Diabetes-Related Mechanisms of Action Involved in the Therapeutic Effect of Croton Species: A Systematic Review
Abstract
:1. Introduction
2. Results
2.1. C. bonplandianus Baill
2.2. C. cajucara Benth
2.3. C. cuneatus Klotzsch
2.4. C. ferrugineus Kunth
2.5. C. gratissimus Burch. var. gratissimus
2.6. C. grewioides Baill
2.7. C. guatemalensis Lotsy
2.8. C. heterodoxus Baill
- Compound 1: 3β-hydroxyhop-22(29)ene (Figure 1c). This compound decreased the postprandial peak by 35% at a dose of 1 mg/kg after 15 min in oral glucose tolerance tests (OGTTs), as well as increased insulin and glucagon-like peptide-1 (GLP-1) levels in rats co-administrated with 10 mg/kg of the compound and an oral glucose load. Moreover, this triterpene significantly increased the glycogen content of liver rats, stimulated glucose uptake, and promoted insulin vesicle translocation to the plasma membrane through the involvement of potassium and calcium channels and the modulation of calcium influx via protein kinase A (PKA) and protein kinase C (PKC) activation in pancreatic β-cells [34];
- Compound 2: fern-9(11)-ene-2α,3β-diol. This triterpene was shown to have an acute antihyperglycemic effect in OGTTs at a dose of 10 mg/kg, diminishing the hyperglycemic peak by 15% in rats. In this same study, the authors synthetized a p-nitrobenzoyl derivative of this compound (Figure 1d) that exhibited a better antihyperglycemic effect at a dose of 1 mg/kg, inhibiting the hyperglycemic peak by 36% after the glucose load. This decrease was associated with an increase in insulin secretion since the derivative increased insulin concentration by 30% compared with the control group 15 min after the glucose load. In addition, the effect of the derivative compound on this mechanism was investigated in vitro in pancreatic islets, displaying an increased glucose uptake by 310% and influx of calcium by 360% compared with the controls. All these results suggest that this derivative compound has a direct effect on pancreatic β-cells, by promoting insulin secretion. One of the pathways involved in the promotion of insulin secretion is PKC activation; therefore, an inhibitor of this kinase (stearoylcarnitine chloride) was used to verify that the effects observed by the compound were due to the activation of PKC. The results show that the inhibitor abolishes the stimulatory effect of the compound on calcium influx and, as a result, it is concluded that the derivative triterpene stimulates insulin secretion through PKC activation [35];
- Compound 3: 2α,3β,23-trihydroxyolean-12-ene (Figure 1e). The compound was shown to have the best antihyperglycemic effect at a dose of 10 mg/kg in OGTTs in rats where insulin levels were also quantified. The compound inhibited the hyperglycemic peak by 30% after the first 15 min of the study and enhanced the glucose-stimulated insulin secretion (GSIS) at 30 min after the glucose load. Additionally, it promoted a four-fold increase in glycogen content measured in rat livers and glucose uptake in isolated adipocytes via GLUT4 translocation [36].
2.9. C. hirtus L’Hér
2.10. C. klotzschianus (Wight) Thwaites
2.11. C. krabas Gagnep
2.12. C. lechleri Müll.Arg
2.13. C. membranaceus Müll.Arg
2.14. C. persimilis Müll.Arg
2.15. C. thurifer Kunth
2.16. C. tiglium L.
2.17. C. yunnanensis W.W.Sm
3. Discussion
4. Methods
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs over the Nearly Four Decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83, 770–803. [Google Scholar] [CrossRef] [PubMed]
- Omale, S.; Amagon, K.I.; Johnson, T.O.; Bremner, S.K.; Gould, G.W. A Systematic Analysis of Anti-Diabetic Medicinal Plants from Cells to Clinical Trials. PeerJ 2023, 11, e14639. [Google Scholar] [CrossRef] [PubMed]
- Willcox, M.L.; Elugbaju, C.; Al-Anbaki, M.; Lown, M.; Graz, B. Effectiveness of Medicinal Plants for Glycaemic Control in Type 2 Diabetes: An Overview of Meta-Analyses of Clinical Trials. Front. Pharmacol. 2021, 12, 777561. [Google Scholar] [CrossRef] [PubMed]
- International Diabetes Federation. IDF Diabetes Atlas, 10th ed.; International Diabetes Federation: Brussels, Belgium, 2021; ISBN 9782930229874. [Google Scholar]
- ElSayed, N.A.; Aleppo, G.; Aroda, V.R.; Bannuru, R.R.; Brown, F.M.; Bruemmer, D.; Collins, B.S.; Hilliard, M.E.; Isaacs, D.; Johnson, E.L.; et al. 2. Classification and Diagnosis of Diabetes: Standards of Care in Diabetes—2023. Diabetes Care 2023, 46, S19–S40. [Google Scholar] [CrossRef]
