Evidence That PbrSAUR72 Contributes to Iron Deficiency Tolerance in Pears by Facilitating Iron Absorption
Abstract
:1. Introduction
2. Results
2.1. Exogenous IAA Treatment Slightly Induces Re-Greening of Fe-Deficienty Chlorotic Pear Leaves
2.2. Identification of the Pear Fe Deficiency-Responsive Gene PbrSAUR72
2.3. Sequence Analysis, Subcellular Localization, Phylogenetic Analysis, and Expression Pattern of PbrSAUR72
2.4. Heterologous Expression of PbrSAUR72 Confers Strong Tolerance to Fe Deficiency of Both Solanum lycopersicum and A. thaliana
2.5. Expression of Genes Related to Fe Deficiency Adaptive Response Were Elevated in PbrSAUR72-Overexpressing Transgenic Plants under Fe Deficiency Stress
2.6. PbrSAUR72 Overexpression Enhanced Fe Accumulation under Fe-Limited Conditions
2.7. ROS and Hydrogen Peroxide (H2O2) Levels Were Decreased in PbrSAUR72-Overexpressing Plants under Fe-Deficient Stress
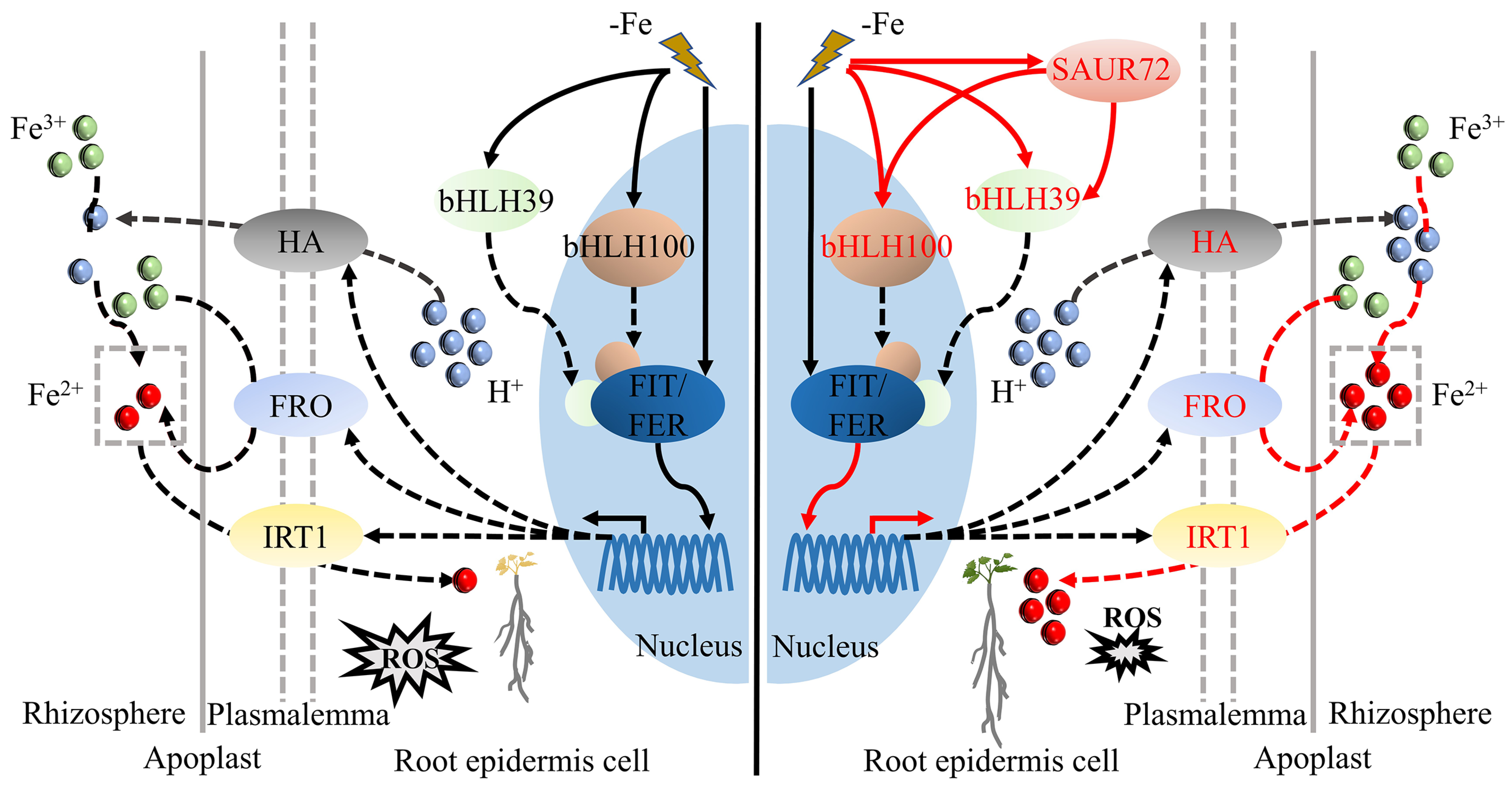
3. Discussion
4. Materials and Methods
4.1. Plant Materials, Growth Conditions, and Treatments
4.2. Determination of IAA Content, Enzyme Activities, and Fe Concentration
4.3. FCR and Rhizosphere pH Location Assays
4.4. Chlorophyll Content, Root Length, and ROS Accumulation Assays
4.5. Phylogenetic Analysis and Sequence Alignment
4.6. Plasmid Construction and Pear Leaf Transient Transformation
4.7. Plant Transformation and Subcellular Localization
4.8. RNA Extraction and qRT-PCR Analysis
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Guerinot, M.-L.; Yi, Y. Iron: Nutritious, noxious, and not readily available. Plant Physiol. 1994, 104, 815–820. [Google Scholar] [CrossRef] [PubMed]
- Briat, J.-F.; Dubos, C.; Gaymard, F. Iron nutrition, biomass production, and plant product quality. Trends Plant Sci. 2015, 20, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Q.; Zhong, Y.; Lu, S. Assessment of heavy metal pollution and human health risk in urban soils of steel industrial city (Anshan), Liaoning, Northeast China. Ecotox. Environ. Safe 2015, 120, 377–385. [Google Scholar]
- Curie, C.; Mari, S. New routes for plant iron mining. New Phytol. 2017, 214, 521–525. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Fernandez, A.; Melgar, J.-C.; Abadia, J.; Abadia, A. Effects of moderate and severe iron deficiency chlorosis on fruit yield, appearance and composition in pear (Pyrus communis L.) and peach (Prunus persica (L.) Batsch). Environ. Exp. Bot. 2011, 71, 280–286. [Google Scholar] [CrossRef]
- Brumbarova, T.; Bauer, P.; Ivanov, R. Molecular mechanisms governing Arabidopsis iron uptake. Trends Plant Sci. 2015, 20, 124–133. [Google Scholar] [CrossRef]
- Merry, R.; Dobbels, A.-A.; Sadol, W.; Naeve, S.; Stupar, R.-M.; Lorenz, A.-J. Iron deficiency in soybean. Crop Sci. 2022, 62, 136–152. [Google Scholar] [CrossRef]
