Cassava Witches’ Broom Disease in Southeast Asia: A Review of Its Distribution and Associated Symptoms
Abstract
:1. Introduction
2. Symptoms and Pathogens Identified in CWBD-Affected Plants
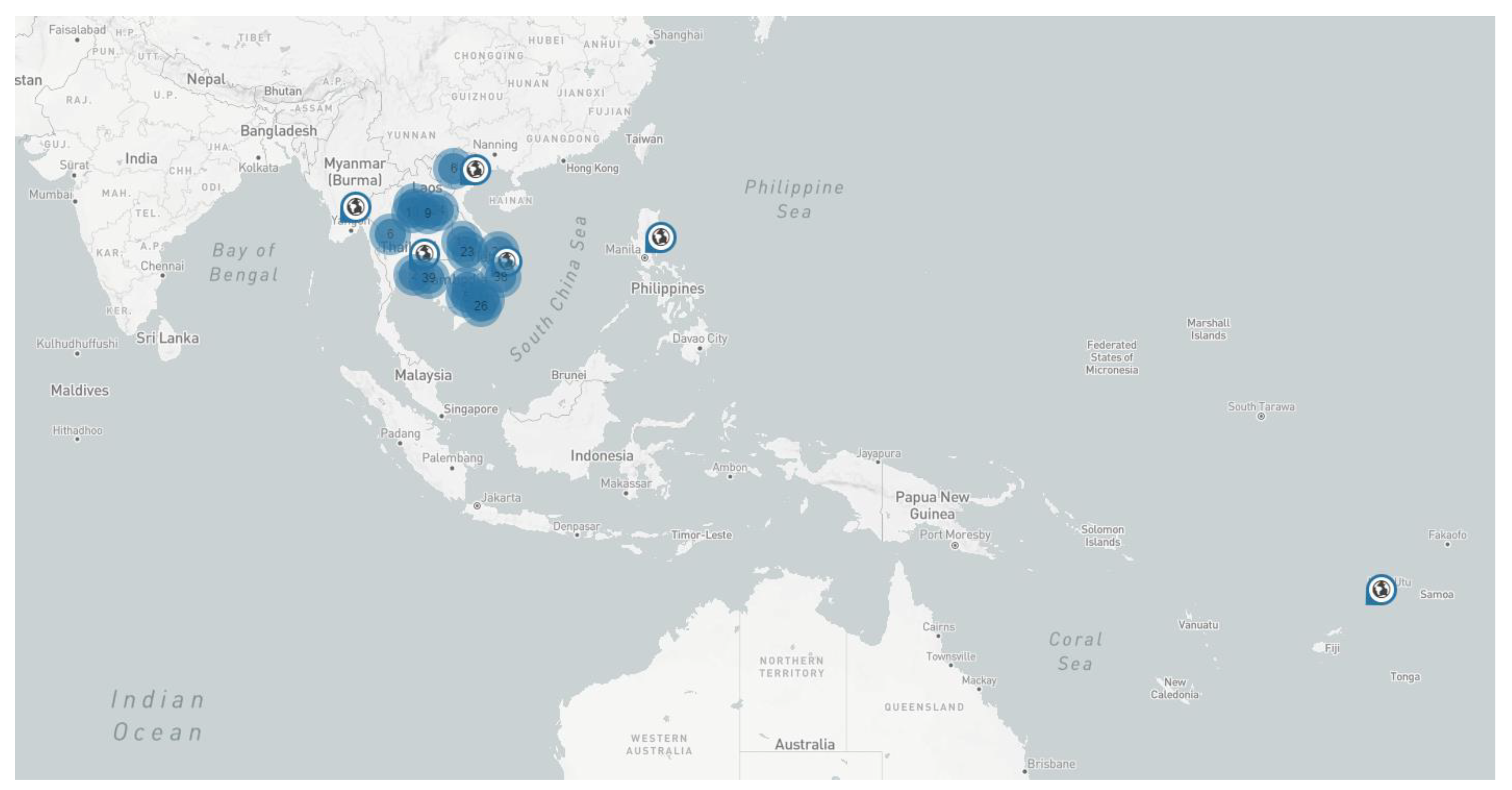
3. CWBD in the Field
4. Phytoplasma Sequences Detected in Cassava Plants
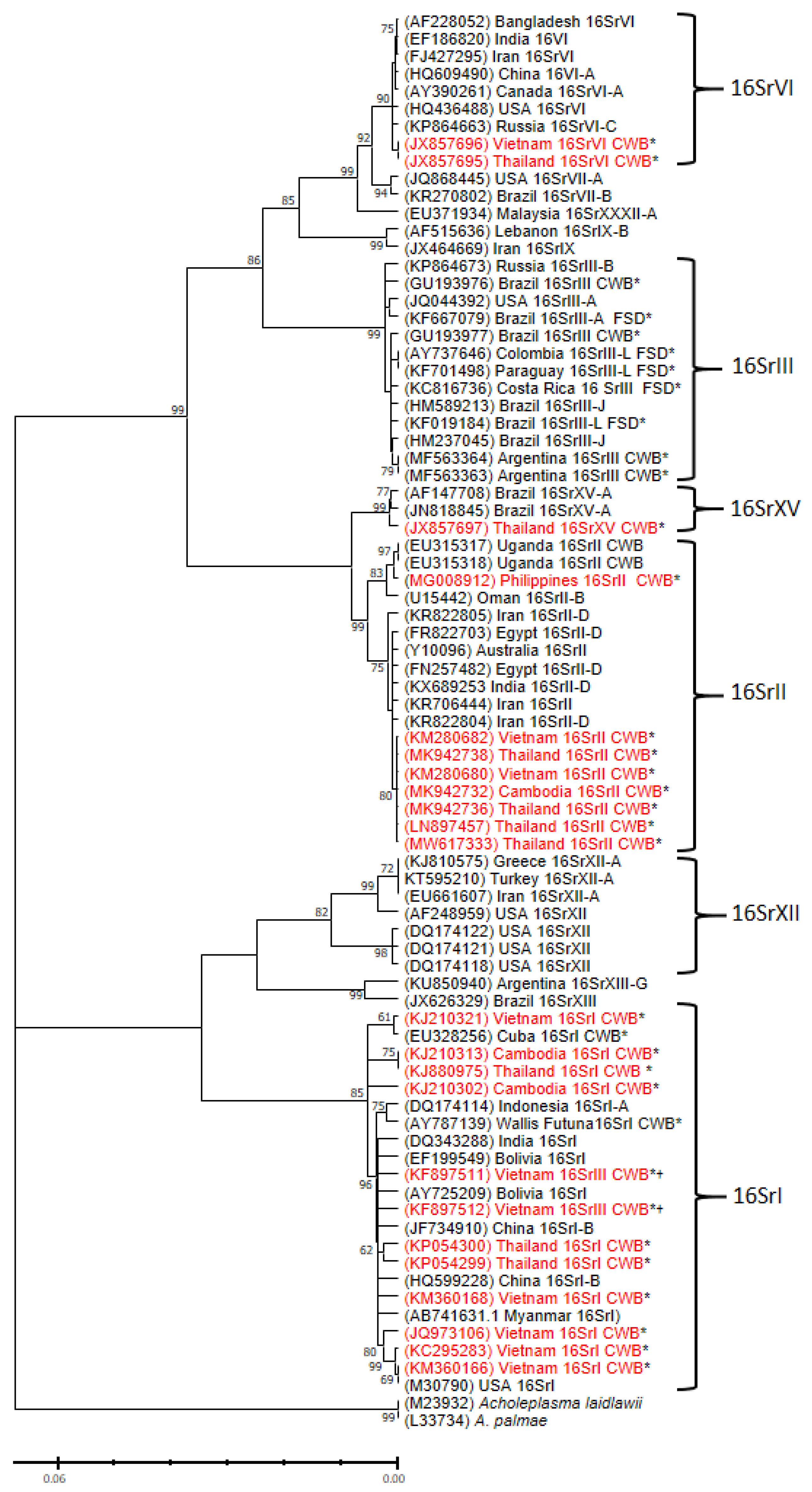
5. Conclusions and Future Insights
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Malik, A.I.; Kongsil, P.; Nguyễn, V.A.; Ou, W.; Srean, P.; López-Lavalle, L.A.B.; Utsumi, Y.; Lu, C.; Kittipadakul, P.; Nguyễn, H.H.; et al. Cassava breeding and agronomy in Asia: 50 years of history and future directions. Breed. Sci. 2020, 70, 18180. [Google Scholar] [CrossRef] [Green Version]
- Winotai, A.; Goergen, G.; Tamò, M.; Neuenschwander, P. Cassava mealybug has reached Asia. Biocontrol. News Inf. 2010, 31, 10N–11N. [Google Scholar]
- Muniappan, R.; Shepard, B.M.; Watson, G.W.; Carner, G.R.; Rauf, A.; Sartiami, D.; Hidayat, P.; Afun, J.V.K.; Goergen, G.; Rahman, A.K.M.Z. New Records of Invasive Insects (Hemiptera: Sternorrhyncha) in Southeast Asia and West Africa. J. Agric. Urban Entomol. 2009, 26, 167–174. [Google Scholar] [CrossRef]
- Graziosi, I.; Minato, N.; Alvarez, E.; Ngo, D.T.; Hoat, T.X.; Aye, T.M.; Pardo, J.M.; Wongtiem, P.; Wyckhuys, K.A. Emerging pests and diseases of South-east Asian cassava: A comprehensive evaluation of geographic priorities, management options and research needs. Pest Manag. Sci. 2016, 72, 1071–1089. [Google Scholar] [CrossRef]
- Siriwan, W.; Jimenez, J.; Hemniam, N.; Saokham, K.; Lopez-Alvarez, D.; Leiva, A.M.; Martinez, A.; Mwanzia, L.; Lopez-Lavalle, L.A.B.; Cuellar, W.J. Surveillance and Diagnostics of the emergent Sri Lankan cassava mosaic virus (Fam. Geminiviridae) in Southeast Asia. Virus Res. 2020, 285, 197959. [Google Scholar] [CrossRef]
- Delaquis, E.; Andersen, K.F.; Minato, N.; Cu, T.T.L.; Karssenberg, M.E.; Sok, S.; Wyckhuys, K.A.G.; Newby, J.C.; Burra, D.D.; Srean, P.; et al. Raising the Stakes: Cassava Seed Networks at Multiple Scales in Cambodia and Vietnam. Front. Sustain. Food Syst. 2008, 2, 73. [Google Scholar] [CrossRef]