- Galicia-Garcia, U.; Benito-Vicente, A.; Jebari, S.; Larrea-Sebal, A.; Siddiqi, H.; Uribe, K.B.; Ostolaza, H.; Martín, C. Pathophysiology of Type 2 Diabetes Mellitus. Int. J. Mol. Sci. 2020, 21, 6275. [Google Scholar] [CrossRef] [PubMed]
- Espinoza-Hernández, F.A.; Andrade-Cetto, A. Chronic Antihyperglycemic Effect Exerted by Traditional Extracts of Three Mexican Medicinal Plants. Evid.-Based Complement. Altern. Med. 2022, 2022, 5970358. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO Global Report on Traditional and Complementary Medicine 2019; (CC BY-NC-SA 3.0 IGO); World Health Organization: Geneva, Switzerland, 2019; ISBN 9789241515436. [Google Scholar]
- Ramalho, S.; Pinto, M.; Ferreira, D.; Bolzani, V. Biologically Active Orbitides from the Euphorbiaceae Family. Planta Med. 2018, 84, 558–567. [Google Scholar] [CrossRef] [PubMed]
- Salatino, A.; Salatino, M.L.F.; Negri, G. Traditional Uses, Chemistry and Pharmacology of Croton Species (Euphorbiaceae). J. Braz. Chem. Soc. 2007, 18, 11–33. [Google Scholar] [CrossRef]
- Mohammed, A.; Tajuddeen, N.; Ibrahim, M.A.; Isah, M.B.; Aliyu, A.B.; Islam, M.S. Potential of Diterpenes as Antidiabetic Agents: Evidence from Clinical and Pre-Clinical Studies. Pharmacol. Res. 2022, 179, 106158. [Google Scholar] [CrossRef]
- Maroyi, A. Review of Croton Membranaceus Mûll. Arg.: Phytochemical, Pharmacological and Toxicological Perspective. J. Complement. Med. Res. 2018, 9, 24. [Google Scholar] [CrossRef]
- Moremi, M.P.; Makolo, F.; Viljoen, A.M.; Kamatou, G.P. A Review of Biological Activities and Phytochemistry of Six Ethnomedicinally Important South African Croton Species. J. Ethnopharmacol. 2021, 280, 114416. [Google Scholar] [CrossRef] [PubMed]
- Magwilu, K.D.; Nguta, J.M.; Mapenay, I.; Matara, D. Phylogeny, Phytomedicines, Phytochemistry, Pharmacological Properties, and Toxicity of Croton Gratissimus Burch (Euphorbiaceae). Adv. Pharmacol. Pharm. Sci. 2022, 2022, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Liu, Z.; Sun, X.; Liu, Z.; Zhang, L.; Zhang, Q.; Peng, W.; Wu, C. Botany, Traditional Uses, Phytochemistry, Pharmacological and Toxicological Effects of Croton Tiglium Linn.: A Comprehensive Review. J. Pharm. Pharmacol. 2022, 74, 1061–1084. [Google Scholar] [CrossRef] [PubMed]
- Scarpa, G.F. Medicinal Plants Used by the Criollos of Northwestern Argentine Chaco. J. Ethnopharmacol. 2004, 91, 115–135. [Google Scholar] [CrossRef] [PubMed]
- Qaisar, M.; Chaudhary, B.; Sajid, M.; Hussain, N. Evaluation of α-Glucosidase Inhibitory Activity of Dichloromethane and Methanol Extracts of Croton bonplandianum Baill. Trop. J. Pharm. Res. 2014, 13, 1833. [Google Scholar] [CrossRef]
- Qaisar, M.N.; Uzair, M.; Imran, M.; Chaudhary, B.A.; Hussain, S.N. New α-Glucosidase Inhibitors from Croton bonplandianum Croton bonplandianum Baill (Euphorbiaceae). Trop. J. Pharm. Res. 2016, 15, 319. [Google Scholar] [CrossRef]
- Karuppiah Vijayamuthuramalingam, U.D.; Rajaram, R.; Kuppusamy, K.M.; Jonnalagadda, B.; Arokiasamy, S. Anti-Hyperglycemic and Antioxidant Potential of Croton Bonplandianus. Bail Fractions in Correlation with Polyphenol Content. Iran. J. Basic. Med. Sci. 2017, 20, 1390–1397. [Google Scholar] [CrossRef]
- Farias, R.; Rao, V.; Viana, G.; Silveira, E.; Maciel, M.; Pino, A. Hypoglycemic Effect of Trans -Dehydrocrotonin, a Nor-Clerodane Diterpene from Croton Cajucara. Planta Med. 1997, 63, 558–560. [Google Scholar] [CrossRef]
- Silva, R.M.; Santos, F.A.; Rao, V.S.N.; Maciel, M.A.; Pinto, A.C. Blood Glucose- and Triglyceride-Lowering Effect of Trans-Dehydrocrotonin, a Diterpene from Croton Cajucara Benth., in Rats. Diabetes Obes. Metab. 2001, 3, 452–456. [Google Scholar] [CrossRef]
- Silva, R.M.; Oliveira, F.A.; Cunha, K.M.A.; Maia, J.L.; Maciel, M.A.M.; Pinto, A.C.; Nascimento, N.R.F.; Santos, F.A.; Rao, V.S.N. Cardiovascular Effects of Trans-Dehydrocrotonin, a Diterpene from Croton Cajucara in Rats. Vasc. Pharmacol. 2005, 43, 11–18. [Google Scholar] [CrossRef]
