Phenotypic, Physiological, and Molecular Response of Loropetalum chinense var. rubrum under Different Light Quality Treatments Based on Leaf Color Changes
Abstract
:1. Introduction
2. Results
2.1. Changes in Leaf Color Phenotype under Different Light Quality Treatments
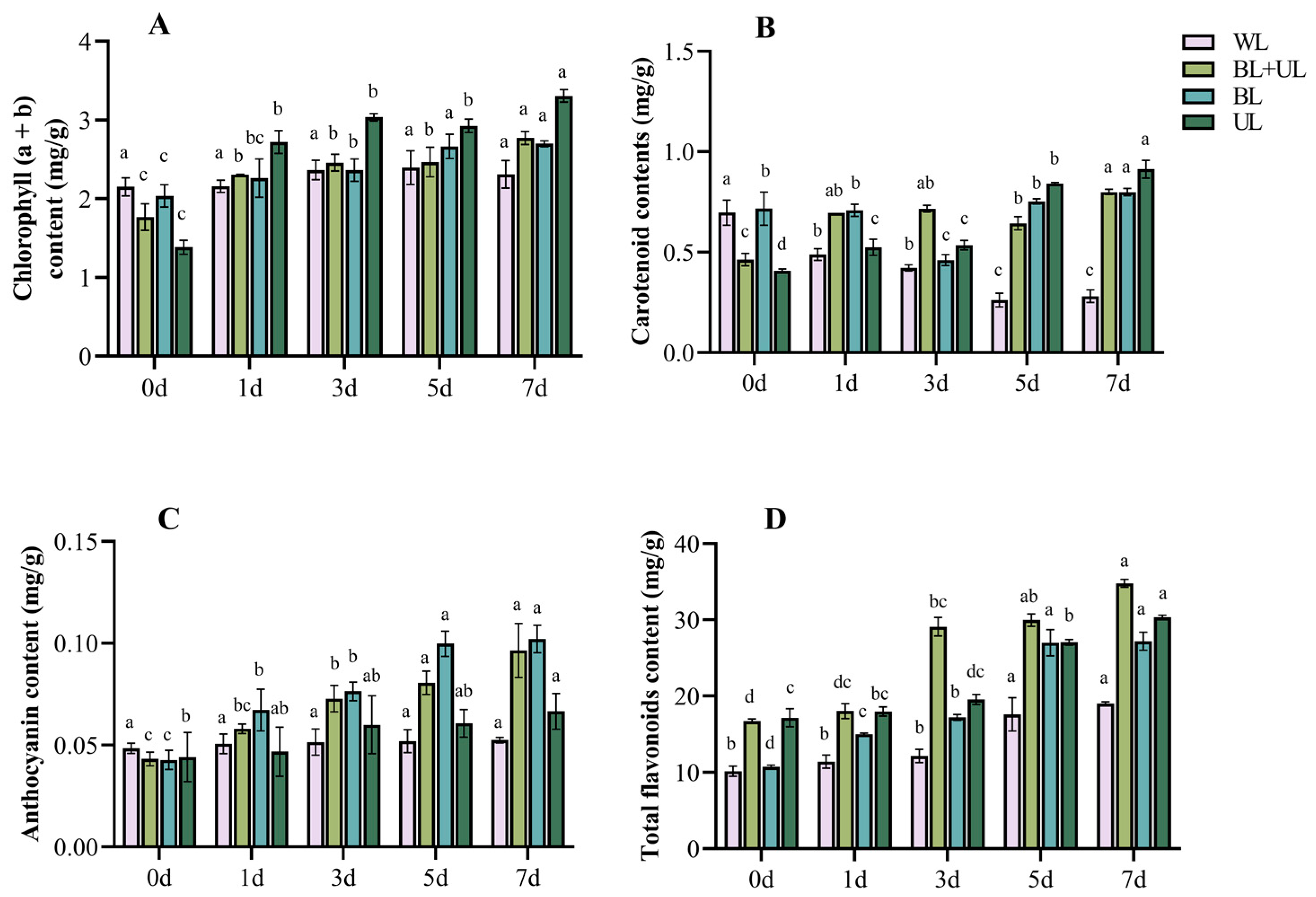
2.2. Changes in Pigment Contents under Different Light Qualities with Time
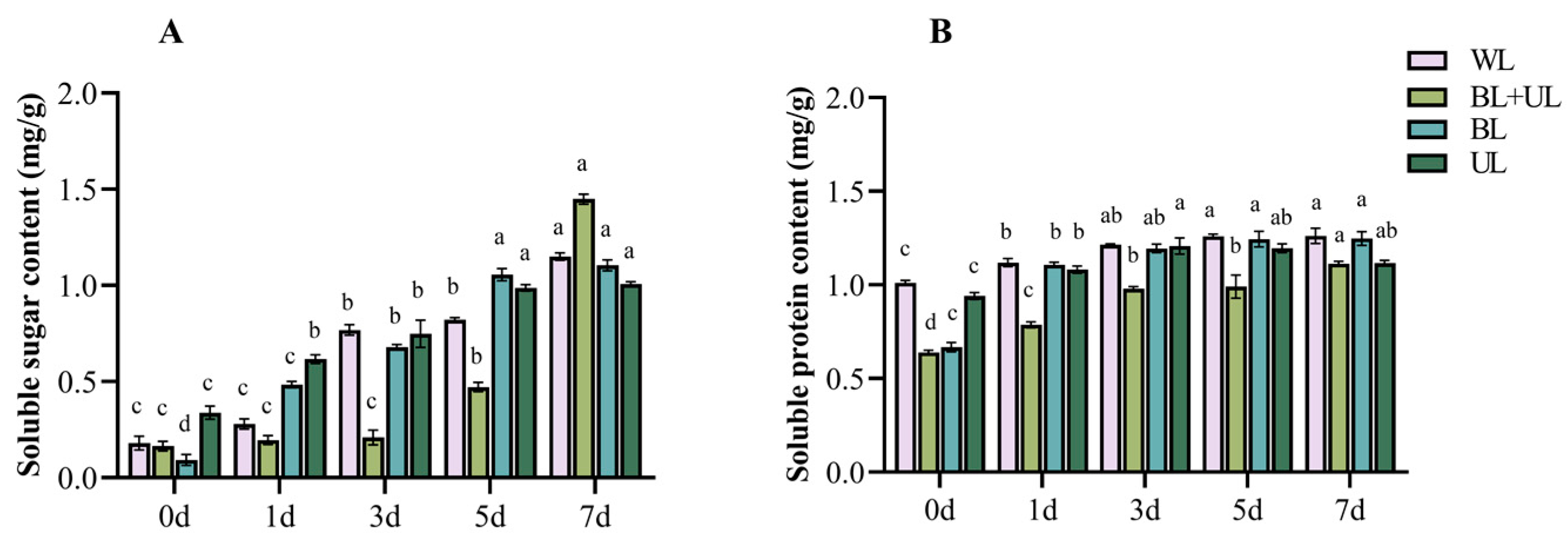
2.3. Changes in Soluble Sugar and Soluble Protein Content with Time under Different Light Qualities
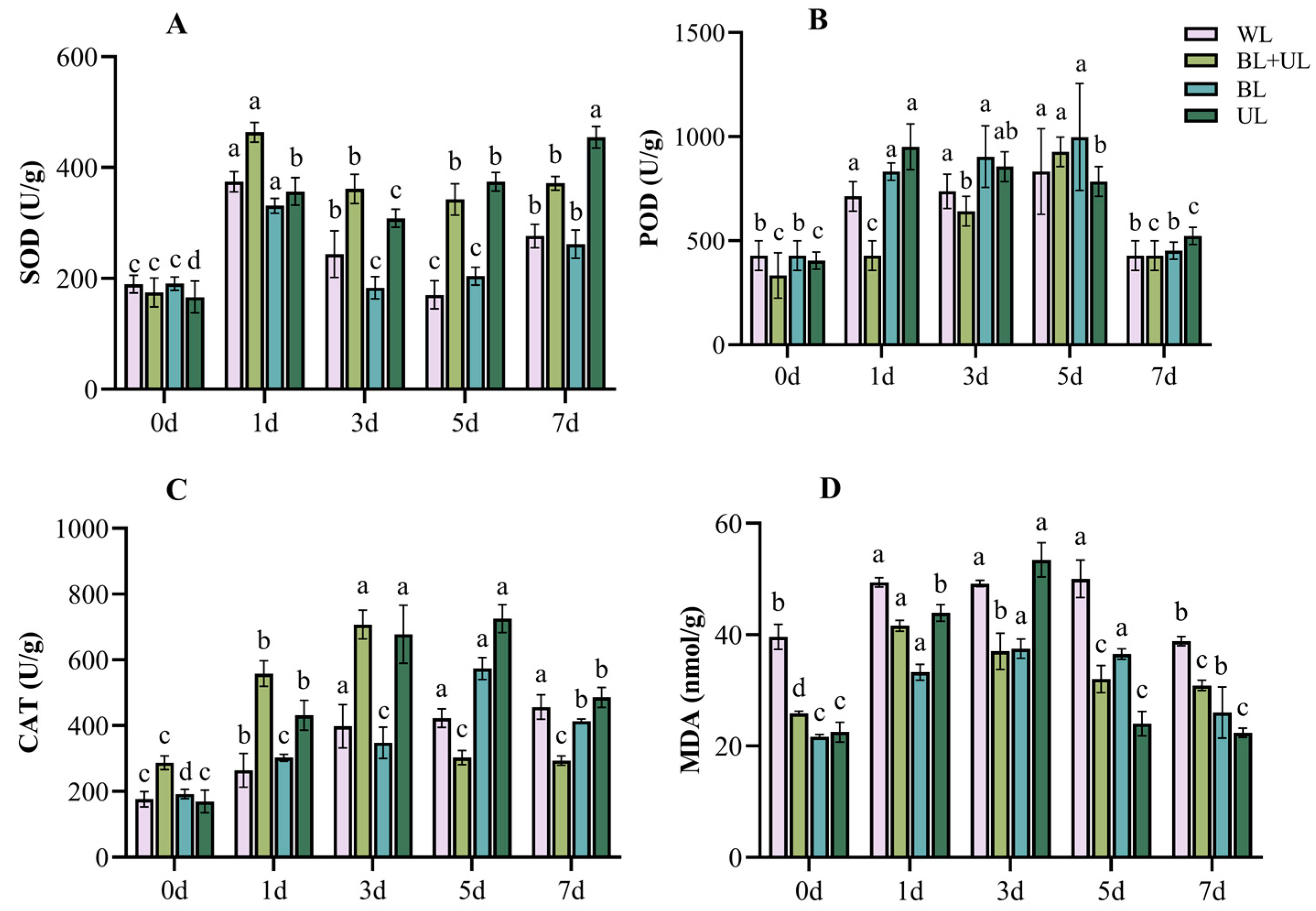
2.4. Stress Injury and Self-Repair of the Antioxidant System during Leaf Growth under Different Light Qualities
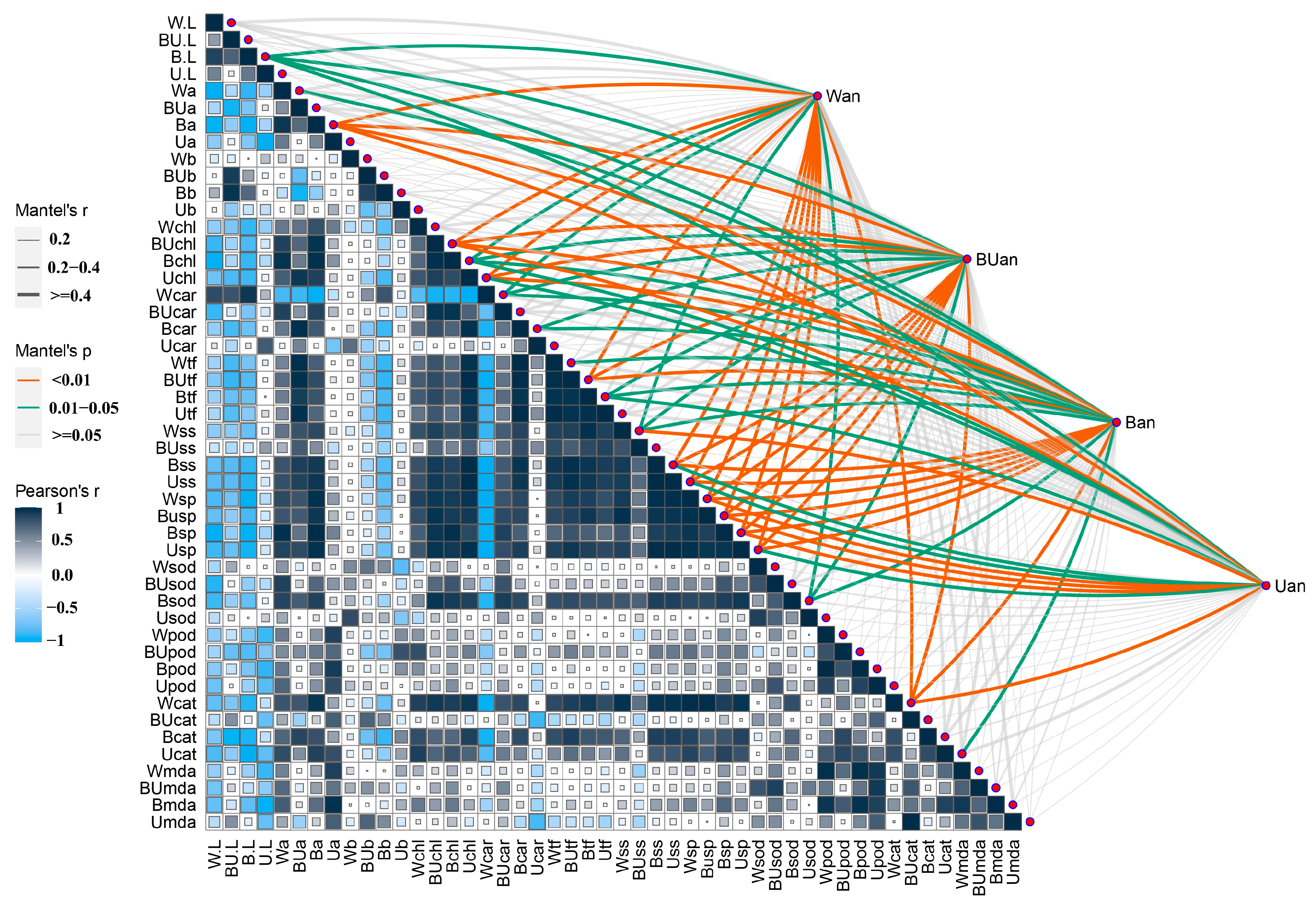
2.5. Correlation between Physiological and Biochemical Indicators and Anthocyanin Content in the Leaves
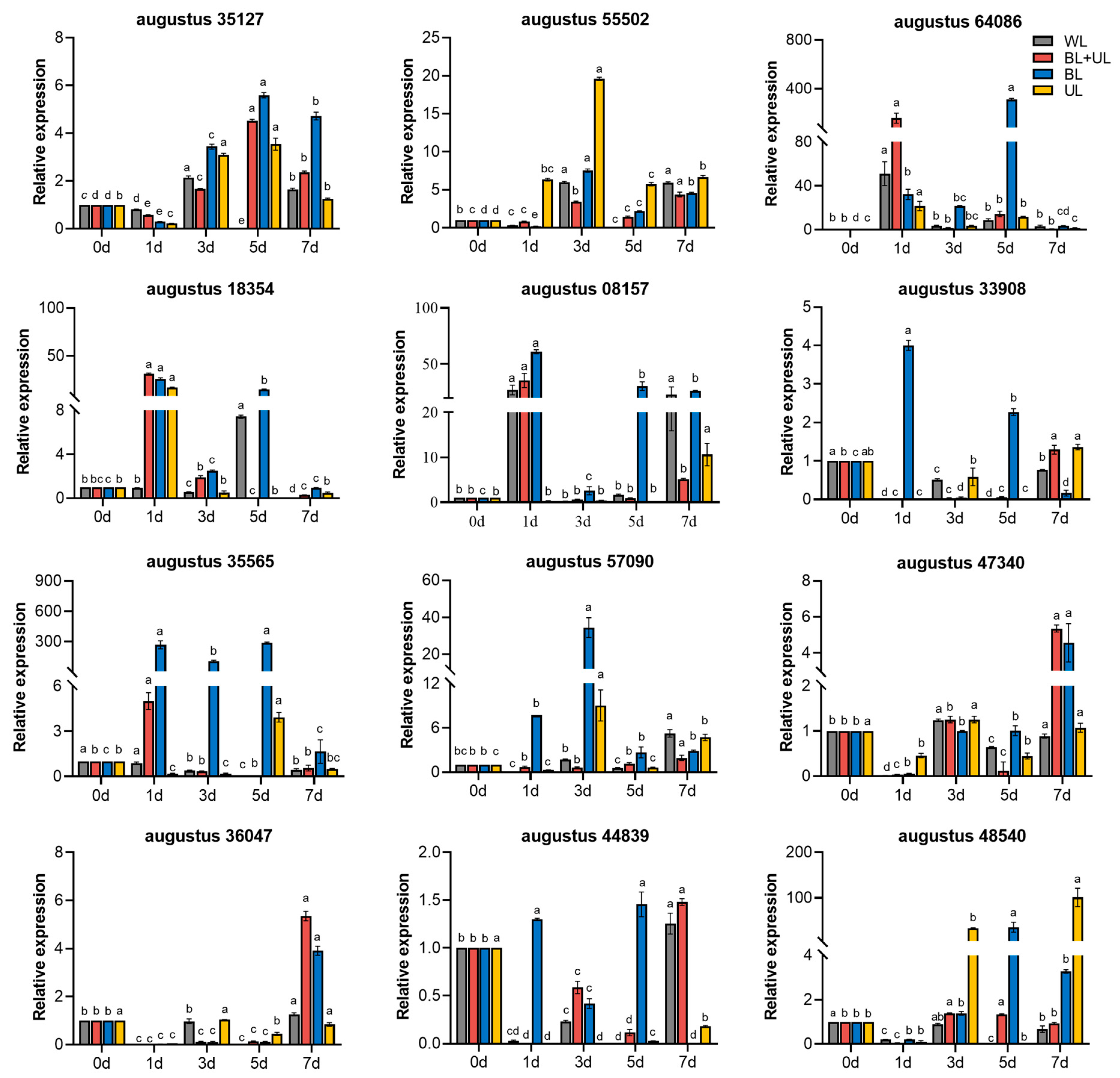
2.6. The Gene Expression of Related Anthocyanin Synthesis under Different Light Qualities
2.7. The Gene Expression of Related Antioxidant Enzymes under Different Light Qualities
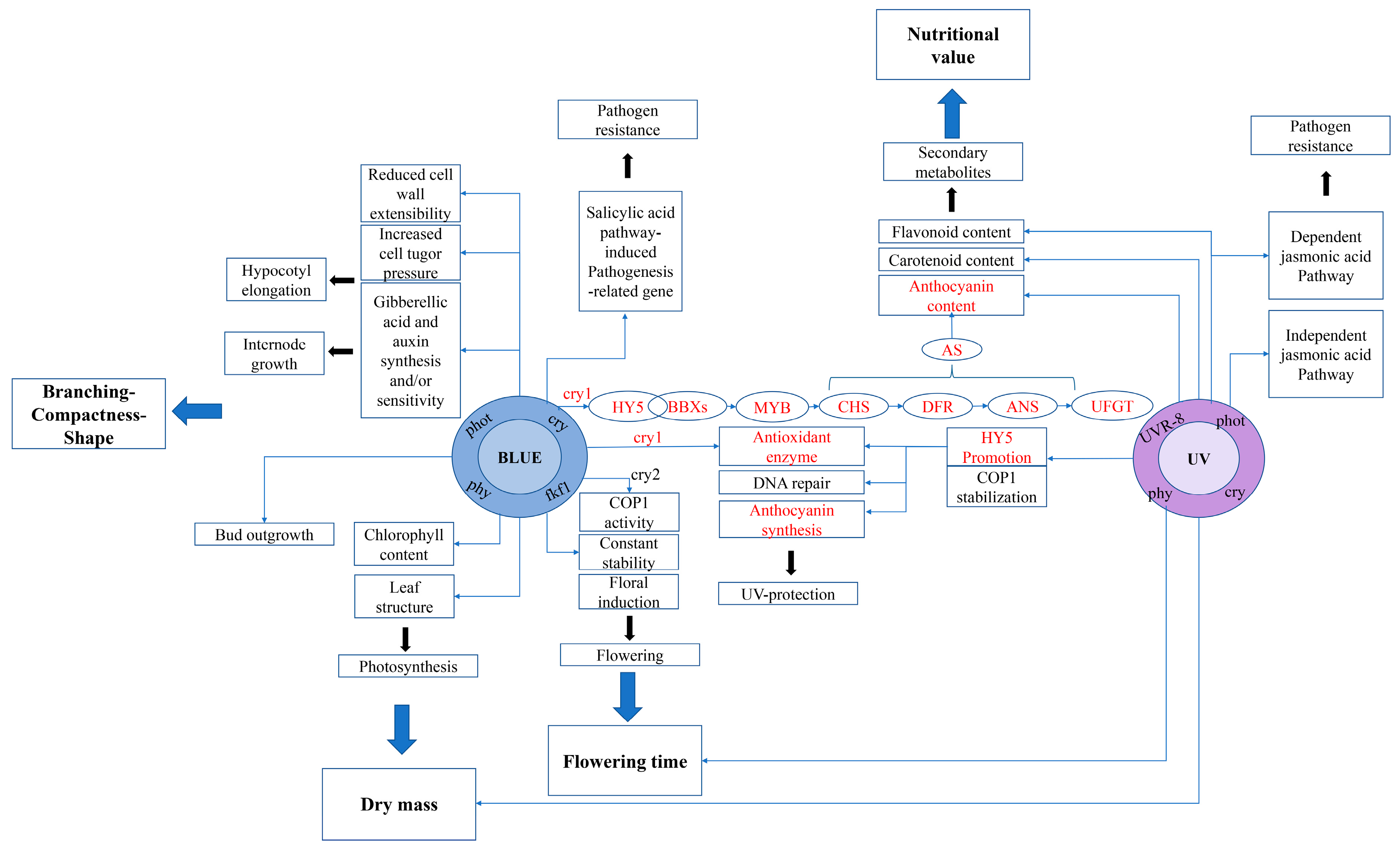
3. Discussion
3.1. Different Changes in Leaf Phenotype with Time under Different Light Qualities
3.2. Changes in the Physicochemical Properties of Leaves Induced by Different Light Quality Treatments with Different Times
3.3. Anthocyanin Content under Different Light Quality Conditions Has a Different Correlation with Other Physicochemical Indexes
3.4. Anthocyanin Synthesis-Related Genes and Antioxidant Enzyme Synthesis-Related Genes Respond to the Expression Regulation of Different Light Qualities
4. Materials and Methods
4.1. Plant Materials
4.2. Light Quality Settings
4.3. Leaf Observation
4.4. Determination of Pigment Content
4.5. Determination of Total Flavonoids, Soluble Sugar, and Soluble Protein Content
4.6. Determination of MDA Content and Antioxidant Enzyme Activity
4.7. Analysis of Relationship
4.8. Detection of Gene Expression
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Altman, A.; Shennan, S.; Odling-Smee, J. Ornamental plant domestication by aesthetics-driven human cultural niche construction. Trends Plant Sci. 2021, 27, 124–138. [Google Scholar] [CrossRef] [PubMed]
- Dong, S.L.; Ma, J.M.; Mo, Y.H.; Li, L. Review of research on germplasm resources and application of Loropetalum chinense var. rubrum. Guangxi For. Sci. 2022, 51, 290–297. [Google Scholar]
- Xiao, F.; Fan, J.L.; Zhang, Q.Y. Isolation and Identification of Flavonoids from Leaves of Loropetalum chinense var. rubrum. J. China Pharm. 2012, 23, 1021–1022. [Google Scholar]