- Alvarez, E.; Pardo, J.M.; Mejia, J.F.; Bertaccini, A.; Thanh, N.D.; Hoat, T.X. Detection and identification of ‘Candidatus Phytoplasma asteris’-related phytoplasmas associated with a witches’ broom disease of cassava in Vietnam. Phytopathogenic Mollicutes 2013, 3, 77–81. [Google Scholar] [CrossRef]
- Alvarez, E.; Pardo, J.M.; Truke, M.J. Detection and identification of ‘Candidatus Phytoplasma asteris’-related phytoplasma associated with a witches’ broom disease of cassava in Cambodia. Phytopathology 2014, 104, S3.7. [Google Scholar]
- Alvarez, E.; Pardo, J.M. Detection and Identification of ‘Candidatus Phytoplasma Asteris’-Related Phytoplasma Associated with a Witches’ Broom Disease of Cassava in Thailand; Abstract presented at the APS. In Proceedings of the Northeastern Division meeting, Quebec City, QC, Canada, 1–3 November 2017. [Google Scholar]
- Davis, R.I.; Arocha, Y.; Jones, P.; Malay, A. First report of the association of phytoplasmas with plant diseases in the territory of Wallis and Futuna. Australas. Plant Pathol. 2005, 34, 417–418. [Google Scholar]
- Kumari, S.; Nagendran, K.; Rai, A.B.; Singh, B.; Rao, G.P.; Bertaccini, A. Global status of phytoplasma diseases in vegetable crops. Front. Microbiol. 2019, 10, 1349. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weintraub, P.G.; Beanland, L. Insect vectors of phytoplasmas. Ann. Rev. Entomol. 2006, 51, 91–111. [Google Scholar] [CrossRef]
- Wei, W.; Trivellone, V.; Dietrich, C.H.; Zhao, Y.; Bottner-Parker, K.D.; Ivanauskas, A. Identification of phytoplasmas representing multiple new genetic lineages from phloem-feeding leafhoppers highlights the diversity of phytoplasmas and their potential vectors. Pathogens 2021, 10, 352. [Google Scholar] [CrossRef]
- IRPCM. ‘Candidatus Phytoplasma’, a taxon for the wall-less, non-helical prokaryotes that colonize plant phloem and insects. Int. J. Syst. Evol. Microbiol. 2004, 54, 1243–1255. [Google Scholar] [CrossRef] [Green Version]
- Lee, I.M.; Gundersen-Rindal, D.E.; Davis, R.E.; Bartoszyk, I.M. Revised classification scheme of phytoplasmas based on RFLP analyses of 16S rRNA and ribosomal protein gene sequences. Int. J. Syst Evol. Microbial. 1998, 48, 1153–1169. [Google Scholar] [CrossRef] [Green Version]
