Wild vs. Cultivated Zingiber striolatum Diels: Nutritional and Biological Activity Differences
Abstract
1. Introduction
2. Results
2.1. Chemical Composition
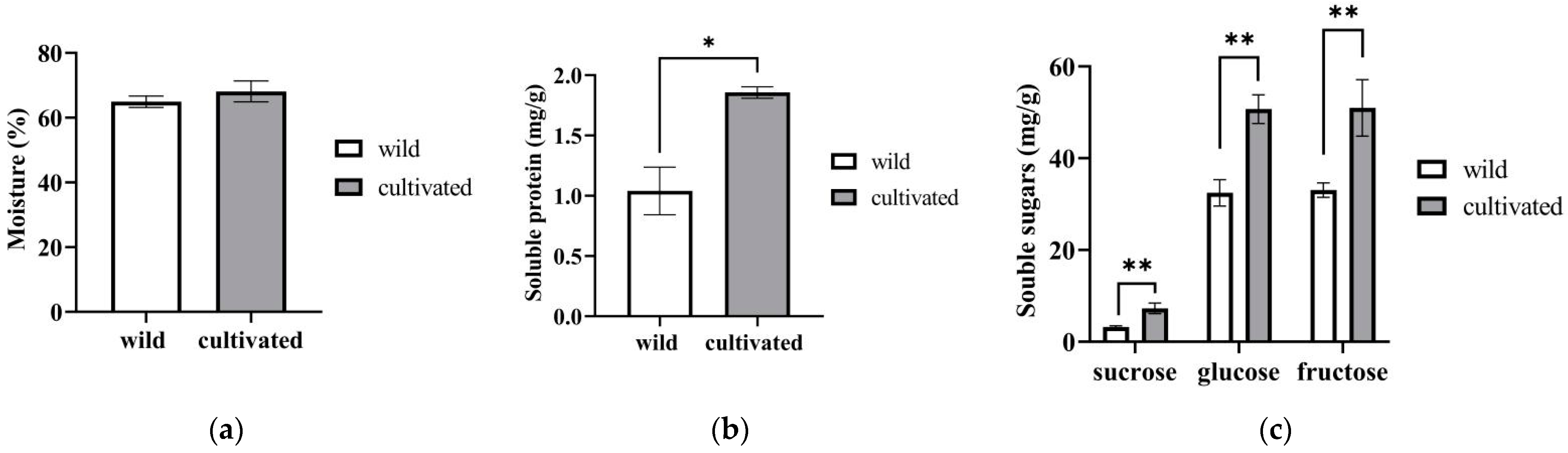
2.1.1. Moisture, Soluble Protein, and Soluble Sugars
2.1.2. Minerals, Potentially Toxic Elements and Nitrate
2.1.3. Free Amino Acid
2.1.4. Vitamins
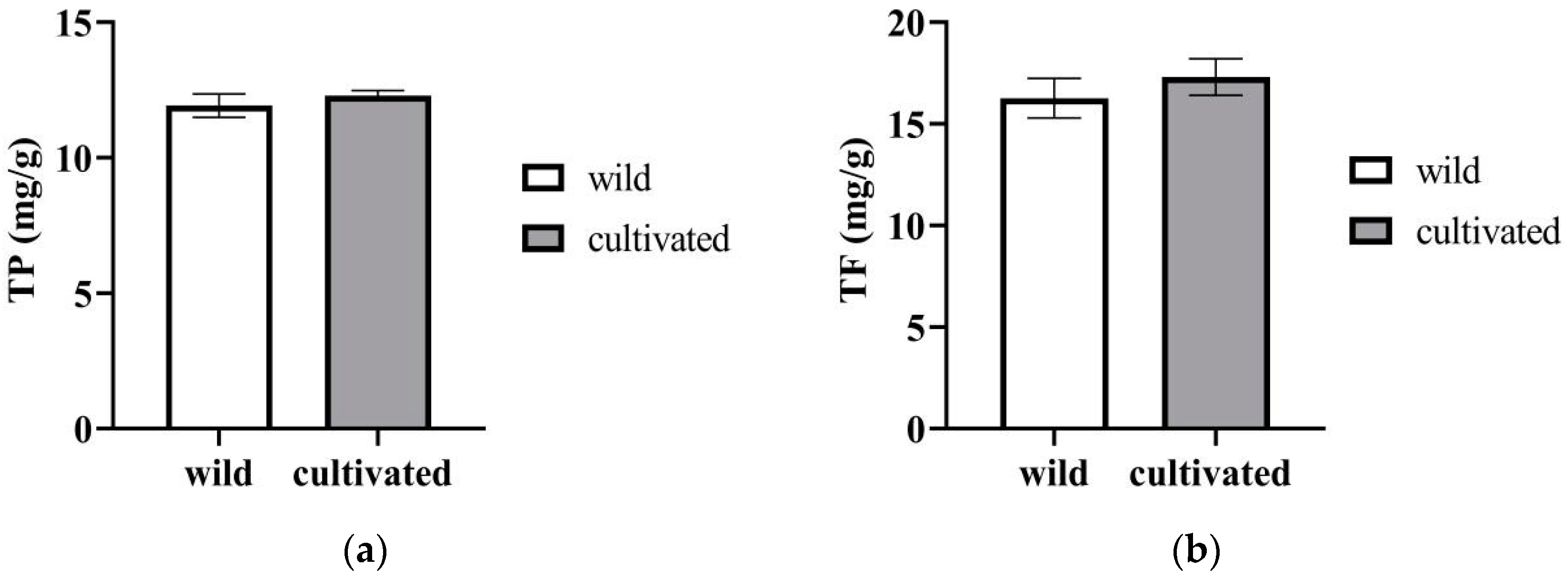
2.1.5. TP (Total Phenols) and TF (Total Flavonoids)
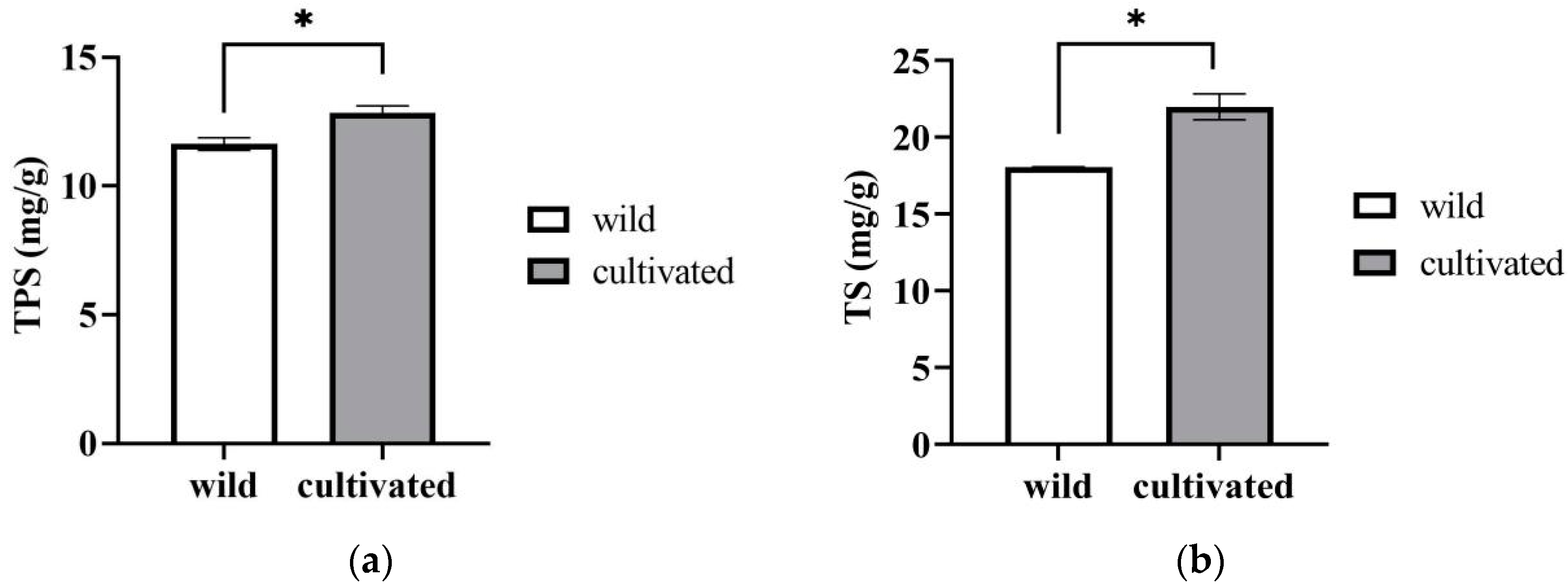
2.1.6. TPS (Total Polysaccharides) and TS (Total Saponins)
2.2. Bioactive Properties
2.2.1. Antioxidant Activity
2.2.2. α-Glucosidase and α-Amylase Inhibition
2.3. Volatie Composition
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Chemical Composition Measurement
4.2.1. Moisture
4.2.2. Soluble Protein
4.2.3. Soluble Sugar
4.2.4. Minerals and Potentially Toxic Elements
4.2.5. Nitrate
4.2.6. Free Amino Acid
4.2.7. Vitamin C
4.2.8. Vitamin E
4.2.9. Total Phenol (TP), Total Flavonoid (TF), Total Polysaccharides (TPS), and Saponin (TS)
4.3. Antioxidant Activity Evaluation
4.4. Hypoglycemic Activity Evaluation
4.4.1. Inhibitory Effect on α-Glucosidase Activity
4.4.2. Inhibitory Effect on α-Amylase Activity
4.5. Volatie Composition Identification
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Han, X.Z.; Ma, R.; Chen, Q.; Jin, X.; Jin, Y.Z.; An, R.B.; Piao, X.M.; Lian, M.L.; Quan, L.H.; Jiang, J. Anti-inflammatory action of Athyrium multidentatum extract suppresses the LPS-induced TLR4 signaling pathway. J. Ethnopharmacol. 2018, 217, 220–227. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.K.; Rana, Z.H.; Islam, S.N.; Akhtaruzzaman, M. Comparative assessment of nutritional composition, polyphenol profile, antidiabetic and antioxidative properties of selected edible wild plant species of Bangladesh. Food Chem. 2020, 320, 126646. [Google Scholar] [PubMed]
- Song, W.; Si, L.; Ji, S.; Wang, H.; Fang, X.M.; Yu, L.Y.; Li, R.Y.; Liang, L.N.; Zhou, D.; Ye, M. Uralsaponins M-Y, antiviral triterpenoid saponins from the roots of Glycyrrhiza uralensis. J. Nat. Prod. 2014, 77, 1632–1643. [Google Scholar] [CrossRef]