- Rodrigues, G.; Marcolin, É.; Bona, S.; Porawski, M.; Lehmann, M.; Marroni, N.P. Hepatics Alterations and Genotoxic Effects of Croton Cajucara Benth (SACACA) in Diabetic Rats. Arq. Gastroenterol. 2010, 47, 301–305. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, G.R.; Di Naso, F.C.; Porawski, M.; Marcolin, E.; Kretzmann, N.A.; Ferraz, A.D.B.F.; Richter, M.F.; Marroni, C.A.; Marroni, N.P. Treatment with Aqueous Extract from Croton Cajucara Benth Reduces Hepatic Oxidative Stress in Streptozotocin-Diabetic Rats. J. Biomed. Biotechnol. 2012, 2012, 902351. [Google Scholar] [CrossRef] [PubMed]
- Torrico, F.; Cepeda, M.; Guerrero, G.; Melendez, F.; Blanco, Z.; Canelón, D.J.; Diaz, B.; Compagnone, R.S.; Suarez, A.I. Hypoglycaemic Effect of Croton Cuneatus in Streptozotocin-Induced Diabetic Rats. Rev. Bras. Farmacogn. 2007, 17, 166–169. [Google Scholar] [CrossRef]
- Valarezo, E.; Gaona-Granda, G.; Morocho, V.; Cartuche, L.; Calva, J.; Meneses, M.A. Chemical Constituents of the Essential Oil from Ecuadorian Endemic Species Croton Ferrugineus and Its Antimicrobial, Antioxidant and α-Glucosidase Inhibitory Activity. Molecules 2021, 26, 4608. [Google Scholar] [CrossRef]
- Okokon, J.; Bassey, A.; Obot, J. Antidiabetic Activity of Ethanolic Leaf Extract of Croton Zambesicus Muell.(Thunder Plant) in Alloxan Diabetic Rats. Afr. J. Tradit. Complement. Altern. Med. 2006, 3, 21–26. [Google Scholar] [CrossRef]
- Ofusori, D.A.; Komolafe, O.A.; Adewole, O.S.; Obuotor, E.M.; Fakunle, J.B.; Ayoka, A.O. Effect of Ethanolic Leaf Extract of Croton Zambesicus (Müll. Arg.) on Lipid Profile in Streptozotocin-Induced Diabetic Rats. Diabetol. Croat. 2012, 41, 69–76. [Google Scholar]
- Ofusori, D.; Komolafe, O.; Adewole, O.; Obuotor, E.; Fakunle, J. Antihyperglycemic and Antioxidative Effects of Ethanolic Leaf Extract of Croton Zambesicus in Streptozotocin Induced Diabetic Rats [C. Zambesicus’un Etanolik Yaprak Özütünün Streptozotocin Ile Indüklenmiş Diyabetik s?Çanlarda Antihiperglisemik ve Aktioksi. J. Cell. Mol. Biol. 2014, 12, 19–30. [Google Scholar]
- Ayanniyi, R.O.; Olumoh-Abdul, H.A.; Ojuade, F.I.; Asogwa, N. Protective Effect of Croton Zambesicus Leaf Extract against Carbon Tetrachloride-Induced Cardiac Toxicity in Rats. Thai J. Pharm. Sci. 2020, 44, 216–219. [Google Scholar]
- Silva-Alves, K.S.; Ferreira-da-Silva, F.W.; Coelho-de-Souza, A.N.; Leal-Cardoso, J.H.J.H. Essential Oil of Croton Zehntneri Prevents Conduction Alterations Produced by Diabetes Mellitus on Vagus Nerve. Plants 2021, 10, 893. [Google Scholar] [CrossRef]
- Andrade-Cetto, A.; Cruz, E.C.; Cabello-Hernández, C.A.; Cárdenas-Vázquez, R. Hypoglycemic Activity of Medicinal Plants Used among the Cakchiquels in Guatemala for the Treatment of Type 2 Diabetes. Evid.-Based Complement. Altern. Med. 2019, 2019, 2168603. [Google Scholar] [CrossRef]
- Escandón-Rivera, S.M.; Andrade-Cetto, A.; Rosas-Ramírez, D.G.; Arreguín-Espinosa, R. Phytochemical Screening and Isolation of New Ent-Clerodane Diterpenoids from Croton Guatemalensis Lotsy. Plants 2022, 11, 3159. [Google Scholar] [CrossRef] [PubMed]
- Gomes Castro, A.J.; Cazarolli, L.H.; de Carvalho, F.K.; da Luz, G.; Altenhofen, D.; dos Santos, A.R.S.; Pizzolatti, M.G.; Silva, F.R.M.B. Acute Effect of 3β-Hidroxihop-22(29)Ene on Insulin Secretion Is Mediated by GLP-1, Potassium and Calcium Channels for the Glucose Homeostasis. J. Steroid Biochem. Mol. Biol. 2015, 150, 112–122. [Google Scholar] [CrossRef] [PubMed]
- da Luz, G.; Frederico, M.J.S.; Castro, A.J.G.; Moraes, A.L.L.; de Carvalho, F.K.; Espíndola, L.; Schmidt, É.C.; Bouzon, Z.L.; Pizzolatti, M.G.; Silva, F.R.M.B. Triterpene Derivative: A Potential Signaling Pathway for the Fern-9(11)-Ene-2α,3β-Diol on Insulin Secretion in Pancreatic Islet. Life Sci. 2016, 154, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Gomes Castro, A.J.; Cazarolli, L.H.; Silva Frederico, M.J.; Dambrós, B.F.; de Carvalho, F.K.; de Medeiros Pinto, V.A.; da Fonte Ramos, C.; Filippin Monteiro, F.B.; Pizzolatti, M.G.; Mena Barreto Silva, F.R. Biological Activity of 2α,3β,23-Trihydroxyolean-12-Ene on Glucose Homeostasis. Eur. J. Pharmacol. 2021, 907, 174250. [Google Scholar] [CrossRef]
- Fuentes, J.C.; Castro, V.; Jakupovic, J.; Murillo, R. Diterpenos y Otros Constituyentes de Croton Hirtus (Euphorbiaceae). Rev. Biol. Trop. 2004, 52, 269–285. [Google Scholar] [CrossRef]
- Kim, M.J.; Kim, J.G.; Sydara, K.M.; Lee, S.W.; Jung, S.K. Croton Hirtus L’Hér Extract Prevents Inflammation in RAW264.7 Macrophages Via Inhibition of NF-ΚB Signaling Pathway. J. Microbiol. Biotechnol. 2020, 30, 490–496. [Google Scholar] [CrossRef]
