Mechanism of Mepiquat Chloride Regulating Soybean Response to Drought Stress Revealed by Proteomics
Abstract
:1. Introduction
2. Results
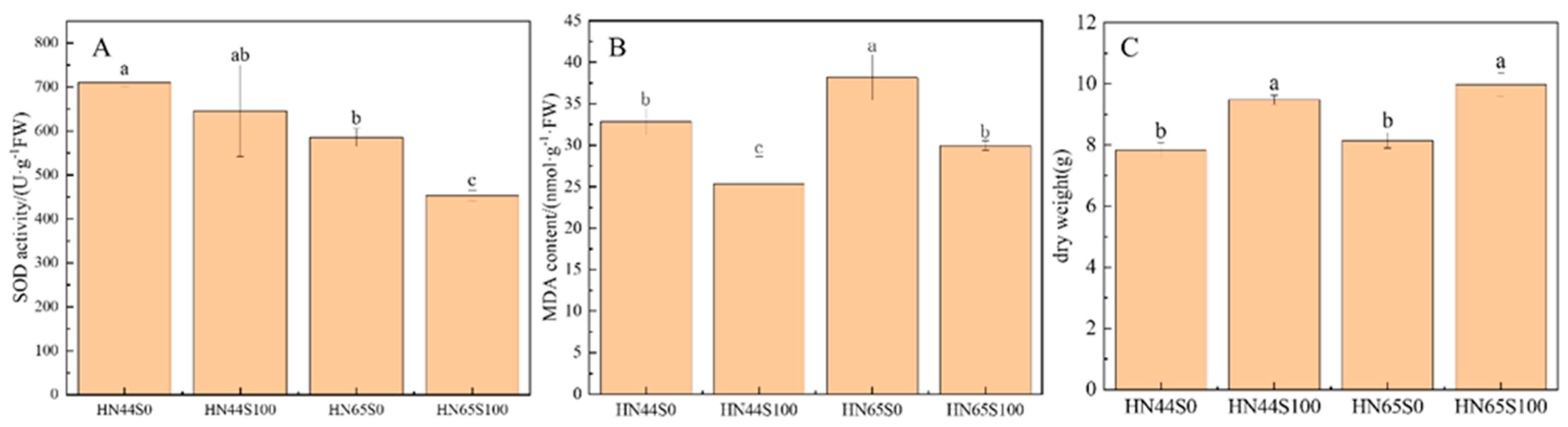
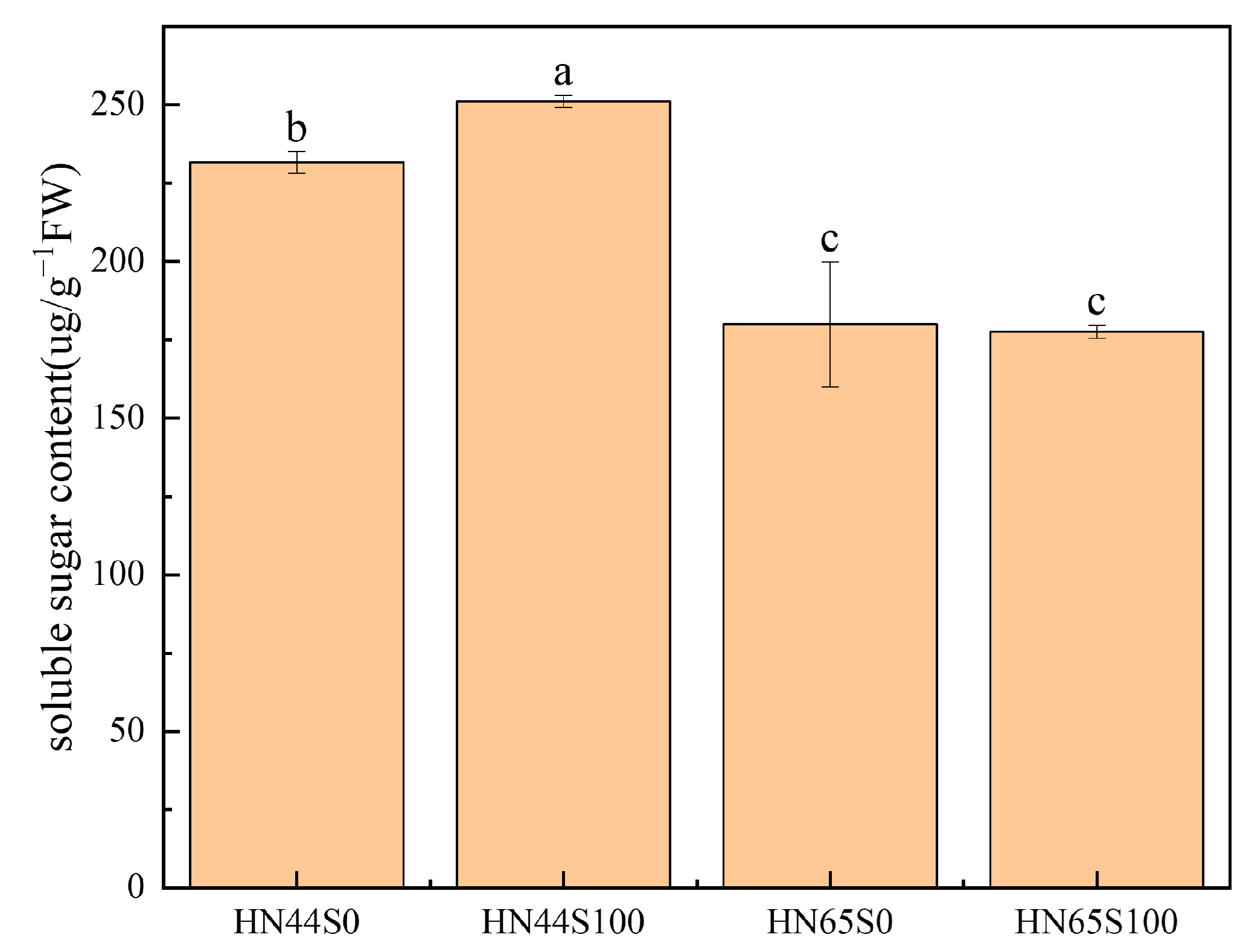
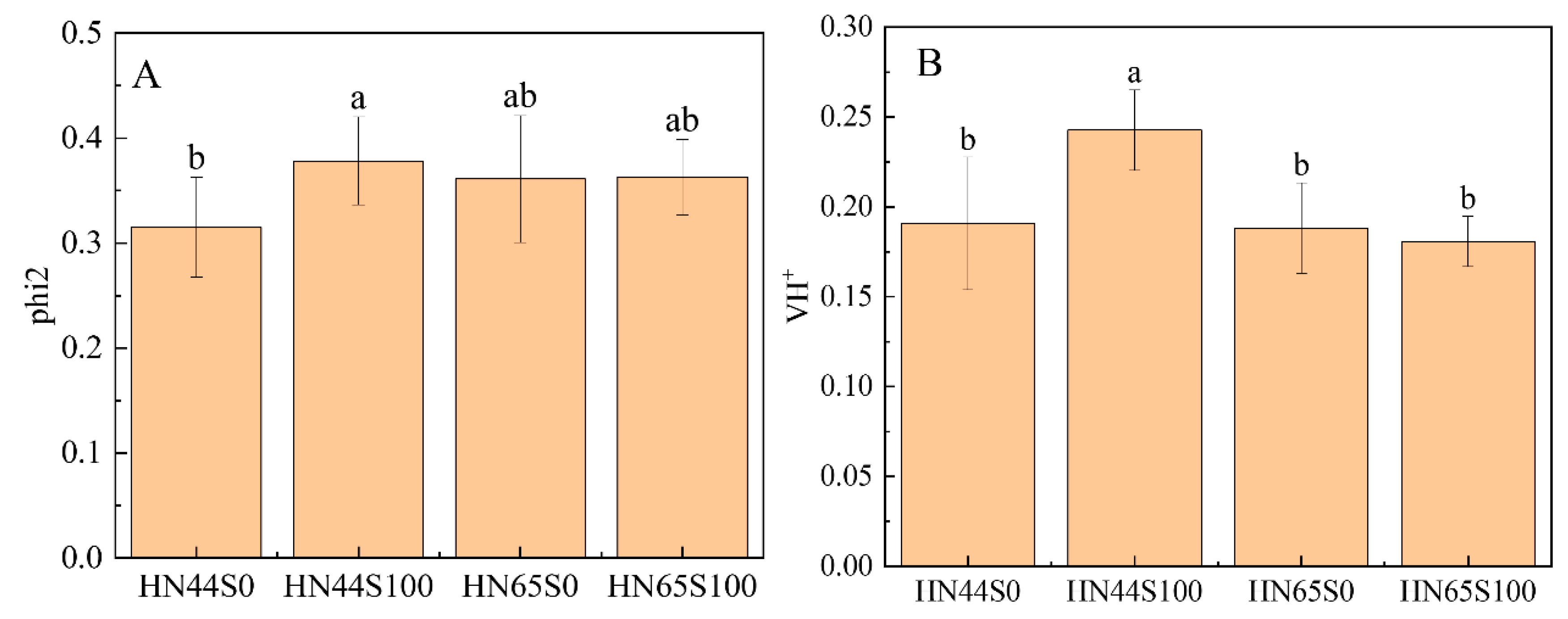
2.1. Physiological and Biochemical Response in Soybean after MC Pretreatment
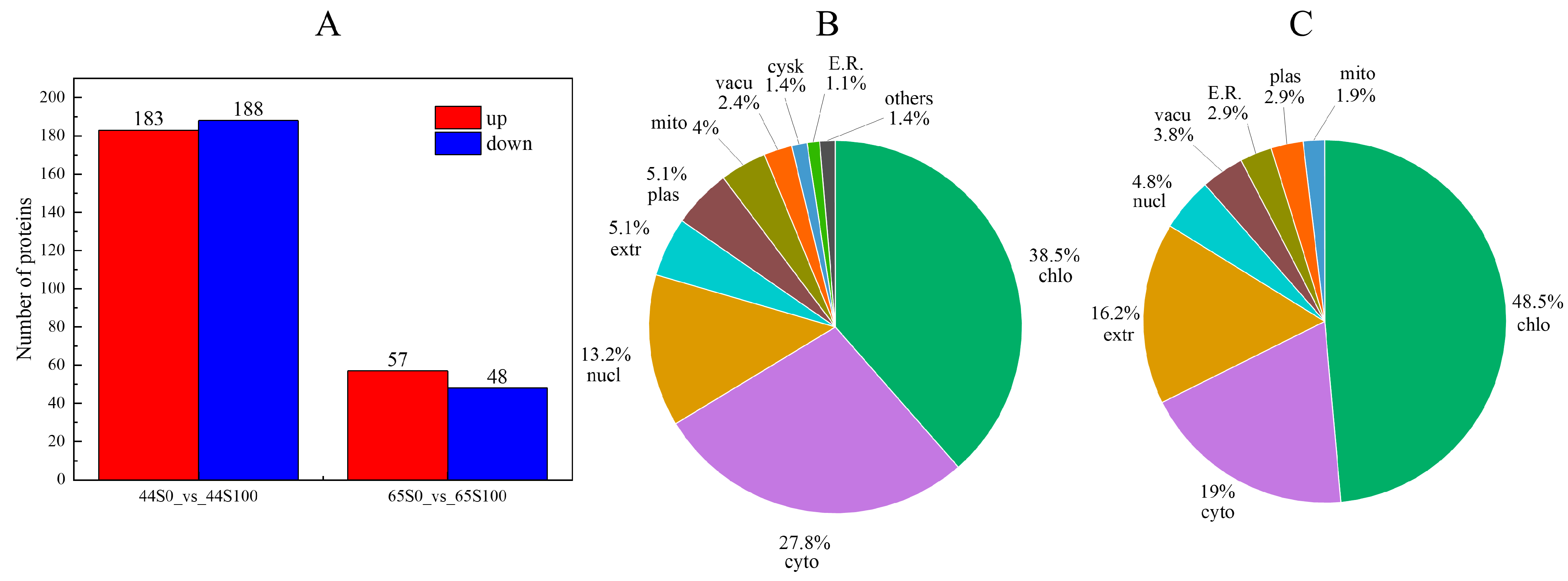
2.2. Differential Accumulation Proteins of Drought Stress after MC Pretreatment
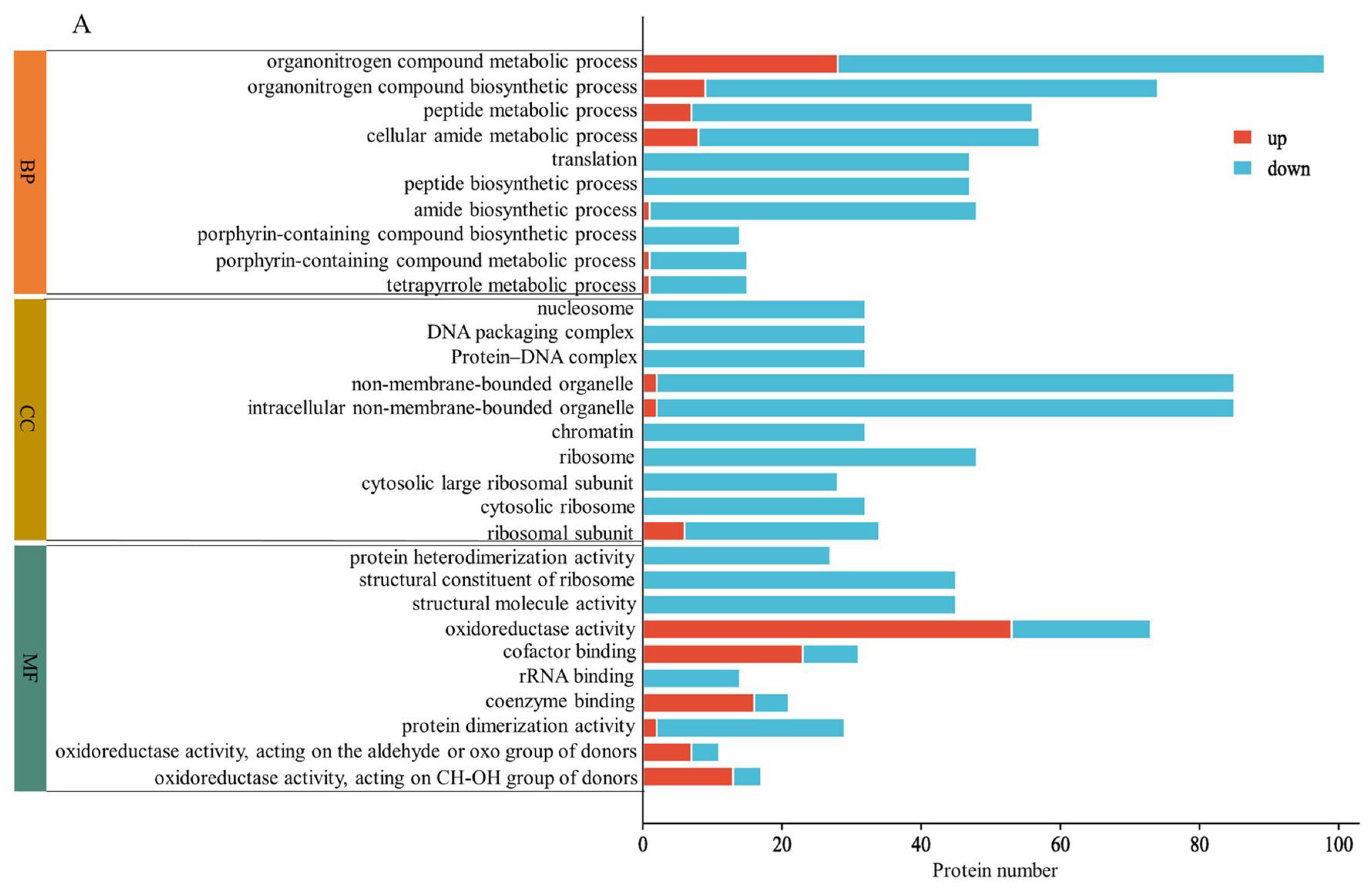
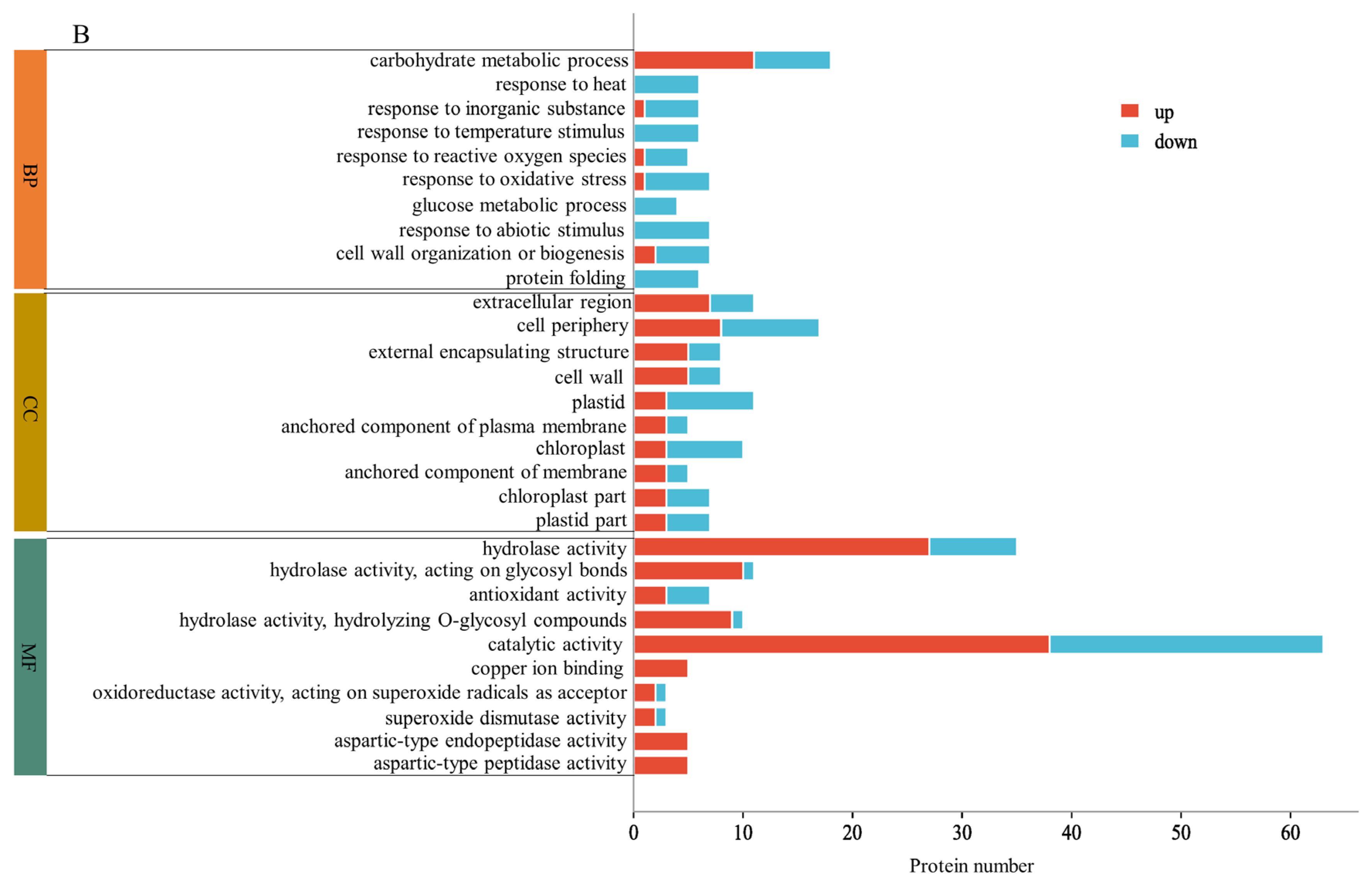
2.3. GO Enrichment Analysis
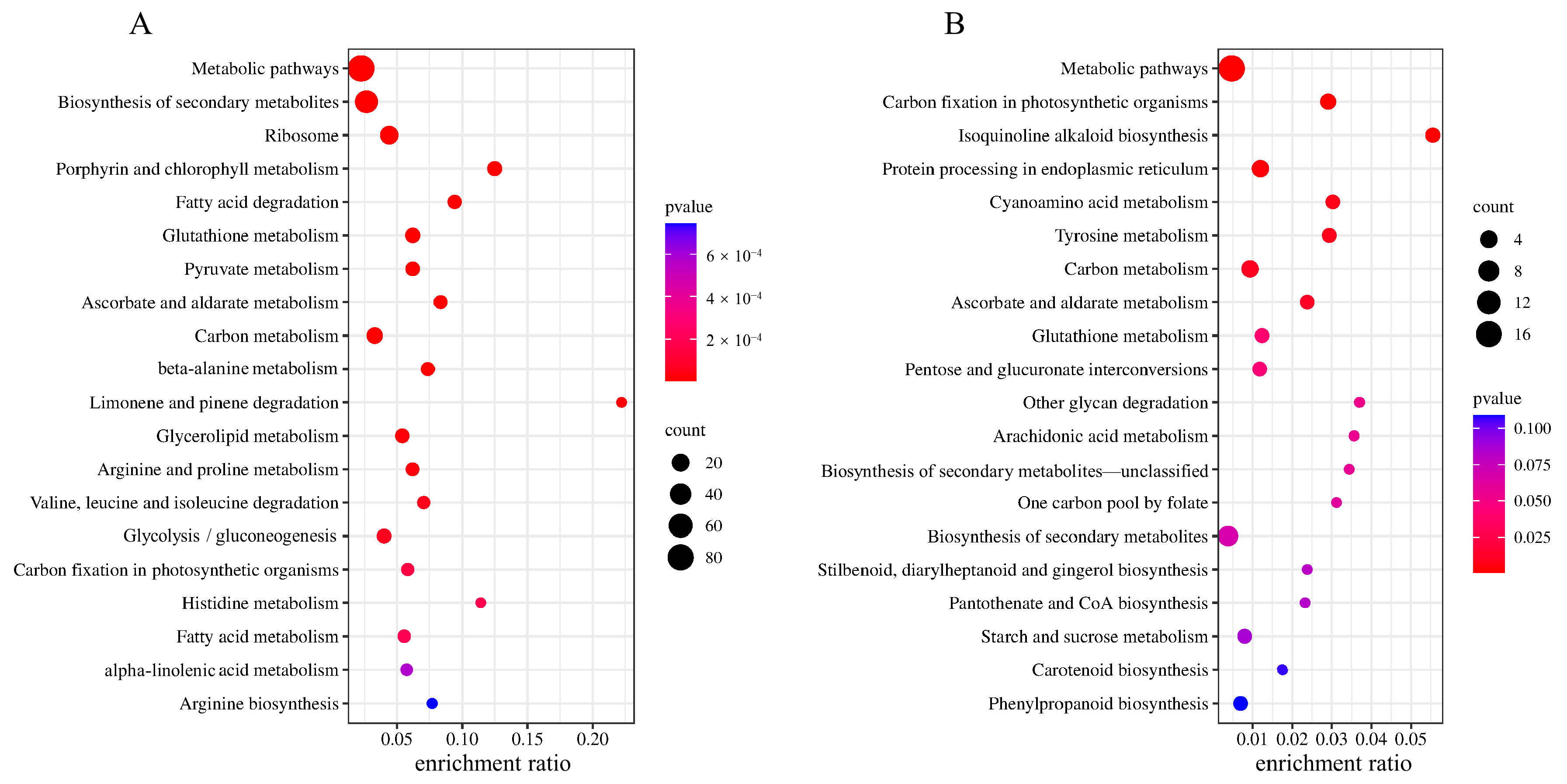
2.4. KEGG Enrichment Analysis
2.5. MC Pretreatment Improved the Glutathione–Ascorbic Acid Cycle and Alleviated Oxidative Damage
2.6. MC Pretreatment Affects Carbon Metabolism in Soybean
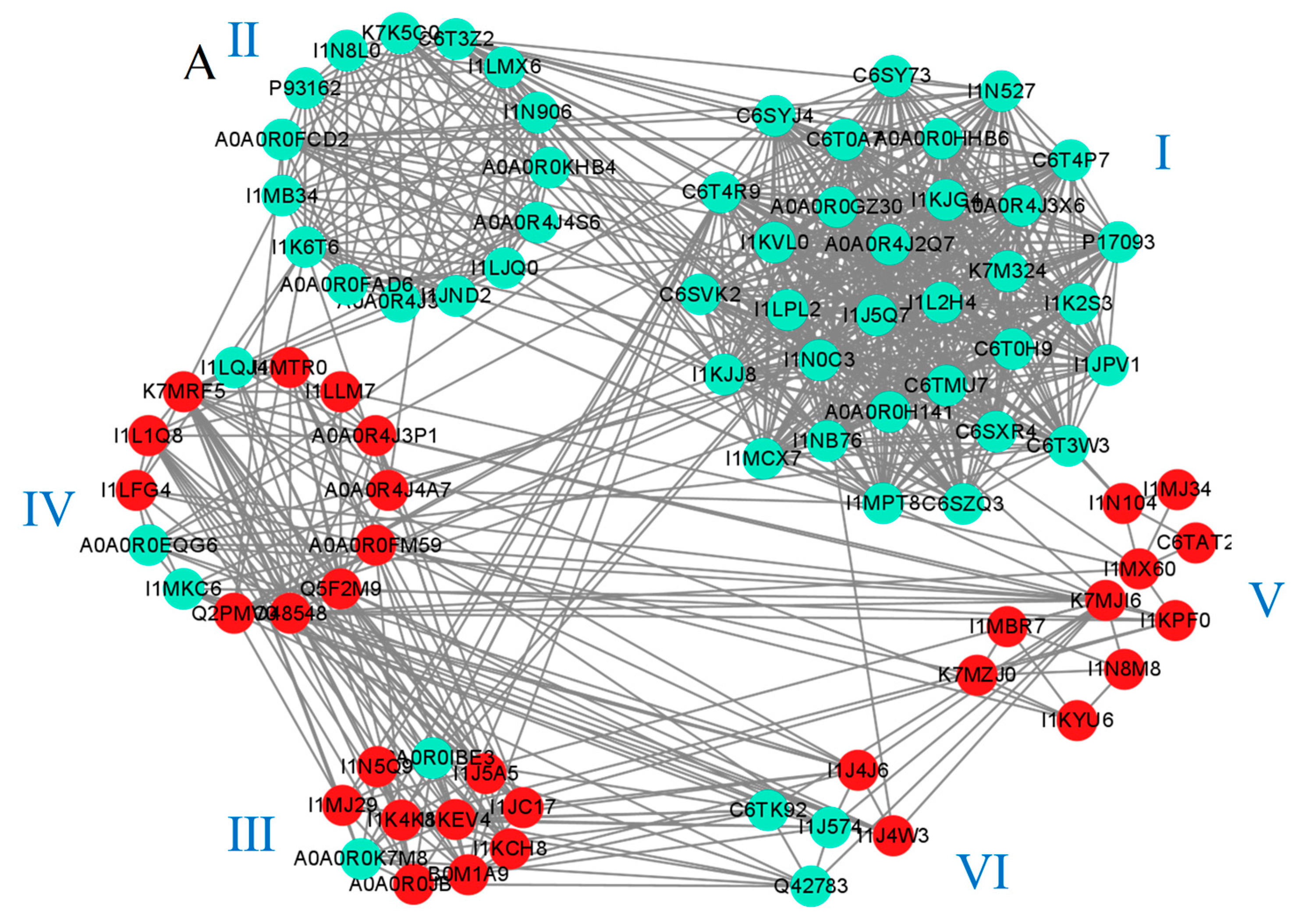
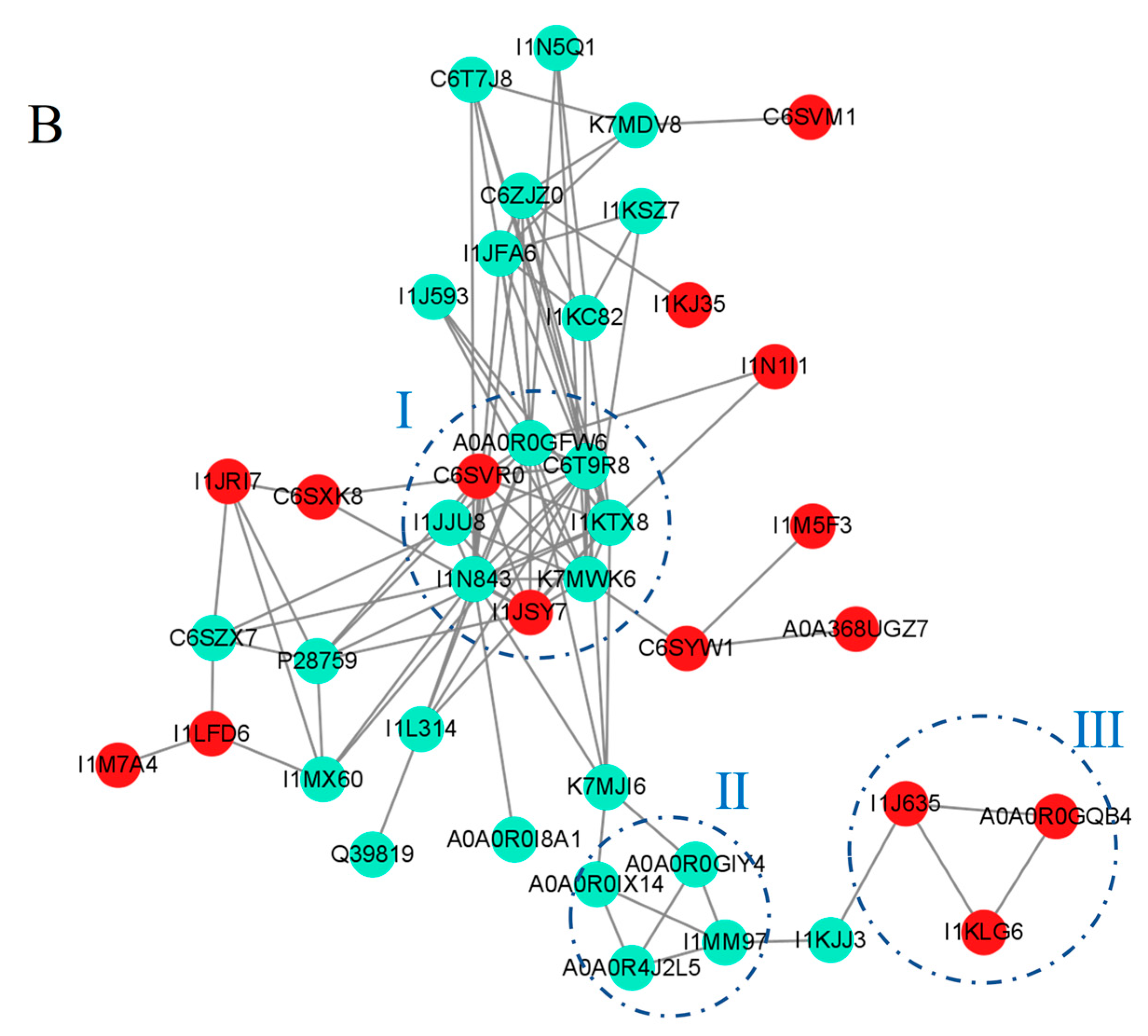
2.7. The Unique Response of the Two Varieties Reveals a Protein–Protein Interaction Network
2.8. Validation of Proteomic Analysis
3. Discussion
4. Materials and Methods
4.1. Experimental Materials and Treatment
4.2. The Proteomics Determination Method
4.3. Mass Spectrometry Detection and Data Analysis
4.4. Determination of Physiological and Biochemical Indexes
4.5. Real-Time Quantitative Polymerase Chain Reaction Detection Method
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Cunicelli, M.J.; Bhandari, H.S.; Chen, P.Y.; Sams, C.E.; Mian, M.A.R.; Mozzoni, L.A.; Smallwood, C.J.; Pantalone, V.R. Effect of a Mutant Danbaekkong Allele on Soybean Seed Yield, Protein, and Oil Concentration. J. Am. Oil Chem. Soc. 2019, 96, 927–935. [Google Scholar] [CrossRef]
- Anderson, N.R.; Irizarry, M.D.; Bloomingdale, C.A.; Smith, D.L.; Bradley, C.A.; Delaney, D.P.; Kleczewski, N.M.; Sikora, E.J.; Mueller, D.S.; Wise, K.A. Effect of soybean vein necrosis on yield and seed quality of soybean. Can. J. Plant Pathol. 2017, 39, 334–341. [Google Scholar] [CrossRef]
