Phytochemical Compounds Involved in the Bone Regeneration Process and Their Innovative Administration: A Systematic Review
Abstract
1. Introduction
1.1. Bone Metabolism
1.1.1. The Main Cells of Bone Metabolism
1.1.2. The Main Biomarkers of Bone Metabolism
1.1.3. The Main Signaling Pathways Specific to Bone Metabolism
1.2. Biomaterials for Bone Regeneration
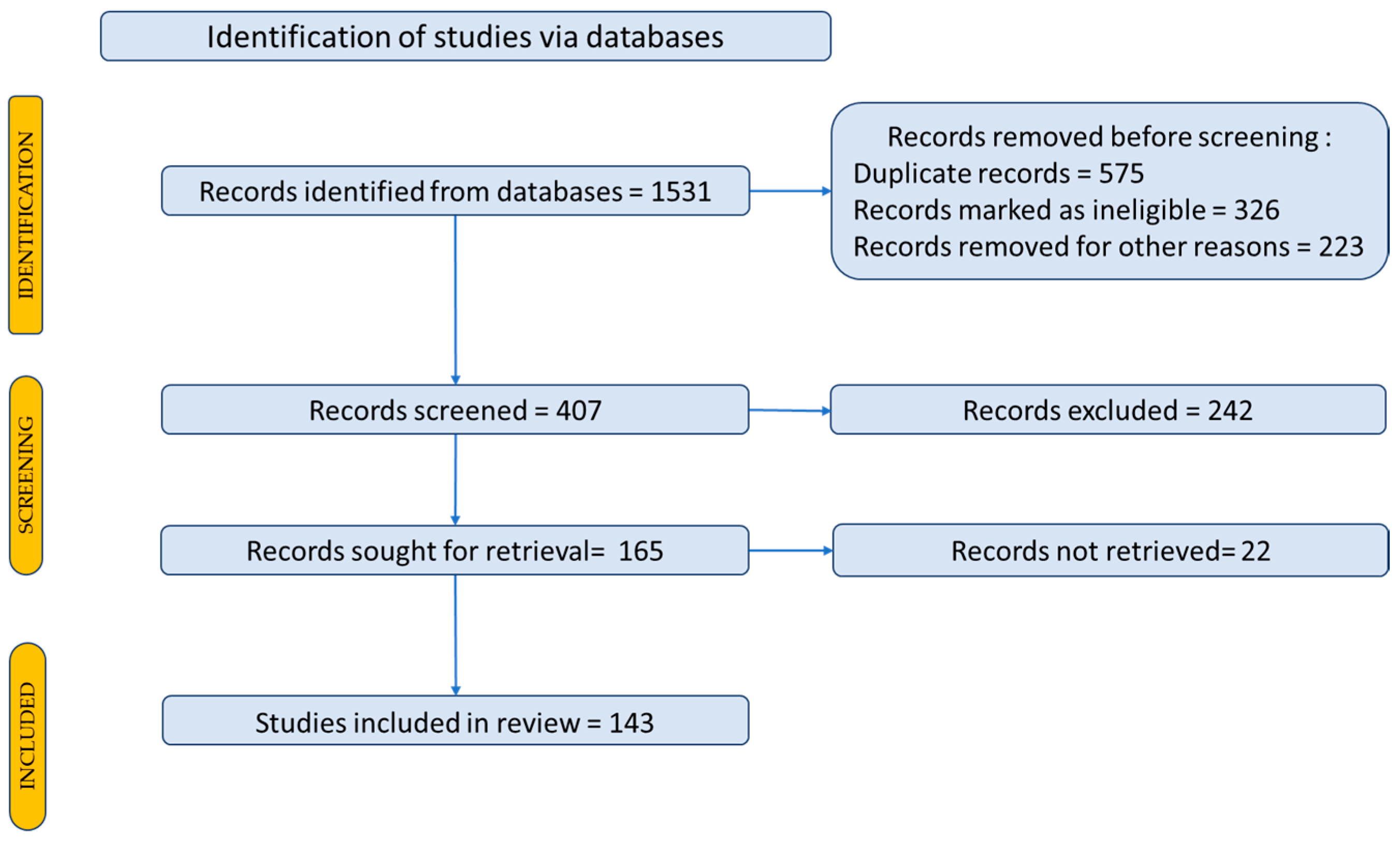
2. Research Methodology
3. Plant Extracts and Phytochemical Compounds with a Positive Effect on the Bone Regeneration Process
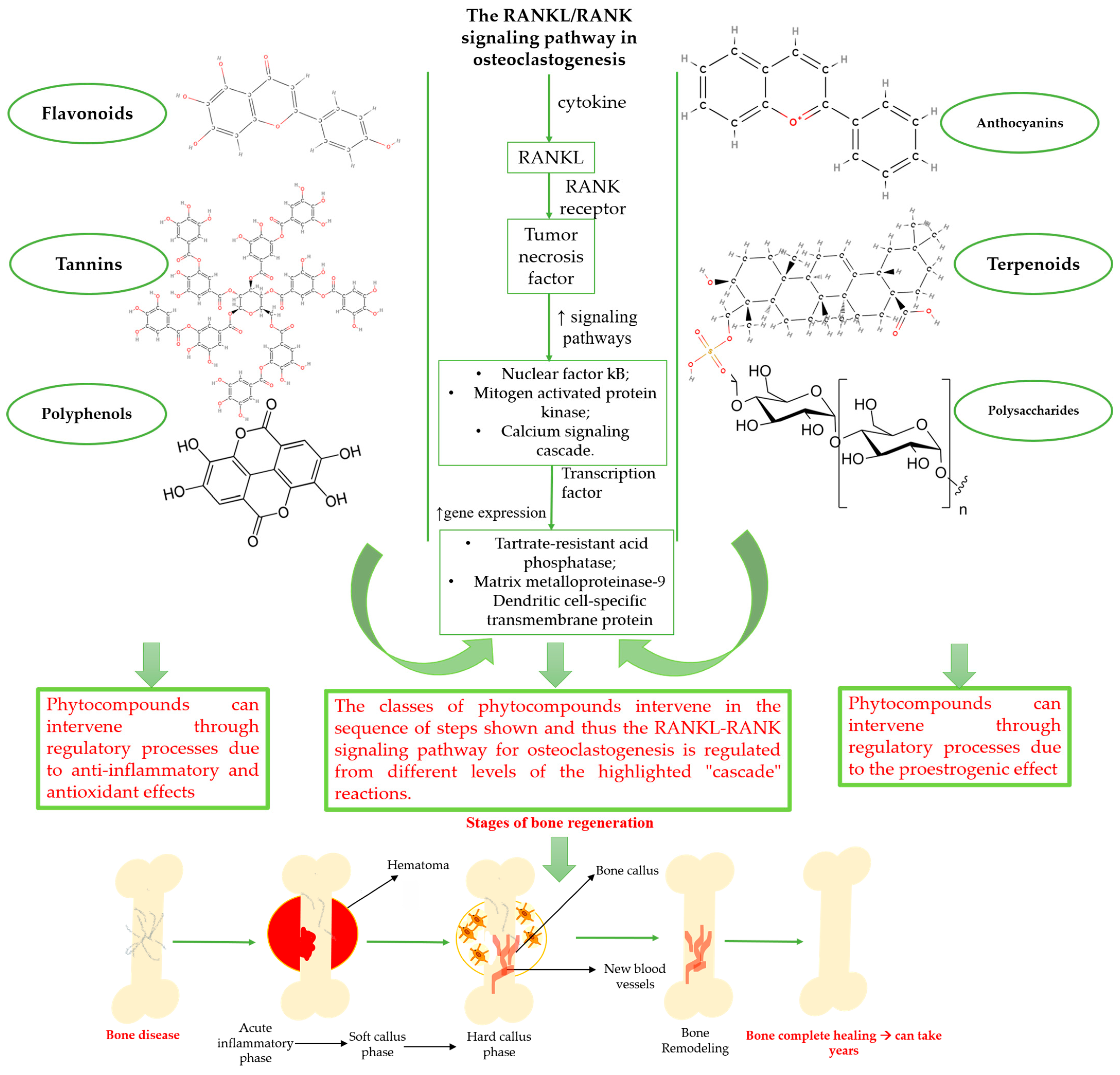
3.1. Classes of Phytochemical Compounds Involved in the Bone Regeneration Process
3.2. Phytocompounds Used in the Bone Regeneration Process—State of the Art
4. The Innovative Administration and Application of Plant Extracts in the Process of Bone Regeneration
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Infante, A.; Rodríguez, C.I. Osteogenesis and Aging: Lessons from Mesenchymal Stem Cells. Stem Cell Res. 2018, 9, 244. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Kim, N. Bone Cell Communication Factors Provide a New Therapeutic Strategy for Osteoporosis. Chonnam. Med. J. 2020, 56, 94. [Google Scholar] [CrossRef]
- Hannah, S.S.; McFadden, S.; McNeilly, A.; McClean, C. “Take My Bone Away?” Hypoxia and Bone: A Narrative Review. J. Cell Physiol. 2021, 236, 721–740. [Google Scholar] [CrossRef] [PubMed]
- Wojda, S.J.; Donahue, S.W. Parathyroid Hormone for Bone Regeneration: Parathyroid Hormone For Bone Regeneration. J. Orthop. Res. 2018, 36, 2586–2594. [Google Scholar] [CrossRef]
- Leder, B.Z. Parathyroid Hormone and Parathyroid Hormone-Related Protein Analogs in Osteoporosis Therapy. Curr. Osteoporos. Rep. 2017, 15, 110–119. [Google Scholar] [CrossRef]
- Kužma, M.; Jackuliak, P.; Killinger, Z.; Payer, J. Parathyroid Hormone-Related Changes of Bone Structure. Physiol. Res. 2021, 70 (Suppl. S1), S3–S11. [Google Scholar] [CrossRef] [PubMed]
- Schwetz, V.; Trummer, C.; Pandis, M.; Grübler, M.; Verheyen, N.; Gaksch, M.; Zittermann, A.; März, W.; Aberer, F.; Lang, A.; et al. Effects of Vitamin D Supplementation on Bone Turnover Markers: A Randomized Controlled Trial. Nutrients 2017, 9, 432. [Google Scholar] [CrossRef] [PubMed]
- Lerchbaum, E.; Trummer, C.; Theiler-Schwetz, V.; Kollmann, M.; Wölfler, M.; Pilz, S.; Obermayer-Pietsch, B. Effects of Vitamin D Supplementation on Bone Turnover and Bone Mineral Density in Healthy Men: A Post-Hoc Analysis of a Randomized Controlled Trial. Nutrients 2019, 11, 731. [Google Scholar] [CrossRef]
- Muresan, G.C.; Hedesiu, M.; Lucaciu, O.; Boca, S.; Petrescu, N. Effect of Vitamin D on Bone Regeneration: A Review. Medicina 2022, 58, 1337. [Google Scholar] [CrossRef]
- Robling, A.G.; Bonewald, L.F. The Osteocyte: New Insights. Annu. Rev. Physiol. 2020, 82, 485–506. [Google Scholar] [CrossRef]
- Pathak, J.L.; Bravenboer, N.; Klein-Nulend, J. The Osteocyte as the New Discovery of Therapeutic Options in Rare Bone Diseases. Front. Endocrinol. 2020, 11, 405. [Google Scholar] [CrossRef]
- Li, M.C.M.; Chow, S.K.H.; Wong, R.M.Y.; Qin, L.; Cheung, W.H. The Role of Osteocytes-Specific Molecular Mechanism in Regulation of Mechanotransduction—A Systematic Review. J. Orthop. Transl. 2021, 29, 1–9. [Google Scholar] [CrossRef]
- Qin, L.; Liu, W.; Cao, H.; Xiao, G. Molecular Mechanosensors in Osteocytes. Bone Res. 2020, 8, 23. [Google Scholar] [CrossRef]
- Amarasekara, D.S.; Kim, S.; Rho, J. Regulation of Osteoblast Differentiation by Cytokine Networks. Int. J. Mol. Sci. 2021, 22, 2851. [Google Scholar] [CrossRef]
- Al-Bari, A.A.; Al Mamun, A. Current Advances in Regulation of Bone Homeostasis. FASEB BioAdvances 2020, 2, 668–679. [Google Scholar] [CrossRef] [PubMed]
- Ghorbaninejad, M.; Khademi-Shirvan, M.; Hosseini, S.; Baghaban Eslaminejad, M. Epidrugs: Novel Epigenetic Regulators That Open a New Window for Targeting Osteoblast Differentiation. Stem Cell Res. 2020, 11, 456. [Google Scholar] [CrossRef]
- Astleford, K.; Campbell, E.; Norton, A.; Mansky, K.C. Epigenetic Regulators Involved in Osteoclast Differentiation. Int. J. Mol. Sci. 2020, 21, 7080. [Google Scholar] [CrossRef]
- Hayashibara, T.; Hiraga, T.; Sugita, A.; Wang, L.; Hata, K.; Ooshima, T.; Yoneda, T. Regulation of Osteoclast Differentiation and Function by Phosphate: Potential Role of Osteoclasts in the Skeletal Abnormalities in Hypophosphatemic Conditions. J. Bone Min. Res. 2007, 22, 1743–1751. [Google Scholar] [CrossRef] [PubMed]
- Bilezikian, J.P. Hypoparathyroidism. J. Clin. Endocrinol. Metab. 2020, 105, 1722–1736. [Google Scholar] [CrossRef] [PubMed]
- Lou, Y.-R.; Toh, T.C.; Tee, Y.H.; Yu, H. 25-Hydroxyvitamin D3 Induces Osteogenic Differentiation of Human Mesenchymal Stem Cells. Sci. Rep 2017, 7, 42816. [Google Scholar] [CrossRef]
- Dempster, D.W.; Cauley, J.A.; Bouxsein, M.L.; Cosman, F. Marcus and Feldman’s Osteoporosis, 5th ed.; Dempster, D.W., Cauley, J.A., Bouxsein, M.L., Cosman, F., Eds.; Academic Press: London, UK, 2020; Volume 1, ISBN 978-0-12-813074-2. [Google Scholar]
- Millán, J.L.; Whyte, M.P. Alkaline Phosphatase and Hypophosphatasia. Calcif Tissue Int. 2016, 98, 398–416. [Google Scholar] [CrossRef]
- Moriishi, T.; Ozasa, R.; Ishimoto, T.; Nakano, T.; Hasegawa, T.; Miyazaki, T.; Liu, W.; Fukuyama, R.; Wang, Y.; Komori, H.; et al. Osteocalcin Is Necessary for the Alignment of Apatite Crystallites, but Not Glucose Metabolism, Testosterone Synthesis, or Muscle Mass. PLoS Genet. 2020, 16, e1008586. [Google Scholar] [CrossRef] [PubMed]
- Shan, C.; Ghosh, A.; Guo, X.; Wang, S.; Hou, Y.; Li, S.; Liu, J. Roles for Osteocalcin in Brain Signalling: Implications in Cognition- and Motor-Related Disorders. Mol. Brain 2019, 12, 23. [Google Scholar] [CrossRef]
- Si, J.; Wang, C.; Zhang, D.; Wang, B.; Hou, W.; Zhou, Y. Osteopontin in Bone Metabolism and Bone Diseases. Med. Sci. Monit. 2020, 26, e919159. [Google Scholar] [CrossRef]
- Tobeiha, M.; Moghadasian, M.H.; Amin, N.; Jafarnejad, S. RANKL/RANK/OPG Pathway: A Mechanism Involved in Exercise-Induced Bone Remodeling. BioMed Res. Int. 2020, 2020, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Baud’huin, M.; Duplomb, L.; Teletchea, S.; Lamoureux, F.; Ruiz-Velasco, C.; Maillasson, M.; Redini, F.; Heymann, M.-F.; Heymann, D. Osteoprotegerin: Multiple Partners for Multiple Functions. Cytokine Growth Factor Rev. 2013, 24, 401–409. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Li, M.; Wang, S.; Xiao, Z.; Xiong, Y.; Wang, G. Recent Advances of Osterix Transcription Factor in Osteoblast Differentiation and Bone Formation. Front. Cell Dev. Biol. 2020, 8, 601224. [Google Scholar] [CrossRef]
