Differences in Phytobenthic Diatom Community between Natural and Channelized River Sections
Abstract
1. Introduction
2. Results
2.1. Differences between Natural and Regulated River Sections
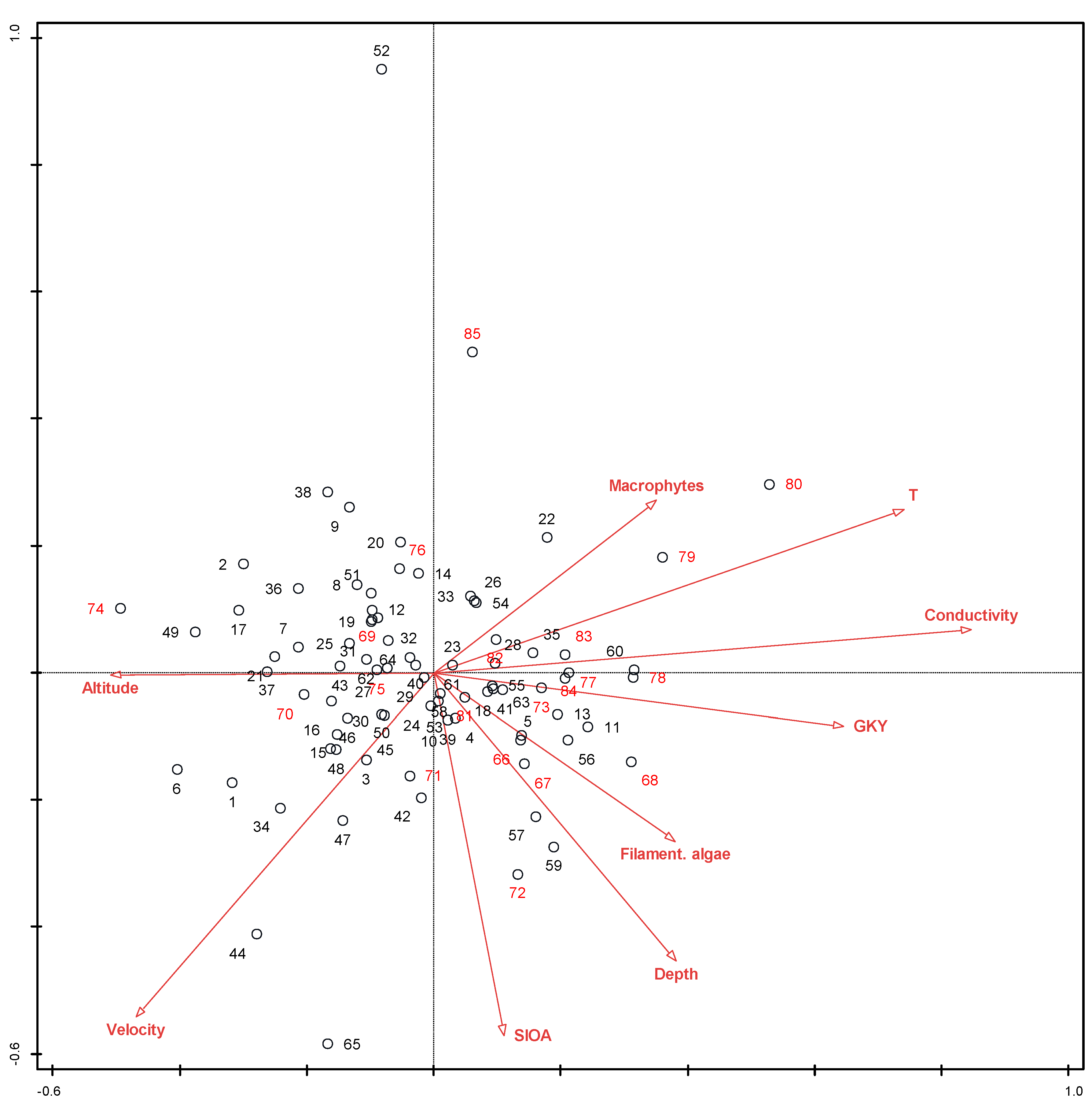
2.2. Influence of Selected Environmental Factors on the Structure of the Diatom Community
3. Discussion
3.1. Differences between Natural and Regulated River Sections
3.2. Influence of Selected Environmental Factors on the Structure of the Diatom Community
4. Materials and Methods
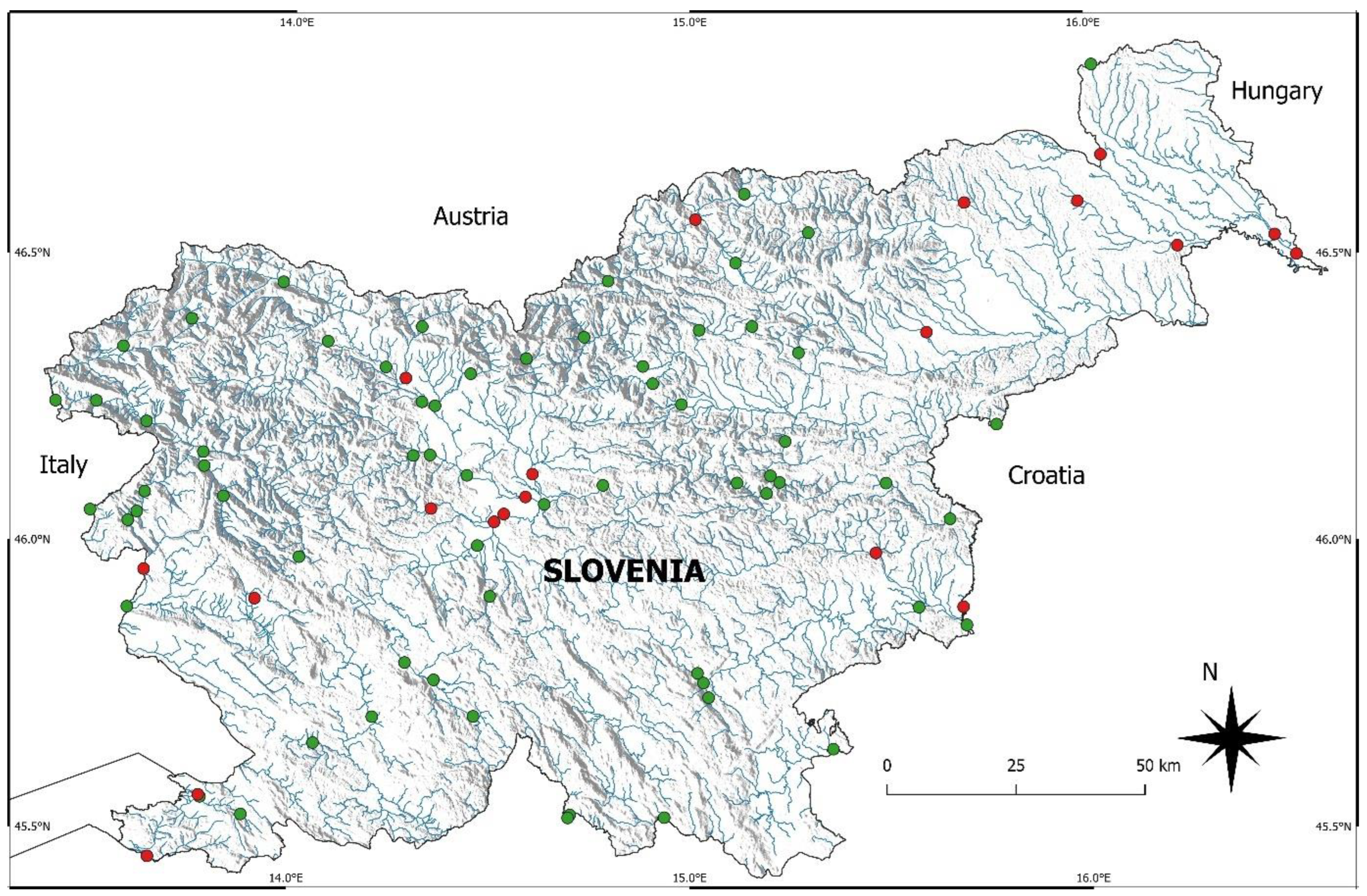
4.1. Study Area
4.2. Phytobenthos Sampling
4.3. Assessment of Environmental Parameters
4.4. Laboratory Analyses
4.5. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- EEA European Environment Agency. European Waters: Assessment of Status and Pressures; EEA Report No 7/2018; EEA European Environment Agency: Copenhagen, Denmark, 2018. [Google Scholar]
- González del Tánago, M.; Gurnell, A.M.; Belletti, B.; García de Jalón, D. Indicators of River System Hydromorphological Character and Dynamics: Understanding Current Conditions and Guiding Sustainable River Management. Aquat. Sci. 2016, 78, 35–55. [Google Scholar] [CrossRef]
- Feld, C.K.; Hering, D. Community Structure or Function: Effects of Environmental Stress on Benthic Macroinvertebrates at Different Spatial Scales. Freshw. Biol. 2007, 52, 1380–1399. [Google Scholar] [CrossRef]
- Erba, S.; Cazzola, M.; Belfiore, C.; Buffagni, A. Macroinvertebrate Metrics Responses to Morphological Alteration in Italian Rivers. Hydrobiologia 2020, 847, 2169–2191. [Google Scholar] [CrossRef]
- Lau, J.K.; Lauer, T.E.; Weinman, M.L. Impacts of Channelization on Stream Habitats and Associated Fish Assemblages in East Central Indiana. Am. Midl. Nat. 2006, 156, 319–330. [Google Scholar] [CrossRef]
- Tockner, K.; Stanford, J.A. Riverine Flood Plains: Present State and Future Trends. Environ. Conserv. 2002, 29, 308–330. [Google Scholar] [CrossRef]