- Wei, W.; Davis, R.E.; Lee, M.; Zhao, Y. Computer-simulated RFLP analysis of 16S rRNA genes: Identification of ten new phytoplasma groups. Int. J. Syst. Evol. Microbiol. 2007, 57, 1855–1867. [Google Scholar] [CrossRef]
- Zhao, Y.; Wei, W.; Lee, M.; Shao, J.; Suo, X.; Davis, R.E. Construction of an interactive online phytoplasma classification tool, iPhyClassifier, and its application in analysis of the peach X-disease phytoplasma group (16SrIII). Int. J. Syst. Evol. Microbiol. 2009, 59 Pt 10, 2582. [Google Scholar] [CrossRef] [PubMed]
- Bertaccini, A.; Fiore, N.; Zamorano, A.; Tiwari, A.K.; Rao, G.P. Molecular and serological approaches in detection of phytoplasmas in plants and insects. In Phytoplasmas: Plant Pathogenic Bacteria-III; Springer: Singapore, 2019; pp. 105–136. [Google Scholar]
- Hemmati, C.; Nikooei, M.; Al-Sadi, A.M. Five decades of research on phytoplasma-induced witches’ broom diseases. CABI Rev. 2021, 16, 1–16. [Google Scholar] [CrossRef]
- Lee, I.M.; Bottner, K.D.; Sun, M. An Emerging Potato Purple Top Disease Associated with a New 16SrIII Group Phytoplasma in Montana. Plant Dis. 2009, 93, 970. [Google Scholar] [CrossRef] [PubMed]
- Brooks, D.R.; Hoberg, E.P.; Boeger, W.A.; Trivellone, V. Emerging infectious disease: An underappreciated area of strategic concern for food security. Transbound. Emerg. Dis. 2021, 69, 254–267. [Google Scholar] [CrossRef]
- Alvarez, E.; Mejía, J.F.; Llano, G.A.; Loke, J.B.; Calari, A.; Duduk, B.; Bertaccini, A. Characterization of a phytoplasma associated with frogskin disease in cassava. Plant Dis. 2009, 93, 1139–1145. [Google Scholar] [CrossRef] [Green Version]
- Pardo, J.M.; Alvarez, E.B.; Becerra Lopez-Lavalle, L.A.; Olaya, C.; Leiva, A.M.; Cuellar, W.J. Cassava Frogskin Disease: Current knowledge on a re-emerging disease in the Americas. Plants 2022, 11, 1841. [Google Scholar] [CrossRef]
- Oliveira, S.A.S.D.; Abreu, E.F.M.; Araújo, T.S.; Oliveira, E.J.; Andrade, E.C.; Garcia, J.M.P.; Alvarez, E. First report of a 16SrIII-L phytoplasma associated with frogskin disease in cassava (Manihot esculenta Crantz) in Brazil. Plant Dis. 2014, 98, 153. [Google Scholar] [CrossRef] [PubMed]