- Komori, T. Regulation of Proliferation, Differentiation and Functions of Osteoblasts by Runx2. Int. J. Mol. Sci. 2019, 20, 1694. [Google Scholar] [CrossRef] [PubMed]
- Majidinia, M.; Sadeghpour, A.; Yousefi, B. The Roles of Signaling Pathways in Bone Repair and Regeneration. J. Cell Physiol. 2018, 233, 2937–2948. [Google Scholar] [CrossRef]
- Karner, C.M.; Long, F. Wnt Signaling and Cellular Metabolism in Osteoblasts. Cell. Mol. Life Sci. 2017, 74, 1649–1657. [Google Scholar] [CrossRef]
- Ballhause, T.M.; Jiang, S.; Baranowsky, A.; Brandt, S.; Mertens, P.R.; Frosch, K.-H.; Yorgan, T.; Keller, J. Relevance of Notch Signaling for Bone Metabolism and Regeneration. Int. J. Mol. Sci. 2021, 22, 1325. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Chen, D. The BMP Signaling and in Vivo Bone Formation. Gene 2005, 357, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Lowery, J.W.; Rosen, V. The BMP Pathway and Its Inhibitors in the Skeleton. Physiol. Rev. 2018, 98, 2431–2452. [Google Scholar] [CrossRef]
- Fang, H.; Zhu, D.; Yang, Q.; Chen, Y.; Zhang, C.; Gao, J.; Gao, Y. Emerging Zero-Dimensional to Four-Dimensional Biomaterials for Bone Regeneration. J. Nanobiotechnol. 2022, 20, 26. [Google Scholar] [CrossRef]
- Battafarano, G.; Rossi, M.; De Martino, V.; Marampon, F.; Borro, L.; Secinaro, A.; Del Fattore, A. Strategies for Bone Regeneration: From Graft to Tissue Engineering. Int. J. Mol. Sci. 2021, 22, 1128. [Google Scholar] [CrossRef]
- Girón, J.; Kerstner, E.; Medeiros, T.; Oliveira, L.; Machado, G.M.; Malfatti, C.F.; Pranke, P. Biomaterials for Bone Regeneration: An Orthopedic and Dentistry Overview. Braz. J. Med. Biol. Res. 2021, 54, e11055. [Google Scholar] [CrossRef]
- Qi, J.; Yu, T.; Hu, B.; Wu, H.; Ouyang, H. Current Biomaterial-Based Bone Tissue Engineering and Translational Medicine. Int. J. Mol. Sci. 2021, 22, 10233. [Google Scholar] [CrossRef]
- Guo, L.; Liang, Z.; Yang, L.; Du, W.; Yu, T.; Tang, H.; Li, C.; Qiu, H. The Role of Natural Polymers in Bone Tissue Engineering. J. Control. Release 2021, 338, 571–582. [Google Scholar] [CrossRef]
- Bharadwaz, A.; Jayasuriya, A.C. Recent Trends in the Application of Widely Used Natural and Synthetic Polymer Nanocomposites in Bone Tissue Regeneration. Mater. Sci. Eng. C 2020, 110, 110698. [Google Scholar] [CrossRef]
- Zhu, Y.; Goh, C.; Shrestha, A. Biomaterial Properties Modulating Bone Regeneration. Macromol. Biosci. 2021, 21, 2000365. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Yang, G.; Johnson, B.N.; Jia, X. Three-Dimensional (3D) Printed Scaffold and Material Selection for Bone Repair. Acta Biomater. 2019, 84, 16–33. [Google Scholar] [CrossRef]
- Su, X.; Xian, C.; Gao, M.; Liu, G.; Wu, J. Edible Materials in Tissue Regeneration. Macromol. Biosci. 2021, 21, 2100114. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, n71, e112. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Moher, D. Updating Guidance for Reporting Systematic Reviews: Development of the PRISMA 2020 Statement. J. Clin. Epidemiol. 2021, 134, 103–112. [Google Scholar] [CrossRef]
- An, J.; Hao, D.; Zhang, Q.; Chen, B.; Zhang, R.; Wang, Y.; Yang, H. Natural Products for Treatment of Bone Erosive Diseases: The Effects and Mechanisms on Inhibiting Osteoclastogenesis and Bone Resorption. Int. Immunopharmacol. 2016, 36, 118–131. [Google Scholar] [CrossRef] [PubMed]
- Torre, E. Molecular Signaling Mechanisms behind Polyphenol-Induced Bone Anabolism. Phytochem. Rev. 2017, 16, 1183–1226. [Google Scholar] [CrossRef]
- Chircov, C.; Miclea, I.I.; Grumezescu, V.; Grumezescu, A.M. Essential Oils for Bone Repair and Regeneration—Mechanisms and Applications. Materials 2021, 14, 1867. [Google Scholar] [CrossRef]
- Schilling, T.; Ebert, R.; Raaijmakers, N.; Schütze, N.; Jakob, F. Effects of Phytoestrogens and Other Plant-Derived Compounds on Mesenchymal Stem Cells, Bone Maintenance and Regeneration. J. Steroid Biochem. Mol. Biol. 2014, 139, 252–261. [Google Scholar] [CrossRef]
- Singh, P.; Gupta, A.; Qayoom, I.; Singh, S.; Kumar, A. Orthobiologics with Phytobioactive Cues: A Paradigm in Bone Regeneration. Biomed. Pharmacother. 2020, 130, 110754. [Google Scholar] [CrossRef]
- Khosla, S.; Oursler, M.J.; Monroe, D.G. Estrogen and the Skeleton. Trends Endocrinol. Metab. 2012, 23, 576–581. [Google Scholar] [CrossRef]
- Domazetovic, V. Oxidative Stress in Bone Remodeling: Role of Antioxidants. CCMBM 2017, 14, 209. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Shen, S.; Zhang, S.; Huang, M.; Zhang, L.; Chen, X. Autophagy in Bone Remodeling: A Regulator of Oxidative Stress. Front. Endocrinol. 2022, 13, 898634. [Google Scholar] [CrossRef] [PubMed]
- Vuolteenaho, K.; Moilanen, T.; Moilanen, E. Non-Steroidal Anti-Inflammatory Drugs, Cyclooxygenase-2 and the Bone Healing Process. Basic Clin. Pharm. Toxicol 2007, 0, 149. [Google Scholar] [CrossRef]
- Schett, G. Effects of Inflammatory and Anti-Inflammatory Cytokines on the Bone: Cytokine Effects On Bone. Eur. J. Clin. Investig. 2011, 41, 1361–1366. [Google Scholar] [CrossRef] [PubMed]
- Dhawan, U.; Jaffery, H.; Salmeron-Sanchez, M.; Dalby, M.J. An Ossifying Landscape: Materials and Growth Factor Strategies for Osteogenic Signalling and Bone Regeneration. Curr. Opin. Biotechnol. 2022, 73, 355–363. [Google Scholar] [CrossRef] [PubMed]
- Han, Q.-Q.; Du, Y.; Yang, P.-S. The Role of Small Molecules in Bone Regeneration. Future Med. Chem. 2013, 5, 1671–1684. [Google Scholar] [CrossRef]
- Maeda, A. Recruitment of Mesenchymal Stem Cells to Damaged Sites by Plant-Derived Components. Front. Cell Dev. Biol. 2020, 8, 437. [Google Scholar] [CrossRef]
- Kornicka, K.; Kocherova, I.; Marycz, K. The Effects of Chosen Plant Extracts and Compounds on Mesenchymal Stem Cells-a Bridge between Molecular Nutrition and Regenerative Medicine- Concise Review. Phytother. Res. 2017, 31, 947–958. [Google Scholar] [CrossRef]