- Poff, L.N.; Hart, D.D. How Dams Vary and Why It Matters for the Emerging Science of Dam Removal: An Ecological Classification of Dams Is Needed to Characterize How the Tremendous Variation in the Size, Operational Mode, Age, and Number of Dams in a River Basin Influences the Potential for Restoring Regulated Rivers via Dam Removal. Bioscience 2002, 52, 659–668. [Google Scholar]
- Kennedy, T.L.; Turner, T.F. River Channelization Reduces Nutrient Flow and Macroinvertebrate Diversity at the Aquatic Terrestrial Transition Zone. Ecosphere 2011, 2, 1–13. [Google Scholar] [CrossRef]
- Schmutz, S.; Bakken, T.H.; Friedrich, T.; Greimel, F.; Harby, A.; Jungwirth, M.; Melcher, A.; Unfer, G.; Zeiringer, B. Response of Fish Communities to Hydrological and Morphological Alterations in Hydropeaking Rivers of Austria. River Res. Appl. 2015, 31, 919–930. [Google Scholar] [CrossRef]
- Wyzga, B.; Amirowicz, A.; Oglecki, P.; Hajdukiewicz, H.; Radecki-Pawlik, A.; Zawiejska, J.; Mikuś, P. Response of Fish and Benthic Invertebrate Communities to Constrained Channel Conditions in a Mountain River: Case Study of the Biała, Polish Carpathians. Limnologica 2014, 46, 58–69. [Google Scholar] [CrossRef]
- Zelnik, I.; Muc, T. Relationship between Environmental Conditions and Structure of Macroinvertebrate Community in a Hydromorphologically Altered Pre-Alpine River. Water 2020, 12, 2987. [Google Scholar] [CrossRef]
- Gebler, D.; Szoszkiewicz, K. Response of Aquatic Plants to Extreme Alterations in River Morphology. Water 2022, 14, 3746. [Google Scholar] [CrossRef]
- Friberg, N.; Bonada, N.; Bradley, D.C.; Dunbar, M.J.; Edwards, F.K.; Grey, J.; Hayes, R.B.; Hildrew, A.G.; Lamouroux, N.; Trimmer, M.; et al. Biomonitoring of Human Impacts in Freshwater Ecosystems: The Good, the Bad and the Ugly. Adv. Ecol. Res. 2011, 44, 1–68. [Google Scholar] [CrossRef]