- Wang, L.B.; Liu, L.J.; Ma, Y.L.; Li, S.; Dong, S.K.; Zu, W. Transcriptome profilling analysis characterized the gene expression patterns responded to combined drought and heat stresses in soybean. Comput. Biol. Chem. 2018, 77, 413–429. [Google Scholar] [CrossRef] [PubMed]
- Deshmukh, R.; Sonah, H.; Patil, G.; Chen, W.; Prince, S.; Mutava, R.; Vuong, T.; Valliyodan, B.; Nguyen, H.T. Integrating omic approaches for abiotic stress tolerance in soybean. Front. Plant Sci. 2014, 5, 244. [Google Scholar] [CrossRef] [PubMed]
- Zou, J.N.; Qi, Y.U.; Jin, X.J.; Wang, M.Y.; Qin, B.; Ren, C.Y.; Wang, M.X.; Zhang, Y.X. Effects of exogenous melatonin on physiology and yield of soybean during seed filling stage under drought stress. Acta Agron. Sin. 2020, 46, 745–758. [Google Scholar] [CrossRef]
- Bailey-Serres, J.; Parker, J.E.; Ainsworth, E.A.; Oldroyd, G.E.D.; Schroeder, J.I. Genetic strategies for improving crop yields. Nature 2019, 575, 109–118. [Google Scholar] [CrossRef]
- Lesk, C.; Rowhani, P.; Ramankutty, N. Influence of extreme weather disasters on global crop production. Nature 2016, 529, 84–87. [Google Scholar] [CrossRef]
- Bartlett, M.K.; Klein, T.; Jansen, S.; Choat, B.; Sack, L. The correlations and sequence of plant stomatal, hydraulic, and wilting responses to drought. Proc. Natl. Acad. Sci. USA 2016, 113, 13098–13103. [Google Scholar] [CrossRef]
- Kim, K.; Wang, M.C.; Ranjitkar, S.; Liu, S.H.; Xu, J.C.; Zomer, R.J. Using leaf area index (LAI) to assess vegetation response to drought in Yunnan province of China. J. Mt. Sci. 2017, 14, 1863–1872. [Google Scholar] [CrossRef]
- Kerchev, P.; Waszczak, C.; Lewandowska, A.; Willems, P.; Shapiguzov, A.; Li, Z.; Alseekh, S.; Mühlenbock, P.; Hoeberichts, F.A.; Huang, J.; et al. Lack of GLYCOLATE OXIDASE1, but Not GLYCOLATE OXIDASE2, Attenuates the Photorespiratory Phenotype of CATALASE2-Deficient Arabidopsis. Plant Physiol. 2016, 171, 1704–1719. [Google Scholar] [CrossRef]
