Leaf Color Classification and Expression Analysis of Photosynthesis-Related Genes in Inbred Lines of Chinese Cabbage Displaying Minor Variations in Dark-Green Leaves
Abstract
:1. Introduction
2. Results
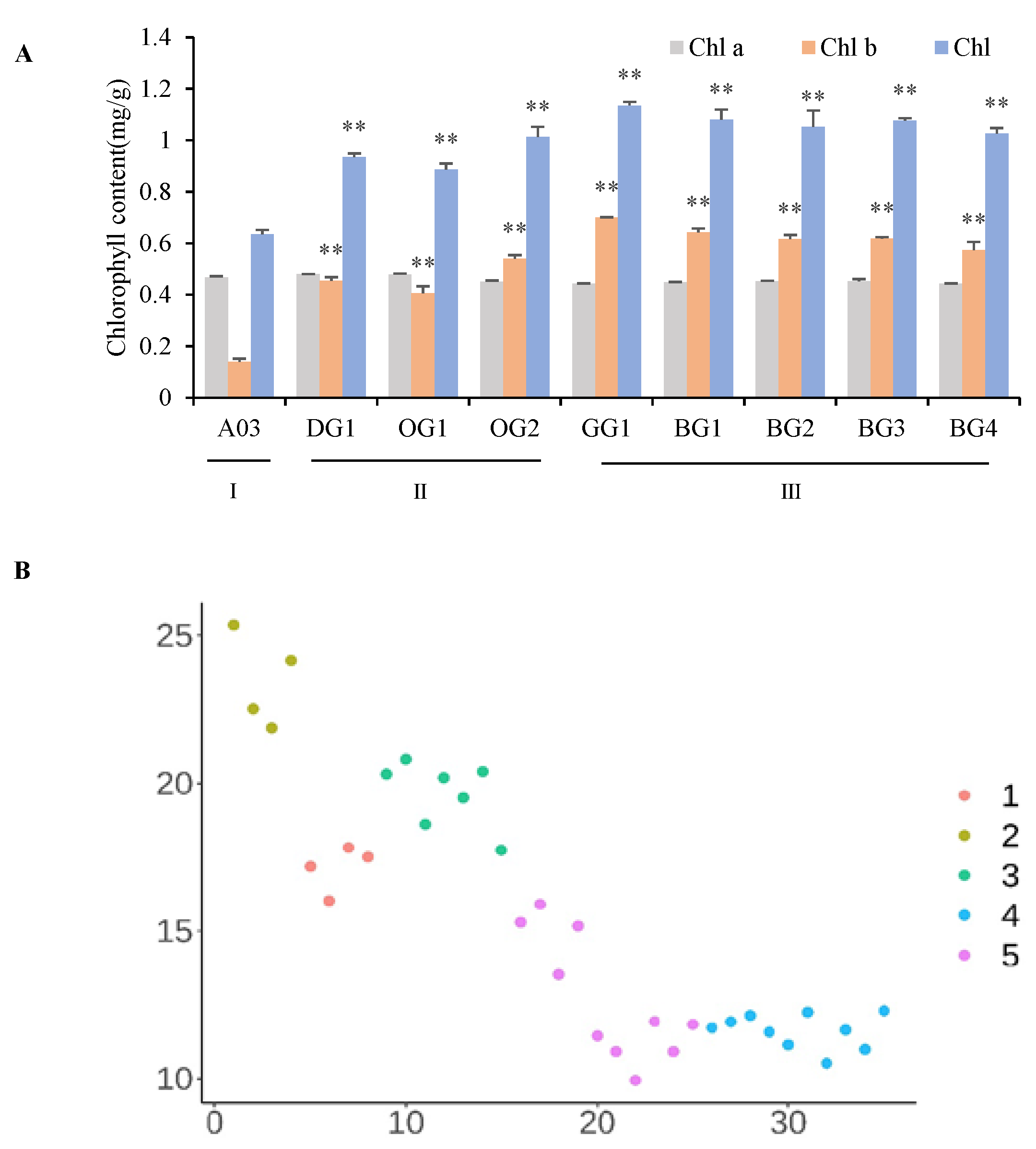
2.1. Phenotypic Identification of Chinese Cabbage Inbred Lines with Slight Differences in Leaf Color
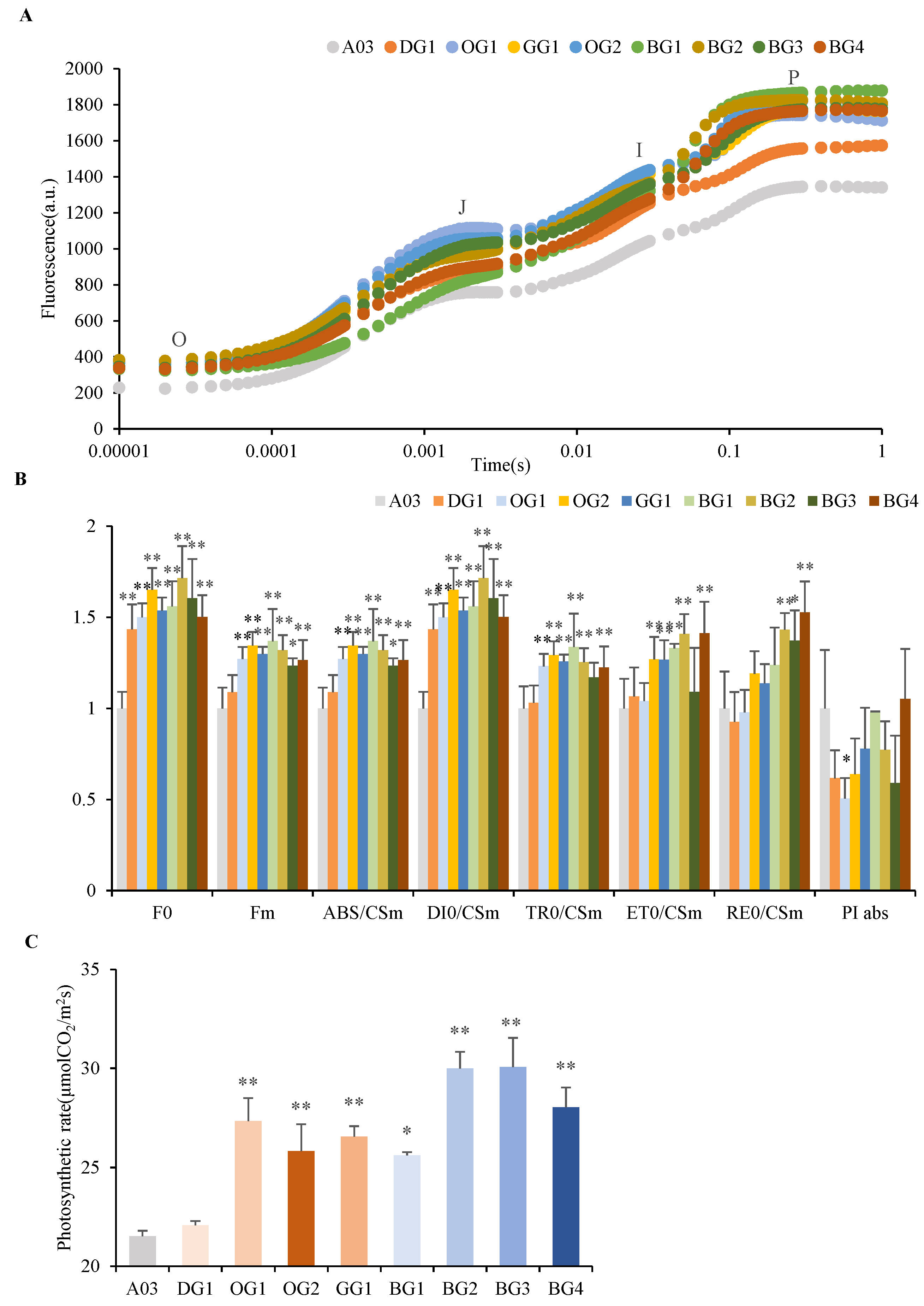
2.2. Photosynthetic Parameters
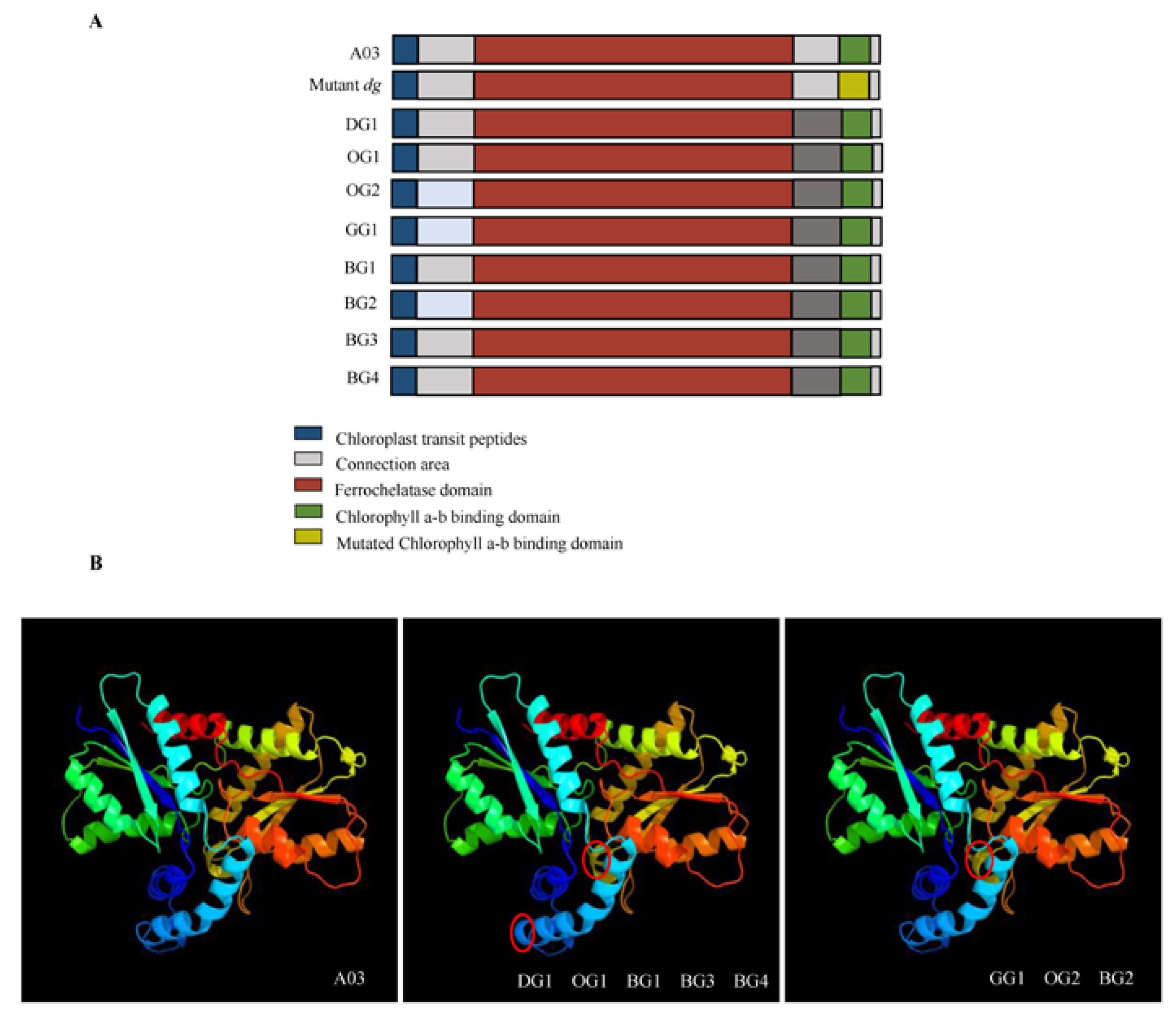
2.3. Gene Sequence and Structural Analysis of BrFC2
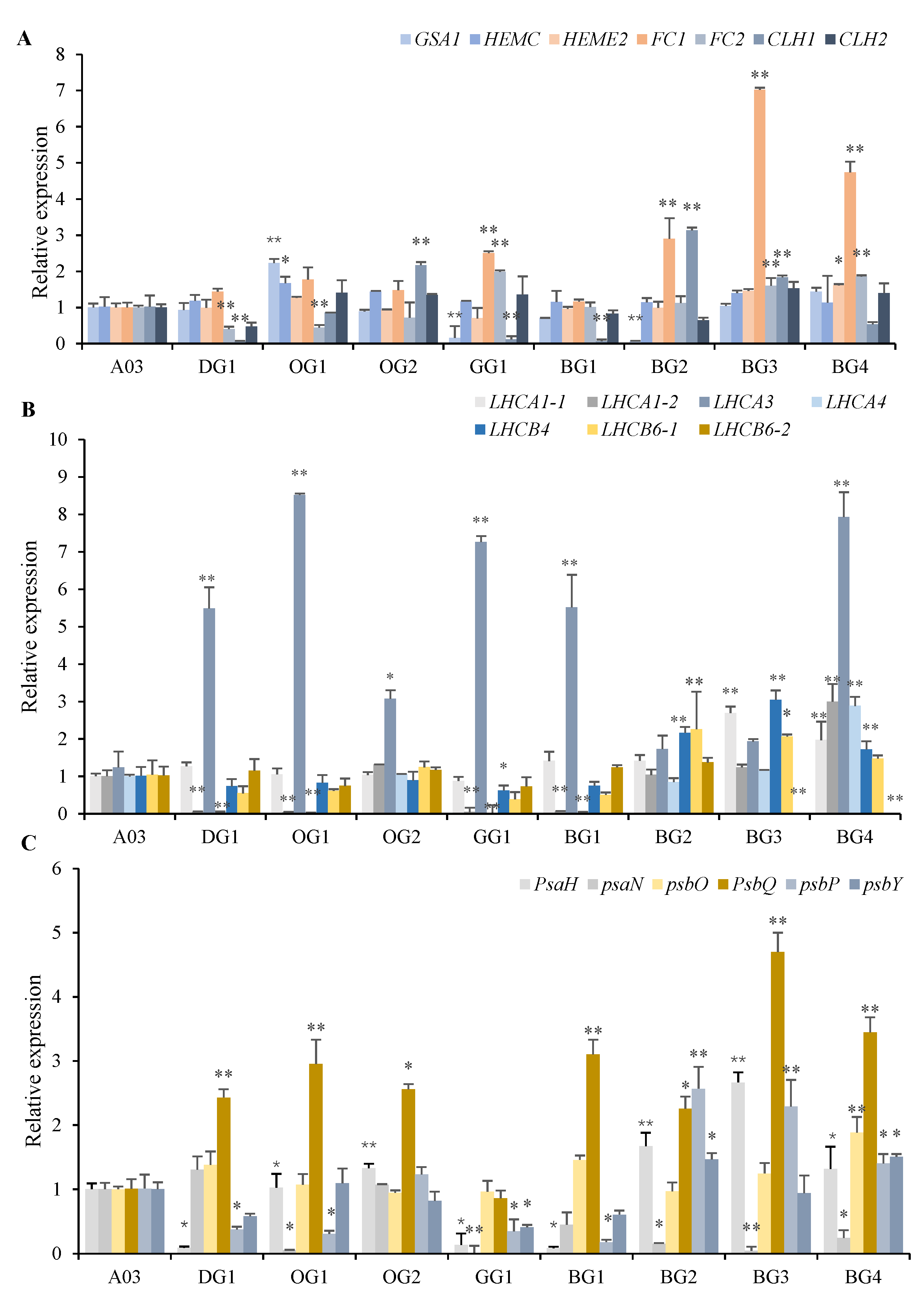
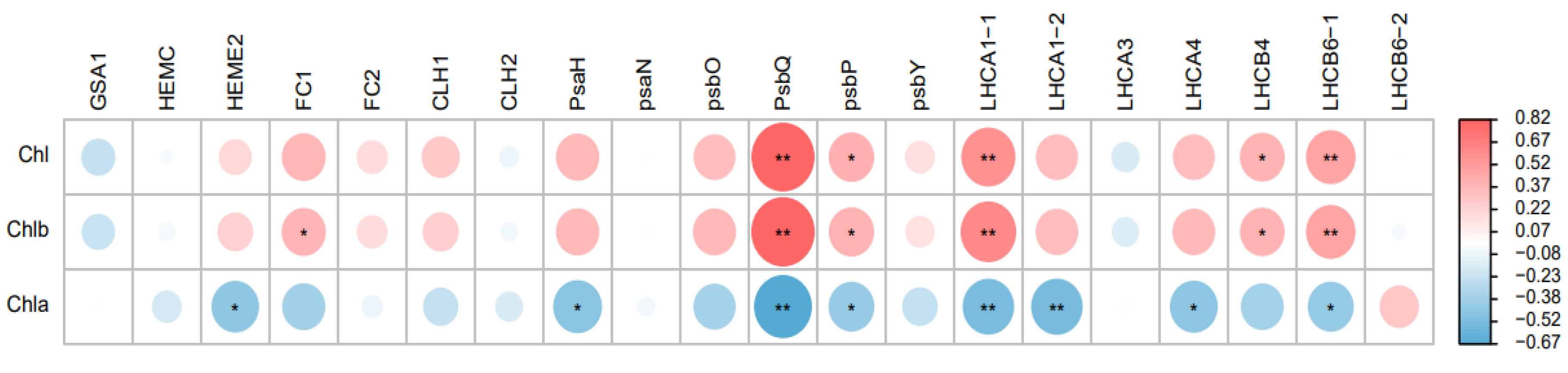
2.4. Expression Analysis of Photosynthesis-Related Genes
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Growth Conditions
4.2. Chlorophyll Measurement and Multispectral Analysis
4.3. Measurements of Photosynthetic Parameters
4.4. RNA Extraction and Quantitative Real-Time PCR (qRT-PCR)
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Block, M.A.; Joyard, J.; Douce, R. Site of synthesis of geranylgeraniol derivatives in intact spinach chloroplasts. Biochim. Biophys. Acta 1980, 631, 210–219. [Google Scholar] [CrossRef] [PubMed]
- Espineda, C.E.; Linford, A.S.; Devine, D.; Brusslan, J.A. The AtCAO gene, encoding chlorophyll a oxygenase, is required for chlorophyll b synthesis in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 1999, 96, 10507–10511. [Google Scholar] [CrossRef] [PubMed]