- Li, Q.; Chen, L.; Yang, A. The molecular mechanisms underlying iron deficiency responses in rice. Int. J. Mol. Sci. 2020, 21, 43. [Google Scholar] [CrossRef]
- Valentinuzzi, F.; Venuti, S.; Pii, Y.; Marroni, F.; Cesco, S.; Hartmann, F.; Mimmo, T.; Morgante, M.; Pinton, R.; Tomasi, N.; et al. Common and specific responses to iron and phosphorus deficiencies in roots of apple tree (Malus × domestica). Plant Mol. Biol. 2019, 101, 129–148. [Google Scholar] [CrossRef]
- Zamboni, A.; Zanin, L.; Tomasi, N.; Pezzotti, M.; Pinton, R.; Varanini, Z.; Cesco, S. Genome-wide microarray analysis of tomato roots showed defined responses to iron deficiency. BMC Genom. 2012, 13, 101. [Google Scholar] [CrossRef]
- Briat, J.-F.; Fobis-Loisy, I.; Grignon, N.; Lobreaux, S.; Pascal, N.; Savino, G.; Thoiron, S.; Wiren, N.; Wuytswinkel, O. Cellular and molecular aspects of iron metabolism in plants. Biol. Cell 1995, 84, 69–81. [Google Scholar] [CrossRef]
- James, M.-C.; Janneke, B.; Jorge, R.-C. Iron homeostasis in plants—A brief overview. Metallomics 2017, 9, 813–823. [Google Scholar]
- Ivanov, R.; Brumbarova, T.; Bauer, P. Fitting into the harsh reality: Regulation of iron-deficiency responses in dicotyledonous plants. Mol. Plant 2012, 5, 27–42. [Google Scholar] [CrossRef] [PubMed]
- Robinson, N.-J.; Procter, C.-M.; Connolly, E.-L.; Guerinot, M.-L. A ferric-chelate reductase for iron uptake from soils. Nature 1999, 397, 694–697. [Google Scholar] [CrossRef]
- Vert, G.; Grotz, N.; Dedaldechamp, F.; Gaymard, F.; Guerinot, M.-L.; Briat, J.-F.; Curie, C. IRT1, an Arabidopsis transporter essential for iron uptake from the soil and for plant growth. Plant Cell 2002, 14, 1223–1233. [Google Scholar] [CrossRef] [PubMed]
- Tong, H.; Madison, I.; Long, T.-A.; Williams, C.-M. Computational solutions for modeling and controlling plant response to abiotic stresses: A review with focus on iron deficiency. Curr. Opin. Plant Biol. 2020, 57, 8–15. [Google Scholar] [CrossRef]
- Tissot, N.; Robe, K.; Gao, F.; Grant-Grant, S.; Boucherez, J.; Bellegarde, F.; Maghiaoui, A.; Marcelin, R.; Izquierdo, E.; Benhamed, M.; et al. Transcriptional integration of the responses to iron availability in Arabidopsis by the bHLH factor ILR3. New Phytol. 2019, 223, 1443–1446. [Google Scholar] [CrossRef]
- Gao, F.; Robe, K.; Gaymard, F.; Izquierdo, E.; Dubos, C. The transcriptional control of iron homeostasis in plants: A tale of bHLH transcription factors. Front. Plant Sci. 2019, 10, 6. [Google Scholar] [CrossRef]
- Riaz, N.; Guerinot, M.-L. All together now: Regulation of the iron deficiency. J. Exp. Bot. 2021, 72, 2045–2055. [Google Scholar] [CrossRef]
