Efficient Plant Regeneration System from Leaf Explant Cultures of Daphne genkwa via Somatic Embryogenesis
Abstract
:1. Introduction
2. Results
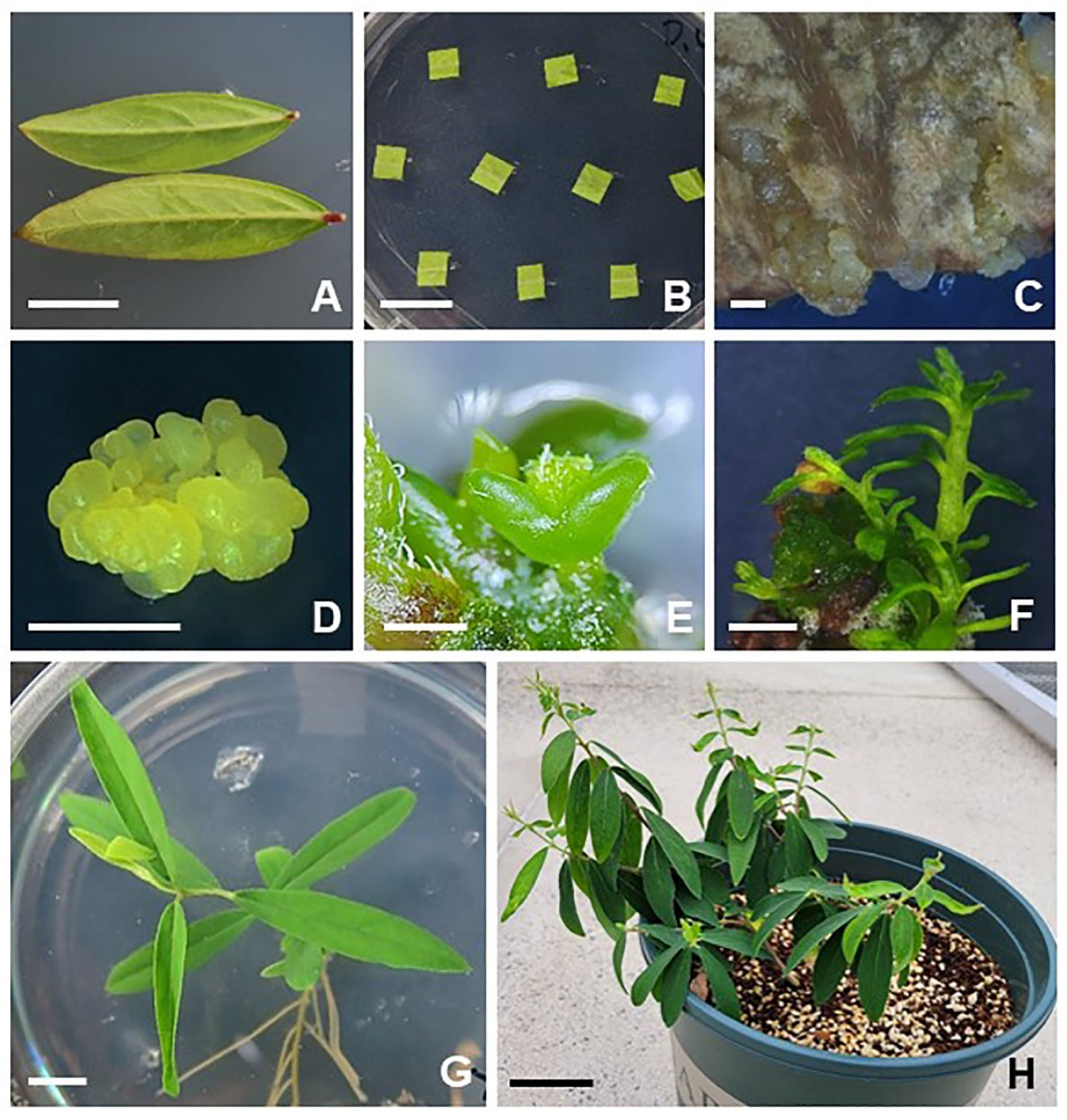
2.1. Plant Regeneration from Leaf-Derived Embryogenic Structures of D. genkwa
2.2. 2,4-D Promotes Embryogenic Structure Formation from Leaf Explants of D. genkwa
2.3. Both IBA and NAA Promote Embryogenic Structure Formation from Leaf Explants of D. genkwa
2.4. BA Promotes Somatic Embryo Development from Embryogenic Callus and of Embryogenic Structures of D. genkwa
2.5. Conversion of Somatic Embryos into Plantlets
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Surface Sterilization of Leaf Explants
4.2. Effect of Auxins (2,4-D, IBA, NAA) and Type of Culture Media on Embryogenic Structure Formation from Leaf Explants of D. genkwa
4.3. Effect of Cytokinin on Development of Somatic Embryos and Shoot Differentiation from Embryogenic Callus and Embryonic Structures of D. genkwa
4.4. Rooting and Acclimatization of Regenerated Plantlets
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Halda, J.J. Some taxonomic problems in the genus Daphne L. Acta Mus. Richnoviensis Sect. Nat. 1998, 5, 133–160. [Google Scholar]
- Moshiashvili, G.; Tabatadze, N.; Mshvildadze, V. The genus Daphne: A review of its traditional uses, phytochemistry and pharmacology. Fitoterapia 2020, 143, 104540. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.-Y.; Park, B.-Y.; Kwon, O.-K.; Yuk, J.-E.; Oh, S.-R.; Kim, H.-S.; Lee, H.-K.; Ahn, K.-S. Anti-inflammatory activity of (−)-aptosimon isolated from Daphne genkwa in RAW264. 7 cells. Int. Immunopharmacol. 2009, 9, 878–885. [Google Scholar] [CrossRef]
- Li, S.; Chou, G.; Hseu, Y.; Yang, H.; Kwan, H.; Yu, Z. Isolation of anticancer constituents from flos genkwa (Daphne genkwa Sieb. et Zucc.) through bioassay-guided procedures. Chem. Cent. J. 2013, 7, 159. [Google Scholar] [CrossRef] [PubMed]
- Park, B.-Y.; Min, B.-S.; Oh, S.-R.; Kim, J.-H.; Bae, K.-H.; Lee, H.-K. Isolation of flavonoids, a biscoumarin and an amide from the flower buds of Daphne genkwa and the evaluation of their anti-complement activity. Phytother. Res. 2006, 20, 610–613. [Google Scholar] [CrossRef]
- Zhou, D.-C.; Zheng, G.; Jia, L.-Y.; He, X.; Zhang, C.-F.; Wang, C.-Z.; Yuan, C.-S. Comprehensive evaluation on anti-inflammatory and anti-angiogenic activities in vitro of fourteen flavonoids from Daphne Genkwa based on the combination of efficacy coefficient method and principal component analysis. J. Ethnopharmacol. 2021, 268, 113683. [Google Scholar] [CrossRef]