- Wang, L.; Leister, D.; Guan, L.; Zheng, Y.; Schneider, K.; Lehmann, M.; Apel, K.; Kleine, T. The Arabidopsis SAFEGUARD1 suppresses singlet oxygen-induced stress responses by protecting grana margins. Proc. Natl. Acad. Sci. USA 2020, 117, 6918–6927. [Google Scholar] [CrossRef]
- Zia, A.; Walker, B.J.; Oung, H.M.; Charuvi, D.; Jahns, P.; Cousins, A.B.; Farrant, J.M.; Reich, Z.; Kirchhoff, H. Protection of the photosynthetic apparatus against dehydration stress in the resurrection plant Craterostigma pumilum. Plant J. Cell Mol. Biol. 2016, 87, 664–680. [Google Scholar] [CrossRef]
- Zhao, D.; Oosterhuis, D.M. Pix plus and mepiquat chloride effects on physiology, growth, and yield of field-grown cotton. J. Plant Growth Regul. 2000, 19, 415–422. [Google Scholar] [CrossRef]
- Kamran, M.; Ahmad, I.; Wang, H.Q.; Wu, X.R.; Xu, J.; Liu, T.N.; Ding, R.X.; Han, Q.F. Mepiquat chloride application increases lodging resistance of maize by enhancing stem physical strength and lignin biosynthesis. Field Crop. Res. 2018, 224, 148–159. [Google Scholar] [CrossRef]
- de Souza, M.J.L.; Viana, A.E.S.; Matsumoto, S.N.; de Vasconcelos, R.C.; Sediyama, T.; Morais, O.M. Cassava agronomical characteristics related to interaction among irrigation, harvest time and mepiquat chloride. Acta Sci. Agron. 2010, 32, 45–53. [Google Scholar] [CrossRef]
- Wang, N.; Wang, X.R.; Qi, Q.; Iqbal, A.; Zhang, H.H.; Shi, J.B.; Dong, Q.; Xu, Q.H.; Liu, X.H.; Gui, H.P.; et al. Analysis of the effects of mepiquat chloride priming on the seedling growth-promoting in cotton under salt stress by multi-omics. Ind. Crop. Prod. 2022, 186, 115296. [Google Scholar] [CrossRef]