- Qian, Y.-C.; Zhang, T.-Y.; Yu, Y.; Gou, L.-P.; Yang, J.-T.; Xu, J.; Pi, E.-X. Regulatory mechanisms of bHLH transcription factors in plant adaptive responses to various abiotic stresses. Front. Plant Sci. 2021, 12, 677611. [Google Scholar] [CrossRef]
- Wu, T.; Zhang, H.-T.; Wang, Y.; Jia, W.-S.; Xu, X.F.; Zhang, X.-Z.; Han, Z.-H. Induction of root Fe(III) reductase activity and proton extrusion by iron deficiency is mediated by auxin-based systemic signaling in Malus xiaojinensis. J. Exp. Bot. 2012, 63, 859–870. [Google Scholar] [CrossRef]
- Bacaicoa, E.; Mora, V.; Zamarreno, A.-M.; Fuentes, M.; Casanova, E.; Garcia-Mina, J.-M. Auxin: A major player in the shoot-to-root regulation of root Fe-stress physiological responses to Fe deficiency in cucumber plants. Plant Physiol. Biochem. 2011, 49, 545–556. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.-X.; Du, N.-X.; Li, Y.-N.; Zheng, S.-X.; Shen, S.-S.; Piao, F.-Z. Gamma-aminobutyric acid enhances tolerance to iron deficiency by stimulating auxin signaling in cucumber (Cucumis sativus L.). Ecotox. Environ. Safe 2020, 192, 110285. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.-W.; Feng, F.; Liu, J.; Zhao, Q.-Z. The interaction between auxin and nitric oxide regulates root growth in response to iron deficiency in rice. Front. Plant Sci. 2018, 8, 2169. [Google Scholar] [CrossRef] [PubMed]
- Hou, K.; Wu, W.; Gan, S.-S. SAUR36, a small auxin up gene, is involved in the promotion of leaf senescence in Arabidopsis. Plant Physiol. 2013, 161, 1002–1009. [Google Scholar] [CrossRef] [PubMed]
- Kant, S.; Bi, Y.-M.; Zhu, T.; Rothstein, S.-J. SAUR39, a small auxin up RNA gene, acts as a negative regulator of auxin synthesis and transport in rice. Plant Physiol. 2009, 151, 691–701. [Google Scholar] [CrossRef] [PubMed]
- Bemer, M.; Van, M.-H.; Muino, J.-M.; Ferrandiz, C.; Kaufmann, K.; Angenent, G.-C. FRUITFULL controls SAUR10 expression and regulates Arabidopsis growth and architecture. J. Exp. Bot. 2017, 68, 3391–3403. [Google Scholar] [CrossRef]
- Wen, W.-Z.; Mei, Y.-Y.; Zhou, J.; Cui, Y.-J.; Wang, D.; Wang, N.-N. SAUR49 can positively regulate leaf senescence by suppressing SSPP in Arabidopsis. Plant Cell Physiol. 2020, 61, 644–658. [Google Scholar] [CrossRef]
- Wang, Z.; Yang, L.; Liu, Z.; Lu, M.; Wang, M.; Sun, Q.; Lan, Y.-H.; Shi, T.-L.; Wu, D.-X.; Hua, J. Natural variations of growth thermo-responsiveness determined by SAUR26/27/28 proteins in Arabidopsis thaliana. New Phytol. 2019, 224, 291–305. [Google Scholar] [CrossRef]
- Qiu, T.; Qi, M.; Ding, X.; Zheng, Y.; Zhou, T.; Chen, Y.; Han, N.; Zhu, M.-Y.; Bian, H.-W.; Wang, J.-H. The SAUR41 subfamily of small auxin up RNA genes is abscisic acid-inducible to modulate cell expansion and salt tolerance in Arabidopsis thaliana seedlings. Ann. Bot. 2020, 125, 805–819. [Google Scholar] [CrossRef]