- Li, H.W.; Duan, Z.G.; Feng, J.T.; Liu, J.J. Characteristics and cultivation management techniques of Loropetalum chinense var. rubrum. Agric. Dev. Equip. 2017, 8, 185. [Google Scholar]
- Xu, G. Test on the Cutting Rootage Factors of Loropetalum chinense. Chin. Landsc. Archit. 2002, 3, 97–98. [Google Scholar]
- Fei, F.; Wang, H.Y.; Tang, Q.R.; Yin, H.; He, S.H.; Zhao, Q.Y.; Tang, Q.R. An Experiment on the Impact of Different Humidity on Loropetalum chinese var. rubrum Leaves Color. J. Hunan Inst. Sci. Technol. Nat. Sci. 2006, 22, 61–65. [Google Scholar]
- Li, X.L.; Liu, J.H.; Chen, S.Y. Effects of Light Intensity on Leaf-color Expression of Loropetalum Chinense var. rubrum. Shaanxi For. Sci. Technol. 2013, 1–5. [Google Scholar]
- Lin, K.-H.; Huang, M.-Y.; Huang, W.-D.; Hsu, M.-H.; Yang, Z.-W.; Yang, C.-M. The effects of red, blue, and white light-emitting diodes on the growth, development, and edible quality of hydroponically grown lettuce (Lactuca sativa L. var. capitata). Sci. Hortic. 2013, 150, 86–91. [Google Scholar] [CrossRef]
- Cheng, R.F.; Rong, Z.Z.; Jun, W. Study on Photosynthesis of Greenhouse Cucumber under Supplemental Light. Shanxi J. Agric. Sci. 2004, 3, 17–18. [Google Scholar]
- Fu, C.M.; Huang, N.Z.; Zhao, Z.G.; Tang, F.L.; Huang, Z.M. Effects of different light quantities and illumination supplement on growth and photosynthetic rate of rice seedlings. Guihaia 2007, 27, 255–259. [Google Scholar]
- Ilić, Z.S.; Fallik, E. Light quality manipulation improves vegetable quality at harvest and postharvest: A review. Environ. Exp. Bot. 2017, 139, 79–90. [Google Scholar] [CrossRef]
- Zhang, Y.; Jiang, L.; Li, Y.; Chen, Q.; Ye, Y.; Zhang, Y.; Luo, Y.; Sun, B.; Wang, X.; Tang, H. Effect of Red and Blue Light on Anthocyanin Accumulation and Differential Gene Expression in Strawberry (Fragaria × ananassa). Molecules 2018, 23, 820. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.; Wang, L.; Wang, F.; Xi, Z. Effects of UVA disappearance and presence on the acylated anthocyanins formation in grape berries. Food Chem. Mol. Sci. 2022, 5, 100142. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Li, Y.; He, R.; Tan, J.; Liu, K.; Chen, Y.; Liu, H. Effect of Supplemental UV-A Intensity on Growth and Quality of Kale under Red and Blue Light. Int. J. Mol. Sci. 2022, 23, 6819. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Liu, X.; Yang, Q.; Lei, B.; Zheng, Y.; Bian, Z.; Wang, S.; Li, W.; Mao, P.; Xu, Y. UVA Enhanced Promotive Effects of Blue Light on the Antioxidant Capacity and Anthocyanin Biosynthesis of Pak Choi. Horticulturae 2022, 8, 850. [Google Scholar] [CrossRef]
- Wang, D.X.; Sun, H.J.; De, Y.J.; Shi, J.X. Change of leaf color of Liquidambar formosana seedlings under different light quality treatments. For. Res. 2019, 32, 158–164. [Google Scholar]