- Jafari, M.; Saadatmand, S.; Zangi, M.R.; Iranbakhsh, A.R. Effect of pix (mepiquat chloride) on the salinity resistance of new cultivars of cotton (Gossypium hirsutum L.) in the seedling stage. Appl. Ecol. Environ. Res. 2018, 16, 4097–4114. [Google Scholar] [CrossRef]
- Wang, X.Y.; Zhou, Q.; Wang, X.; Song, S.; Liu, J.; Dong, S.K. Mepiquat chloride inhibits soybean growth but improves drought resistance. Front. Plant Sci. 2022, 13, 982415. [Google Scholar] [CrossRef]
- Sappl, P.G.; Onate-Sanchez, L.; Singh, K.B.; Millar, A.H. Proteomic analysis of glutathione S-transferases of Arabidopsis thaliana reveals differential salicylic acid-induced expression of the plant-specific phi and tau classes. Plant Mol. Biol. 2004, 54, 205–219. [Google Scholar] [CrossRef]
- Rezaei, M.K.; Shobbar, Z.S.; Shahbazi, M.; Abedini, R.; Zare, S. Glutathione S-transferase (GST) family in barley: Identification of members, enzyme activity, and gene expression pattern. J. Plant Physiol. 2013, 170, 1277–1284. [Google Scholar] [CrossRef]
- Bashir, K.; Todaka, D.; Rasheed, S.; Matsui, A.; Ahmad, Z.; Sako, K.; Utsumi, Y.; Vu, A.T.; Tanaka, M.; Takahashi, S.; et al. Ethanol-Mediated Novel Survival Strategy against Drought Stress in Plants. Plant Cell Physiol. 2022, 63, 1181–1192. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Gu, L.L.; Song, J.X.; Li, C.J.; Zhang, Y.H.; Wang, Y.X.; Pang, Y.Z.; Zhang, B. Physiological and transcriptome analyses highlight multiple pathways involved in drought stress in Medicago falcata. PLoS ONE 2022, 17, e0266542. [Google Scholar] [CrossRef] [PubMed]
- Zeevaart, J.A. Changes in the levels of abscisic acid and its metabolites in excised leaf blades of Xanthium strumarium during and after water stress. Plant Physiol. 1980, 66, 672–678. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.L.; Zheng, Z.H.; Zhou, H.B.; Qu, H.; Gao, H.Y. Proteomics Reveals the Mechanism Underlying the Autolysis of Postharvest Coprinus comatus Fruiting Bodies. J. Agric. Food Chem. 2022, 70, 1346–1357. [Google Scholar] [CrossRef]