- Huang, Z.; Xie, L.; Xu, Y.; Zhao, K.; Li, X.; Zhong, J.; Lu, Y.; Xu, X.; Goodin, S.; Zhang, K.; et al. Essential oils from Zingiber striolatum Diels attenuate inflammatory response and oxidative stress through regulation of MAPK and NF-κB signaling pathways. Antioxidants 2021, 10, 2019. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Cai, J.; Ni, J.; Yang, F. An UPLC-MS/MS application to investigate chemical compositions in the ethanol extract with hypoglycemic activity from Zingiber striolatum Diels. J. Chin. Pharm. Sci. 2016, 25, 116–121. [Google Scholar]
- Kim, H.W.; Murakami, A.; Abe, M.; Ozawa, Y.; Morimitsu, Y.; Williams, M.V.; Ohigashi, H. Suppressive effects of mioga ginger and ginger constituents on reactive oxygen and nitrogen species generation, and the expression of inducible pro-inflammatory genes in macrophages. Antioxid. Redox Signal. 2005, 7, 1621–1629. [Google Scholar] [CrossRef]
- Tian, M.; Liu, T.; Wu, X.; Hong, Y.; Zhou, Y. Chemical composition, antioxidant, antimicrobial and anticancer activities of the essential oil from the rhizomes of Zingiber striolatum Diels. Nat. Prod. Res. 2020, 34, 2621–2625. [Google Scholar] [CrossRef]
- Vulin, M.; Magušić, L.; Metzger, A.M.; Muller, A.; Drenjančević, I.; Jukić, I.; Šijanović, S.; Lukić, M.; Stanojević, L.; Davidović, C.E.; et al. Sodium-to-potassium ratio as an indicator of diet quality in healthy pregnant women. Nutrients 2022, 14, 5052. [Google Scholar] [CrossRef]
- Chowaniak, M.; Niemiec, M.; Zhu, Z.; Rashidov, N.; Gródek, S.Z.; Szeląg, S.A.; Sikora, J.; Kuboń, M.; Fayzullo, S.A.; Mahmadyorzoda, U.M.; et al. Quality assessment of wild and cultivated green tea from different regions of china. Molecules 2021, 26, 3620. [Google Scholar] [CrossRef]
- Jones, A.M. Dietary nitrate supplementation and exercise performance. Sports Med. 2014, 44, 35–45. [Google Scholar] [CrossRef]