- Brickell, C.; White, R. A quartet of new Daphnes. New Plantsman 2000, 7, 6–18. [Google Scholar]
- Noshad, D.; Miresmaili, S.; Riseman, A.; Ekramoddoullah, A. In Vitro propagation of seven Daphne L. species. Plant Cell Tissue Organ Cult. 2009, 96, 201–209. [Google Scholar] [CrossRef]
- Marks, T.R.; Simpson, S.E. Interaction of explant type and indole-3-butyric acid during rooting in vitro in a range of difficult and easy-to-root woody plants. Plant Cell Tissue Organ Cult. 2000, 62, 65–74. [Google Scholar] [CrossRef]
- Noshad, D.; Riseman, A.; Punja, Z. Evaluation of Daphne germplasm for resistance to Daphne sudden death syndrome caused by the soil-borne pathogen Thielaviopsis basicola. HortScience 2007, 42, 1639–1643. [Google Scholar] [CrossRef]
- Moran, J. Daphne viral diseases. Agric. Notes 1995, 175, 1–3. [Google Scholar]
- Moraes, R.M.; Cerdeira, A.L.; Lourenço, M.V. Using micropropagation to develop medicinal plants into crops. Molecules 2021, 26, 1752. [Google Scholar] [CrossRef]
- Hanus-Fajerska, E.; Wiszniewska, A.; Czaicki, P. Effectiveness of Daphne L. (Thymelaeaceae) in vitro propagation, rooting of microshoots and acclimatization of plants. Acta Agrobot. 2012, 65, 21–28. [Google Scholar] [CrossRef]
- Wiszniewska, A.; Hanus-Fajerska, E.; Grabski, K.; Tukaj, Z. Promoting effects of organic medium supplements on the micropropagation of promising ornamental Daphne species (Thymelaeaceae). In Vitro Cell. Dev. Biol.-Plant 2013, 49, 51–59. [Google Scholar] [CrossRef]
- Nowakowska, K.; Pacholczak, A.; Tepper, W. The effect of selected growth regulators and culture media on regeneration of Daphne mezereum L. ‘Alba’. Rend. Lincei Sci. Fis. Nat. 2019, 30, 197–205. [Google Scholar] [CrossRef]
- Nowakowska, K.; Pacholczak, A. Comparison of the effect of meta-topolin and benzyladenine during Daphne mezereum L. micropropagation. Agronomy 2020, 10, 1994. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol. Plant 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Lloyd, G.; McCown, B. Commercially-feasible micropropagation of mountain laurel, Kalmia latifolia, by use of shoot-tip culture. Comb. Proc. Int. Plant Propag. Soc. 1980, 30, 421–427. [Google Scholar]
- Giri, C.; Shyamkumar, B.; Anjaneyulu, C. Progress in tissue culture, genetic transformation and applications of biotechnology to trees: An overview. Trees 2004, 18, 115–135. [Google Scholar] [CrossRef]
- Isah, T. Induction of somatic embryogenesis in woody plants. Acta Physiol. Plant 2016, 38, 118. [Google Scholar] [CrossRef]