- Govindarajan, R.; Vijayakumar, M.; Rao, C.V.; Pushpangadan, P.; Asare-Anane, H.; Persaud, S.; Jones, P.; Houghton, P.J. Antidiabetic Activity of Croton Klozchianus in Rats and Direct Stimulation of Insulin Secretion In-Vitro. J. Pharm. Pharmacol. 2008, 60, 371–376. [Google Scholar] [CrossRef]
- Rajachan, O.; Lakornwong, W.; Pitchuanchom, S.; Suchaichit, N.P.; Boonmak, J.; Youngme, S.; Kanokmedhakul, K.; Kanokmedhakul, S. Ent-Clerodane Diterpenoids from the Stems of Croton Krabas. Fitoterapia 2021, 152, 104912. [Google Scholar] [CrossRef]
- Chen, Z.; Bertin, R.; Marin, R.; Medjiofack Djeujo, F.; Froldi, G. Effects of Croton Lechleri Sap (Sangre de Drago) on AGEs Formation, LDL Oxidation and Oxidative Stress Related to Vascular Diseases. Nat. Prod. Res. 2022, 36, 4165–4169. [Google Scholar] [CrossRef]
- Sarkodie, J.A.; Appiah, A.A.; Edoh, D.A.; Aboagye, F.A.; Asiedu-Larbi, J.; Tandoh, M.; Sakyiamah, M.; Donkor, K. Antihyperglycaemic and Antioxidant Effects of Croton Membranaceus Mull. Arg (Euphorbiaceae). Int. J. Pharm. Sci. Res. 2014, 5, 110. [Google Scholar] [CrossRef]
- Asare, G.A.; Adjei, S.; Afriyie, D.; Appiah-Danquawh, A.B.; Asia, J.; Asiedu, B.; Santa, S.; Doku, D. Croton Membranaceus Improves Some Biomarkers of Cardiovascular Disease and Diabetes in Genetic Animal Models. J. Clin. Diagn. Res. 2015, 9, OF01–OF05. [Google Scholar] [CrossRef] [PubMed]
- Srisongkram, T.; Waithong, S.; Thitimetharoch, T.; Weerapreeyakul, N. Machine Learning and In Vitro Chemical Screening of Potential Alpha-Amylase and Alpha-Glucosidase Inhibitors from Thai Indigenous Plants. Nutrients 2022, 14, 267. [Google Scholar] [CrossRef] [PubMed]
- Morocho, V.; Sarango, D.; Cruz-Erazo, C.; Cumbicus, N.; Cartuche, L.; Suárez, A.I. Chemical Constituents of Croton Thurifer Kunth as α-Glucosidase Inhibitors. Rec. Nat. Prod. 2020, 14, 31–41. [Google Scholar] [CrossRef]
- Zhang, D.; Arunachalam, K.; Wang, Y.; Zhang, Y.; Yang, J.; Hein, P.P.; Mon, A.M.; Li, J.; Inta, A.; Yang, X. Evaluation on Antidiabetic Properties of Medicinal Plants from Myanmar. Sci. World J. 2021, 2021, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.-H.; Liu, W.-Y.; Liang, Q. Chemical Constituents from Croton Species and Their Biological Activities. Molecules 2018, 23, 2333. [Google Scholar] [CrossRef]
- Jiang, Z.-Y.; Liu, C.-J.; Niu, Q.; Yan, X.-Y.; Xiao, D.; Zhang, H.-L.; Huang, C.-Q.; Shi, S.-L.; Zuo, A.-X.; He, H.-P. In Vitro Hypoglycemic Diterpenoids from the Roots of Croton Yunnanensis. J. Nat. Prod. 2023, 86, 199–208. [Google Scholar] [CrossRef]
- Maciel, M.A.M.; Pinto, A.C.; Arruda, A.C.; Pamplona, S.G.S.R.; Vanderlinde, F.A.; Lapa, A.J.; Echevarria, A.; Grynberg, N.F.; Côlus, I.M.S.; Farias, R.A.F.; et al. Ethnopharmacology, Phytochemistry and Pharmacology: A Successful Combination in the Study of Croton Cajucara. J. Ethnopharmacol. 2000, 70, 41–55. [Google Scholar] [CrossRef]
- Morais, W.A.; Costa, M.P.; Paixão, A.D.O.; MacIel, M.A.M.; Santos-Magalhes, N.S. Encapsulation and Release Characteristics of DCTN/PLGA Microspheres. J. Microencapsul. 2009, 26, 529–534. [Google Scholar] [CrossRef]
- Herrera, C.; Pérez, Y.; Morocho, V.; Armijos, C.; Malagón, O.; Brito, B.; Tacán, M.; Cartuche, L.; Gilardoni, G. Preliminary Phytochemical Study of the Ecuadorian Plant Croton Elegans Kunth (Euphorbiaceae). J. Chil. Chem. Soc. 2018, 63, 3875–3877. [Google Scholar] [CrossRef]
- Quattrocchi, U. CRC World Dictionary of Medicinal and Poisonous Plants Common Names, Scientific Names, Eponyms, Synonyms, and Etymology; CRC Press: Boca Raton, FL, USA, 2012; ISBN 9781482250640. [Google Scholar]
- Shama, I.Y.A.; Ebtihal, A.S. Investigations on the Effects of Various Oral Doses of Croton Zambesicus Seeds’ in Wistar Rats. J. Pharmacol. Toxicol. 2012, 8, 19–27. [Google Scholar] [CrossRef]
- Agra, M.D.F.; Silva, K.N.; Basílio, I.J.L.D.; Freitas, P.F.D.; Barbosa-Filho, J.M. Survey of Medicinal Plants Used in the Region Northeast of Brazil. Rev. Bras. Farmacogn. 2008, 18, 472–508. [Google Scholar] [CrossRef]
- Cruz, E.C.; Andrade-Cetto, A. Ethnopharmacological Field Study of the Plants Used to Treat Type 2 Diabetes among the Cakchiquels in Guatemala. J. Ethnopharmacol. 2015, 159, 238–244. [Google Scholar] [CrossRef] [PubMed]
- WFO. World Flora Online. Available online: http://www.worldfloraonline.org/ (accessed on 29 January 2023).