- Feld, C.K.; de Bello, F.; Dolédec, S. Biodiversity of Traits and Species Both Show Weak Responses to Hydromorphological Alteration in Lowland River Macroinvertebrates. Freshw. Biol. 2014, 59, 233–248. [Google Scholar] [CrossRef]
- Villeneuve, B.; Souchon, Y.; Usseglio-Polatera, P.; Ferréol, M.; Valette, L. Can We Predict Biological Condition of Stream Ecosystems? A Multi-Stressors Approach Linking Three Biological Indices to Physico-Chemistry, Hydromorphology and Land Use. Ecol. Indic. 2015, 48, 88–98. [Google Scholar] [CrossRef]
- Hohensinner, S.; Hauer, C.; Muhar, S.; Hohensinner, S.; Muhar, S.; Hauer, C. River Morphology, Channelization, and Habitat Restoration. Riverine Ecosyst. Manag. 2018, 8, 41–65. [Google Scholar] [CrossRef]
- Jungwirth, M.; Muhar, S.; Schmutz, S. Fundamentals of Fish Ecological Integrity and Their Relation to the Extended Serial Discontinuity Concept. Hydrobiologia 2000, 422, 85–97. [Google Scholar] [CrossRef]
- Szczepocka, E.; Żelazna-Wieczorek, J.; Nowicka-Krawczyk, P. Critical Approach to Diatom-Based Bioassessment of the Regulated Sections of Urban Flowing Water Ecosystems. Ecol. Indic. 2019, 104, 259–267. [Google Scholar] [CrossRef]
- Booman, G.C.; Laterra, P. Channelizing Streams for Agricultural Drainage Impairs Their Nutrient Removal Capacity. J. Environ. Qual. 2019, 48, 459–468. [Google Scholar] [CrossRef]
- Lynch, L.M.; Sutfin, N.A.; Fegel, T.S.; Boot, C.M.; Covino, T.P.; Wallenstein, M.D. River Channel Connectivity Shifts Metabolite Composition and Dissolved Organic Matter Chemistry. Nat. Commun. 2019, 10, 459. [Google Scholar] [CrossRef]
- Godillot, R.; Caussade, B.; Ameziane, T.; Capblancq, J. Interplay between Turbulence and Periphyton in Rough Open-Channel Flow. J. Hydraul. Res. 2001, 39, 227–239. [Google Scholar] [CrossRef]
- EU Directive 2000/60 Water Framework Directive (WFD) 2000/60/EC—European Environment Agency. Available online: https://www.eea.europa.eu/policy-documents/water-framework-directive-wfd-2000 (accessed on 17 January 2023).