- Mao, W.; Huang, G.; Chen, H.; Xu, L.; Qin, S.; Li, A. Research Progress of the Role of Anthocyanins on Bone Regeneration. Front. Pharm. 2021, 12, 773660. [Google Scholar] [CrossRef]
- Martiniakova, M.; Babikova, M.; Omelka, R. Pharmacological Agents and Natural Compounds: Available Treatments for Osteoporosis. J. Physiol. Pharm. 2020, 71, 1–4. [Google Scholar] [CrossRef]
- Sarkar, N.; Bose, S. Controlled Release of Soy Isoflavones from Multifunctional 3D Printed Bone Tissue Engineering Scaffolds. Acta Biomater. 2020, 114, 407–420. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Sun, Y.; Dai, H.; Ma, Y.; Bing, H. Engineered 3D-Printed Polyvinyl Alcohol Scaffolds Incorporating β-Tricalcium Phosphate and Icariin Induce Bone Regeneration in Rat Skull Defect Model. Molecules 2022, 27, 4535. [Google Scholar] [CrossRef]
- Burim, R.A.; Sendyk, D.I.; Hernandes, L.S.; de Souza, D.F.M.; Correa, L.; Deboni, M.C.Z. Repair of Critical Calvarias Defects With Systemic Epimedium Sagittatum Extract. J. Craniofac. Surg. 2016, 27, 799–804. [Google Scholar] [CrossRef] [PubMed]
- You, M.; Jing, J.; Tian, D.; Qian, J.; Yu, G. Dioscin Stimulates Differentiation of Mesenchymal Stem Cells towards Hypertrophic Chondrocytes in Vitro and Endochondral Ossification in Vivo. Am J Transl Res 2016, 8, 3930–3938. [Google Scholar]
- Adhikary, S.; Choudhary, D.; Ahmad, N.; Karvande, A.; Kumar, A.; Banala, V.T.; Mishra, P.R.; Trivedi, R. Dietary Flavonoid Kaempferol Inhibits Glucocorticoid-Induced Bone Loss by Promoting Osteoblast Survival. Nutrition 2018, 53, 64–76. [Google Scholar] [CrossRef]
- Zhao, J.; Wu, J.; Xu, B.; Yuan, Z.; Leng, Y.; Min, J.; Lan, X.; Luo, J. Kaempferol Promotes Bone Formation in Part via the MTOR Signaling Pathway. Mol. Med. Rep. 2019, 20, 5197–5207. [Google Scholar] [CrossRef]
- Kulkarni, C.; Sharma, S.; Bora, P.S.; Verma, S.; Rajput, S.; Porwal, K.; Rath, S.K.; Gayen, J.R.; Sharma, U.; Chattopadhyay, N. A Novel Extraction Method Enhanced the Osteogenic and Anti-Osteoporosis Effect of Tea Extract without Any Hepatotoxicity in Ovariectomized Rats. Front. Endocrinol. 2022, 13. [Google Scholar] [CrossRef]
- Wei, Y.; Fu, J.; Wu, W.; Ma, P.; Ren, L.; Yi, Z.; Wu, J. Quercetin Prevents Oxidative Stress-Induced Injury of Periodontal Ligament Cells and Alveolar Bone Loss in Periodontitis. Drug. Des. Devel. 2021, 15, 3509–3522. [Google Scholar] [CrossRef] [PubMed]
- Khedgikar, V.; Kushwaha, P.; Ahmad, N.; Gautam, J.; Kumar, P.; Maurya, R.; Trivedi, R. Ethanolic Extract of Dalbergia Sissoo Promotes Rapid Regeneration of Cortical Bone in Drill-Hole Defect Model of Rat. Biomed Pharm. 2017, 86, 16–22. [Google Scholar] [CrossRef]
- Gong, W.; Zhang, N.; Cheng, G.; Zhang, Q.; He, Y.; Shen, Y.; Zhang, Q.; Zhu, B.; Zhang, Q.; Qin, L. Rehmannia Glutinosa Libosch Extracts Prevent Bone Loss and Architectural Deterioration and Enhance Osteoblastic Bone Formation by Regulating the IGF-1/PI3K/MTOR Pathway in Streptozotocin-Induced Diabetic Rats. Int. J. Mol. Sci. 2019, 20, 3964. [Google Scholar] [CrossRef]
- Tan, B.; Wu, Y.; Wu, Y.; Shi, K.; Han, R.; Li, Y.; Qian, Z.; Liao, J. Curcumin-Microsphere/IR820 Hybrid Bifunctional Hydrogels for In Situ Osteosarcoma Chemo-Co-Thermal Therapy and Bone Reconstruction. ACS Appl. Mater. Interfaces 2021, 13, 31542–31553. [Google Scholar] [CrossRef] [PubMed]
- Verma, A.H.; Kumar, T.S.S.; Madhumathi, K.; Rubaiya, Y.; Ramalingan, M.; Doble, M. Curcumin Releasing Eggshell Derived Carbonated Apatite Nanocarriers for Combined Anti-Cancer, Anti-Inflammatory and Bone Regenerative Therapy. J. Nanosci. Nanotechnol. 2019, 19, 6872–6880. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Dånmark, S.; Edlund, U.; Finne-Wistrand, A.; He, X.; Norgård, M.; Blomén, E.; Hultenby, K.; Andersson, G.; Lindgren, U. Resveratrol-Conjugated Poly-ε-Caprolactone Facilitates in vitro Mineralization and in vivo Bone Regeneration. Acta Biomater. 2011, 7, 751–758. [Google Scholar] [CrossRef] [PubMed]
- Murgia, D.; Mauceri, R.; Campisi, G.; De Caro, V. Advance on Resveratrol Application in Bone Regeneration: Progress and Perspectives for Use in Oral and Maxillofacial Surgery. Biomolecules 2019, 9, 94. [Google Scholar] [CrossRef]
- Bhattarai, G.; Poudel, S.B.; Kook, S.-H.; Lee, J.-C. Resveratrol Prevents Alveolar Bone Loss in an Experimental Rat Model of Periodontitis. Acta Biomater. 2016, 29, 398–408. [Google Scholar] [CrossRef]
- Choi, Y.; Yoon, D.S.; Lee, K.-M.; Choi, S.M.; Lee, M.-H.; Park, K.H.; Han, S.H.; Lee, J.W. Enhancement of Mesenchymal Stem Cell-Driven Bone Regeneration by Resveratrol-Mediated SOX2 Regulation. Aging Dis. 2019, 10, 818–833. [Google Scholar] [CrossRef]
- Kim, J.-S.; Takanche, J.S.; Kim, J.-E.; Jeong, S.-H.; Han, S.-H.; Yi, H.-K. Schisandra Chinensis Extract Ameliorates Age-Related Muscle Wasting and Bone Loss in Ovariectomized Rats. Phytother. Res. 2019, 33, 1865–1877. [Google Scholar] [CrossRef]
- Suliman, S.; Mieszkowska, A.; Folkert, J.; Rana, N.; Mohamed-Ahmed, S.; Fuoco, T.; Finne-Wistrand, A.; Dirscherl, K.; Jørgensen, B.; Mustafa, K.; et al. Immune-Instructive Copolymer Scaffolds Using Plant-Derived Nanoparticles to Promote Bone Regeneration. Inflamm. Regen. 2022, 42, 12. [Google Scholar] [CrossRef]
- Folkert, J.; Meresta, A.; Gaber, T.; Miksch, K.; Buttgereit, F.; Detert, J.; Pischon, N.; Gurzawska, K. Nanocoating with Plant-Derived Pectins Activates Osteoblast Response in vitro. Int. J. Nanomed. 2016, 12, 239–249. [Google Scholar] [CrossRef]
- Soares, I.M.V.; Fernandes, G.V. de O.; Cavalcante, L.C.; Leite, Y.K.P. de C.; Bezerra, D. de O.; de Carvalho, M.A.M.; Carvalho, C.M.R.S. The Influence of Aloe Vera with Mesenchymal Stem Cells from Dental Pulp on Bone Regeneration: Characterization and Treatment of Non-Critical Defects of the Tibia in Rats. J. Appl Oral Sci. 2019, 27, e20180103. [Google Scholar] [CrossRef]
- Lin, Z.; Lin, C.; Fu, C.; Lu, H.; Jin, H.; Chen, Q.; Pan, J. The Protective Effect of Ellagic Acid (EA) in Osteoarthritis: An in vitro and in Vivo Study. Biomed. Pharm. 2020, 125, 109845. [Google Scholar] [CrossRef] [PubMed]
- Fikry, E.M.; Gad, A.M.; Eid, A.H.; Arab, H.H. Caffeic Acid and Ellagic Acid Ameliorate Adjuvant-Induced Arthritis in Rats via Targeting Inflammatory Signals, Chitinase-3-like Protein-1 and Angiogenesis. Biomed Pharm. 2019, 110, 878–886. [Google Scholar] [CrossRef] [PubMed]