- Flôres, D.; Haas, I.C.; Canale, M.C.; Bedendo, I.P. Molecular identification of a 16SrIII-B phytoplasma associated with cassava witches’ broom disease. Eur. J. Plant Pathol. 2013, 137, 237–242. [Google Scholar] [CrossRef]
- Fernández, F.; Uset, A.; Baumgratz, G.; Conci, L. Detection and identification of a 16SrIII-J phytoplasma affecting cassava (Manihot esculenta Crantz) in Argentina. Australas. Plant Dis. Notes 2018, 13, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Vu, N.T.; Pardo, J.M.; Alvarez, E.; Le, H.H.; Wyckhuys, K.; Nguyen, K.L.; Le, D.T. Establishment of a loop-mediated isothermal amplification (LAMP) assay for the detection of phytoplasma-associated cassava witches’ broom disease. Appl. Biol. Chem. 2016, 59, 151–156. [Google Scholar] [CrossRef]
- Meinhardt, L.W.; Rincones, J.; Bailey, B.A.; Aime, M.C.; Griffith, G.W.; Zhang, D.; Pereira, G.A. Moniliophthora perniciosa, the causal agent of witches’ broom disease of cacao: What’s new from this old foe? Mol. Plant Pathol. 2008, 9, 577–588. [Google Scholar] [CrossRef]
- Komatsu, M.; Taniguchi, M.; Matsushita, N.; Takahashi, Y.; Hogetsu, T. Overwintering of Taphrina wiesneri within cherry shoots monitored with species-specific PCR. J. Gen. Plant Pathol. 2010, 76, 363–369. [Google Scholar] [CrossRef]
- Chen, J.Y.; Xu, X.D. Advances in research of Longan Witches’ Broom Disease. Acta Hortic. 2000, 558, 413–416. [Google Scholar] [CrossRef]
- Seo, J.K.; Kim, M.K.; Kwak, H.R.; Kim, J.S.; Choi, H.S. Complete genome sequence of longan witches’ broom-associated virus, a novel member of the family Potyviridae. Arch. Virol. 2017, 162, 2885–2889. [Google Scholar] [CrossRef]
- Doi, Y.; Teranaka, M.; Yora, K.; Asuyama, H. Mycoplasma-or PLT group-like microorganisms found in the phloem elements of plants infected with mulberry dwarf, potato witches’ broom, aster yellows, or paulownia witches’ broom. Jpn. J. Phytopathol. 1967, 33, 259–266. [Google Scholar] [CrossRef]
- Ayman, O.F.; Kumar, Y.; Hallan, V.; Zaidi, A.A. Molecular characterization of the phytoplasmas associated with toon trees and periwinkle in India. J. Gen. Plant Pathol. 2010, 76, 351–354. [Google Scholar] [CrossRef]