- Sakuraba, Y.; Rahman, M.L.; Cho, S.H.; Kim, Y.S.; Koh, H.J.; Yoo, S.C.; Paek, N.C. The rice faded green leaf locus encodes protochlorophyllide oxidoreductase B and is essential for chlorophyll synthesis under high light conditions. Plant J. Cell Mol. Biol. 2013, 74, 122–133. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Wang, P.; Gao, L.; Yang, R.; Li, L.; Zhang, E.; Wang, Q.; Li, Y.; Yin, Z. Characterization and Complementation of a Chlorophyll-Less Dominant Mutant GL1 in Lagerstroemia indica. DNA Cell Biol. 2017, 36, 354–366. [Google Scholar] [CrossRef]
- Jansson, S. The light-harvesting chlorophyll a/b-binding proteins. Biochim. Biophys. Acta 1994, 1184, 1–19. [Google Scholar] [CrossRef]
- Kim, E.H.; Li, X.P.; Razeghifard, R.; Anderson, J.M.; Niyogi, K.K.; Pogson, B.J.; Chow, W.S. The multiple roles of light-harvesting chlorophyll a/b-protein complexes define structure and optimize function of Arabidopsis chloroplasts: A study using two chlorophyll b-less mutants. Biochim. Biophys. Acta 2009, 1787, 973–984. [Google Scholar] [CrossRef]
- Liu, M.; Ma, W.; Su, X.; Zhang, X.; Lu, Y.; Zhang, S.; Yan, J.; Feng, D.; Ma, L.; Taylor, A.; et al. Mutation in a chlorophyll-binding motif of Brassica ferrochelatase enhances both heme and chlorophyll biosynthesis. Cell Rep. 2022, 41, 111758. [Google Scholar] [CrossRef]
- Sobotka, R.; Mclean, S.; Zuberova, M.; Hunter, C.N.; Tichy, M. The C-Terminal Extension of Ferrochelatase Is Critical for Enzyme Activity and for Functioning of the Tetrapyrrole Pathway in Synechocystis Strain PCC 6803. J. Bacteriol. 2008, 190, 2086–2095. [Google Scholar] [CrossRef]