- De Keyser, E.; Dhooghe, E.; Christiaens, A.; Van Labeke, M.-C.; Van Huylenbroeck, J. LED light quality intensifies leaf pigmentation in ornamental pot plants. Sci. Hortic. 2019, 253, 270–275. [Google Scholar] [CrossRef]
- Li, W.; Mu, X.; Wu, X.; He, W.; Liu, Y.; Liu, Y.; Deng, J.; Nie, X. Dendrobium nobile Lindl. Polysaccharides protect fibroblasts against UVA-induced photoaging via JNK/c-Jun/MMPs pathway. J. Ethnopharmacol. 2022, 298, 115590. [Google Scholar] [CrossRef]
- Fujii, J.; Homma, T.; Osaki, T. Superoxide Radicals in the Execution of Cell Death. Antioxidants 2022, 11, 501. [Google Scholar] [CrossRef]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef]
- Gusti, A.M.T.; Qusti, S.Y.; Alshammari, E.M.; Toraih, E.A.; Fawzy, M.S. Antioxidants-Related Superoxide Dismutase (SOD), Catalase (CAT), Glutathione Peroxidase (GPX), Glutathione-S-Transferase (GST), and Nitric Oxide Synthase (NOS) Gene Variants Analysis in an Obese Population: A Preliminary Case-Control Study. Antioxidants 2021, 10, 595. [Google Scholar] [CrossRef]
- Jiang, L. Research on Leaf Color Change and Physiological Characteristics in Some Species of Quercus. Master’s Thesis, Shandong Agriculture University, Shandong, China, 2016. [Google Scholar]
- Möglich, A.; Yang, X.; Ayers, R.A.; Moffat, K. Structure and function of plant photoreceptors. Annu. Rev. Plant Biol. 2010, 61, 21–47. [Google Scholar] [CrossRef]
- Guo, J.; Wang, M.-H. Ultraviolet A-specific induction of anthocyanin biosynthesis and PAL expression in tomato (Solanum lycopersicum L.). Plant Growth Regul. 2010, 62, 1–8. [Google Scholar] [CrossRef]
- Hoecker, U. The activities of the E3 ubiquitin ligase COP1/SPA, a key repressor in light signaling. Curr. Opin. Plant Biol. 2017, 37, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Gangappa, S.N.; Botto, J.F. The BBX family of plant transcription factors. Trends Plant Sci. 2014, 19, 460–470. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Chu, L.; Zhang, Y.; Bian, Y.; Xiao, J.; Xu, D. HY5: A Pivotal Regulator of Light-Dependent Development in Higher Plants. Front. Plant Sci. 2022, 12, 800989. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.; Ren, L.; Lian, H.; Liu, Y.; Chen, H. Novel insight into the mechanism underlying light-controlled anthocyanin accumulation in eggplant (Solanum melongena L.). Plant Sci. 2016, 249, 46–58. [Google Scholar] [CrossRef] [PubMed]
- Gangappa, S.N.; Botto, J.F. The Multifaceted Roles of HY5 in Plant Growth and Development. Mol. Plant 2016, 9, 1353–1365. [Google Scholar] [CrossRef]