- Guan, Y.; Li, S.-G.; Fan, X.-F.; Su, Z.-H. Application of somatic embryogenesis in woody plants. Front. Plant Sci. 2016, 7, 938. [Google Scholar] [CrossRef] [PubMed]
- Gaj, M.D. Factors influencing somatic embryogenesis induction and plant regeneration with particular reference to Arabidopsis thaliana (L.) Heynh. Plant Growth Regul. 2004, 43, 27–47. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, A.; Qin, M.; Qin, X.; Yang, S.; Su, S.; Sun, Y.; Zhang, L. Direct and indirect somatic embryogenesis induction in Camellia oleifera Abel. Front. Plant Sci. 2021, 12, 644389. [Google Scholar] [CrossRef] [PubMed]
- Zou, S.; Yao, X.; Zhong, C.; Li, D.; Wang, Z.; Huang, H. Recurrent somatic embryogenesis and development of somatic embryos in Akebia trifoliata (Thunb.) Koidz (Lardizabalaceae). Plant Cell Tissue Organ Cult. 2019, 139, 493–504. [Google Scholar] [CrossRef]
- Kuo, H.-L.; Chen, J.-T.; Chang, W.-C. Efficient plant regeneration through direct somatic embryogenesis from leaf explants of Phalaenopsis ‘Little Steve’. In Vitro Cell. Dev. Biol.-Plant 2005, 41, 453–456. [Google Scholar] [CrossRef]
- Zhao, J.; Cui, J.; Liu, J.; Liao, F.; Henny, R.J.; Chen, J. Direct somatic embryogenesis from leaf and petiole explants of Spathiphyllum ‘Supreme’ and analysis of regenerants using flow cytometry. Plant Cell Tissue Organ Cult. 2012, 110, 239–249. [Google Scholar] [CrossRef]
- Ramakrishna, D.; Shasthree, T. High efficient somatic embryogenesis development from leaf cultures of Citrullus colocynthis (L.) Schrad for generating true type clones. Physiol. Mol. Biol. Plants 2016, 22, 279–285. [Google Scholar] [CrossRef]
- Venkataiah, P.; Bhanuprakash, P.; Kalyan, S.S.; Subhash, K. Somatic embryogenesis and plant regeneration of Capsicum baccatum L. J. Genet. Eng. Biotechnol. 2016, 14, 55–60. [Google Scholar] [CrossRef]
- Shen, H.-J.; Chen, J.-T.; Chung, H.-H.; Chang, W.-C. Plant regeneration via direct somatic embryogenesis from leaf explants of Tolumnia Louise Elmore ‘Elsa’. Bot. Stud. 2018, 59, 4. [Google Scholar] [CrossRef]
- Liang, H.; Xiong, Y.; Guo, B.; Yan, H.; Jian, S.; Ren, H.; Zhang, X.; Li, Y.; Zeng, S.; Wu, K. Shoot organogenesis and somatic embryogenesis from leaf and root explants of Scaevola sericea. Sci. Rep. 2020, 10, 11343. [Google Scholar] [CrossRef]
- Xiong, Y.; Chen, S.; Wu, T.; Wu, K.; Li, Y.; Zhang, X.; da Silva, J.A.T.; Zeng, S.; Ma, G. Shoot organogenesis and somatic embryogenesis from leaf and petiole explants of endangered Euryodendron excelsum. Sci. Rep. 2022, 12, 20506. [Google Scholar] [CrossRef] [PubMed]