- Jia, B.; Guo, G.-L.; Yu, T.; Dong, W.-Y.; Zhang, S.-Q.; Cheng, M.; Liu, L. Analysis of endogenous IAA content and signaling genes expression in retrieved leaves of ‘Dangshansuli’ Pear (Pyrus bretschneideri Rehd.). Acta Bot. Boreali-Occident. Sin. 2021, 41, 595–605. (In Chinese) [Google Scholar]
- Mittler, R.; Suzuki, N.; Miller, G.; Tognetti, V.-B.; Vandepoele, K.; Gollery, M.; Shulaev, V.; Breusegem, F.-V. ROS signaling: The new wave. Trends Plant Sci. 2011, 16, 300–309. [Google Scholar] [CrossRef]
- Mittler, R.; Vanderauwera, S.; Gollery, M.; Breusegem, F.-V. Reactive oxygen gene network of plants. Trends Plant Sci. 2004, 9, 490–498. [Google Scholar] [CrossRef] [PubMed]
- Verbon, E.-H.; Trapet, P.-L.; Stringlis, I.-A.; Kruijs, K.; Bakker, P.-A.-H.-M.; Pieterse, C.-M.-J. Iron and immunity. Annu. Rev. Phytopathol. 2017, 55, 355–375. [Google Scholar] [CrossRef]
- Chen, W.-W.; Yang, J.-L.; Qin, C.; Jin, C.-W.; Mo, J.-H.; Ye, T.; Zheng, S.-J. Nitric oxide acts downstream of auxin to trigger root ferric-chelate reductase activity in response to iron deficiency in Arabidopsis. Plant Physiol. 2010, 154, 810–819. [Google Scholar] [CrossRef] [PubMed]
- Romera, F.-J.; Garcia, M.-J.; Alcantara, E.; Perez-Vicente, R. Latest findings about the interplay of auxin, ethylene and nitric oxide in the regulation of Fe deficiency responses by strategy I plants. Plant Signal. Behav. 2011, 6, 167–170. [Google Scholar] [CrossRef]
- Stortenbeker, N.; Bemer, M. The SAUR gene family: The plant’s toolbox for adaptation of growth and development. J. Exp. Bot. 2019, 70, 17–27. [Google Scholar] [CrossRef]
- Guo, Y.; Xu, C.-N.; Sun, X.-J.; Hu, Z.; Fan, S.-H.; Jiang, Q.-Y. TaSAUR78 enhances multiple abiotic stress tolerance by regulating the interacting gene TaVDAC1. J. Integr. Agric. 2019, 18, 2682–2690. [Google Scholar] [CrossRef]
- He, Y.-J.; Liu, Y.; Li, M.-Z.; Lamin-samu, A.-T.; Yang, D.-D.; Yu, X.-L.; Izhar, M.; Jan, I.; Ali, M.; Lu, G. The Arabidopsis small auxin up RNA32 protein regulates ABA-mediated responses to drought stress. Front. Plant Sci. 2021, 12, 625493. [Google Scholar] [CrossRef]
- Zhu, X.-F.; Wu, Q.; Meng, Y.-T.; Tao, Y.; Shen, R.-F. AtHAP5A regulates iron translocation in iron-deficient Arabidopsis thaliana. J. Integr. Plant Biol. 2020, 62, 1910–1924. [Google Scholar] [CrossRef]
- Li, D.-Y.; Sun, Q.-R.; Zhang, G.-F.; Zhai, L.-M.; Li, K.-T.; Feng, Y. MxMPK6-2-bHLH104 interaction is involved in reactive oxygen species signaling in response to iron deficiency in apple rootstock. J. Exp. Bot. 2021, 72, 1919–1932. [Google Scholar] [CrossRef]
- Zhu, H.-H.; Wang, J.-Y.; Jiang, D.; Hong, Y.-G.; Xu, J.-M.; Zheng, S.J.; Yang, J.-L.; Chen, W.-W. The miR157-SPL-CNR module acts upstream of bHLH101 to negatively regulate iron deficiency responses in tomato. J. Integr. Plant Biol. 2022, 64, 1059–1075. [Google Scholar] [CrossRef] [PubMed]