- Fang, H.; Dong, Y.; Yue, X.; Hu, J.; Jiang, S.; Xu, H.-f.; Wang, Y.; Su, M.-y.; Zhang, J.; Zhang, Z.; et al. The B-box zinc finger protein MdBBX20 integrates anthocyanin accumulation in response to ultraviolet radiation and low temperature. Plant Cell Environ. 2019, 42, 2090–2104. [Google Scholar] [CrossRef]
- Bai, S.; Tao, R.; Tang, Y.; Yin, L.; Ma, Y.; Ni, J.; Yan, X.; Yang, Q.; Wu, Z.; Zeng, Y.; et al. BBX16, a B-box protein, positively regulates light-induced anthocyanin accumulation by activating MYB10 in red pear. Plant Biotechnol. J. 2019, 17, 1985–1997. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, J.; Tian, S.; Hao, W.; Du, L. Two B-Box Proteins, MaBBX20 and MaBBX51, Coordinate Light-Induced Anthocyanin Biosynthesis in Grape Hyacinth. Int. J. Mol. Sci. 2022, 23, 5678. [Google Scholar] [CrossRef] [PubMed]
- Fu, Z.; Shang, H.; Jiang, H.; Gao, J.; Dong, X.; Wang, H.; Li, Y.; Wang, L.; Zhang, J.; Shu, Q.; et al. Systematic Identification of the Light-quality Responding Anthocyanin Synthesis-related Transcripts in Petunia Petals. Hortic. Plant J. 2020, 6, 428–438. [Google Scholar] [CrossRef]
- Nakai, A.; Tanaka, A.; Yoshihara, H.; Murai, K.; Watanabe, T.; Miyawaki, K. Blue LED light promotes indican accumulation and flowering in indigo plant, Polygonum tinctorium. Ind. Crops Prod. 2020, 155, 112774. [Google Scholar] [CrossRef]
- Kubra, G.; Khan, M.; Hussain, S.; Iqbal, T.; Muhammad, J.; Ali, H.; Gul, A.; Munir, F.; Amir, R. Molecular characterization of Leucoanthocyanidin reductase and Flavonol synthase gene in Arachis hypogaea. Saudi J. Biol. Sci. 2021, 28, 2301–2315. [Google Scholar] [CrossRef]
- Li, M.L.; Hong, Y.; Dai, S.Y.; Shen, R.Y.; Zhou, Y. Effects of Different Light Qualities on Anthocyanin Biosynthesis and Coloration in Chrysanthemum (Chrysanthemum × morifolium Ramat). In Advances in Omamental Horticulture of China; Changsha, China, 2016. [Google Scholar]
- Tohge, T.; Nishiyama, Y.; Hirai, M.; Yano, M.; Nakajima, J.-i.; Awazuhara, M.; Inoue, E.; Takahashi, H.; Goodenowe, D.; Kitayama, M.; et al. Functional genomics by integrated analysis of metabolome and transcriptome of Arabidopsis plants over-expressing an MYB transcription factor. Plant J. Cell Mol. Biol. 2005, 42, 218–235. [Google Scholar] [CrossRef]
- Albert, N.W.; Davies, K.M.; Schwinn, K.E. Gene regulation networks generate diverse pigmentation patterns in plants. Plant Signal. Behav. 2014, 9, e29526. [Google Scholar] [CrossRef]
- Mol, J.; Jenkins, G.; Schäfer, E.; Weiss, D.; Walbot, V. Signal perception, transduction, and gene expression involved in anthocyanin biosynthesis. Crit. Rev. Plant Sci. 1996, 15, 525–557. [Google Scholar] [CrossRef]