- Sun, H.; Liu, F.; Sun, L.; Liu, J.; Wang, M.; Chen, X.; Xu, X.; Ma, R.; Feng, K.; Jiang, R. Proteomic analysis of amino acid metabolism differences between wild and cultivated Panax ginseng. J. Ginseng. Res. 2016, 40, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.C.; Zhang, X.X.; Jiang, N.; Wang, J.; Li, W.Y.; Zhou, D.Z.; Zheng, X.J. Analysis of amino acid composition and evaluation of nutritional. Value of Zingiber strioatum Diels. J. Hubei Inst. Natl. (Nat. Sci. Ed.) 2014, 32, 380–383. (In Chinese) [Google Scholar]
- Murillo, E.; Britton, G.B.; Durant, A.A. Antioxidant activity and polyphenol content in cultivated and wild edible fruits grown in Panama. J. Pharm. Bioallied. Sci. 2012, 4, 313–317. [Google Scholar]
- Luby, C.H.; Maeda, H.A.; Goldman, I.L. Genetic and phenological variation of tocochromanol (vitamin E) content in wild (Daucus carota L. var. carota) and domesticated carrot (D. carota L. var. sativa). Hortic. Res. 2014, 1, 14015. [Google Scholar] [CrossRef]
- Goławska, S.; Łukasik, I.; Chojnacki, A.A.; Chrzanowski, G. Flavonoids and phenolic acids content in cultivation and wild collection of european cranberry bush Viburnum opulus L. Molecules 2023, 28, 2285. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Wang, L.; Lin, X.; Shen, L.; Feng, Y. Drug delivery for bioactive polysaccharides to improve their drug-like properties and curative efficacy. Drug Deliv. 2017, 24, 70–80. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Laval, S.; Yu, B. Chemical synthesis of saponins. Adv. Carbohydr. Chem. Biochem. 2021, 79, 63–150. [Google Scholar]
- Juang, Y.; Liang, P. Biological and pharmacological effects of synthetic saponins. Molecules 2020, 25, 4974. [Google Scholar] [CrossRef]
- Melini, V.; Melini, F. Functional components and anti-nutritional factors in gluten-free grains: A focus on quinoa seeds. Foods. 2021, 10, 351. [Google Scholar] [CrossRef]
- Riyaphan, J.; Pham, D.C.; Leong, M.K.; Weng, C.F. In silico approaches to identify polyphenol compounds as α-glucosidase and α-amylase inhibitors against type-II diabetes. Biomolecules 2021, 11, 1877. [Google Scholar] [CrossRef]