- Ding, L.; Gu, S.; Zhou, B.; Wang, M.; Zhang, Y.; Wu, S.; Zou, H.; Zhao, G.; Gao, Z.; Xu, L. Ginsenoside Compound K Enhances Fracture Healing via Promoting Osteogenesis and Angiogenesis. Front Pharm. 2022, 13, 855393. [Google Scholar] [CrossRef]
- Zhang, Y.; Ma, J.; Zhang, W. Berberine for Bone Regeneration: Therapeutic Potential and Molecular Mechanisms. J. Ethnopharmacol. 2021, 277, 114249. [Google Scholar] [CrossRef]
- Dong, B.; Liu, X.; Li, J.; Wang, B.; Yin, J.; Zhang, H.; Liu, W. Berberine Encapsulated in Exosomes Derived from Platelet-Rich Plasma Promotes Chondrogenic Differentiation of the Bone Marrow Mesenchymal Stem Cells via the Wnt/β-Catenin Pathway. Biol. Pharm. Bull. 2022, 45, 1444–1451. [Google Scholar] [CrossRef]
- Shaban, N.Z.; Kenawy, M.Y.; Taha, N.A.; Abd El-Latif, M.M.; Ghareeb, D.A. Cellulose Acetate Nanofibers: Incorporating Hydroxyapatite (HA), HA/Berberine or HA/Moghat Composites, as Scaffolds to Enhance In vitro Osteoporotic Bone Regeneration. Polymers 2021, 13, 4140. [Google Scholar] [CrossRef]
- Zhang, R.; Yang, J.; Wu, J.; Xiao, L.; Miao, L.; Qi, X.; Li, Y.; Sun, W. Berberine Promotes Osteogenic Differentiation of Mesenchymal Stem Cells with Therapeutic Potential in Periodontal Regeneration. Eur. J. Pharmacol. 2019, 851, 144–150. [Google Scholar] [CrossRef]
- Talebi, A.; Hayati Roodbari, N.; Reza Sameni, H.; Zarbakhsh, S. Impact of Coadministration of Apigenin and Bone Marrow Stromal Cells on Damaged Ovaries Due to Chemotherapy in Rat: An Experimental Study. Int. J. Reprod. Biomed. 2020, 18, 551–560. [Google Scholar] [CrossRef]
- Mao, Y.-W.; Lin, R.-D.; Hung, H.-C.; Lee, M.-H. Stimulation of Osteogenic Activity in Human Osteoblast Cells by Edible Uraria Crinita. J. Agric. Food Chem. 2014, 62, 5581–5588. [Google Scholar] [CrossRef]
- Pal, S.; Kumar, P.; Ramakrishna, E.; Kumar, S.; Porwal, K.; Kumar, B.; Arya, K.R.; Maurya, R.; Chattopadhyay, N. Extract and Fraction of Cassia Occidentalis L. (a Synonym of Senna Occidentalis) Have Osteogenic Effect and Prevent Glucocorticoid-Induced Osteopenia. J. Ethnopharmacol. 2019, 235, 8–18. [Google Scholar] [CrossRef] [PubMed]
- Pal, S.; Mittapelly, N.; Husain, A.; Kushwaha, S.; Chattopadhyay, S.; Kumar, P.; Ramakrishna, E.; Kumar, S.; Maurya, R.; Sanyal, S.; et al. A Butanolic Fraction from the Standardized Stem Extract of Cassia Occidentalis L Delivered by a Self-Emulsifying Drug Delivery System Protects Rats from Glucocorticoid-Induced Osteopenia and Muscle Atrophy. Sci. Rep. 2020, 10, 195. [Google Scholar] [CrossRef]
- Zhou, R.P.; Lin, S.J.; Wan, W.B.; Zuo, H.L.; Yao, F.F.; Ruan, H.B.; Xu, J.; Song, W.; Zhou, Y.C.; Wen, S.Y.; et al. Chlorogenic Acid Prevents Osteoporosis by Shp2/PI3K/Akt Pathway in Ovariectomized Rats. PLoS ONE 2016, 11, e0166751. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Hu, X. Mechanism of Chlorogenic Acid Treatment on Femoral Head Necrosis and Its Protection of Osteoblasts. Biomed. Rep. 2016, 5, 57–62. [Google Scholar] [CrossRef]
- Na, W.; Kang, M.-K.; Park, S.-H.; Kim, D.Y.; Oh, S.Y.; Oh, M.-S.; Park, S.; Kang, I.-J.; Kang, Y.-H. Aesculetin Accelerates Osteoblast Differentiation and Matrix-Vesicle-Mediated Mineralization. Int. J. Mol. Sci. 2021, 22, 12391. [Google Scholar] [CrossRef]
- Kurian, I.G.; Dileep, P.; Ipshita, S.; Pradeep, A.R. Comparative Evaluation of Subgingivally-Delivered 1% Metformin and Aloe Vera Gel in the Treatment of Intrabony Defects in Chronic Periodontitis Patients: A Randomized, Controlled Clinical Trial. J Investig. Clin. Dent. 2018, 9, e12324. [Google Scholar] [CrossRef]
- Tanık, A.; Güler Doğru, A.; Akpolat, V.; Acun Kaya, F.; Sarıbaş, E.; Gül, M.; İrtegün Kandemir, S.; Deveci, E. Investigation of the Effect of Combined Use of Alloplastic-Based Tricalcium Phosphate Bone Graft and Antihemorrhagic Plant Extract (ABS) on Bone Regeneration in Surgically Induced Bone Defects in Nondiabetic Rats: An Experimental Animal Study. Turk. J. Med. Sci. 2018, 48, 1302–1314. [Google Scholar] [CrossRef] [PubMed]
- Khedgikar, V.; Kushwaha, P.; Gautam, J.; Verma, A.; Changkija, B.; Kumar, A.; Sharma, S.; Nagar, G.K.; Singh, D.; Trivedi, P.K.; et al. Withaferin A: A Proteasomal Inhibitor Promotes Healing after Injury and Exerts Anabolic Effect on Osteoporotic Bone. Cell Death Dis. 2013, 4, e778. [Google Scholar] [CrossRef]
- Yan, C.-P.; Wang, X.-K.; Jiang, K.; Yin, C.; Xiang, C.; Wang, Y.; Pu, C.; Chen, L.; Li, Y.-L. β-Ecdysterone Enhanced Bone Regeneration through the BMP-2/SMAD/RUNX2/Osterix Signaling Pathway. Front. Cell Dev. Biol. 2022, 10. [Google Scholar] [CrossRef]
- Li, F.; Yang, X.; Yang, Y.; Guo, C.; Zhang, C.; Yang, Z.; Li, P. Antiosteoporotic Activity of Echinacoside in Ovariectomized Rats. Phytomedicine 2013, 20, 549–557. [Google Scholar] [CrossRef] [PubMed]
- Madhurakkat, P.S.K.; Lee, S.M.; Lee, J.; Ahmad, T.; Lee, M.S.; Yang, H.S.; Shin, H. Oxidative Epigallocatechin Gallate Coating on Polymeric Substrates for Bone Tissue Regeneration. Macromol. Biosci. 2019, 19, e1800392. [Google Scholar] [CrossRef]
- Sasayama, S.; Hara, T.; Tanaka, T.; Honda, Y.; Baba, S. Osteogenesis of Multipotent Progenitor Cells Using the Epigallocatechin Gallate-Modified Gelatin Sponge Scaffold in the Rat Congenital Cleft-Jaw Model. Int. J. Mol. Sci. 2018, 19, 3803. [Google Scholar] [CrossRef]
- Naumenko, E.; Guryanov, I.; Zakirova, E.; Fakhrullin, R. Forskolin-Loaded Halloysite Nanotubes as Osteoconductive Additive for the Biopolymer Tissue Engineering Scaffolds. Polymers 2021, 13, 3949. [Google Scholar] [CrossRef]
- Douglas, T.E.L.; Keppler, J.K.; Vandrovcová, M.; Plencner, M.; Beranová, J.; Feuereisen, M.; Parakhonskiy, B.V.; Svenskaya, Y.; Atkin, V.; Ivanova, A.; et al. Enhancement of Biomimetic Enzymatic Mineralization of Gellan Gum Polysaccharide Hydrogels by Plant-Derived Gallotannins. Int. J. Mol. Sci. 2020, 21, 2315. [Google Scholar] [CrossRef]
- Porwal, K.; Pal, S.; Dev, K.; China, S.P.; Kumar, Y.; Singh, C.; Barbhuyan, T.; Sinha, N.; Sanyal, S.; Trivedi, A.K.; et al. Guava Fruit Extract and Its Triterpene Constituents Have Osteoanabolic Effect: Stimulation of Osteoblast Differentiation by Activation of Mitochondrial Respiration via the Wnt/β-Catenin Signaling. J. Nutr. Biochem. 2017, 44, 22–34. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Chen, L.; Chen, Y.; Zhang, Z.; Wang, X.; Zhou, B. Cyanidin-3-Glucoside Regulates Osteoblast Differentiation via the ERK1/2 Signaling Pathway. ACS Omega 2021, 6, 4759–4766. [Google Scholar] [CrossRef] [PubMed]