- Boulêtreau, S.; Garabétian, F.; Sauvage, S.; Sánchez-Pérez, J.M. Assessing the Importance of a Self-Generated Detachment Process in River Biofilm Models. Freshw. Biol. 2006, 51, 901–912. [Google Scholar] [CrossRef]
- Connell, J.H. Diversity in Tropical Rain Forests and Coral Reefs. Science 1978, 199, 1302–1310. [Google Scholar] [CrossRef]
- Izagirre, O.; Elosegi, A. Environmental Control of Seasonal and Inter-Annual Variations of Periphytic Biomass in a North Iberian Stream. Ann. Limnol. Int. J. Limnol. 2005, 41, 35–46. [Google Scholar] [CrossRef]
- Hill, W.R.; Ryon, M.G.; Smith, J.G.; Adams, S.M.; Boston, H.L.; Stewart, A.J. The Role of Periphyton in Mediating the Effects of Pollution in a Stream Ecosystem. Environ. Manag. 2010, 45, 563–576. [Google Scholar] [CrossRef] [PubMed]
- Biggs, B.J.F.; Stokseth, S. Hydraulic Habitat Preferences for Periphyton in Rivers. Regul. Rivers Res. Manag. 1996, 12, 251–261. [Google Scholar] [CrossRef]
- Law, R.J.; Elliott, J.A.; Thackeray, S.J. Do Functional or Morphological Classifications Explain Stream Phytobenthic Community Assemblages? Diatom Res. 2014, 29, 309–324. [Google Scholar] [CrossRef]
- Virtanen, L.K.; Köngäs, P.; Aitto-Oja, S.; Soininen, J. Is Temporal Occurrence of Diatoms Related to Species Traits, Local Abundance, and Regional Distribution? J. Phycol. 2011, 47, 1445–1453. [Google Scholar] [CrossRef]
- Rimet, F.; Bouchez, A. Life-Forms, Cell-Sizes and Ecological Guilds of Diatoms in European Rivers. Knowl. Manag. Aquat. Ecosyst. 2012, 406, 1–12. [Google Scholar] [CrossRef]
- Passy, S.I. Diatom Ecological Guilds Display Distinct and Predictable Behavior along Nutrient and Disturbance Gradients in Running Waters. Aquat. Bot. 2007, 86, 171–178. [Google Scholar] [CrossRef]
- Lengyel, E.; Padisák, J.; Stenger-Kovács, C. Establishment of Equilibrium States and Effect of Disturbances on Benthic Diatom Assemblages of the Torna-Stream, Hungary. Hydrobiologia 2015, 750, 43–56. [Google Scholar] [CrossRef]
- B-Béres, V.; Lukács, Á.; Török, P.; Kókai, Z.; Novák, Z.; T-Krasznai, E.; Tóthmérész, B.; Bácsi, I. Combined Eco-Morphological Functional Groups Are Reliable Indicators of Colonisation Processes of Benthic Diatom Assemblages in a Lowland Stream. Ecol. Indic. 2016, 64, 31–38. [Google Scholar] [CrossRef]
- Hardin, G. The Competitive Exclusion Principle. Science 1960, 131, 1292–1297. [Google Scholar] [CrossRef]
- Berthon, V.; Bouchez, A.; Rimet, F. Using Diatom Life-Forms and Ecological Guilds to Assess Organic Pollution and Trophic Level in Rivers: A Case Study of Rivers in South-Eastern France. Hydrobiologia 2011, 673, 259–271. [Google Scholar] [CrossRef]
- Brooker, M.P. The Ecological Effects of Channelization. Geogr. J. 1985, 151, 63–69. [Google Scholar] [CrossRef]
- Tapolczai, K.; Bouchez, A.; Stenger-Kovács, C.; Padisák, J.; Rimet, F. Trait-Based Ecological Classifications for Benthic Algae: Review and Perspectives. Hydrobiologia 2016, 776, 1–17. [Google Scholar] [CrossRef]
- B-Béres, V.; Török, P.; Kókai, Z.; Lukács, Á.; T-Krasznai, E.; Tóthmérész, B.; Bácsi, I. Ecological Background of Diatom Functional Groups: Comparability of Classification Systems. Ecol. Indic. 2017, 82, 183–188. [Google Scholar] [CrossRef]
- Stenger-Kovács, C.; Lengyel, E.; Crossetti, L.O.; Üveges, V.; Padisák, J. Diatom Ecological Guilds as Indicators of Temporally Changing Stressors and Disturbances in the Small Torna-Stream, Hungary. Ecol. Indic. 2013, 24, 138–147. [Google Scholar] [CrossRef]