- Ahmed, M.; Kheir, A.M.S.; Mehmood, M.Z.; Ahmad, S.; Hasanuzzaman, M. Changes in Germination and Seedling Traits of Sesame under Simulated Drought. Phyton-Int. J. Exp. Bot. 2022, 91, 713–726. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, F.; Zhou, J.J.; Chen, F.; Wang, B.S.; Xie, X.Z. Phytochrome B control of total leaf area and stomatal density affects drought tolerance in rice. Plant Mol. Biol. 2012, 78, 289–300. [Google Scholar] [CrossRef]
- Brdar-Jokanovic, M.; Girek, Z.; Pavlovic, S.; Ugrinovic, M.; Zdravkovic, J. Shoot and Rooy Dry Weight in Drought Exposed Tomato Populations. Genetika 2014, 46, 495–504. [Google Scholar] [CrossRef]
- Chen, Y.K.; Li, C.H.; Yi, J.; Yang, Y.; Lei, C.X.; Gong, M. Transcriptome Response to Drought, Rehydration and Re-Dehydration in Potato. Int. J. Mol. Sci. 2020, 21, 159. [Google Scholar] [CrossRef]
- Xing, X.H.; Fang, C.W.; Li, L.; Jiang, H.Q.; Zhou, Q.; Jiang, H.D.; Wang, S.H. Improved drought tolerance by alpha-naphthaleneacetic acid-induced ROS accumulation in two soybean cultivars. J. Integr. Agric. 2016, 15, 1770–1784. [Google Scholar] [CrossRef]
- Zhang, S.; Chen, H.F.; Liu, J.X.; Liu, D.M.; Yan, H.L.; Wang, F.G. Effects of drought on Physiological Responses of Bretschneidera sinensis. Int. J. Agric. Biol. 2019, 22, 545–552. [Google Scholar] [CrossRef]
- Bianchi, M.W.; Roux, C.; Vartanian, N. Drought regulation of GST8, encoding the Arabidopsis homologue of ParC/Nt107 glutathione transferase/peroxidase. Physiol. Plant 2002, 116, 96–105. [Google Scholar] [CrossRef]
- Aleem, M.; Aleem, S.; Sharif, I.; Wu, Z.Y.; Aleem, M.; Tahir, A.; Atif, R.M.; Cheema, H.M.N.; Shakeel, A.; Lei, S.; et al. Characterization of SOD and GPX Gene Families in the Soybeans in Response to Drought and Salinity Stresses. Antioxidants 2022, 11, 460. [Google Scholar] [CrossRef]