- Subramoniam, A. Plants with Anti-Diabetes Mellitus Properties; CRC Press: Boca Raton, FL, USA, 2016; ISBN 9781482249903. [Google Scholar]
- DeFilipps, R.A.; Krupnick, G.A. The Medicinal Plants of Myanmar. PhytoKeys 2018, 102, 1–341. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO Traditional Medicine Strategy: 2014–2023; World Health Organization: Geneva, Switzerland, 2013; ISBN 9789241506090. [Google Scholar]
- Leonti, M.; Stafford, G.I.; Cero, M.D.; Cabras, S.; Castellanos, M.E.; Casu, L.; Weckerle, C.S. Reverse Ethnopharmacology and Drug Discovery. J. Ethnopharmacol. 2017, 198, 417–431. [Google Scholar] [CrossRef]
- ElSayed, N.A.; Aleppo, G.; Aroda, V.R.; Bannuru, R.R.; Brown, F.M.; Bruemmer, D.; Collins, B.S.; Hilliard, M.E.; Isaacs, D.; Johnson, E.L.; et al. 9. Pharmacologic Approaches to Glycemic Treatment: Standards of Care in Diabetes—2023. Diabetes Care 2023, 46, S140–S157. [Google Scholar] [CrossRef]
- Barre, D.E.; Mizier-Barre, K.A. The Polypharmacy Reduction Potential of Cinnamic Acids and Some Related Compounds in Pre- and Post-Onset Management of Type 2 Diabetes Mellitus. Endocr. Regul. 2020, 54, 137–155. [Google Scholar] [CrossRef]
- Mata-Torres, G.; Andrade-Cetto, A.; Espinoza-Hernández, F. Approaches to Decrease Hyperglycemia by Targeting Impaired Hepatic Glucose Homeostasis Using Medicinal Plants. Front. Pharmacol. 2021, 12, 809994. [Google Scholar] [CrossRef]
- Shi, M.; Loftus, H.; McAinch, A.J.; Su, X.Q. Blueberry as a Source of Bioactive Compounds for the Treatment of Obesity, Type 2 Diabetes and Chronic Inflammation. J. Funct. Foods 2017, 30, 16–29. [Google Scholar] [CrossRef]
- Daryabor, G.; Atashzar, M.R.; Kabelitz, D.; Meri, S.; Kalantar, K. The Effects of Type 2 Diabetes Mellitus on Organ Metabolism and the Immune System. Front. Immunol. 2020, 11, 1582. [Google Scholar] [CrossRef]
- Luc, K.; Schramm-Luc, A.; Guzik, T.J.; Mikolajczyk, T.P. Oxidative Stress and Inflammatory Markers in Prediabetes and Diabetes. J. Physiol. Pharmacol. 2019, 70, 809–824. [Google Scholar] [CrossRef]
- Dimitriadis, G.D.; Maratou, E.; Kountouri, A.; Board, M.; Lambadiari, V. Regulation of Postabsorptive and Postprandial Glucose Metabolism by Insulin-Dependent and Insulin-Independent Mechanisms: An Integrative Approach. Nutrients 2021, 13, 159. [Google Scholar] [CrossRef] [PubMed]
- Maffettone, A.; Rinaldi, M.; Fontanella, A. Postprandial Hyperglycemia: A New Frontier in Diabetes Management? Ital. J. Med. 2018, 12, 108–115. [Google Scholar] [CrossRef]
- Andrade-Cetto, A.; Heinrich, M. Mexican Plants with Hypoglycaemic Effect Used in the Treatment of Diabetes. J. Ethnopharmacol. 2005, 99, 325–348. [Google Scholar] [CrossRef] [PubMed]
- ElSayed, N.A.; Aleppo, G.; Aroda, V.R.; Bannuru, R.R.; Brown, F.M.; Bruemmer, D.; Collins, B.S.; Hilliard, M.E.; Isaacs, D.; Johnson, E.L.; et al. 6. Glycemic Targets: Standards of Care in Diabetes—2023. Diabetes Care 2023, 46, S97–S110. [Google Scholar] [CrossRef] [PubMed]