- Vimolmangkang, S.; Zheng, D.; Han, Y.; Khan, M.A.; Soria-Guerra, R.E.; Korban, S.S. Transcriptome analysis of the exocarp of apple fruit identifies light-induced genes involved in red color pigmentation. Gene 2014, 534, 78–87. [Google Scholar] [CrossRef]
- Chen, Z.; Yu, L.; Liu, W.; Zhang, J.; Wang, N.; Chen, X. Research progress of fruit color development in apple (Malus domestica Borkh). Plant Physiol. Biochem. 2021, 162, 267–279. [Google Scholar] [CrossRef]
- Song, B.; Xu, H.; Chen, L.; Fan, X.; Jing, Z.; Chen, S.; Xu, Z. Study of the Relationship between Leaf Color Formation and Anthocyanin Metabolism among Different Purple Pakchoi Lines. Molecules 2020, 25, 4809. [Google Scholar] [CrossRef]
- Liu, C.C.; Chi, C.; Jin, L.J.; Zhu, J.; Yu, J.Q.; Zhou, Y.H. The bZip transcription factor HY5 mediates CRY1a-induced anthocyanin biosynthesis in tomato. Plant Cell Environ. 2018, 41, 1762–1775. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Kaiser, E.; Zhang, Y.; Zou, J.; Bian, Z.; Yang, Q.; Li, T. UVA radiation promotes tomato growth through morphological adaptation leading to increased light interception. Environ. Exp. Bot. 2020, 176, 104073. [Google Scholar] [CrossRef]
- Kataoka, I.; Beppu, K. UV Irradiance Increases Development of Red Skin Color and Anthocyanins in “Hakuho” Peach. HortScience HortSci 2004, 39, 1234–1237. [Google Scholar] [CrossRef]
- Jiang, W.B.; Zhuang, M.; Han, H.Z.; Dai, M.S.; Hua, G.P. Progress on Color Emerging Mechanism and Photosynthetic Characteristics of Colored-leaf Plants. Acta Hortic. Sin. 2005, 32, 352–358. [Google Scholar]
- Zhao, X.; Zhang, Y.; Long, T.; Wang, S.; Yang, J. Regulation Mechanism of Plant Pigments Biosynthesis: Anthocyanins, Carotenoids, and Betalains. Metabolites 2022, 12, 871. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Cao, S.; Shi, L.; Chen, W.; Su, X.; Yang, Z. Blue light irradiation affects anthocyanin content and enzyme activities involved in postharvest strawberry fruit. J. Agric. Food Chem. 2014, 62, 4778–4783. [Google Scholar] [CrossRef]
- Shi, L.; Shi, L.; Cao, S.; Shao, J.; Chen, W.; Yang, Z.; Zheng, Y. Chinese bayberry fruit treated with blue light after harvest exhibit enhanced sugar production and expression of cryptochrome genes. Postharvest Biol. Technol. 2016, 111, 197–204. [Google Scholar] [CrossRef]
- Izzo, L.G.; Arena, C.; De Micco, V.; Capozzi, F.; Aronne, G. Light quality shapes morpho-functional traits and pigment content of green and red leaf cultivars of Atriplex hortensis. Sci. Hortic. 2019, 246, 942–950. [Google Scholar] [CrossRef]