- Xu, L.P.; Hu, Y.B.; Jin, G.Z.; Lei, P.; Sang, L.Q.; Luo, Q.X.; Liu, Z.; Guan, F.C.; Meng, F.J.; Zhao, X.Y. Physiological and Proteomic Responses to Drought in Leaves of Amygdalus mira (Koehne) Yu et Lu. Front. Plant Sci. 2021, 12, 620499. [Google Scholar] [CrossRef]
- Gonias, E.D.; Oosterhuis, D.M.; Bibi, A.C. Cotton radiation use efficiency response to plant growth regulators. J. Agric. Sci. 2012, 150, 595–602. [Google Scholar] [CrossRef]
- Tung, S.A.; Huang, Y.; Ali, S.; Hafeez, A.; Shah, A.N.; Song, X.; Ma, X.; Luo, D.; Yang, G. Mepiquat chloride application does not favor leaf photosynthesis and carbohydrate metabolism as well as lint yield in late-planted cotton at high plant density. Field Crop. Res. 2018, 221, 108–118. [Google Scholar] [CrossRef]
- Reddy, A.R.; Reddy, K.; Hodges, H. Mepiquat chloride (PIX)-induced changes in photosynthesis and growth of cotton. Plant Growth Regul. 1996, 20, 179–183. [Google Scholar] [CrossRef]
- Dos Santos, J.A.; Matsumoto, S.N.; Trazzi, P.A.; Ramos, P.A.S.; de Oliveira, L.S.; Campoe, O.C. Morphophysiological changes by mepiquat chloride application in Eucalyptus clones. Trees 2021, 35, 189–198. [Google Scholar] [CrossRef]
- Helm, L.T.; Shi, H.Y.; Lerdau, M.T.; Yang, X. Solar-induced chlorophyll fluorescence and short-term photosynthetic response to drought. Ecol. Appl. 2020, 30, e02101. [Google Scholar] [CrossRef]
- Herms, D.A.; Mattson, W.J. The dilemma of plants: To grow or defend. Q. Rev. Biol. 1992, 67, 283–335. [Google Scholar] [CrossRef]
- Figueroa-Macías, J.P.; García, Y.C.; Núñez, M.; Díaz, K.; Olea, A.F.; Espinoza, L. Plant Growth-Defense Trade-Offs: Molecular Processes Leading to Physiological Changes. Int. J. Mol. Sci. 2021, 22, 693. [Google Scholar] [CrossRef]