- Bernale, M.; Monsalve, L.; Ayala-Raso, A.; Valdenegro, M.; Martinez, J.-P.; Travisany, D.; Defilippi, B.; Gonzalez-Aguero, M.; Cherian, S.; Fuentes, L. Expression of two indole-3-acetic acid (IAA)-amido synthetase (GH3) genes during fruit development of raspberry (Rubus idaeus Heritage). Sci. Hortic. 2019, 246, 168–175. [Google Scholar] [CrossRef]
- Yue, P.-T.; Wang, Y.-N.; Bu, H.-N.; Li, X.-Y.; Yuan, H.; Wang, A.-D. Ethylene promotes IAA reduction through PuERFs-activated PuGH3.1 during fruit ripening in pear (Pyrus ussuriensis). Postharvest Biol. Technol. 2019, 157, 110955. [Google Scholar] [CrossRef]
- Ling, H.-Q.; Bauer, P.; Bereczky, Z.; Keller, B.; Ganal, M. The tomato fer gene encoding a bHLH protein controls iron-uptake responses in roots. Proc. Natl. Acad. Sci. USA 2002, 99, 13938–13943. [Google Scholar] [CrossRef] [PubMed]
- Colangelo, E.-P.; Guerinot, M.-L. The essential basic helix-loop-helix protein FIT1 is required for the iron deficiency response. Plant Cell 2004, 16, 3400–3412. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.-X.; Zhang, J.; Wang, D.-W.; Ling, H.-Q. AtbHLH29 of Arabidopsis thaliana is a functional ortholog of tomato FER involved in controlling iron acquisition in strategy I plants. Cell Res. 2005, 15, 613–621. [Google Scholar] [CrossRef]
- Wu, H.; Ling, H.-Q. FIT-binding proteins and their functions in the regulation of Fe homeostasis. Front. Plant Sci. 2019, 10, 844. [Google Scholar] [CrossRef]
- Cai, Y.-R.; Yang, Y.-J.; Ping, H.-Q.; Lu, C.-K.; Lei, R.-H.; Li, Y.; Liang, G. Why FIT and bHLH Ib interdependently regulate Fe-uptake. Mol. Biol. 2022, 2, 480172. [Google Scholar]
- Yuan, Y.; Wu, H.; Wang, N.; Li, J.; Zhao, W.; Du, J.; Wang, D.-W.; Ling, H.-Q. FIT interacts with AtbHLH38 and AtbHLH39 in regulating iron uptake gene expression for iron homeostasis in Arabidopsis. Cell Res. 2008, 18, 385–397. [Google Scholar] [CrossRef]
- Wang, N.; Cui, Y.; Liu, Y.; Fan, H.-J.; Du, J.; Huang, Z.-J.; Yuan, Y.-X.; Wu, H.-L.; Ling, H.-Q. Requirement and functional redundancy of Ib subgroup bHLH proteins for iron deficiency responses and uptake in Arabidopsis thaliana. Mol. Plant 2013, 6, 503–513. [Google Scholar] [CrossRef] [PubMed]
- Palmgren, M.-G. Plant plasma membrane H+-ATPases: Powerhouses for nutrient uptake. Annu. Rev. Plant Physiol. Plant Mol. Biol. 2001, 52, 817–845. [Google Scholar] [CrossRef] [PubMed]
- Henriques, R.; Jasik, J.; Klein, M.; Martinoia, E.; Feller, U.; Schell, J.; Pais, M.-S.; Koncz, K. Knock-out of Arabidopsis metal transporter gene IRT1 results in iron deficiency accompanied by cell differentiation defects. Plant Mol. Biol. 2002, 50, 587–597. [Google Scholar] [CrossRef] [PubMed]