- Goldenberg Vilar, A.; Vonk, J.A.; Bichebois, S.; van Dam, H.; Admiraal, W.; van der Geest, H.G. Suspended Organic Particles Drive the Development of Attached Algal Communities in Degraded Peatlands. Hydrobiologia 2015, 744, 211–221. [Google Scholar] [CrossRef]
- Leira, M.; Filippi, M.L.; Cantonati, M. Diatom Community Response to Extreme Water-Level Fluctuations in Two Alpine Lakes: A Core Case Study. J. Paleolimnol. 2015, 53, 289–307. [Google Scholar] [CrossRef]
- Szczepocka, E.; Żelazna-Wieczorek, J. Diatom Biomonitoring—Scientific Foundations, Commonly Discussed Issues and Frequently Made Errors. Oceanol. Hydrobiol. Stud. 2018, 47, 313–325. [Google Scholar] [CrossRef]
- Salmaso, N.; Naselli-Flores, L.; Padisák, J. Functional Classifications and Their Application in Phytoplankton Ecology. Freshw. Biol. 2015, 60, 603–619. [Google Scholar] [CrossRef]
- Zelnik, I.; Sušin, T. Epilithic Diatom Community Shows a Higher Vulnerability of the River Sava to Pollution during the Winter. Diversity 2020, 12, 465. [Google Scholar] [CrossRef]
- Toman, M.J.; Grošelj, A.M.; Zelnik, I. The Influence of Selected Factors on the Distribution of Epilithic Diatoms in a Torrential River the Kamniška Bistrica (Slovenia). Acta Bot. Croat. 2014, 73, 447–463. [Google Scholar] [CrossRef]
- Soininen, J. Environmental and Spatial Control of Freshwater Diatoms—A Review. Diatom Res. 2007, 22, 473–490. [Google Scholar] [CrossRef]
- Cantonati, M. Diatom Communities of Springs in the Southern Alps. Diatom Res. 1998, 13, 201–220. [Google Scholar] [CrossRef]
- Beltrami, M.E.; Ciutti, F.; Cappelletti, C.; Lösch, B.; Alber, R.; Ector, L. Diatoms from Alto Adige/Südtirol (Northern Italy): Characterization of Assemblages and Their Application for Biological Quality Assessment in the Context of the Water Framework Directive. Hydrobiologia 2012, 695, 153–170. [Google Scholar] [CrossRef]
- Wraber, M. Pflanzengeographische Stellung und Gliedernung Sloweniens. Vegetatio 1969, 17, 176–199. [Google Scholar] [CrossRef]
- Illies, J. Limnofauna Europaea: Eine Zusammensstellung Aller Die Europäischen Binnengewässer Bewohnenden Mehrzelligen Tierarten Mit Angaben Über Ihre Verbreitung und Ökologie; Fischer: Stuttgart, Germany, 1978; ISBN 3-437-30246-9. [Google Scholar]
- Mršić, N. Biotic Diversity in Slovenia: Slovenia—The “Hot Spot” of Europe; Ministrvo za Okolje in Prostor: Ljubljana, Slovenia, 1997. [Google Scholar]
- Kolbezen, M.; Pristov, J.; Bat, M.; Klemenc, B.; Hrček, D. Surface Streams and Water Balance of Slovenia; Ministrstvo za Okolje in Proctor, Hidrometeorološki Zavod Republike Slovenije: Ljubljana, Slovenia, 1998. [Google Scholar]
- Šraj-Kržič, N.; Germ, M.; Urbanc-Berčič, O.; Kuhar, U.; Janauer, G.A.; Gaberščik, A. The Quality of the Aquatic Environment and Macrophytes of Karstic Watercourses. Plant. Ecol. 2007, 192, 107–118. [Google Scholar] [CrossRef]
- Hrvatin, M.; Tičar, J.; Zorn, M. Rocks and Tectonic Structure of Slovenia BT—The Geography of Slovenia: Small but Diverse; Perko, D., Ciglič, R., Zorn, M., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 23–34. ISBN 978-3-030-14066-3. [Google Scholar]
- Komac, B.; Pavšek, M.; Topole, M. Climate and Weather of Slovenia. In The Geography of Slovenia; Perko, D., Ciglič, R., Matija, Z., Eds.; Springer Nature Switzerland AG: Cham, Switzerland, 2020; pp. 71–89. [Google Scholar]
- MOP. Metodologija Vrednotenja Ekološkega Stanja Vodotokov Na Podlagi Bentoških Nevretenčarjev. Available online: https://www.gov.si/assets/ministrstva/MOP/Dokumenti/Voda/Ekolosko_stanje/metod_vredn_ekoloskega_st_vodotokov_fitobentos.pdf (accessed on 8 December 2022).