- Vespermann, K.A.; Paulino, B.N.; Barcelos, M.C.; Pessôa, M.G.; Pastore, G.M.; Molina, G. Biotransformation of α- and β-pinene into flavor compounds. Appl. Microbiol. Biotechnol. 2017, 101, 1805–1817. [Google Scholar] [CrossRef] [PubMed]
- Petropoulos, S.A.; Levizou, E.; Ntatsi, G.; Fernandes, Â.; Petrotos, K.; Akoumianakis, K.; Barros, L.; Ferreira, I.C.F.R. Salinity effect on nutritional value, chemical composition and bioactive compounds content of Cichorium spinosum L. Food Chem. 2017, 214, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Petropoulos, S.A.; Fernandes, Â.; Dias, M.I.; Pereira, C.; Calhelha, R.; Di Gioia, F.; Tzortzakis, N.; Ivanov, M.; Sokovic, M.; Barros, L.; et al. Wild and cultivated Centaurea raphanina subsp. mixta: A valuable source of bioactive compounds. Antioxidants 2020, 9, 314. [Google Scholar] [PubMed]
- Abdalla, M.; Li, F.; Wenzel-Storjohann, A.; Sulieman, S.; Tasdemir, D.; Mühling, K. Comparative metabolite profile, biological activity and overall quality of three lettuce (Lactuca sativa L., Asteraceae) cultivars in response to sulfur nutrition. Pharmaceutics 2021, 13, 713. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Yuan, F.; Fu, X.; Zhu, D. Profiling and relationship of water-soluble sugar and protein compositions in soybean seeds. Food Chem. 2016, 196, 776–782. [Google Scholar] [CrossRef] [PubMed]
- Hayat, S.; Hayat, Q.; Alyemeni, M.N.; Wani, A.S.; Pichtel, J.; Ahmad, A. Role of proline under changing environments: A review. Plant Signal. Behav. 2012, 7, 1456–1466. [Google Scholar] [CrossRef]
- Nkobole, N.; Prinsloo, G. 1H-NMR and LC-MS Based Metabolomics Analysis of Wild and Cultivated Amaranthus spp. Molecules 2021, 26, 795. [Google Scholar] [CrossRef]
- Bowne, J.B.; Erwin, T.A.; Juttner, J.; Schnurbusch, T.; Langridge, P.; Bacic, A.; Roessner, U. Drought responses of leaf tissues from wheat cultivars of differing drought tolerance at the metabolite level. Mol. Plant 2012, 5, 418–429. [Google Scholar] [CrossRef]
- USDA. National Nutrient Database for Standard Reference, Legacy Release; United States 694 Department of Agriculture–Agricultural Research Service: New York, NY, USA, 2018.