- Zeng, L.; Zhang, G.; Liao, Y.; Gong, D. Inhibitory Mechanism of Morin on α-Glucosidase and Its Anti-Glycation Properties. Food Funct. 2016, 7, 3953–3963. [Google Scholar] [CrossRef] [PubMed]
- Holst, J.J.; Gasbjerg, L.S.; Rosenkilde, M.M. The Role of Incretins on Insulin Function and Glucose Homeostasis. Endocrinology 2021, 162, bqab065. [Google Scholar] [CrossRef]
- Perreault, L.; Skyler, J.S.; Rosenstock, J. Novel Therapies with Precision Mechanisms for Type 2 Diabetes Mellitus. Nat. Rev. Endocrinol. 2021, 17, 364–377. [Google Scholar] [CrossRef]
- Holst, J.J. Incretin Therapy for Diabetes Mellitus Type 2. Curr. Opin. Endocrinol. Diabetes Obes. 2020, 27, 2–10. [Google Scholar] [CrossRef]
- Sultana, R.; Sissoho, F.; Kaushik, V.P.; Raji, M.A. The Case for Early Use of Glucagon-like Peptide-1 Receptor Agonists in Obstructive Sleep Apnea Patients with Comorbid Diabetes and Metabolic Syndrome. Life 2022, 12, 1222. [Google Scholar] [CrossRef]
- Nirmalan, N.; Nirmalan, M. Hormonal Control of Metabolism: Regulation of Plasma Glucose. Anaesth. Intensive Care Med. 2017, 18, 502–507. [Google Scholar] [CrossRef]
- Singh, A.; Singh, K.; Sharma, A.; Kaur, K.; Kaur, K.; Chadha, R.; Bedi, P.M.S. Recent Developments in Synthetic α-Glucosidase Inhibitors: A Comprehensive Review with Structural and Molecular Insight. J. Mol. Struct. 2023, 1281, 135115. [Google Scholar] [CrossRef]
- Lyu, H.; Chen, J.; Li, W.L. Natural Triterpenoids for the Treatment of Diabetes Mellitus: A Review. Nat. Prod. Commun. 2016, 11, 1579–1586. [Google Scholar] [CrossRef] [PubMed]
- Ansari, P.; Akther, S.; Hannan, J.M.A.; Seidel, V.; Nujat, N.J.; Abdel-Wahab, Y.H.A. Pharmacologically Active Phytomolecules Isolated from Traditional Antidiabetic Plants and Their Therapeutic Role for the Management of Diabetes Mellitus. Molecules 2022, 27, 4278. [Google Scholar] [CrossRef] [PubMed]
- Guerra, S.; Gastaldelli, A. The Role of the Liver in the Modulation of Glucose and Insulin in Non Alcoholic Fatty Liver Disease and Type 2 Diabetes. Curr. Opin. Pharmacol. 2020, 55, 165–174. [Google Scholar] [CrossRef]
- Mata-Torres, G.; Andrade-Cetto, A.; Espinoza-Hernández, F.A.; Cárdenas-Vázquez, R. Hepatic Glucose Output Inhibition by Mexican Plants Used in the Treatment of Type 2 Diabetes. Front. Pharmacol. 2020, 11, 215. [Google Scholar] [CrossRef] [PubMed]
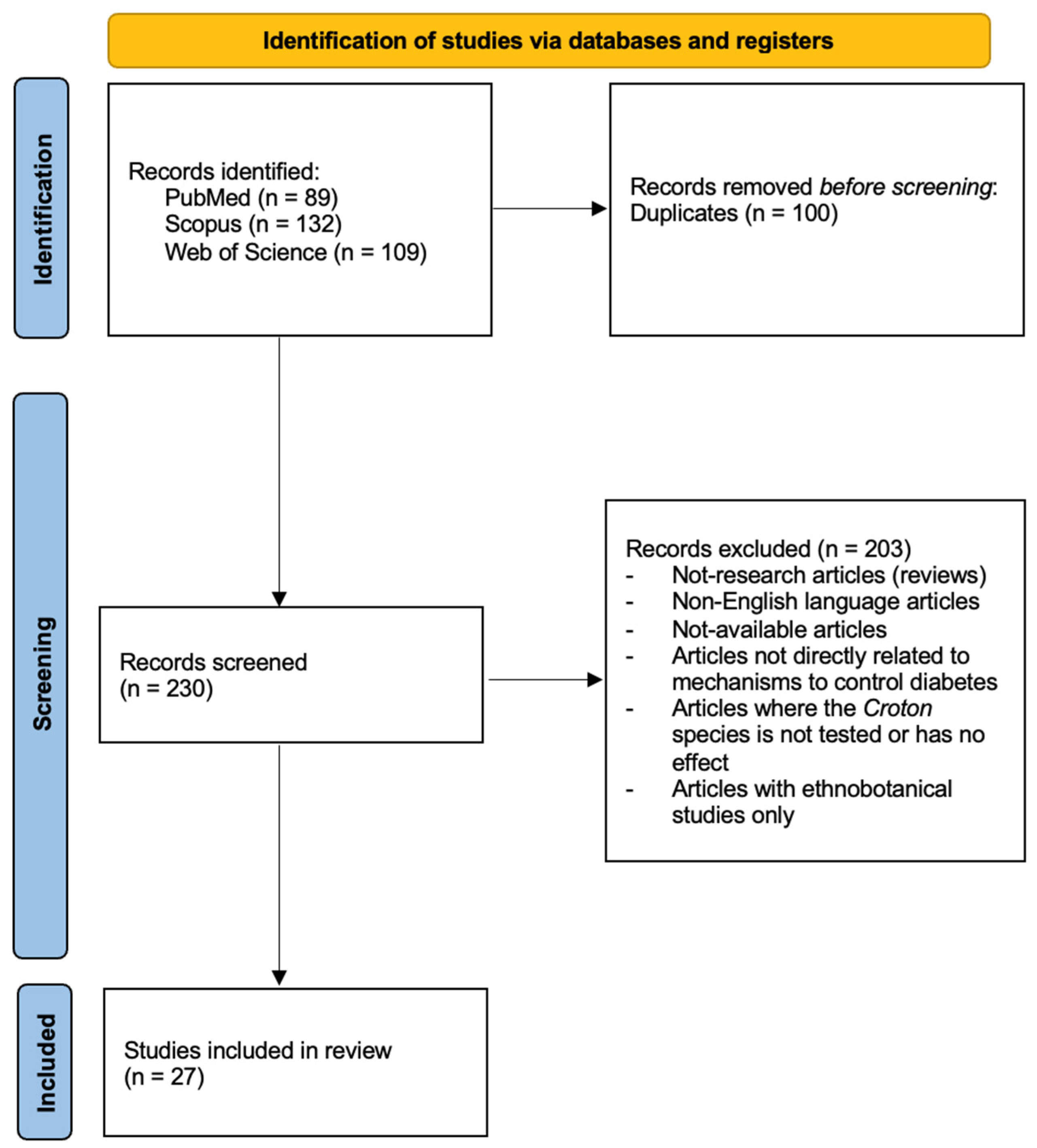
- Page, M.J.; Moher, D.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. PRISMA 2020 Explanation and Elaboration: Updated Guidance and Exemplars for Reporting Systematic Reviews. BMJ 2021, 372, n160. [Google Scholar] [CrossRef] [PubMed]
- Tropicos. Missouri Botanical Garden. Available online: https://tropicos.org (accessed on 29 January 2023).
- POWO. Plants of the World Online. Facilitated by the Royal Botanic Gardens, Kew. Available online: http://www.plantsoftheworldonline.org/ (accessed on 29 January 2023).