- Underhill, A.N.; Hirsch, C.D.; Clark, M.D. Evaluating and Mapping Grape Color Using Image-Based Phenotyping. Plant Phenomics 2020, 2020, 8086309. [Google Scholar] [CrossRef]
- Ishikita, H.; Saenger, W.; Biesiadka, J.; Loll, B.; Knapp, E.W. How photosynthetic reaction centers control oxidation power in chlorophyll pairs P680, P700, and P870. Proc. Natl. Acad. Sci. USA 2006, 103, 9855–9860. [Google Scholar] [CrossRef]
- Tanaka, R.; Tanaka, A. Chlorophyll cycle regulates the construction and destruction of the light-harvesting complexes. Biochim. Biophys. Acta 2011, 1807, 968–976. [Google Scholar] [CrossRef]
- Leverenz, J.W.; Öquist, G.; Wingsle, G.J.P.P. Photosynthesis and photoinhibition in leaves of chlorophyll b-less barley in relation to absorbed light. Physiol. Plant. 1992, 85, 495–502. [Google Scholar] [CrossRef]
- Hirashima, M.; Satoh, S.; Tanaka, R.; Tanaka, A. Pigment shuffling in antenna systems achieved by expressing prokaryotic chlorophyllide a oxygenase in Arabidopsis. J. Biol. Chem. 2006, 281, 15385–15393. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, R.; Koshino, Y.; Sawa, S.; Ishiguro, S.; Okada, K.; Tanaka, A. Overexpression of chlorophyllide a oxygenase (CAO) enlarges the antenna size of photosystem II in Arabidopsis thaliana. Plant J. Cell Mol. Biol. 2001, 26, 365–373. [Google Scholar] [CrossRef] [PubMed]