- Gu, H.; Boonanantanasarn, K.; Kang, M.; Kim, I.; Woo, K.M.; Ryoo, H.-M.; Baek, J.-H. Morinda Citrifolia Leaf Extract Enhances Osteogenic Differentiation Through Activation of Wnt/β-Catenin Signaling. J. Med. Food. 2018, 21, 57–69. [Google Scholar] [CrossRef]
- Boonanantanasarn, K.; Janebodin, K.; Suppakpatana, P.; Arayapisit, T.; Rodsutthi, J.; Chunhabundit, P.; Boonanuntanasarn, S.; Sripairojthikoon, W. Morinda Citrifolia Leaves Enhance Osteogenic Differentiation and Mineralization of Human Periodontal Ligament Cells. Dent. Mater. J. 2014, 33, 157–165. [Google Scholar] [CrossRef][Green Version]
- Shalan, N.A.A.M.; Mustapha, N.M.; Mohamed, S. Noni Leaf and Black Tea Enhance Bone Regeneration in Estrogen-Deficient Rats. Nutrition 2017, 33, 42–51. [Google Scholar] [CrossRef]
- Kalalinia, F.; Ghasim, H.; Amel Farzad, S.; Pishavar, E.; Ramezani, M.; Hashemi, M. Comparison of the Effect of Crocin and Crocetin, Two Major Compounds Extracted from Saffron, on Osteogenic Differentiation of Mesenchymal Stem Cells. Life Sci. 2018, 208, 262–267. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.S.; Kim, N.K.; Seo, S.R. Cynanchi Atrati and Its Phenolic Constituent Sinapic Acid Target Regulator of Calcineurin 1 (RCAN1) to Control Skin Inflammation. Antioxid 2022, 11, 205. [Google Scholar] [CrossRef]
- Balagangadharan, K.; Trivedi, R.; Vairamani, M.; Selvamurugan, N. Sinapic Acid-Loaded Chitosan Nanoparticles in Polycaprolactone Electrospun Fibers for Bone Regeneration in vitro and in vivo. Carbohydr. Polym. 2019, 216, 1–16. [Google Scholar] [CrossRef]
- Abiramasundari, G.; Gowda, C.M.M.; Pampapathi, G.; Praveen, S.; Shivamurugan, S.; Vijaykumar, M.; Devi, A.; Sreepriya, M. Ethnomedicine Based Evaluation of Osteoprotective Properties of Tinospora Cordifolia on in Vitro and in Vivo Model Systems. Biomed. Pharm. 2017, 87, 342–354. [Google Scholar] [CrossRef] [PubMed]
- Cheng, W.-X.; Liu, Y.-Z.; Meng, X.-B.; Zheng, Z.-T.; Li, L.-L.; Ke, L.-Q.; Li, L.; Huang, C.-S.; Zhu, G.-Y.; Pan, H.-D.; et al. PLGA/β-TCP Composite Scaffold Incorporating Cucurbitacin B Promotes Bone Regeneration by Inducing Angiogenesis. J. Orthop. Transl. 2021, 31, 41–51. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Jiang, Z.; Yang, R.; Ye, Y.; Pei, L.; Xiong, S.; Wang, S.; Wang, L.; Liu, S. Polysaccharide-Rich Extract from Polygonatum Sibiricum Protects Hematopoiesis in Bone Marrow Suppressed by Triple Negative Breast Cancer. Biomed. Pharm. 2021, 137, 111338. [Google Scholar] [CrossRef] [PubMed]
- Wardhana, A.S.; Nirwana, I.; Budi, H.S.; Surboyo, M.D.C. Role of Hydroxyapatite and Ellagic Acid in the Osteogenesis. Eur. J. Dent. 2021, 15, 8–12. [Google Scholar] [CrossRef]
- Xu, W.; Xu, J.; Wang, T.; Liu, W.; Wei, H.; Yang, X.; Yan, W.; Zhou, W.; Xiao, J. Ellagic Acid and Sennoside B Inhibit Osteosarcoma Cell Migration, Invasion and Growth by Repressing the Expression of c-Jun. Oncol. Lett. 2018, 16, 898–904. [Google Scholar] [CrossRef]
- Nirwana, I.; Munadziroh, E.; Yuliati, A.; Fadhila, A.I.; Nurliana; Wardhana, A.S.; Shariff, K.A.; Surboyo, M.D.C. Ellagic Acid and Hydroxyapatite Promote Angiogenesis Marker in Bone Defect. J. Oral Biol. Craniofac. Res. 2022, 12, 116–120. [Google Scholar] [CrossRef]
- Primasari, D.N.; Nirwana, I.; Budi, H.S.; Wardhana, A.S.; Sari, A.F.; Novita, N.; Setyawan, A.P.; Surboyo, M.D.C.; Shariff, K.A. The Cytokine and Bone Protein Expression by Ellagic Acid-Hydroxyapatite in Bone Remodelling Model. Sci. World J. 2022, 2022, 6740853. [Google Scholar] [CrossRef]
- Pang, X.; Zhong, Z.; Jiang, F.; Yang, J.; Nie, H. Juglans Regia L. Extract Promotes Osteogenesis of Human Bone Marrow Mesenchymal Stem Cells through BMP2/Smad/Runx2 and Wnt/β-Catenin Pathways. J. Orthop. Surg. Res. 2022, 17, 88. [Google Scholar] [CrossRef]
- Vandenbroucke, A.; Luyten, F.P.; Flamaing, J.; Gielen, E. Pharmacological Treatment of Osteoporosis in the Oldest Old. Clin Interv. Aging 2017, 12, 1065–1077. [Google Scholar] [CrossRef]
- Brown, J.P. Long-Term Treatment of Postmenopausal Osteoporosis. Endocrinol. Metab. 2021, 36, 544–552. [Google Scholar] [CrossRef] [PubMed]
- Du, Z.; Wang, C.; Zhang, R.; Wang, X.; Li, X. Applications of Graphene and Its Derivatives in Bone Repair: Advantages for Promoting Bone Formation and Providing Real-Time Detection, Challenges and Future Prospects. Int. J. Nanomed. 2020, 15, 7523–7551. [Google Scholar] [CrossRef] [PubMed]
- Masne, N.; Ambade, R.; Bhugaonkar, K. Use of Nanocomposites in Bone Regeneration. Cureus 2022, 14, e31346. [Google Scholar] [CrossRef]
- Qaseem, A.; Forciea, M.A.; McLean, R.M.; Denberg, T.D.; Clinical Guidelines Committee of the American College of Physicians; Barry, M.J.; Cooke, M.; Fitterman, N.; Harris, R.P.; Humphrey, L.L.; et al. Treatment of Low Bone Density or Osteoporosis to Prevent Fractures in Men and Women: A Clinical Practice Guideline Update From the American College of Physicians. Ann. Intern. Med. 2017, 166, 818–839. [Google Scholar] [CrossRef] [PubMed]
- An, J.; Yang, H.; Zhang, Q.; Liu, C.; Zhao, J.; Zhang, L.; Chen, B. Natural Products for Treatment of Osteoporosis: The Effects and Mechanisms on Promoting Osteoblast-Mediated Bone Formation. Life Sci. 2016, 147, 46–58. [Google Scholar] [CrossRef]
- Raut, N.; Wicks, S.M.; Lawal, T.O.; Mahady, G.B. Epigenetic Regulation of Bone Remodeling by Natural Compounds. Pharm. Res. 2019, 147, 104350. [Google Scholar] [CrossRef]
- Shanmugavadivu, A.; Balagangadharan, K.; Selvamurugan, N. Angiogenic and Osteogenic Effects of Flavonoids in Bone Regeneration. Biotechnol. Bioeng. 2022, 119, 2313–2330. [Google Scholar] [CrossRef]
- Qiao, K.; Xu, L.; Tang, J.; Wang, Q.; Lim, K.S.; Hooper, G.; Woodfield, T.B.F.; Liu, G.; Tian, K.; Zhang, W.; et al. The Advances in Nanomedicine for Bone and Cartilage Repair. J. Nanobiotechnology 2022, 20, 141. [Google Scholar] [CrossRef]
- Jin, G.-Z. Current Nanoparticle-Based Technologies for Osteoarthritis Therapy. Nanomaterials 2020, 10, 2368. [Google Scholar] [CrossRef]