- Chen, Q.R.; Cai, W.Q.; Zhang, X.; Zhang, D.M.; LI, W.D.; Xu, L.; Yu, X.Y.; Li, Y.L. The Comparative Studies on Phytochemicals of Leaf Coloration of Loropetalum chinense var. rubrum. Acta Hortic. Sin. 2021, 48, 1969–1982. [Google Scholar]
- Zhou, B.; Li, Y.; Xu, Z.; Yan, H.; Homma, S.; Kawabata, S. Ultraviolet A-specific induction of anthocyanin blosynthesis in the swollen hypocotyls of turnip (Brassica rapa). J. Exp. Bot. 2007, 58, 1771–1781. [Google Scholar] [CrossRef]
- Mao, P.; Duan, F.; Zheng, Y.; Yang, Q. Blue and UV-A light wavelengths positively affected accumulation profiles of healthy compounds in pak-choi. J. Sci. Food Agric. 2021, 101, 1676–1684. [Google Scholar] [CrossRef]
- Albert, N.W.; Lewis, D.H.; Zhang, H.; Schwinn, K.E.; Jameson, P.E.; Davies, K.M. Members of an R2R3-MYB transcription factor family in Petunia are developmentally and environmentally regulated to control complex floral and vegetative pigmentation patterning. Plant J. Cell Mol. Biol. 2011, 65, 771–784. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Koes, R.; Shang, H.; Fu, Z.; Wang, L.; Dong, X.; Zhang, J.; Passeri, V.; Li, Y.; Jiang, H.; et al. Identification and functional analysis of three new anthocyanin R2R3-MYB genes in Petunia. Plant Direct 2019, 3, e00114. [Google Scholar] [CrossRef]
- An, J.P.; Wang, X.F.; Espley, R.V.; Lin-Wang, K.; Bi, S.Q.; You, C.X.; Hao, Y.J. An Apple B-Box Protein MdBBX37 Modulates Anthocyanin Biosynthesis and Hypocotyl Elongation Synergistically with MdMYBs and MdHY5. Plant Cell Physiol. 2020, 61, 130–143. [Google Scholar] [CrossRef] [PubMed]
- Nakabayashi, R.; Yonekura-Sakakibara, K.; Urano, K.; Suzuki, M.; Yamada, Y.; Nishizawa, T.; Matsuda, F.; Kojima, M.; Sakakibara, H.; Shinozaki, K.; et al. Enhancement of oxidative and drought tolerance in Arabidopsis by overaccumulation of antioxidant flavonoids. Plant J. Cell Mol. Biol. 2014, 77, 367–379. [Google Scholar] [CrossRef]
- Tanaka, Y.; Brugliera, F.; Kalc, G.; Senior, M.; Dyson, B.; Nakamura, N.; Katsumoto, Y.; Chandler, S. Flower color modification by engineering of the flavonoid biosynthetic pathway: Practical perspectives. Biosci. Biotechnol. Biochem. 2010, 74, 1760–1769. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Cheng, F.; Zhong, Y.; Cheng, X.; Tao, X. The Quantitative Classification of Flower Color Phenotype in Paeonia rockii (Flare Tree Peony). Acta Hortic. Sin. 2022, 49, 86–99. [Google Scholar]
- Mudalige, R.G.; Kuehnle, A.R.; Amore, T.D. Pigment Distribution and Epidermal Cell Shape in Dendrobium Species and Hybrids. Hortscience 2003, 38, 573–577. [Google Scholar] [CrossRef]