- Bouzari, A.; Holstege, D.; Barrett, D.M. Vitamin retention in eight fruits and vegetables: A comparison of refrigerated and frozen storage. J. Agric. Food Chem. 2015, 63, 957–962. [Google Scholar] [CrossRef]
- Rahmani, R.; Bouajila, J.; Jouaidi, M.; Debouba, M. African mustard (Brassica tournefortii) as source of nutrients and nutraceuticals properties. J. Food Sci. 2020, 85, 1856–1871. [Google Scholar] [CrossRef]
- Kaulmann, A.; Jonville, M.C.; Schneider, Y.J.; Hoffmann, L.; Bohn, T. Carotenoids, polyphenols and micronutrient profiles of Brassica oleraceae and plum varieties and their contribution to measures of total antioxidant capacity. Food Chem. 2014, 155, 240–250. [Google Scholar] [CrossRef] [PubMed]
- Cao, Q.; Yan, J.; Sun, Z.; Gong, L.; Wu, H.; Tan, S.; Lei, Y.; Jiang, B.; Wang, Y. Simultaneous optimization of ultrasound-assisted extraction for total flavonoid content and antioxidant activity of the tender stem of Triarrhena lutarioriparia using response surface methodology. Food Sci. Biotechnol. 2021, 30, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Mustafa, I.; Chin, N.L.; Fakurazi, S.; Palanisamy, A. Comparison of phytochemicals, antioxidant and anti-inflammatory properties of sun-, oven- and freeze-dried ginger extracts. Foods 2019, 8, 456. [Google Scholar] [CrossRef] [PubMed]
- Iyda, J.H.; Fernandes, Â.; Ferreira, F.D.; Alves, M.J.; Pires, T.C.S.P.; Barros, L.; Amaral, J.S.; Ferreira, I.C.F.R. Chemical composition and bioactive properties of the wild edible plant Raphanus raphanistrum L. Food Res. Int. 2019, 121, 714–722. [Google Scholar] [CrossRef]
- Rahman, M.M.; Abdullah, A.T.M.; Sharif, M.; Jahan, S.; Kabir, M.A.; Motalab, M.; Khan, T.A. Relative evaluation of in-vitro antioxidant potential and phenolic constituents by HPLC-DAD of Brassica vegetables extracted in different solvents. Heliyon 2022, 8, e10838. [Google Scholar] [CrossRef] [PubMed]
- Thilavech, T.; Marnpae, M.; Mäkynen, K.; Adisakwattana, S. Phytochemical composition, antiglycation, antioxidant activity and methylglyoxal-trapping action of brassica vegetables. Plant Foods Hum. Nutr. 2021, 76, 340–346. [Google Scholar] [CrossRef]
- Dai, J.; Mumperm, R.J. Plant phenolics: Extraction, analysis and their antioxidant and anticancer properties. Molecules 2010, 15, 7313–7352. [Google Scholar] [CrossRef]
- Lockowandt, L.; Pinela, J.; Roriz, C.L.; Pereira, C.; Abreu, R.M.V.; Calhelha, R.C.; Alves, M.J.; Barros, L.; Bredol, M.; Ferreira, I.C.F.R. Chemical features and bioactivities of cornflower (Centaurea cyanus L.) capitula: The blue flowers and the unexplored non-edible part. Ind. Crops Prod. 2019, 128, 496–503. [Google Scholar] [CrossRef]
- Asraoui, F.; Kounnoun, A.; Cacciola, F.; El, M.F.; Kabach, I.; Oulad, E.M.Y.; Alibrando, F.; Arena, K.; Trovato, E.; Mondello, L.; et al. Phytochemical profile, antioxidant capacity, α-amylase and α-glucosidase inhibitory potential of wild Moroccan Inula viscosa (L.) Aiton leaves. Molecules 2021, 26, 3134. [Google Scholar] [CrossRef]
- Hossain, U.; Das, A.K.; Ghosh, S.; Sil, P.C. An overview on the role of bioactive α-glucosidase inhibitors in ameliorating diabetic complications. Food Chem. Toxicol. 2020, 145, 111738. [Google Scholar] [CrossRef]