- Phillips, G.C.; Garda, M. Plant tissue culture media and practices: An overview. In Vitro Cell. Dev. Biol.-Plant 2019, 55, 242–257. [Google Scholar] [CrossRef]
- Zhang, Y.; Cao, Y.; Wang, Y.; Cai, X. Somatic embryogenesis induction and genetic stability assessment of plants regenerated from immature seeds of Akebia trifoliate (Thunb.) Koidz. Forests 2023, 14, 473. [Google Scholar] [CrossRef]
- Thorpe, T.A.; Stasolla, C. Somatic embryogenesis. In Current Trends in the Embryology of Angiosperms; Bhojwani, S.S., Soh, W.Y., Eds.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2001; pp. 279–336. [Google Scholar]
- Raghavan, V. Role of 2,4-dichlorophenoxyacetic acid (2,4-D) in somatic embryogenesis on cultured zygotic embryos of Arabidopsis: Cell expansion, cell cycling, and morphogenesis during continuous exposure of embryos to 2,4-D. Am. J. Bot. 2004, 91, 1743–1756. [Google Scholar] [CrossRef]
- Nic-Can, G.I.; Loyola-Vargas, V.M. The role of the auxins during somatic embryogenesis. In Somatic Embryogenesis: Fundamental Aspects and Applications; Loyola-Vargas, V.M., Ochoa-Alejo, N., Eds.; Springer: Cham, Switzerland, 2016; pp. 171–182. [Google Scholar]
- Wójcik, A.M.; Wójcikowska, B.; Gaj, M.D. Current perspectives on the auxin-mediated genetic network that controls the induction of somatic embryogenesis in plants. Int. J. Mol. Sci. 2020, 21, 1333. [Google Scholar] [CrossRef]
- Li, M.; Wrobel-Marek, J.; Heidmann, I.; Horstman, A.; Chen, B.; Reis, R.; Angenent, G.C.; Boutilier, K. Auxin biosynthesis maintains embryo identity and growth during BABY BOOM-induced somatic embryogenesis. Plant Physiol. 2022, 188, 1095–1110. [Google Scholar] [CrossRef]
- Karami, O.; Philipsen, C.; Rahimi, A.; Nurillah, A.R.; Boutilier, K.; Offringa, R. Endogenous auxin maintains embryonic cell identity and promotes somatic embryo development in Arabidopsis. Plant J. 2023, 113, 7–22. [Google Scholar] [CrossRef]
- Hazubska-Przybył, T.; Ratajczak, E.; Obarska, A.; Pers-Kamczyc, E. Different roles of auxins in somatic embryogenesis efficiency in two Picea species. Int. J. Mol. Sci. 2020, 21, 3394. [Google Scholar] [CrossRef]
- Michler, C.H.; Lineberger, R.D. Effects of light on somatic embryo development and abscisic levels in carrot suspension cultures. Plant Cell Tissue Organ Cult. 1987, 11, 189–207. [Google Scholar] [CrossRef]
- Kintzios, S.E.; Taravira, N. Effect of genotype and light intensity on somatic embryogenesis and plant regeneration in melon (Cucumis melo L.). Plant Breed. 1997, 116, 359–362. [Google Scholar] [CrossRef]