- Yu, X.L.; Hu, Q.L.; Huang, Y. Study on Extracting Methods and Characteristics of Chlorophyll in Peony. Luoyang Norm. Univ. 2005, 113–115. [Google Scholar]
- Romero, I.; Teresa Sanchez-Ballesta, M.; Maldonado, R.; Isabel Escribano, M.; Merodio, C. Anthocyanin, antioxidant activity and stress-induced gene expression in high CO2-treated table grapes stored at low temperature. J. Plant Physiol. 2008, 165, 522–530. [Google Scholar] [CrossRef]
- Zhang, Y.L.; Fang, Z.Z.; Ye, X.F.; Pan, S.L. Identification of candidate genes involved in anthocyanin accumulation in the peel of jaboticaba (Myrciaria cauliflora) fruits by transcriptomic analysis. Gene 2018, 676, 202–213. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Chen, Q.; Zhang, X.; Lin, L.; Cai, M.; Cai, W.; Liu, Y.; Xiang, L.-S.; Sun, M.; Yu, X.; et al. Effects of low temperature on flowering and the expression of related genes in Loropetalum chinense var. rubrum. Front. Plant Sci. 2022, 13, 1000160. [Google Scholar] [CrossRef] [PubMed]


| Treatment Number | Treatment Time | Munsell Color System | Color Group According to CIELab | ||
|---|---|---|---|---|---|
| H | V | C | |||
| WL | 0 d | 2.4 GY | 4.90 | 2.00 | Olive green |
| 1 d | 0.05 Y | 3.90 | 2.00 | Olive brown | |
| 3 d | 0.0034 Y | 3.90 | 1.90 | Olive brown | |
| 5 d | 7.1 R | 3.10 | 1.50 | Red | |
| 7 d | 9.9 YR | 3.90 | 2.00 | Yellowish-brown | |
| BL + UL | 0 d | 1.2 GY | 4.90 | 2.10 | Olive green |
| 1 d | 8.9 Y | 4.70 | 2.60 | Olive | |
| 3 d | 8.9 Y | 4.70 | 2.60 | Olive | |
| 5 d | 4.4 YR | 3.10 | 0.94 | Brown | |
| 7 d | 9.9 YR | 3.90 | 1.90 | Yellowish-brown | |
| BL | 0 d | 2.5 GY | 4.80 | 1.80 | Olive green |
| 1 d | 0.14 Y | 3.90 | 2.00 | Olive brown | |
| 3 d | 5.1 YR | 3.40 | 2.00 | Brown | |
| 5 d | 6.5 R | 3.00 | 1.50 | Red | |
| 7 d | 0.53 YR | 3.50 | 1.80 | Reddish-brown | |
| UL | 0 d | 1.5 GY | 4.80 | 2.10 | Olive green |
| 1 d | 2 Y | 4.20 | 1.50 | Olive brown | |
| 3 d | 7 YR | 3.40 | 2.50 | Brown | |
| 5 d | 1.2 Y | 3.90 | 2.80 | Olive brown | |
| 7 d | 1.5 GY | 4.80 | 1.60 | Olive green | |
| Gene Type | Gene Name | Primer Sequence (5′-3′) | |
|---|---|---|---|
| Internal reference genes | β-actin 2 | F | CCACAAGGCTTATTGATAGAAT |
| R | CAATGGTTGAACCTGAATACT | ||
| CRY-like gene-1 | augustus 35127 | F | CTGGCGTCATCGATTCCATC |
| R | AGTGGGTCTCTTCAGCAACA | ||
| CRY-like gene-2 | augustus 55502 | F | GCTGGCATGAGAGAGTTGTG |
| R | TCTCCATGGAAGCTGCAGAA | ||
| HY5-like gene-1 | augustus 64086 | F | CAGCAAGCCCGAGAAAGAAA |
| R | ACCTGGACCTTGATCGTGTT | ||
| HY5-like gene-2 | augustus 18354 | F | TTCCTCCCTCACTGCTCAAG |
| R | AAACTCTGCCTCATCACCCA | ||
| BBX-like gene | augustus 08157 | F | TGCAGATTCATTGCCGTCAG |
| R | AACGTTTCTTGGGTGGCTTC | ||