- AQEM Consortium. Manual for the Application of the AQEM Method: A Comprehensive Method to Assess European Streams Using Benthic Macroinvertebrates, Developed for the Purpose of the Water Framework Directive; Version 1.0.; European Commission: Brussels, Belgium, 2002. [Google Scholar]
- Krammer, K.; Lange-Bertalot, H. Bacillariophyceae. 1. Teil. Naviculaceae. In Subwasserflora von Mitteleuropa; Ett, J., Gerloff, J., Heymig, H., Mollenhauer, D., Eds.; Gustav Fischer Verlag: Stuttgart, NY, USA, 1986; Volume 2/1, pp. 1–876. [Google Scholar]
- Krammer, K.; Lange-Bertalot, H. Bacillariophyceae. 2. Teil: Bacillariaceae, Epithemiaceae, Surirellaceae. In Süsswasserflora von Mitteleuropa; Ettl, H., Gerloff, J., Heynig, H., Mollenhauer, D., Eds.; VEB Gustav Fischer Verlag: Jena, Germany, 1988; Volume 2/2. [Google Scholar]
- Krammer, K.; Lange-Bertalot, H. Bacillariophyceae. 3. Teil: Centrales, Fragilariaceae, Eunotiaceae. In Susswasserflora von Mitteleuropa; Ettl, H., Gerloff, J., Heynig, H., Mollenhauer, D., Eds.; Gustav Fisher Verlag: Stuttgart, Germany, 1991; pp. 1–576. [Google Scholar]
- Krammer, K.; Lange-Bertalot, H. Bacillariophyceae. 4 Teil, Achnanthaceae, Kritische Ergänzungen Zu Navicula (Lineolatae) und Gomphonema Gesamtliteraturverzeichnis; Gustav Fischer: Stuttgart, Germany, 1991. [Google Scholar]
- Hofmann, G.; Werum, M.; Lange-Bertalot, H. Diatomeen Im Süsswasser-Benthos von Mitteleuropa. Bestimmungsflora Kieselalgen Für Die Ökologische Praxis. Über 700 der Häufigsten Arten und Ihre Ökologie; A.R.G. Gantner Verlag K.G.: Ruggell, Liechtenstein, 2011. [Google Scholar]
- Susswasserflora von Mitteleuropa. Band 3: Xanthophyceae, Band 4: Xanthophyceae 2, Band 6: Dinophyceae, Band 7: Rhodophyta and Phaeophyceae, Band 9-16: Chlorophyta I-VIII, Band 19: Cyanoprokaryota; Ettl, H., Gerloff, J., Heynig, H., Mollenhauer, D., Eds.; Gustav Fisher Verlag: Stuttgart, Germany, 1999–2013. [Google Scholar]
- John, D.M.; Whitton, B.A.; Brook, A.J. The Freshwater Algal Flora of the British Isles: An Identification Guide to Freshwater and Terrestrial Algae; Cambridge University Press: Cambridge, UK, 2002. [Google Scholar]
- Rott, E.; Pipp, E.; Pfister, P.; Van Dahm, H.; Ortler, K.; Binder, N.; Pall, K. Indikationslisten Fur Aufwuchsalgen in Östereichen Fließgevessern, Teil 2: Trophienindikation So Vie Geochemische Präferenz, Taxonomische und Toxicologische Anmerkungen; Bundesministerium für Land und Forstwirtschaft: Wien, Australia, 1999. [Google Scholar]
- ter Braak, C.J.F.; Smilauer, P. Canoco Reference Manual and User’s Guide: Software for Ordination, Version 5.0; Microcomputer Power: Ithaca, NY, USA, 2012. [Google Scholar]
- ter Braak, C.J.F.; Verdonschot, P.F.M. Canonical Correspondence Analysis and Related Multivariate Methods in Aquatic Ecology. Aquat. Sci. 1995, 57, 255–289. [Google Scholar] [CrossRef]
- Urrea-Clos, G.; Sabater, S. Identifying Reference Benthic Diatom Communities in an Agricultural Watershed (Guadiana River, SW Spain). Hydrobiologia 2012, 695, 171–184. [Google Scholar] [CrossRef]




| Ecological Type | N | R | ||
|---|---|---|---|---|
| Proportion [%] | Average | p-Value | Average | |
| Low profile | 57.3 | 0.199 | 49.6 | |