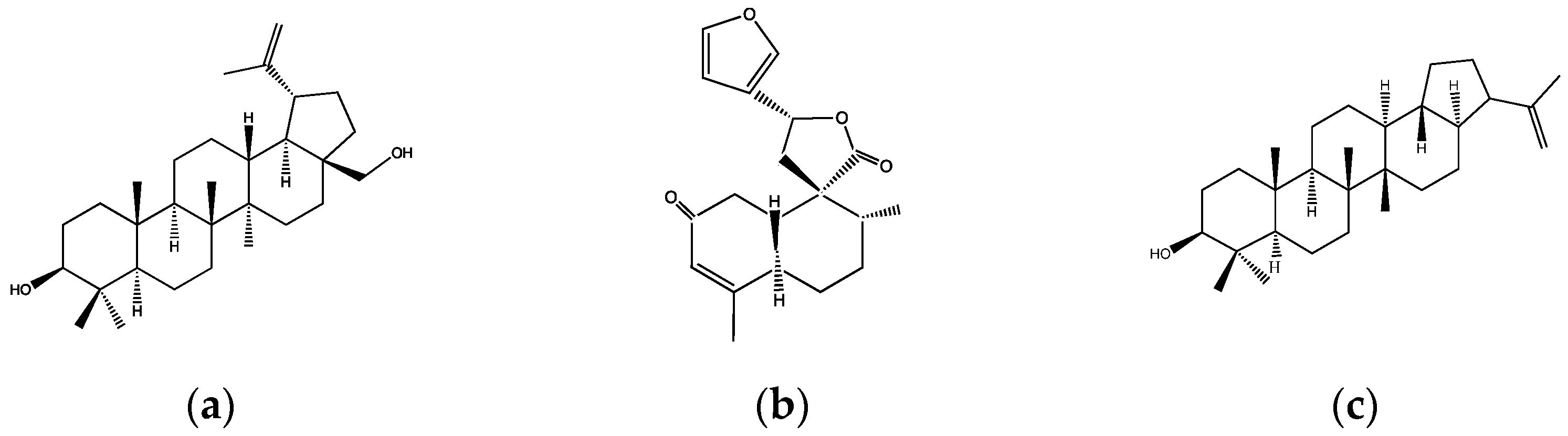


| Species | Common Names | Places Where It Is Used | Type of Extracts and Parts Used | Identified Compounds | Pharmacological Studies Related to Diabetes | References |
|---|---|---|---|---|---|---|
| C. bonplandianus Baill. | “Escoba negra” “Ban tulsi” | South America (Argentina) Asia (India) | Dichlorometane extract of the whole plant. Chloroform fraction obtained from methanol extract of the leaves. | 2 H-1-benzopyran-2-one Betulin (Figure 1a) 3,5-Dimethoxy 4-hydroxy cinnamic acid Z-5-Nonadecene Cyclotetracosane N-Nonadecenol-1 Cycloeicosane 3-eicosene Z-8—Hexadecene 6-5heptadecenal Phenol,2,4-bis(1,1-dimethyl) | In vitro: Inhibition of α-glucosidase and α-amylase activities. In vitro: Antioxidant activity. | [16,17,18,19] |
| C. cajucara Benth. | “Sacaca” | South America (Brazil) | Aqueous extract of the bark. | Trans-Crotonin trans-Dehydrocrotonin (Figure 1b) Acetyl aleuritolic acid | In vivo: Hypoglycemic and antihyperglycemic effects of t-DCTN in alloxan-induced hyperglycemic rats. In vivo: Antihypertriglyceridemic effect of t-DCTN in ethanol-induced hypertriglyceridemic rats. In vivo: Antihypertensive effect of t-DCTN in normotensive rats. In vivo: Reduction in hepatic OxS markers in STZ-induced hyperglycemic rats. | [10,20,21,22,23,24] |
| C. cuneatus Klotzsch | “Arapurima” | South America (Venezuela) | Aqueous extract of the stem bark. | A-11 Eudesmene Methyleugenol 4-α-Seleniol Cedryl propyl ether τ-Cadinol Cubenol | In vivo: Hypoglycemic effect in STZ-induced hyperglycemic rats. | [25] |
| C. ferrugineus Kunth | “Mosquera” | South America (Ecuador) | Essential oil of the leaves. | Caryophyllene Myrcene β-Phellandrene Germacrene D Linalool α-Humulene | In vitro: Inhibition of α-glucosidase activity. In vitro: Antioxidant activity. | [26] |
| C. gratissimus Burch. Var. gratissimus | “Koriba” | Africa (Nigeria) | Ethanolic and aqueous extract of the leaves. Aqueous extract of the leaves. | Trachylobane ent-trachyloban-3β-ol ent-18-Hydroxy-trachyloban-3-one Isopimara-7,15-dien-3β-ol ent-18-hydroxytrachyloban-3β-ol ent-18-hydroxyisopimara-7,15-diene-3β-ol | In vivo: Acute and chronic hypoglycemic effects in alloxan-induced hyperglycemic rats. In vivo: Improvement in lipid profile in STZ-induced hyperglycemic rats. In vivo: Cardioprotective effect on carbon tetrachloride-induced cardiac toxicity in rats. In vivo: Decreased lipid peroxidation and antioxidant markers in STZ-induced hyperglycemic rats. | [10,27,28,29,30] |
| C. grewioides Baill. | “Canela-de-cunhã” | South America (Brazil) | Essential oil of the leaves. | Anethole Estragole | In vivo: Protection against decreased excitability and conduction velocity of myelinated vagus nerve fibers in STZ-induced hyperglycemic rats. | [31] |
| C. guatemalensis Lotsy | “Copalchí” | Central America (Guatemala) | Aqueous and ethanol-water extracts of the bark. | Junceic acid Crotoguatenoic acid A and B Bartsiifolic acid Formosin F Rutin Epicatechin Quercetin | In vivo: Acute hypoglycemic effect in STZ-NA-induced hyperglycemic rats. | [32,33] |
| C. heterodoxus Baill. | No records available | No reports on medicinal uses | Ethanol–water extract of the aerial parts. | 3β-Hydroxyhop-22(29)ene (Figure 1c) p-nitrobenzoyl derivative of fern-9(11)-ene-2α,3β-diol (Figure 1d) 2α,3β,23-Trihydroxyolean-12-ene (Figure 1e) | In vivo: Decreased postprandial hyperglycemia due to increased insulin and/or GLP-1 levels in OGTTs performed in healthy rats. In vivo: Increased glycogen content in liver rats. In vitro: Promoted glucose uptake in pancreatic islets. In vitro: Promoted insulin vesicle translocation via PKA and/or PKC activation in pancreatic islets. In vitro: Promoted glucose uptake via GLUT4 translocation in adipocytes. | [34,35,36] |
| C. hirtus L’Hér. | No records available | No reports on medicinal uses | Methanol extract of the whole plant. | Bis-nordolabradane Dolabradanes Kauranes Cyclopropakauranes Hirtusanes Germacradiene esters | In vitro: Suppressed lipopolysaccharide-induced production of nitric oxide and inflammatory cytokines in RAW264.7 macrophages. | [37,38] |