- Gyugos, M.; Ahres, M.; Gulyas, Z.; Szalai, G.; Darko, E.; Mednyanszky, Z.; Dey, N.; Kar, R.K.; Simon-Sarkadi, L.; Kocsy, G. Light spectrum modifies the drought-induced changes of glutathione and free amino acid levels in wheat. Acta Physiol. Plant. 2021, 43, 90. [Google Scholar] [CrossRef]
- Diamond, A.; Desgagne-Penix, I. Metabolic engineering for the production of plant isoquinoline alkaloids. Plant Biotechnol. J. 2016, 14, 1319–1328. [Google Scholar] [CrossRef] [PubMed]
- Cui, G.C.; Zhang, Y.; Zhang, W.J.; Lang, D.Y.; Zhang, X.J.; Li, Z.X.; Zhang, X.H. Response of Carbon and Nitrogen Metabolism and Secondary Metabolites to Drought Stress and Salt Stress in Plants. J. Plant Biol. 2019, 62, 387–399. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, X.Y.; Chen, J.; Chen, A.J.; Wang, L.Y.; Guo, X.Y.; Niu, Y.L.; Liu, S.R.; Mi, G.H.; Gao, Q. Reducing basal nitrogen rate to improve maize seedling growth, water and nitrogen use efficiencies under drought stress by optimizing root morphology and distribution. Agric. Water Manag. 2019, 212, 328–337. [Google Scholar] [CrossRef]
- Shao, Q.S.; Shu, S.; Du, J.; Xing, W.W.; Guo, S.R.; Sun, J. Effects of NaCl stress on nitrogen metabolism of cucumber seedlings. Russ. J. Plant Physiol. 2015, 62, 595–603. [Google Scholar] [CrossRef]
- Wu, G.Q.; Wang, C.M.; Su, Y.Y.; Zhang, J.J.; Feng, R.J.; Liang, N. Assessment of drought tolerance in seedlings of sugar beet (Beta vulgaris L.) cultivars using inorganic and organic solutes accumulation criteria. Soil Sci. Plant Nutr. 2014, 60, 565–576. [Google Scholar] [CrossRef]
- Wang, X.Y.; Song, S.; Wang, X.; Liu, J.; Dong, S.K. Transcriptomic and Metabolomic Analysis of Seedling-Stage Soybean Responses to PEG-Simulated Drought Stress. Int. J. Mol. Sci. 2022, 23, 6869. [Google Scholar] [CrossRef]
- Lan, P.; Li, W.; Schmidt, W. Complementary Proteome and Transcriptome Profiling in Phosphate-deficient Arabidopsis Roots Reveals Multiple Levels of Gene Regulation. Mol. Cell. Proteom. 2012, 11, 1156–1166. [Google Scholar] [CrossRef]