| High profile | 25.8 | 0.063 | 19.5 | |
| Motile | 15.0 | 0.024 | 25.1 | |
| Number of diatom taxa | ||||
| Low profile | 7 | 0.187 | 7.5 | |
| High profile | 8 | 0.167 | 7 | |
| Motile | 9.3 | 0.018 | 13 | |
| Shannon–Wiener DI | ||||
| Low profile | 1.07 | 0.026 | 1.26 | |
| High profile | 1.36 | 0.634 | 1.29 | |
| Motile | 1.53 | 0.029 | 1.88 | |
| All taxa | Number of diatom taxa | 24.9 | 0.071 | 28.6 |
| All taxa | Shannon–Wiener DI | 2.02 | 0.020 | 2.33 |
| All taxa | SI | 1.76 | 0.012 | 1.97 |
| All taxa | TI | 2.06 | 0.002 | 2.57 |
| All taxa | SI EQR | 1.19 | 0.037 | 0.90 |
| All taxa | TI EQR | 1.07 | 0.003 | 0.77 |
| Environmental Parameter | N | R | |
|---|---|---|---|
| Average | p-Value | Average | |
| Altitude [m a.s.l.] | 311.95 | 0.002 | 213.65 |
| Temperature [°C] | 16.40 | n.s. | 18.09 |
| Conductivity [µS/cm] | 377.04 | 0.005 | 492.40 |
| Width of the channel [m] | 56.14 | 0.030 | 33.85 |
| Width of the channel under water [m] | 40.17 | 0.043 | 22.15 |
| Current velocity (4-level scale) | 1.93 | 0.017 | 1.61 |
| Turbidity (3-level scale) | 1.2 | n.s. | 1.5 |
| Sand proportion [%] | 21.46 | 0.045 | 34.50 |
| Gravel proportion [%] | 55.08 | 0.003 | 36.25 |
| Substrate coarseness (5-level scale) | 3.01 | n.s. | 2.73 |
| Shadiness of the channel [%] | 15.77 | n.s. | 8.00 |
| Filamentous algae cover [6-level scale] | 4.00 | n.s. | 4.55 |
| Ecological Type | Name | N | t-Test | R |
|---|---|---|---|---|
| Average | p-Value | Average | ||
| LP | Achnanthidium pyrenaicum (Hustedt) Kobayasi | 19.16 | 0.042 | 8.66 |
| LP | Planothidium dubium (Grunow) Round and Bukhtiyarova | 0.33 | 0.037 | 1.71 |
| LP | Cymbella affinis Kützing | 0.71 | 0.049 | 0.19 |
| HP | Diatoma moniliformis (Kützing) Williams | 0.11 | 0.045 | 0.00 |
| HP | Diatoma vulgaris Bory | 5.55 | 0.006 | 1.17 |
| HP | Didymosphenia geminata (Lyngbye) Schmidt | 0.10 | 0.017 | 0.00 |
| HP | Gomphonema pumilum (Grunow) Reichardt and Lange-Bertalot | 3.05 | 0.037 | 0.95 |
| M | Denticula tenuis Kützing | 1.90 | 0.002 | 0.05 |
| M | Nitzschia fonticola Grunow | 2.22 | 0.042 | 0.82 |
| M | Nitzschia pura Hustedt | 0.25 | 0.024 | 0.00 |
| M | Surirella minuta Brébisson ex Kützing | 0.02 | 0.026 | 0.09 |
| Ecol. Type | Name | Order of Frequency | Average Proportions (%) | Order of Frequency | |
|---|---|---|---|---|---|
| N | N | R | R | ||
| LP | Achnanthidium minutissimum (Kütz.) Czarn. | 1 | 21.9 | 15.2 | 1 |
| LP | Achnanthidium pyrenaicum (Hustedt) Kobayasi | 2 | 19.2 | 8.7 | 3 |
| LP | Cocconeis cf. placentula Ehrenberg | 3 | 6.3 | 11.3 | 2 |
| HP | Diatoma vulgaris Bory | 4 | 5.5 | 1.2 | 23 |
| HP | Melosira varians Agardh | 13 | 1.6 | 5.4 | 4 |
| LP | Cocconeis pediculus Ehrenberg | 7 | 2.4 | 4.3 | 5 |
| P | Stephanocyclus meneghinianus (Kützing) Kulikovskiy, Genkal, and Kociolek | 0.9 | 3.8 | 6 | |
| HP | Gomphonema pumilum (Grunow) Reichardt and Lange-Bertalot | 5 | 3.1 | 0.9 | |
| HP | Encyonema minutum (Hilse in Rabh.) D.G. Mann | 6 | 2.9 | 2.5 | 10 |
| M | Navicula gregaria Donkin | 0.7 | 2.8 | 7 | |
| M | Nitzschia palea (Kützing) W.Smith var. debilis (Kützing) Grunow | 15 | 1.5 | 2.5 | 8 |