- Mu, P.; Feng, J.; Hu, Y.; Xiong, F.; Ma, X.; Tian, L. Botanical Drug Extracts Combined With Biomaterial Carriers for Osteoarthritis Cartilage Degeneration Treatment: A Review of 10 Years of Research. Front. Pharm. 2021, 12, 789311. [Google Scholar] [CrossRef]
- Song, H.; Li, X.; Zhao, Z.; Qian, J.; Wang, Y.; Cui, J.; Weng, W.; Cao, L.; Chen, X.; Hu, Y.; et al. Reversal of Osteoporotic Activity by Endothelial Cell-Secreted Bone Targeting and Biocompatible Exosomes. Nano Lett. 2019, 19, 3040–3048. [Google Scholar] [CrossRef] [PubMed]
- Paesa, M.; Alejo, T.; Garcia-Alvarez, F.; Arruebo, M.; Mendoza, G. New Insights in Osteoarthritis Diagnosis and Treatment: Nano-Strategies for an Improved Disease Management. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2023, 15, e1844. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.-R.; Feng, Y.-Z.; Zhao, Y.-Q.; Zhao, J.; Zhou, Y.-H.; Ye, Q.; Chen, Y.; Tan, L.; Zhang, S.-H.; Feng, Y.; et al. Traditional Chinese Medicine Promotes Bone Regeneration in Bone Tissue Engineering. Chin Med 2022, 17, 86. [Google Scholar] [CrossRef] [PubMed]
- Bose, S.; Sarkar, N.; Banerjee, D. Natural Medicine Delivery from Biomedical Devices to Treat Bone Disorders: A Review. Acta Biomater. 2021, 126, 63–91. [Google Scholar] [CrossRef] [PubMed]
- Cavalu, S.; Simon, V.; Goller, G.; Akin, I. Bioactivity And Antimicrobial Properties Of Pmma/Ag2o Acrylic Bone Cement Collagen Coated. Dig. J. Nanomater. Biostruct. 2011, 6, 779–790. [Google Scholar]
- Zhang, X.; Li, Z.; Yang, P.; Duan, G.; Liu, X.; Gu, Z.; Li, Y. Polyphenol Scaffolds in Tissue Engineering. Mater. Horiz. 2021, 8, 145–167. [Google Scholar] [CrossRef] [PubMed]
- Monárrez-Cordero, B.E.; Rodríguez-González, C.A.; Valencia-Gómez, L.E.; Hernández-Paz, J.F.; Martel-Estrada, S.A.; Camacho-Montes, H.; Olivas-Armendáriz, I. The Effect of Allium Cepa Extract on the Chitosan/PLGA Scaffolds Bioactivity. J. Appl. Biomater. Funct. Mater. 2021, 19, 2280800021989701. [Google Scholar] [CrossRef]
- Tahmasebi, A.; Shapouri Moghadam, A.; Enderami, S.E.; Islami, M.; Kaabi, M.; Saburi, E.; Daei Farshchi, A.; Soleimanifar, F.; Mansouri, V. Aloe Vera-Derived Gel-Blended PHBV Nanofibrous Scaffold for Bone Tissue Engineering. ASAIO J. 2020, 66, 966–973. [Google Scholar] [CrossRef]
- Shanmugavel, S.; Reddy, V.J.; Ramakrishna, S.; Lakshmi, B.; Dev, V.G. Precipitation of Hydroxyapatite on Electrospun Polycaprolactone/Aloe Vera/Silk Fibroin Nanofibrous Scaffolds for Bone Tissue Engineering. J. Biomater. Appl. 2014, 29, 46–58. [Google Scholar] [CrossRef]
- Mohammadpour, M.; Samadian, H.; Moradi, N.; Izadi, Z.; Eftekhari, M.; Hamidi, M.; Shavandi, A.; Quéro, A.; Petit, E.; Delattre, C.; et al. Fabrication and Characterization of Nanocomposite Hydrogel Based on Alginate/Nano-Hydroxyapatite Loaded with Linum Usitatissimum Extract as a Bone Tissue Engineering Scaffold. Mar. Drugs 2021, 20, 20. [Google Scholar] [CrossRef]
- Garcia, C.F.; Marangon, C.A.; Massimino, L.C.; Klingbeil, M.F.G.; Martins, V.C.A.; de Guzzi Plepis, A.M. Development of Collagen/Nanohydroxyapatite Scaffolds Containing Plant Extract Intended for Bone Regeneration. Mater. Sci. Eng. C 2021, 123, 111955. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Liu, H.; Luo, W.; Cai, T.; Li, Z.; Liu, Y.; Gao, W.; Wan, Q.; Wang, X.; Wang, J.; et al. Regeneration of Skeletal System with Genipin Crosslinked Biomaterials. J. Tissue Eng. 2020, 11, 2041731420974861. [Google Scholar] [CrossRef] [PubMed]

| Compounds | Type of Activity | Mechanism of Action | Extract Source | In vivo/in vitro Studies | Ref. |
|---|---|---|---|---|---|
Genistein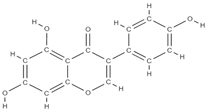 | Proestrogenic activity | ↑ alkaline phosphatase level ↓ urinary excretion of calcium and phosphate, → serum concentration at the appropriate normal level | Erythrina variegate | In vitro (mesenchymal stem cells) | [61] |
Daidzein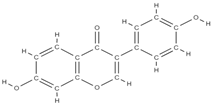 | ↑ osteoclast apoptosis through the mediation of estrogen receptors ↓ the loss of bone density activates tyrosine phosphatase → ↓ membrane depolarization producing changes in intracellular Ca2+ | In vivo (rats) | [62] | ||
Icariin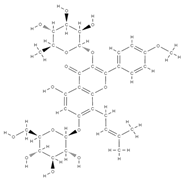 | ↓ bone loss in the median bone area by regulating the ratio between osteoprotegerin and RANKL, which are key mediators of osteoclast genesis. ↑ proliferation, differentiation of osteoblasts, bone mineralization ↓ cell apoptosis direct osteoblast stimulation: activation of the bone morphogenetic protein (BMP) cascade through (promoting Runx2/Cbfa1 expression and the production of BMP-4, BMP-2, and SMAD4 and nitrous oxide release; high levels of ALP suppression of p38 and JNK pathways in the osteoclasts, ↓ release of prostaglandin E2 by osteoblasts => inhibition of osteoclast differentiation | Epimedium | In vivo (rats) | [63] [64] | |
Dioscine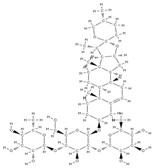 | ↑the proliferation of bone tissue ↓ cell apoptosis by mediating signaling pathways ↓RANKL expression ↓osteoprotegerin/RANKL → inhibits bone reabsorption | Dioscoreaceae family | In vivo (mice) | [65] | |
Kaempferol  | ↑ osteoprotegerin and ↓ RANKL expression → osteoclastogenesis decreases ↑ antiapoptotic expression maintaining bone mass, microarchitecture, and bone strength of the trabecular bones | Ginkgo biloba Camellia sinensis | In vivo (rats) In vivo (rats) In vivo (rats) | [66,67,68] [69] | |
Quercetin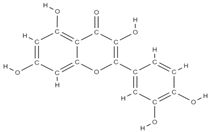 | ↑ the proliferation of bone tissue ↓osteoprotegerin/RANKL → inhibits bone reabsorption | In vitro (mesenchymal stem cells) In vitro (periodontal ligament cells) | [61,69] | ||
Ginkgolic acid | ↑ proliferation, differentiation of osteoblasts, bone mineralization | Ginkgo biloba | In vitro (mesenchymal stem cells) | [61] | |
Caviunin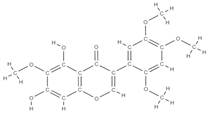 | stimulates BMP-2/Wnt-βcatenin pathway | Dalbergia sissoo | In vivo (rats) | [70] | |
Acteoside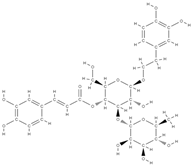 | Antioxidant and anti-inflammatory effect | ↓ the level of pro-inflammatory cytokines such as TNF-α and IL-6, ↓ the differentiation of osteoclasts by reducing free radicals and fighting oxidative stress ↑ cell proliferation ↓ bone demineralization | Verbascum sp. Cistanche sp. | In vivo (rats) | [71] |