- Hodges, D.M.; Andrews, C.J.; Johnson, D.A.; Hamilton, R.I. Antioxidant compound responses to chilling stress in differentially sensitive inbred maize lines. Physiol. Plant. 1996, 98, 685–692. [Google Scholar] [CrossRef]
- Gurrieri, L.; Merico, M.; Trost, P.; Forlani, G.; Sparla, F. Impact of Drought on Soluble Sugars and Free Proline Content in Selected Arabidopsis Mutants. Biology 2020, 9, 36. [Google Scholar] [CrossRef]
- Rashid, U.; Yasmin, H.; Hassan, M.N.; Naz, R.; Nosheen, A.; Sajjad, M.; Ilyas, N.; Keyani, R.; Jabeen, Z.; Mumtaz, S.; et al. Drought-tolerant Bacillus megaterium isolated from semi-arid conditions induces systemic tolerance of wheat under drought conditions. Plant Cell Rep. 2022, 41, 549–569. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dong, S.; Wang, X.; Li, X.; Tian, Y.; Zhou, X.; Qu, Z.; Wang, X.; Liu, L. Mechanism of Mepiquat Chloride Regulating Soybean Response to Drought Stress Revealed by Proteomics. Plants 2023, 12, 2037. https://doi.org/10.3390/plants12102037
Dong S, Wang X, Li X, Tian Y, Zhou X, Qu Z, Wang X, Liu L. Mechanism of Mepiquat Chloride Regulating Soybean Response to Drought Stress Revealed by Proteomics. Plants. 2023; 12(10):2037. https://doi.org/10.3390/plants12102037
Chicago/Turabian StyleDong, Shoukun, Xin Wang, Xiaomei Li, Yumei Tian, Xinyu Zhou, Zhipeng Qu, Xiyue Wang, and Lijun Liu. 2023. "Mechanism of Mepiquat Chloride Regulating Soybean Response to Drought Stress Revealed by Proteomics" Plants 12, no. 10: 2037. https://doi.org/10.3390/plants12102037
APA StyleDong, S., Wang, X., Li, X., Tian, Y., Zhou, X., Qu, Z., Wang, X., & Liu, L. (2023). Mechanism of Mepiquat Chloride Regulating Soybean Response to Drought Stress Revealed by Proteomics. Plants, 12(10), 2037. https://doi.org/10.3390/plants12102037