- Lin, J.J.; Lin, J.H.; Hsu, S.C.; Weng, S.W.; Huang, Y.P.; Tang, N.Y.; Lin, J.G.; Chung, J.G. Alpha-phellandrene promotes immune responses in normal mice through enhancing macrophage phagocytosis and natural killer cell activities. In Vivo 2013, 27, 809–814. [Google Scholar] [PubMed]
- Silva, L.M.A.; Filho, E.G.A.; Rodrigues, T.H.S.; Louredo, F.J.C.; Zocolo, G.J.; Canuto, K.M.; Mikich, S.B.; Liebsch, D.; De, A.A.; De, B.E.S. Metabolomic profiling of phloem sap from different pine species and implications on black capuchin. J. Chem. Ecol. 2022, 48, 660–669. [Google Scholar] [CrossRef] [PubMed]
- Chandra, M.; Prakash, O.; Kumar, R.; Bachheti, R.K.; Bhushan, B.; Kumar, M.; Pant, A.K. β-Selinene-rich essential oils from the parts of callicarpa macrophylla and their antioxidant and pharmacological activities. Medicines 2017, 4, 52. [Google Scholar] [CrossRef] [PubMed]
- Deng, M.; Zhang, Y.; Liu, B.; Chen, Y.; Song, H.; Yu, R.; Che, X.; Qu, X.; Liu, Y.; Hu, X.; et al. β-Elemene inhibits peritoneal metastasis of gastric cancer cells by modulating FAK/Claudin-1 signaling. Phytother. Res. 2019, 33, 2448–2456. [Google Scholar] [CrossRef]
- Salehi, B.; Upadhyay, S.; Erdogan Orhan, I.; Kumar, J.A.; L D Jayaweera, S.; A Dias, D.; Sharopov, F.; Taheri, Y.; Martins, N.; Baghalpour, N.; et al. Therapeutic potential of α- and β-pinene: A miracle gift of nature. Biomolecules 2019, 9, 738. [Google Scholar] [CrossRef]
- Salas-Oropeza, J.; Jimenez-Estrada, M.; Perez-Torres, A.; Castell-Rodriguez, A.E.; Becerril-Millan, R.; Rodriguez-Monroy, M.A.; Jarquin-Yañez, K.; Canales-Martinez, M.M. Wound healing activity of α-pinene and α-phellandrene. Molecules 2021, 26, 2488. [Google Scholar] [CrossRef]
- Zhu, S.; Jiao, W.; Xu, Y.; Hou, L.; Li, H.; Shao, J.; Zhang, X.; Wang, R.; Kong, D. Palmitic acid inhibits prostate cancer cell proliferation and metastasis by suppressing the PI3K/Akt pathway. Life Sci. 2021, 286, 120046. [Google Scholar] [CrossRef]
- Yao, G. Conformation and Characteristics of Sugar and Acid in Pear Fruits of Cultivated Species. Master’s Thesis, Nanjing Agricultural University, Nanjing, China, 2011. (In Chinese). [Google Scholar]
- Yang, J.; Zhu, Z.; Gerendás, J. Interactive effects of phosphorus supply and light intensity on glucosinolates in pakchoi (Brassica campestris L. ssp. chinensis var. communis). Plant Soil. 2009, 323, 323–333. [Google Scholar] [CrossRef]
- Cataldo, D.A.; Maroon, M.; Schrader, L.E. Rapid colorimetric determination of nitrate in plant tissue by nitration of salicylic acid. Commun. Soil Sci. Plant Anal. 1975, 6, 77–80. [Google Scholar] [CrossRef]
- Bartoli, C.G.; Yu, J.; Gómez, F.; Fernández, L.; McIntosh, L.; Foyer, C.H. Inter-relationships between light and respiration in the control of ascorbic acid synthesis and accumulation in Arabidopsis thaliana leaves. J. Exp. Bot. 2006, 57, 1621–1631. [Google Scholar] [CrossRef]
- Tang, L.; Luo, T.M.; Chen, L.F.; Huang, C.S. Preliminary report on the type and content of tocopherol in mulberry leaves and fruits. J. Newsl. Sericultural Sci. 2021, 41, 6–12. (In Chinese) [Google Scholar]
- Colonna, E.; Rouphael, Y.; Barbieri, G.; De Pascale, S. Nutritional quality of ten leafy vegetables harvested at two light intensities. Food Chem. 2016, 199, 702–710. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.F.; Chen, J.X.; Lin, Y.; Qiu, X.M.; Zhuang, Y.H. Optimization of ultrasound-assisted total flavonoid extraction from Brassica juncea and lipid antioxidant activity of the extract. J. Food Res. Dev. 2021, 42, 93–100. (In Chinese) [Google Scholar]