- Bauer, P.; Ling, H.-Q.; Guerinot, M.-L. FIT, the FER-like iron deficiency induced transcription factor in Arabidopsis. Plant Physiol. Biochem. 2007, 45, 260–261. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Chen, C.-L.; Cui, M.; Zhou, W.-J.; Wu, H.-L.; Ling, H.-Q. Four IVa bHLH transcription factors are novel interactors of FIT and mediate JA inhibition of iron uptake in Arabidopsis. Mol. Plant 2018, 11, 1166–1183. [Google Scholar] [CrossRef]
- Mittler, R. ROS are good. Trends Plant Sci. 2017, 22, 11–19. [Google Scholar] [CrossRef]
- Choudhury, F.-K.; Rivero, R.-M.; Blumwald, E.; Mittler, R. Reactive oxygen species, abiotic stress and stress combination. Plant J. 2017, 90, 856–867. [Google Scholar] [CrossRef]
- Marino, D.; Dunand, C.; Puppo, A.; Pauly, N. A burst of plant NADPH oxidases. Trends Plant Sci. 2012, 17, 9–15. [Google Scholar] [CrossRef]
- Mark, C.-V.-D.; Ivanov, R.; Eutebach, M.; Maurino, V.-G.; Bauer, P.; Brumbarova, T. Reactive oxygen species coordinate the transcriptional responses to iron availability in Arabidopsis. J. Exp. Bot. 2021, 72, 2181–2195. [Google Scholar] [CrossRef]
- Song, H.; Chen, F.; Wu, X.; Hu, M.; Geng, Q.-L.; Ye, M.; Zhang, C.; Jiang, L.; Cao, S.-Q. MNB1 gene is involved in regulating the iron-deficiency stress response in Arabidopsis thaliana. BMC Plant Biol. 2022, 22, 151. [Google Scholar] [CrossRef]
- Pence, V.-C.; Caruso, J.-L. Elisa determination of IAA using antibodies against ring-linked IAA. Phytochemistry 1987, 26, 1251–1255. [Google Scholar] [CrossRef]
- Bower, P.-J.; Brown, H.-M.; Purves, W.-K. Cucumber seedling indoleacetaldehyde oxidase. Plant Physiol. 1978, 61, 107–110. [Google Scholar] [CrossRef]
- Koga, J.; Adachi, T.; Hidaka, H. Purification and characterization of indolepyruvate decarboxylase. A novel enzyme for indole–3–acetic acid biosynthesis in Enterobacter cloacae. J. Biol. Chem. 1992, 267, 15823–15828. [Google Scholar] [CrossRef] [PubMed]
- Mao, F.-C.; Zhang, F.-Y.; Zhao, X.-G.; Zhang, K.-L. Relativity of active iron contents and chlorosis in the Kiwis leaf. Acta Agric. Boreali-Occident. Sin. 2002, 11, 54–56. (In Chinese) [Google Scholar]
- Chen, W.-W.; Jin, J.F.; Lou, H.-Q.; Liu, L.; Kochian, L.-V.; Yang, J.-L. LeSPL–CNR negatively regulates Cd acquisition through repressing nitrate reductase-mediated nitric oxide production in tomato. Planta 2018, 248, 893–907. [Google Scholar] [CrossRef]
- Aksoy, E.; Koiwa, H. Determination of ferric chelate reductase activity in the Arabidopsis thaliana root. Bio-Protocol 2013, 3, 15. [Google Scholar] [CrossRef]
- Schmidt, W. Iron solutions: Acquisition strategies and signaling pathways in plants. Trends Plant Sci. 2003, 8, 188–193. [Google Scholar] [CrossRef]