| MYB-like gene | augustus 33908 | F | CTACGCTTCTGCTGACGATG |
| R | ATTGCAGGTTTCCGATGGTG | ||
| CHS-like gene-1 | augustus 35565 | F | AGTCGGTTCGGATCCACTAC |
| R | CCTTCGCTATCCGGGAGAAT | ||
| CHS-like gene-2 | augustus 57090 | F | GACAGTGATGAAGCTCGCAA |
| R | TTCTTCAGCACTCCTTCGGT | ||
| DFR-like gene-1 | augustus 47340 | F | GCCAACAATAGCTGGCATGT |
| R | AGCCGGTCATCTTTACGCTA | ||
| DFR-like gene-2 | augustus 36047 | F | GCCAACAATAGCTGGCATGT |
| R | AGCCGGTCATCTTTACGCTA | ||
| ANS-like gene | augustus 44839 | F | AGTTGGAGGGATGGAAGAGC |
| R | TTGGCCGTGATCCATTTGTC | ||
| UFGT-like gene | augustus 48540 | F | GACGTCGTTCATGCTCCAAA |
| R | CAACTCACCTTCCTCCCTGT | ||
| SOD-like gene-1 | augustus 35171 | F | GCCAAGGGAGATTCGTCAAC |
| R | ACCACCTCCTTCATGGACAG | ||
| SOD-like gene-2 | augustus 13933 | F | ATCCTGCTGGGAAAGAGCAT |
| R | CAACAACAGCCCTTCCAACA | ||
| POD-like gene-1 | augustus 46250 | F | TAGCCTCTCTTGCCACCAAA |
| R | GTGGGACACACGTCGTAAAG | ||
| POD-like gene-2 | augustus 11979 | F | GTGGCCCTGAATACAACGTC |
| R | TTTGGTGGCAAGAGAGGCTA | ||
| CAT-like gene-1 | augustus 68148 | F | GGCGTGAGAAGTGCGTTATT |
| R | GACACGTGGGTCGGTTAAAG | ||
| CAT-like gene-2 | augustus 12226 | F | ATGAGGAGGCTGCAAGGATT |
| R | AGGCTGCAAGGGAAAGAGAT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Liu, Y.; Ling, L.; Huo, W.; Li, Y.; Xu, L.; Xiang, L.; Yang, Y.; Xiong, X.; Zhang, D.; et al. Phenotypic, Physiological, and Molecular Response of Loropetalum chinense var. rubrum under Different Light Quality Treatments Based on Leaf Color Changes. Plants 2023, 12, 2169. https://doi.org/10.3390/plants12112169
Zhang Y, Liu Y, Ling L, Huo W, Li Y, Xu L, Xiang L, Yang Y, Xiong X, Zhang D, et al. Phenotypic, Physiological, and Molecular Response of Loropetalum chinense var. rubrum under Different Light Quality Treatments Based on Leaf Color Changes. Plants. 2023; 12(11):2169. https://doi.org/10.3390/plants12112169
Chicago/Turabian StyleZhang, Yifan, Yang Liu, Lin Ling, Wenwen Huo, Yang Li, Lu Xu, Lili Xiang, Yujie Yang, Xingyao Xiong, Donglin Zhang, and et al. 2023. "Phenotypic, Physiological, and Molecular Response of Loropetalum chinense var. rubrum under Different Light Quality Treatments Based on Leaf Color Changes" Plants 12, no. 11: 2169. https://doi.org/10.3390/plants12112169
APA StyleZhang, Y., Liu, Y., Ling, L., Huo, W., Li, Y., Xu, L., Xiang, L., Yang, Y., Xiong, X., Zhang, D., Yu, X., & Li, Y. (2023). Phenotypic, Physiological, and Molecular Response of Loropetalum chinense var. rubrum under Different Light Quality Treatments Based on Leaf Color Changes. Plants, 12(11), 2169. https://doi.org/10.3390/plants12112169