- Shang, Y.; Wang, D.; Yang, Y. Extraction technology of response surface optimization of polysaccharide from ginseng flower. North. Hortic. 2020, 460, 105–111. (In Chinese) [Google Scholar]
- Le, L.; Gong, X.; An, Q.; Xiang, D.; Zou, L.; Peng, L.; Wu, X.; Tan, M.; Nie, Z.; Wu, Q.; et al. Quinoa sprouts as potential vegetable source: Nutrient composition and functional contents of different quinoa sprout varieties. Food Chem. 2021, 357, 129752. [Google Scholar] [CrossRef]
- Lin, M.Z.; Chai, W.M.; Zheng, Y.L.; Huang, Q.; Ou-Yang, C. Inhibitory kinetics and mechanism of rifampicin on α-glucosidase: Insights from spectroscopic and molecular docking analyses. Int. J. Biol. Macromol. 2019, 122, 1244–1252. [Google Scholar] [CrossRef]
- Zaharudin, N.; Salmeán, A.A.; Dragsted, L.O. Inhibitory effects of edible seaweeds, polyphenolics and alginates on the activities of porcine pancreatic α-amylase. Food Chem. 2018, 245, 1196–1203. [Google Scholar] [CrossRef]



| Compounds | Wild | Cultivated | |
|---|---|---|---|
| Minerals | Calcium (Ca) | 596.38 ± 0.04 b | 613.20 ± 6.22 a |
| Potassium (K) | 7091.76 ± 42.65 a | 6935.63 ± 41.63 b | |
| Magnesium (Mg) | 225.84 ± 1.52 b | 266.95 ± 1.15 a | |
| Phosphorus (P) | 508.15 ± 2.59 b | 622.77 ± 1.25 a | |
| Sodium (Na) | 67.74 ± 0.40 a | 48.60 ± 1.55 b | |
| Cuprum (Cu) | 0.70 ± 0.10 b | 0.86 ± 0.02 a | |
| Ferrum (Fe) | 7.57 ± 0.21 b | 11.64 ± 1.63 a | |
| Manganese (Mn) | 45.49 ± 0.14 b | 84.53 ± 0.62 a | |
| Zinc (Zn) | 7.35 ± 0.05 b | 9.75 ± 0.05 a | |
| Selenium (Se) | 0.07 ± 0.02 a | 0.02 ± 0.02 b | |
| Potentially toxic elements | Arsenic (As) | 0.017 ± 0.007 a | 0.021 ± 0.004 a |
| Chromium (Cr) | 0.095 ± 0.005 a | 0.180 ± 0.079 a | |
| Cadmium (Cd) | 0.007 ± 0.003 a | 0.006 ± 0.002 a | |
| Lead (Pb) | 0.039 ± 0.019 b | 0.123 ± 0.008 a | |
| Nitrate | NO3− | 778.88 ± 54.95 a | 701.2 ± 33.72 a |
| Compounds | Wild | Cultivated |
|---|---|---|
| Aspartic acid (Asp) | 1.070 ± 0.052 a | 0.999 ± 0.096 a |
| Serine (Ser) | 2.199 ± 0.055 a | 1.697 ± 0.051 b |
| Glutamic acid (Glu) | 0.804 ± 0.094 a | 1.082 ± 0.110 a |
| Glycine (Gly) | 0.244 ± 0.006 b | 0.319 ± 0.004 a |
| Histidine (His) | 3.974 ± 0.093 a | 1.557 ± 0.034 b |
| Arginine (Arg) | 0.324 ± 0.040 b | 0.426 ± 0.007 a |
| Threonine (Thr) * | 0.273 ± 0.021 a | 0.177 ± 0.012 b |
| Alanine (Ala) | 0.293 ± 0.012 a | 0.276 ± 0.005 a |
| Proline (Pro) | 1.020 ± 0.091 a | 0.477 ± 0.063 b |
| Cysteine (Cys) | 0.011 ± 0.003 a | 0.013 ± 0.006 a |
| Tyrosine (Tyr) | 0.162 ± 0.010 a | 0.058 ± 0.030 b |
| Valine (Val) * | 0.365 ± 0.006 a | 0.126 ± 0.016 b |
| Methionine (Met) * | 0.053 ± 0.004 a | 0.024 ± 0.005 b |
| Lysine (Lys) * | 0.127 ± 0.006 a | 0.088 ± 0.004 b |
| Isoleucine (Ile) * | 0.238 ± 0.010 a | 0.057 ± 0.001 b |
| Leucine (Leu) * | 0.265 ± 0.003 a | 0.091 ± 0.010 b |
| Phenylalanine (Phe) * | 0.170 ± 0.008 a | 0.053 ± 0.036 b |
| EAA (%) | 12.85 | 8.19 |
| Total | 11.592 ± 0.383 | 7.521 ± 0.172 |
| Activity | Wild | Cultivated |
|---|---|---|
| DPPH (mg/g DW) | 16.47 ± 0.10 a | 17.20 ± 0.15 a |
| ABTS+ (mg/g DW) | 19.98 ± 0.26 a | 21.97 ± 0.10 a |
| FRAP (μmol/L) | 59.69 ± 1.45 b | 72.07 ± 4.78 a |
| Hypoglycemic Activity | Inhibitory Activity (%) | ||||
|---|---|---|---|---|---|