| M | Nitzschia homburgiensis Lange-Bertalot | 20 | 1.0 | 2.5 | 9 |
| HP | Fragilaria capucina Desmazières | 8 | 2.3 | 1.0 | |
| M | Nitzschia fonticola Grunow | 9 | 2.2 | 0.8 | |
| LP | Cymbella microcephala Grunow | 0.7 | 2.2 | 11 | |
| M | Mayamaea atomus (Kützing) Lange-Bertalot | 0.6 | 2.1 | 12 | |
| M | Nitzschia paleacea (Grunow) Grunow | 0.3 | 2.0 | 13 | |
| HP | Gomphonema minutum (Agardh) Agardh | 10 | 1.9 | 1.6 | 17 |
| M | Denticula tenuis Kützing | 11 | 1.9 | 0.0 | |
| LP | Amphora indistincta Levkov | 12 | 1.9 | 1.5 | 19 |
| P | Nitzschia acicularis (Kützing) W. Smith | 0.9 | 1.8 | 14 | |
| HP | Gomphonema exilissimum (Grunow) Lange-Bertalot and Reichardt | 0.5 | 1.8 | 15 | |
| LP | Planothidium dubium (Grunow) Round and Bukhtiyarova | 0.3 | 1.7 | 16 | |
| M | Navicula tripunctata (O.F. Müller) Bory | 19 | 1.0 | 1.6 | 18 |
| HP | Encyonema silesiacum (Bleisch) D.G. Mann | 14 | 1.6 | 0.6 | |
| M | Nitzschia dissipata (Kützing) Grunow ssp. dissipata | 0.9 | 1.5 | 20 | |
| HP | Gomphonema cymbelliclinum Reichardt and Lange-Bertalot | 16 | 1.5 | 0.3 | |
| LP | Achnanthidium saprophilum (Kobayasi and Mayama) Round and Bukhtiyarova | 17 | 1.5 | 0.7 | |
| LP | Rhoicosphenia abbreviata (Agardh) Lange-Bertalot | 0.8 | 1.4 | 21 | |
| HP | Ulnaria ulna (Nitzsch) Compere | 18 | 1.4 | 0.8 | |
| M | Navicula menisculus Schumann | 21 | 1.0 | 1.2 | 22 |
| Entire Species Matrix, CCA | Species Matrix Without A. minutissimum, CCA | Matrix of Ecological Types, RDA | ||||
|---|---|---|---|---|---|---|
| Variable | Explains % | p | Explains % | p | Explains % | p |
| Conductivity | 4.8 | 0.002 | 4.7 | 0.002 | ||
| SI OA | 3.0 | 0.002 | 3.0 | 0.002 | ||
| Temperature | 2.7 | 0.002 | 2.6 | 0.002 | 17.1 | 0.002 |
| Average current velocity | 2.4 | 0.002 | 2.4 | 0.002 | ||
| Altitude | 2.4 | 0.002 | 2.4 | 0.002 | ||
| Macrophyte cover | 2.0 | 0.006 | 2.0 | 0.008 | ||
| Filamentous algae cover | 1.8 | 0.006 | 1.7 | 0.002 | ||
| GKY | 1.8 | 0.032 | ||||
| Average water depth | 1.7 | 0.002 | 1.6 | 0.004 | 5.0 | 0.002 |
| TI OA | 2.0 | 0.002 | ||||
| Sum abundances of other algae | 8.8 | 0.002 | ||||
| GKX | 3.5 | 0.01 | ||||
| Regulated/natural | 1.7 | 0.008 | ||||
| Total | 22.6 | 24.1 | 34.4 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zelnik, I.; Germ, M.; Golob, A.; Krivograd Klemenčič, A. Differences in Phytobenthic Diatom Community between Natural and Channelized River Sections. Plants 2023, 12, 2191. https://doi.org/10.3390/plants12112191
Zelnik I, Germ M, Golob A, Krivograd Klemenčič A. Differences in Phytobenthic Diatom Community between Natural and Channelized River Sections. Plants. 2023; 12(11):2191. https://doi.org/10.3390/plants12112191
Chicago/Turabian StyleZelnik, Igor, Mateja Germ, Aleksandra Golob, and Aleksandra Krivograd Klemenčič. 2023. "Differences in Phytobenthic Diatom Community between Natural and Channelized River Sections" Plants 12, no. 11: 2191. https://doi.org/10.3390/plants12112191
APA StyleZelnik, I., Germ, M., Golob, A., & Krivograd Klemenčič, A. (2023). Differences in Phytobenthic Diatom Community between Natural and Channelized River Sections. Plants, 12(11), 2191. https://doi.org/10.3390/plants12112191