Curcumin | ↓the level of inflammation by decreasing the inflammatory cytokines TNF-a and IL-6 ↓bone loss and demineralization, inhibiting osteoclastogenesis ↑the level of alkaline phosphatase, which leads to an increase in the mineralization process interaction with transcription and growth factors, protein kinases, cytokines and enzymes => apoptosis of cancer cell | Curcuma longa | In vitro (osteosarcoma cells) In vitro (human osteosarcoma cells) | [72] [73] | |
Resveratrol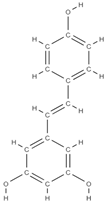 | ↓the level of free radicals from the bone level, neutralizing them ↓bone loss inhibits osteoclastogenesis and the RANKL marker influences the response of estrogen receptors to oxidative stress factors ↑bone differentiation → ↑ bone density ↑the level of morphogenetic protein at the bone level ↓decreases the level of alkaline phosphatase ↓the level of osteocalcin. allows mass production of MSCs; mRNA levels of RUNX2, Collagen Type I Alpha 1 (COL1A1), PPARγ, Adiponectin (APN) were highly expressed, ↑ SIRT1 and SOX2 levels | - | In vivo (rats) In vivo (rats) In vitro (mesenchymal stem cells) | [74,75,76,77] [77] | |
Gomisin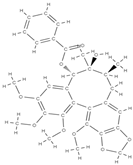 Schisandrin C 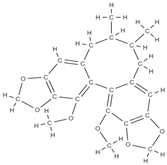 | down-regulation of inflammatory molecules, ROS, and up-regulation of antioxidant molecules | Schisandra chinensis | In vitro (murine macrophage, myoblasts, human diploid fibroblasts, bone marrow macrophages, osteoblasts) In vivo (rats) | [78] | |
Rhamnogalacturonan-I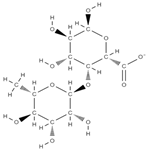 | ↓ intracellular accumulation of galectin-3 down-regulation of RANKL, TNFα, IL-6, and IL-1β | Solanum tuberosum | In vitro (neutrophils and macrophages; osteoblasts) In vivo (rats) | [79,80] | |
Acemannan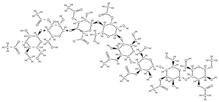 | tissue regeneration, cell proliferation, extracellular matrix synthesis, mineralization. ↑ expression of growth factors; stimulation of bone cementum and periodontal ligament regeneration; induction of bone formation, osteoblast proliferation and differentiation | Aloe vera | In vitro (mesenchymal stem cells) In vivo (rats) | [81] | |
Ellagic acid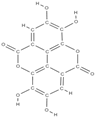 Caffeic acid 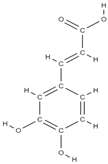 | - inhibition of iNOS, COX-2, NO, TNF-α, PGE2 and IL-6 - down-regulation of IL-1β-stimulated matrix metalloproteinase-13 and thrombospondin motifs 5 - up-regulation of collagen of type II and aggrecan - suppression of NF-κB signaling - ↓ chitinase-3-like protein-1, IL-1β, NF-κB, caspase-3; lipid peroxides, NO - ↑ reduced glutathione | In vivo (mice) In vitro (human chondrocytes) In vivo (rats) | [82] [83] | ||
Ginsenoside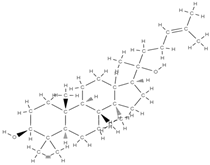 | Modulatory compounds of bone regeneration pathways | ↑ calcium absorption at the intestinal level → thus prevents bone loss ↑ the level of trabecular calcium ↓ C-terminal telopeptide of type I collagen → ↓ resistance to tartrate acid phosphatase at the femoral level | Orchidaceae family | In vitro (mesenchymal stem cells) | [61,84] |
Berberine | ↓ bone loss by preventing decalcification and demineralization inhibits osteoclastogenesis suppresses the activity of the markers involved in the differentiation of acid phosphatase-resistant tartrate bone cells and cathepsin K ↓ the differentiation rate of osteoclasts restore downregulation of osteogenesis-related genes expression; ↑ expression of osteogenesis-related genes such as OSX, COLⅠ, ALP, OCN and OPN ↑ total β-catenin and nuclear β-catenin; activation of the Wnt/β-catenin signaling pathway | Coptis species. Berberis species. Coptidis Rhizoma, Coptis chinensis, Coptis teeta. | In vitro (mesenchymal ctem cells) In vitro (osteoblast and osteoclast) In vitro (mesenchymal stem cells) | [85,86,87,88] [87] | |
Apigenin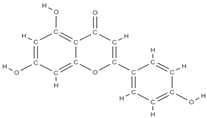 | ↑ the proliferation capacity of osteoblasts inhibits decalcification and osteoclastogenesis modulates intracellular signals → ↓bone loss induced by estrogen hormones ↓ the level of bone inflammation. ↑ mRNA levels of osteogenic genes BMP-2, Runx2 and COL1 downregulation of miR29a, miR17 and miR20a | Olea europaea. Cassia occidentalis | In vivo (rats) In vitro (osteoblasts) In vitro (osteoblasts) In vivo (rats) | [89,90,91,92] | |
Chlorogenic acid | ↑ the level of favorable markers for bone formation ↑ the level of bone morphogenetic protein →↑ the activity of osteoblasts ↓ the level of pro-inflammatory factors ↑ the level of glutathione peroxidase →strong antioxidant effect ↑ the serum activity of alkaline phosphatase, osteoprotegerin ↓ the production of RANKL decreases | Prunus domestica L. | In vivo (rats) In vivo (rats) | [93,94] | |
Aesculetin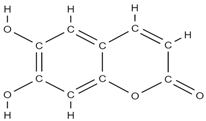 | ↑ expression of bone morphogenetic protein-2, collagen type 1, osteoprotegerin; ALP activation; transcription of Runt-related transcription factor 2; induction of: non-collagenous proteins of bone sialoprotein II, osteopontin, osteocalcin, and osteonectin, of annexin V and PHOSPHO 1. ↑ the production of thrombospondin-1 and tenascin C | - | In vitro (osteoblasts) | [95] | |
Acemannan 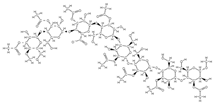 | ↑ mRNA expression of bone morphogenetic protein 2 ↑ mineral deposition | Aloe vera | In vivo (volunteers) | [96] | |
| Antihemorrhagic plant extract | ↑ osteoblastic activity and new bone formation; ↑ osteonectin and osteopontin expression ↓ inflammatory cell infiltration, vascular dilatation and hemorrhage | Glycyrrhiza glabra, Vitis vinifera, Alpinia officinarum Urtica dioica, Thymus vulgaris | In vivo (rats) | [97] | |
Withaferin A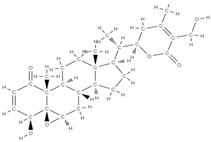 | ↑ expression of osteoblast-specific transcription factor and mineralizing genes, osteoblast survival, ↓ inflammatory cytokines. | Withania somnifera | In vitro (osteoblasts) In vivo (mice, rats) | [98] | |
Ecdysterone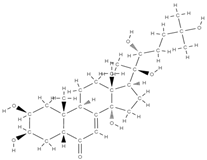 | ↑ gene expression of the BMP-2/Smad/Runx2/Osterix signaling pathway, stimulates MC3T3-E1 cell proliferation | In vitro (osteoblasts) In vivo (rats) | [99] | ||
Echinacoside 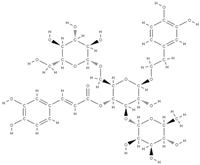 | ↑ the uterine weight and serum E2 levels, ↓ body weight and hydroxyproline serum levels | Cistanche tubulosa | In vivo (rats) | [100] | |