- Chow, K.S.; Singh, D.P.; Walker, A.R.; Smith, A.G. Two different genes encode ferrochelatase in Arabidopsis: Mapping, expression and subcellular targeting of the precursor proteins. Plant J. 2010, 15, 531–541. [Google Scholar] [CrossRef] [PubMed]
- Espinas, N.A.; Kobayashi, K.; Sato, Y.; Mochizuki, N.; Takahashi, K.; Tanaka, R.; Masuda, T. Allocation of Heme Is Differentially Regulated by Ferrochelatase Isoforms in Arabidopsis Cells. Front. Plant Sci. 2016, 7, 1326. [Google Scholar] [CrossRef]
- Scharfenberg, M.; Mittermayr, L.; Roepenack-Lahaye, E.V.; Schlicke, H.; Kleine, T. Functional characterization of the two ferrochelatases in Arabidopsis thaliana. Plant Cell Environ. 2015, 38, 280–298. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Feng, S.J.; Chen, J.; Zhao, W.T.; Yang, Z.M. A cadmium stress-responsive gene AtFC1 confers plant tolerance to cadmium toxicity. BMC Plant Biol. 2017, 17, 187. [Google Scholar] [CrossRef]
- Nagai, S.; Koide, M.; Takahashi, S.; Kikuta, A.; Aono, M.; Sasaki-Sekimoto, Y.; Ohta, H.; Takamiya, K.; Masuda, T. Induction of isoforms of tetrapyrrole biosynthetic enzymes, AtHEMA2 and AtFC1, under stress conditions and their physiological functions in Arabidopsis. Plant Physiol. 2007, 144, 1039–1051. [Google Scholar] [CrossRef]
- Sobotka, R.; Tichy, M.; Hunter, A. Functional Assignments for the Carboxyl-Terminal Domains of the Ferrochelatase from Synechocystis PCC 6803: The CAB Domain Plays a Regulatory Role, and Region II Is Essential for Catalysis. Plant Physiol. 2011, 155, 1735–1747. [Google Scholar] [CrossRef]