- Aono, M.; Kubo, A.; Saji, H.; Tanaka, K.; Kondo, N. Enhanced tolerance to photooxidative stress of transgenic Nicotiana tabacum with high chloroplastic glutathione reductase activity. Plant Cell Physiol. 1993, 34, 129–135. [Google Scholar]
- Kumar, D.; Yusuf, M.-A.; Singh, P.; Sardar, M.; Sari, N.-B. Histochemical detection of superoxide and H2O2 accumulation in Brassica juncea seedlings. Bio-Protocol 2014, 4, 8. [Google Scholar] [CrossRef]
- Lin, C.-C.; Kao, C.-H. Cell wall peroxidase activity, hydrogen peroxidase level and NaCl-inhibited root growth of rice seedling. Plant Soil 2001, 230, 135–143. [Google Scholar] [CrossRef]
- Bai, S.; Tao, R.; Tang, Y.; Yin, L.; Ma, Y.-J.; Ni, J.-B.; Yan, X.-H.; Yang, Q.-S.; Wu, Z.-Y.; Zeng, Y.-L.; et al. BBX16, a B-box protein, positively regulates light-induced anthocyanin accumulation by activating MYB10 in red pear. Plant Biotechol. J. 2019, 17, 1985–1997. [Google Scholar] [CrossRef]
- Fillatti, J.-J.; Kiser, J.; Rose, R.; Comai, L. Efficient transfer of a glyphosate tolerance gene into tomato using a binary agrobacterium tumefaciens vector. Nat. Biotechol. 1987, 5, 726–730. [Google Scholar] [CrossRef]
- Clough, S.-J.; Bent, A.-F. Floral dip: A simplified method for agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 1998, 16, 735–743. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.-J.; Schmittgen, T.-D. Analysis of relative gene expression data using real-time quantitative PCR. Methods 2002, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]








Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, G.; Yu, T.; Zhang, H.; Chen, M.; Dong, W.; Zhang, S.; Tang, X.; Liu, L.; Heng, W.; Zhu, L.; et al. Evidence That PbrSAUR72 Contributes to Iron Deficiency Tolerance in Pears by Facilitating Iron Absorption. Plants 2023, 12, 2173. https://doi.org/10.3390/plants12112173
Guo G, Yu T, Zhang H, Chen M, Dong W, Zhang S, Tang X, Liu L, Heng W, Zhu L, et al. Evidence That PbrSAUR72 Contributes to Iron Deficiency Tolerance in Pears by Facilitating Iron Absorption. Plants. 2023; 12(11):2173. https://doi.org/10.3390/plants12112173
Chicago/Turabian StyleGuo, Guoling, Tao Yu, Haiyan Zhang, Meng Chen, Weiyu Dong, Shuqin Zhang, Xiaomei Tang, Lun Liu, Wei Heng, Liwu Zhu, and et al. 2023. "Evidence That PbrSAUR72 Contributes to Iron Deficiency Tolerance in Pears by Facilitating Iron Absorption" Plants 12, no. 11: 2173. https://doi.org/10.3390/plants12112173
APA StyleGuo, G., Yu, T., Zhang, H., Chen, M., Dong, W., Zhang, S., Tang, X., Liu, L., Heng, W., Zhu, L., & Jia, B. (2023). Evidence That PbrSAUR72 Contributes to Iron Deficiency Tolerance in Pears by Facilitating Iron Absorption. Plants, 12(11), 2173. https://doi.org/10.3390/plants12112173