- Chan, A.; Stasolla, C. Light induction of somatic embryogenesis in Arabidopsis is regulated by PHYTOCHROME E. Plant Physiol. Biochem. 2023, 195, 163–169. [Google Scholar] [CrossRef]
- Armstrong, C.; Green, C. Establishment and maintenance of friable, embryogenic maize callus and the involvement of L-proline. Planta 1985, 164, 207–214. [Google Scholar] [CrossRef]
- Kim, H.S.; Zhang, G.; Juvik, J.A.; Widholm, J.M. Miscanthus × giganteus plant regeneration: Effect of callus types, ages and culture methods on regeneration competence. GCB Bioenergy 2010, 2, 192–200. [Google Scholar] [CrossRef]
- Cai, T.; Butler, L. Plant regeneration from embryogenic callus initiated from immature inflorescences of several high-tannin sorghums. Plant Cell Tissue Organ Cult. 1990, 20, 101–110. [Google Scholar] [CrossRef]
- Brisibe, E.A.; Miyake, H.; Taniguchi, T.; Maeda, E. Regulation of somatic embryogenesis in long-term callus cultures of sugarcane (Saccharum officinarum L.). New Phytol. 1994, 126, 301–307. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, Y.; Yan, Y.; Peng, H.; Long, Y.; Zhang, Y.; Jiang, Z.; Liu, P.; Zou, C.; Peng, H. Transcriptome sequencing analysis of maize embryonic callus during early redifferentiation. BMC Genom. 2019, 20, 159. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ku, S.S.; Woo, H.-A.; Shin, M.J.; Jie, E.Y.; Kim, H.; Kim, H.-S.; Cho, H.S.; Jeong, W.-J.; Lee, M.-S.; Min, S.R.; et al. Efficient Plant Regeneration System from Leaf Explant Cultures of Daphne genkwa via Somatic Embryogenesis. Plants 2023, 12, 2175. https://doi.org/10.3390/plants12112175
Ku SS, Woo H-A, Shin MJ, Jie EY, Kim H, Kim H-S, Cho HS, Jeong W-J, Lee M-S, Min SR, et al. Efficient Plant Regeneration System from Leaf Explant Cultures of Daphne genkwa via Somatic Embryogenesis. Plants. 2023; 12(11):2175. https://doi.org/10.3390/plants12112175
Chicago/Turabian StyleKu, Seong Sub, Hyun-A Woo, Min Jun Shin, Eun Yee Jie, HyeRan Kim, Hyun-Soon Kim, Hye Sun Cho, Won-Joong Jeong, Moon-Soon Lee, Sung Ran Min, and et al. 2023. "Efficient Plant Regeneration System from Leaf Explant Cultures of Daphne genkwa via Somatic Embryogenesis" Plants 12, no. 11: 2175. https://doi.org/10.3390/plants12112175
APA StyleKu, S. S., Woo, H.-A., Shin, M. J., Jie, E. Y., Kim, H., Kim, H.-S., Cho, H. S., Jeong, W.-J., Lee, M.-S., Min, S. R., & Kim, S. W. (2023). Efficient Plant Regeneration System from Leaf Explant Cultures of Daphne genkwa via Somatic Embryogenesis. Plants, 12(11), 2175. https://doi.org/10.3390/plants12112175