- Enami, I.; Okumura, A.; Nagao, R.; Suzuki, T.; Iwai, M.; Shen, J.R. Structures and functions of the extrinsic proteins of photosystem II from different species. Photosynth. Res. 2008, 98, 349–363. [Google Scholar] [CrossRef] [PubMed]
- Caffarri, S.; Kouril, R.; Kereïche, S.; Boekema, E.J.; Croce, R. Functional architecture of higher plant photosystem II supercomplexes. EMBO J. 2009, 28, 3052–3063. [Google Scholar] [CrossRef] [PubMed]
- Ifuku, K.; Ido, K.; Sato, F. Molecular functions of PsbP and PsbQ proteins in the photosystem II supercomplex. J. Photochem. Photobiol. B Biol. 2011, 104, 158–164. [Google Scholar] [CrossRef]
- Allahverdiyeva, Y.; Suorsa, M.; Rossi, F.; Pavesi, A.; Kater, M.M.; Antonacci, A.; Tadini, L.; Pribil, M.; Schneider, A.; Wanner, G.; et al. Arabidopsis plants lacking PsbQ and PsbR subunits of the oxygen-evolving complex show altered PSII super-complex organization and short-term adaptive mechanisms. Plant J. Cell Mol. Biol. 2013, 75, 671–684. [Google Scholar] [CrossRef]
- Amerongen, H.V.; van Grondelle, R. Understanding the Energy Transfer Function of LHCII, the Major Light-Harvesting Complex of Green Plants. J. Phys. Chem. B 2001, 105, 604–617. [Google Scholar] [CrossRef]