Epigallocatechin gallate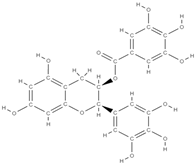 | activation of β-catenin of the Wnt signaling pathway ↑ expression of osteogenic genes, ALP activity, and mineralization in bone marrow-derived mesenchymal stem cells | Grean tea | In vitro (adipose-derived stem cells, dedifferentiated fat cells) In vivo (mice, rats) | [101,102] | |
| Essential oils | blocking nuclear factor kappa B, p38, and c-Jun N-terminal kinase signaling ↓ production of nitric oxide in RAW264.7 cells, inhibited EAhy926 cell proliferation ↑ serum C-telopeptide collagen type I and osteocalcin ↑ plasma calcium and vitamin D3, bone mineral-density Prevention of inflammation and oxidative stress | Hypericum perforatum; Cinnamomum burmanini; Thymus vulgari; Rosmarinus officinalis. Populus alba; | In vitro (macrophages, fibroblasts, osteoblasts) In vivo (rats, mice) | [48] | |
Forskolin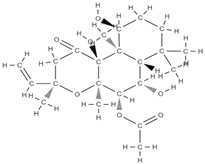 | activation of cyclic adenosine monophosphate (c-AMP) signalling in stem cells | Coleus forskohlii | In vitro (mesenchymal stem cells) | [103] | |
Gallotannin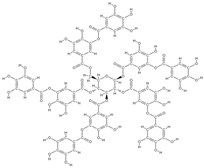 | interaction with ALP growth of Saos-2 cells | Mangifera indica L. | In vitro (osteoblasts) | [104] | |
Ursolic acid 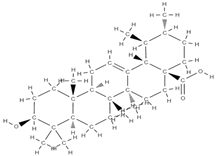 | ↑ trabecular parameters (BV/TV, Tb.Th and conn.D) ↓ SMI ↑ALP activity, osteogenic genes (Runx2, BMP-2, type 1 Col1 and Wnt3a) stimulates Wnt/β-catenin signalling osteoblast differentiation (activation of mitochondrial respiration) | Psidium guajava | In vitro (osteoblasts) In vivo (rats) | [105] | |
Malvidin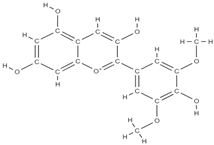 Cyanidin 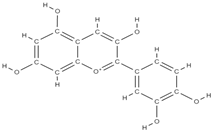 Delphinidin 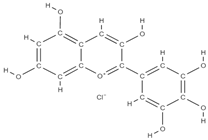 | inhibition of MSC adipogenesis and downregulation of FABP4 and adiponectin genes. ↑ accumulation of calcium deposits upregulation of osteocyte-specific gene BMP-2 and Runx-2 expression | Berries | In vitro (mesenchymal stem cells) | [106] | |
Rutin | activation of Wnt/b-Catenin Signaling ↑ activity of ALP, Runx2, osterix, osteocalcin, bone morphogenetic protein 2, Wnt3a, and b-catenin | Morinda citrifolia (Noni) | In vitro (murine myoblast cell line, human periodontal ligament cells) In vivo (rats) | [107,108,109] | |
Rhamnogalacturonan-I | ↓ intracellular accumulation of galectin-3 up-regulation of collagen type I alpha 1 (COL-Iα1), osteocalcin, sialoprotein. down-regulation of RANKL, TNFα, IL-6, and IL-1β | Solanum tuberosum | In vitro (neutrophils and macrophages; osteoblasts) In vivo (rats) | [79,80] | |
Crocin 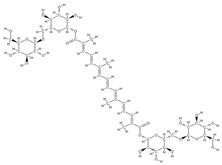 Crocetin 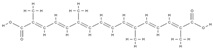 | ↑ ALP activity and ALP mRNA expression in MSCs | Crocus sativus L. | In vitro (mesenchymal stem cells) | [110] | |
Sinapic acid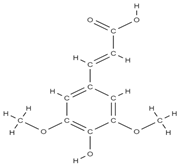 | activation of TGF-β1/BMP/Smads/Runx2 signaling pathways => osteoblast differentiation | Cynanchi atrati | In vitro (macrophags) In vitro (mesenchymal stem cells) In vivo (rats) | [111,112] | |
BetaEcdysone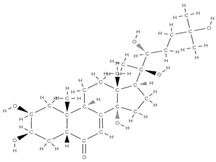 | ↑ collagen deposition, ↑ levels of osteocalcin, ↑ expression of osteogenic genes | Tinospora cordifolia | In vitro (osteoblasts, macrophages) In vivo (rats) | [113] | |
Cucurbitacin B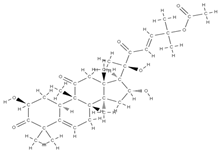 | ↑ expression of ALP and OPN genes, mineralization up-regulation of VEGFR2 and VEGFR-related signaling pathways (induction of angiogenesis) | Cucurbitaceae family plants | In vitro (mesenchymal stem cells) In vivo (rats) | [114] | |
| Polysaccharides | hematopoiesis protection: ↓ myeloid cells within tumor-infiltrating immune cells Inhibition of hematopoietic cell expansion in the spleen ↑ HSPCs (hematopoietic stem and progenitor cells) and common lymphoid progenitors in the bone marrow | Polygonatum sibiricum | In vivo (mice) | [115] | |
Ellagic acid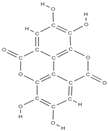 Ellagic acid and Sennoside B 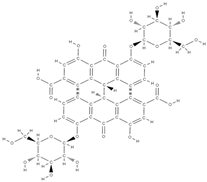 | ↑ number of osteoblasts and expression of OCN and OPG ↓ number of osteoclasts and the expression of RANKL - repression of c-Jun expression at the mRNA level | In vivo (rats) In vitro (human osteosarcoma cells) | [116] [117] | ||
| Ellagic acid and hydroxyapatite | ↑ in the expression of FGF-2, VEGF and ALP ↑ IL-10, BMP-4 and OPN ↓ TNF-α and increasing the expression of | In vivo (rats) | [118,119] | ||
Melibiose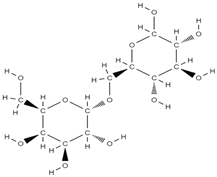 Methylophiopogonanone A 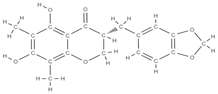 Tubuloside A 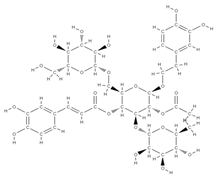 | ↑ expression of ALP, osteocalcin, osterin, osteoprotegerin, and autophagy marker proteins activation of BMP2/Smad/Runx2 and Wnt/β-catenin signaling | Juglans regia | In vitro (mesenchymal stem cells) | [120] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hanga-Farcaș, A.; Miere, F.; Filip, G.A.; Clichici, S.; Fritea, L.; Vicaș, L.G.; Marian, E.; Pallag, A.; Jurca, T.; Filip, S.M.; et al. Phytochemical Compounds Involved in the Bone Regeneration Process and Their Innovative Administration: A Systematic Review. Plants 2023, 12, 2055. https://doi.org/10.3390/plants12102055
Hanga-Farcaș A, Miere F, Filip GA, Clichici S, Fritea L, Vicaș LG, Marian E, Pallag A, Jurca T, Filip SM, et al. Phytochemical Compounds Involved in the Bone Regeneration Process and Their Innovative Administration: A Systematic Review. Plants. 2023; 12(10):2055. https://doi.org/10.3390/plants12102055
Chicago/Turabian StyleHanga-Farcaș, Alina, Florina Miere (Groza), Gabriela Adriana Filip, Simona Clichici, Luminita Fritea, Laura Grațiela Vicaș, Eleonora Marian, Annamaria Pallag, Tunde Jurca, Sanda Monica Filip, and et al. 2023. "Phytochemical Compounds Involved in the Bone Regeneration Process and Their Innovative Administration: A Systematic Review" Plants 12, no. 10: 2055. https://doi.org/10.3390/plants12102055
APA StyleHanga-Farcaș, A., Miere, F., Filip, G. A., Clichici, S., Fritea, L., Vicaș, L. G., Marian, E., Pallag, A., Jurca, T., Filip, S. M., & Muresan, M. E. (2023). Phytochemical Compounds Involved in the Bone Regeneration Process and Their Innovative Administration: A Systematic Review. Plants, 12(10), 2055. https://doi.org/10.3390/plants12102055