| Wild | Cultivated | 60% Ethanol | Acarbose | ||
| α-Glucosidase | Alcohol extraction | 98.72 ± 0.81 a | 98.66 ± 1.72 a | 46.10 | 57.80 |
| Water extraction | - | - | / | 98.98 | |
| α-Amylase | Alcohol extraction | 78.28 ± 4.02 a | 81.52 ± 4.65 a | 52.71 | 87.99 |
| Water extraction | 53.26 ± 3.82 a | 43.97 ± 6.73 a | / | 83.67 | |
| No | Retention Time (min) | Molecular Formula | Compound | Relative Content (%) | |
|---|---|---|---|---|---|
| Wild | Cultivated | ||||
| 1 | 5.541 | C10H16 | α-Pinene | 0.96 | 1.65 |
| 2 | 6.233 | C10H16 | β-Pinene | 2.67 | 5.30 |
| 3 | 6.661 | C10H16 | α-Phellandrene | - | 0.17 |
| 4 | 7.069 | C10H16 | Thujene | 2.54 | - |
| 5 | 7.071 | C10H16 | β-Phellandrene | - | 5.23 |
| 6 | 9.964 | C9H14O | 4-isopropylcyclohex-2-en-1-one | 0.08 | 0.15 |
| 7 | 14.278 | C15H24 | β-Elemene | 1.03 | 2.21 |
| 8 | 14.957 | C15H24 | Caryophyllene | 0.41 | 0.95 |
| 9 | 15.182 | C15H24 | γ-Elemene | - | 0.22 |
| 10 | 15.741 | C15H24 | Humulene | 1.11 | 2.68 |
| 11 | 16.497 | C15H24 | β-Selinene | - | 0.10 |
| 12 | 18.685 | C15H24O | Caryophyllene oxide | - | 0.29 |
| 13 | 19.283 | C15H24O | (-)-Humulene epoxide II | - | 0.46 |
| 14 | 22.792 | C16H32O2 | Ethyl myristate | 0.20 | - |
| 15 | 24.741 | C17H34O2 | Methyl 14-Methylpentadecanoate | 0.31 | - |
| 16 | 24.559 | C15H26O | (-)-Isolongifolol | - | 0.30 |
| 17 | 24.739 | C17H34O2 | Methyl palmitate | - | 0.33 |
| 18 | 25.373 | C16H32O2 | Palmitic acid | 4.07 | 2.65 |
| 19 | 25.59 | C18H36O2 | Palmitic acid ethyl ester | 15.00 | 6.31 |
| 20 | 26.756 | C21H44 | Heneicosane | 0.68 | 0.63 |
| 21 | 26.817 | C19H32O2 | Methyl linolenate | 0.34 | 0.45 |
| 22 | 27.361 | C20H36O2 | Linoleic acid | - | 3.09 |
| 23 | 27.451 | C20H36O2 | Linoleic acid ethyl ester | 14.63 | - |
| 24 | 27.532 | C20H34O2 | Linolenic acid ethyl ester | 21.20 | 16.11 |
| 25 | 27.629 | C20H36O2 | Ethyl linoleate | 3.72 | 1.40 |
| 26 | 27.745 | C20H40O2 | Ethyl stearate | 3.23 | - |
| 27 | 27.944 | C18H32O2 | Linoleicacid | 1.02 | - |
| 28 | 28.559 | C23H46 | cis-9-Tricosene | 0.23 | 0.15 |
| 29 | 28.753 | C23H48 | Tricosane | 5.16 | 4.66 |
| 30 | 29.081 | C19H36 | 5-Butyl-6-hexyloctahydro-1H-indene | 0.15 | - |
| 31 | 30.46 | C25H52 | Pentacosane | 0.30 | - |
| 32 | 30.86 | C20H34O | Thunbergol | 3.77 | 3.56 |
| 33 | 29.608 | C22H38 | 1,1′-Ethylenebisdecalin | - | 19.22 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, J.; Li, Y.; He, Y.; He, H.; Chen, X.; Liu, T.; Zhu, B. Wild vs. Cultivated Zingiber striolatum Diels: Nutritional and Biological Activity Differences. Plants 2023, 12, 2180. https://doi.org/10.3390/plants12112180
Yang J, Li Y, He Y, He H, Chen X, Liu T, Zhu B. Wild vs. Cultivated Zingiber striolatum Diels: Nutritional and Biological Activity Differences. Plants. 2023; 12(11):2180. https://doi.org/10.3390/plants12112180
Chicago/Turabian StyleYang, Jing, Yaochen Li, Yuxin He, Hongying He, Xiaoqi Chen, Tingfu Liu, and Biao Zhu. 2023. "Wild vs. Cultivated Zingiber striolatum Diels: Nutritional and Biological Activity Differences" Plants 12, no. 11: 2180. https://doi.org/10.3390/plants12112180
APA StyleYang, J., Li, Y., He, Y., He, H., Chen, X., Liu, T., & Zhu, B. (2023). Wild vs. Cultivated Zingiber striolatum Diels: Nutritional and Biological Activity Differences. Plants, 12(11), 2180. https://doi.org/10.3390/plants12112180