- Yakushevska, A.E.; Keegstra, W.; Boekema, E.J.; Dekker, J.P.; Andersson, J.; Jansson, S.; Ruban, A.V.; Horton, P. The structure of photosystem II in Arabidopsis: Localization of the CP26 and CP29 antenna complexes. Biochemistry 2003, 42, 608–613. [Google Scholar] [CrossRef] [PubMed]
- Dekker, J.P.; Boekema, E.J. Supramolecular organization of thylakoid membrane proteins in green plants. Biochim. Biophys. Acta 2005, 1706, 12–39. [Google Scholar] [CrossRef]
- De Bianchi, S.; Dall’Osto, L.; Tognon, G.; Morosinotto, T.; Bassi, R. Minor antenna proteins CP24 and CP26 affect the interactions between photosyssstem II subunits and the electron transport rate in grana membranes of Arabidopsis. Plant Cell 2008, 20, 1012–1028. [Google Scholar] [CrossRef]
- Kovács, L.; Damkjaer, J.; Kereïche, S.; Ilioaia, C.; Ruban, A.V.; Boekema, E.J.; Jansson, S.; Horton, P. Lack of the light-harvesting complex CP24 affects the structure and function of the grana membranes of higher plant chloroplasts. Plant Cell 2006, 18, 3106–3120. [Google Scholar] [CrossRef]
- Peng, X.; Deng, X.; Tang, X.; Tan, T.; Zhang, D.; Liu, B.; Lin, H. Involvement of Lhcb6 and Lhcb5 in Photosynthesis Regulation in Physcomitrella patens Response to Abiotic Stress. Int. J. Mol. Sci. 2019, 20, 3665. [Google Scholar] [CrossRef]
- Porra, R.J.; Thompson, W.A.; Kriedemann, P.E. Determination of accurate extinction coefficients and simultaneous equations for assaying chlorophylls a and b extracted with four different solvents: Verification of the concentration of chlorophyll standards by atomic absorption spectroscopy. Biochim. Biophys. Acta–Bioenerg. 1989, 975, 384–394. [Google Scholar] [CrossRef]