| C. klotzschianus (Wight) Thwaites | No records available | Asia (India) | Ethanol–water extract of the aerial parts. | Quercetin Quinic acid | In vivo: Decreased postprandial hyperglycemia in OGTTs performed in healthy rats. In vivo: Sub-chronic hypoglycemic effect in STZ-induced hyperglycemic rats. In vitro: Promoted insulin secretion at low glucose concentration and enhanced GSIS in MIN6 cell line. | [39] |
| C. krabas Gagnep. | “Fai Nam” | Asia (Thailand) | n-Hexane and ethyl acetate extracts of the stems. | Crotonkrabases A–C (crotonkrabas A: Figure 1f) 12-Oxohardwickiic acid Crotonpyrone B | In vitro: Inhibition of α-glucosidase activity. | [40] |
| C. lechleri Müll.Arg. | “Sangre de Drago” | South America (Ecuador) | Bark sap | Crolechinol Crolechinic acid Taspine 3′,4-O-dimethylcedrusin Proanthocyanidin SP–303 | In vitro: Decreased bovine serum albumin glycation, LDL oxidation, and H2O2-induced OxS in human umbilical vein endothelial cells. | [10,41] |
| C. membranaceus Müll.Arg. | “Côte d’Ivoire” | Africa (Ghana) | Ethanolic and aqueous extracts of the roots. | Scopoletin Crotomembranafuran Julocrotine N[N-(2-methylbutanoyl) glutaminoyl]-2-phenylethylamine DL-threitol Gomojoside H β-Sitosterol β-Sitosterol-3-D-glucoside | In vivo: Acute and chronic hypoglycemic effects in STZ-induced hyperglycemic rats. In vivo: Improved cardiovascular profile in spontaneously hypertensive rats. In vivo: Acute hypoglycemic effect in db/db mice. In vitro: Antioxidant activity. | [12,42,43] |
| C. persimilis Müll.Arg. | “Chucka” | Asia (Thailand) | Ethanol–water extract of the stem. | 11-Dehydro (-) hardwickiic acid Labda-7,12(E),14-triene Labda-7,12(E),14-triene-17-al Labda- 7,12(E),14-triene-17-ol Labda-7,12(E),14-triene-17-oic acid Crotocembranoic acid Neocrotocembranal | In vitro: Inhibition of α-glucosidase activity. | [10,44] |
| C. thurifer Kunth | “Mosquera” | South America (Ecuador) | Ethyl acetate extract of the leaves. | (3R, 20S)-3-Palmitate-20-hydroxydammar-24-ene (3R, 20S)-3-Acetoxy-20-hydroxydammar-24-ene (Figure 1g) trans-Phytol Vomifoliol β-Sitosterol trans-Tiliroside Sparsifol | In vitro: Inhibition of α-glucosidase activity. | [45] |
| C. tiglium L. | “Croton-oil-plant” “Kanakho” | Asia (India, China) | Ethanolic extract of the seeds. | 12-O-isobutyrylphorbol-13-decanoate 12-O-(2-methyl)butyrylphorbol-13-octanoate 12-O-(2-methyl)butyrylphorbol-13-tiglate Crotignoid A-K 12-O-tiglylphorbol-4-deoxy-4β-phorbol-13-acetate 12-O-tiglylphorbol-4-deoxy-4β-phorbol-13-hexadecanoate | In vitro: Promoted glucose uptake in 3T3-L1 adipocytes. | [46,47] |
| C. yunnanensis W.W.Sm. | “Ji gu xiang” | Asia (China) | Ethanolic extract of the roots. | Crotonine A-I (crotonine A: Figure 1h, crotonine F: Figure 1i) Crotonyunnan A Hardwickic acid methyl ester 12-Hydroxyhardwickic acid methyl ester 12-Oxohardwickic acid methyl ester Sacacarin Phlorizin | In vitro: Promoted glucose uptake in insulin-resistant 3T3-L1 adipocytes. | [48] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Espinoza-Hernández, F.A.; Moreno-Vargas, A.D.; Andrade-Cetto, A. Diabetes-Related Mechanisms of Action Involved in the Therapeutic Effect of Croton Species: A Systematic Review. Plants 2023, 12, 2014. https://doi.org/10.3390/plants12102014
Espinoza-Hernández FA, Moreno-Vargas AD, Andrade-Cetto A. Diabetes-Related Mechanisms of Action Involved in the Therapeutic Effect of Croton Species: A Systematic Review. Plants. 2023; 12(10):2014. https://doi.org/10.3390/plants12102014
Chicago/Turabian StyleEspinoza-Hernández, Fernanda Artemisa, Angelina Daniela Moreno-Vargas, and Adolfo Andrade-Cetto. 2023. "Diabetes-Related Mechanisms of Action Involved in the Therapeutic Effect of Croton Species: A Systematic Review" Plants 12, no. 10: 2014. https://doi.org/10.3390/plants12102014







