, N.; Lang, D.; Himmelbach, A.; et al. The barley pan-genome reveals the hidden legacy of mutation breeding. Nature 2020, 588, 284–289. [Google Scholar] [CrossRef] [PubMed]
- Thompson M, Ellison SLR, Wood R: Harmonized guidelines for single-laboratory validation of methods of analysis (IUPAC Technical Report). Pure Appl. Chem. 2002, 74, 835–855. [CrossRef]


| Golden Promise | ZDM 01467 | Gramine Standard | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Sample concentration (pmol/µL) | 8 | 25 | 100 | 8 | 25 | 100 | 8 | 25 | 100 |
| Replicate 1 | 8.500 | 29.06 | 112.56 | 9.170 | 29.31 | 123.5 | 8.660 | 26.19 | 99.86 |
| Replicate 2 | 8.340 | 28.71 | 112.70 | 9.200 | 30.35 | 123.3 | 8.740 | 25.96 | 100.1 |
| Replicate 3 | 8.740 | 28.55 | 112.72 | 9.220 | 30.82 | 123.5 | 8.720 | 26.10 | 99.34 |
| Replicate 4 | 8.310 | 28.60 | 112.42 | 9.170 | 29.41 | 123.1 | - | - | - |
| Replicate 5 | 8.430 | 28.62 | 112.59 | 9.230 | 30.46 | 123.5 | - | - | - |
| Replicate 6 | 8.750 | 28.75 | 112.45 | 9.340 | 29.86 | 123.2 | - | - | - |
| Mean | 8.510 | 28.71 | 112.57 | 9.220 | 30.03 | 123.4 | 8.710 | 26.08 | 99.76 |
| Standard deviation | 0.19 | 0.18 | 0.12 | 0.06 | 0.61 | 0.18 | 0.04 | 0.12 | 0.38 |
| Relative standard deviation | 2.26 | 0.64 | 0.11 | 0.70 | 2.02 | 0.15 | 0.47 | 0.46 | 0.38 |
| Injection | Response (Gramine Amount (pmol/µL/mg FW)) | ||
|---|---|---|---|
| L. luteus | L. angustifolius | L. mexicanus | |
| 1 | 667 | 3000 | 251.5 |
| 2 | 671.2 | 3172 | 263.2 |
| 3 | 660.7 | 3065.11 | 232.2 |
| Mean | 666.3 | 3079 | 249.0 |
| Standard deviation | 5.31 | 86.5 | 15.7 |
| RSD | 0.80 | 2.81 | 6.28 |
| Compound | Equation | SE y-Intercept | Coefficient of Determination R2 |
|---|---|---|---|
| Gramine | y = 1,233,973.60x + 192,425.42 | 31,834.10 | 0.9965 |
| Hordenine | y = 88,411.87x − 21,392.30 | 27,264.32 | 0.9963 |
| AMI | y = 400,644.54x − 56,069.36 | 14,840.62 | 0.9973 |
| Tryptophan | y = 148,118.67x − 77,669.45 | 6784.03 | 0.9981 |
| Tyramine | y = 78,928.96x − 25,859.95 | 8311.62 | 0.9968 |
| Fluorescence Detection (1) | Mass Spectrometry (1) | |||
|---|---|---|---|---|
| Compound | LOD (pmol/µL) | LOQ (pmol/µL) | LOD (pmol/µL) | LOQ (pmol/µL) |
| Gramine | 0.09 | 0.26 | 0. 27 * 0.18 ** | 0.83 * 0.54 ** |
| Hordenine | 1.02 | 3.08 | 0.25 | 0.75 |
| AMI | 0.12 | 0.37 | 0.93 | 2.82 |
| Tryptophan | 0.15 | 0.46 | 0.33 | 1.00 |
| Tyramine | 0.35 | 1.05 | 0.48 | 1.46 |
| Name | Common Name | Accession Number | IPK Genebank Code |
|---|---|---|---|
| Lupinus subcarnosus Hook | Texas bluebonnet | LUP 43 | 8839964 |
| Lupinus succulentus Dougl. ex K. Koch | Succulent lupin or Arroyo lupin | LUP 48 | 8839965 |
| Lupinus cosentinii Guss. | Hairy lupin | LUP 76 | 8839966 |
| Lupinus mutabilis Sweet | (White) pearl lupin or Andean lupin | LUP 100 | 8839967 |
| Lupinus albus L. subsp. albus | White lupin | LUP 211 | 8839368 |
| Lupinus luteus L. | Yellow lupin | LUP 344 | 8839969 |
| Lupinus hispanicus Boiss. & Reut. | Spanish lupin | LUP 549 | 8839970 |
| Lupinus polyphyllus Lindl. | Garden lupin | LUP 562 | 8839971 |
| Lupinus mexicanus Cerv. ex Lag. | Mexican lupin | LUP 574 | 8839972 |
| Lupinus atlanticus Gladstones | - | LUP 5152 | 8839973 |
| Lupinus angustifolius L. subsp. angustifolius | Narrowleaf lupin | LUP 5268 | 8839974 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leite Dias, S.; Garibay-Hernández, A.; Brendel, F.L.; Gabriel Chavez, B.; Brückner, E.; Mock, H.-P.; Franke, J.; D’Auria, J.C. A New Fluorescence Detection Method for Tryptophan- and Tyrosine-Derived Allelopathic Compounds in Barley and Lupin. Plants 2023, 12, 1930. https://doi.org/10.3390/plants12101930
Leite Dias S, Garibay-Hernández A, Brendel FL, Gabriel Chavez B, Brückner E, Mock H-P, Franke J, D’Auria JC. A New Fluorescence Detection Method for Tryptophan- and Tyrosine-Derived Allelopathic Compounds in Barley and Lupin. Plants. 2023; 12(10):1930. https://doi.org/10.3390/plants12101930
Chicago/Turabian StyleLeite Dias, Sara, Adriana Garibay-Hernández, Fabian Leon Brendel, Benjamin Gabriel Chavez, Elena Brückner, Hans-Peter Mock, Jakob Franke, and John Charles D’Auria. 2023. "A New Fluorescence Detection Method for Tryptophan- and Tyrosine-Derived Allelopathic Compounds in Barley and Lupin" Plants 12, no. 10: 1930. https://doi.org/10.3390/plants12101930
APA StyleLeite Dias, S., Garibay-Hernández, A., Brendel, F. L., Gabriel Chavez, B., Brückner, E., Mock, H.-P., Franke, J., & D’Auria, J. C. (2023). A New Fluorescence Detection Method for Tryptophan- and Tyrosine-Derived Allelopathic Compounds in Barley and Lupin. Plants, 12(10), 1930. https://doi.org/10.3390/plants12101930