- Strasser, R.J.; Tsimilli-Michael, M.; Qiang, S.; Goltsev, V. Simultaneous in vivo recording of prompt and delayed fluorescence and 820-nm reflection changes during drying and after rehydration of the resurrection plant Haberlea rhodopensis. Biochim. Biophys. Acta 2010, 1797, 1313–1326. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Su, X.; Yue, X.; Kong, M.; Xie, Z.; Yan, J.; Ma, W.; Wang, Y.; Zhao, J.; Zhang, X.; Liu, M. Leaf Color Classification and Expression Analysis of Photosynthesis-Related Genes in Inbred Lines of Chinese Cabbage Displaying Minor Variations in Dark-Green Leaves. Plants 2023, 12, 2124. https://doi.org/10.3390/plants12112124
Su X, Yue X, Kong M, Xie Z, Yan J, Ma W, Wang Y, Zhao J, Zhang X, Liu M. Leaf Color Classification and Expression Analysis of Photosynthesis-Related Genes in Inbred Lines of Chinese Cabbage Displaying Minor Variations in Dark-Green Leaves. Plants. 2023; 12(11):2124. https://doi.org/10.3390/plants12112124
Chicago/Turabian StyleSu, Xiangjie, Xiaonan Yue, Mingyu Kong, Ziwei Xie, Jinghui Yan, Wei Ma, Yanhua Wang, Jianjun Zhao, Xiaomeng Zhang, and Mengyang Liu. 2023. "Leaf Color Classification and Expression Analysis of Photosynthesis-Related Genes in Inbred Lines of Chinese Cabbage Displaying Minor Variations in Dark-Green Leaves" Plants 12, no. 11: 2124. https://doi.org/10.3390/plants12112124