- Tomlinson, J.A.; Boonham, N.; Dickinson, M. Development and evaluation of a one-hour DNA extraction and loop-mediated isothermal amplification assay for rapid detection of phytoplasmas. Plant Pathol. 2010, 59, 465–471. [Google Scholar] [CrossRef]
- Hodgetts, J.; Boonham, N.; Mumford, R.; Harrison, N.; Dickinson, M. Phytoplasma phylogenetics based on analysis of secA and 23S rRNA gene sequences for improved resolution of candidate species of ‘Candidatus Phytoplasma’. Int. J. Syst. Evol. Microbiol. 2008, 58, 1826–1837. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saccardo, F.; Martini, M.; Palmano, S.; Ermacora, P.; Scortichini, M.; Loi, N.; Firrao, G. Genome drafts of four phytoplasma strains of the ribosomal group 16SrIII. Microbiology 2012, 158, 2805–2814. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smart, C.D.; Schneider, B.; Blomquist, C.L.; Guerra, L.J.; Harrison, N.A.; Ahrens, U.; Lorenz, K.H.; Seemüller, E.; Kirkpatrick, B.C. Phytoplasma-specific PCR primers based on sequences of the 16S-23S rRNA spacer region. Appl. Environ. Microbiol. 1996, 62, 2988–2993. [Google Scholar] [CrossRef] [Green Version]
- Martini, M.; Lee, I.M.; Bottner, K.D.; Zhao, Y.; Botti, S.; Bertaccini, A.; Harrison, N.A.; Carraro, L.; Marcone, C.; Khan, A.J.; et al. Ribosomal protein gene-based phylogeny for finer differentiation and classification of phytoplasmas. Int. J. Syst. Evol. Microbiol. 2007, 57, 2037–2051. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.M.; Zhao, Y.; Bottner, K.D. SecY gene sequence analysis for finer differentiation of diverse strains in the aster yellows phytoplasma group. Mol. Cell. Probes 2006, 20, 87–91. [Google Scholar] [CrossRef]
- Al-Subhi, A.M.; Hogenhout, S.; Al-Yahyai, R.A.; Al-Ghaithi, A.G.; Al-Sadi, A.M. Evaluating the effect of tuf and secA gene sequence length for discrimination of phytoplasmas. Can. J. Plant Pathol. 2021, 43, 209–215. [Google Scholar]
- Mitrović, J.; Contaldo, N.; Paltrinieri, S.; Mejia, J.F.; Mori, N.; Bertaccini, A.; Duduk, B. The use of groEL gene for characterization of aster yellows phytoplasmas in field collected samples. Bull. Insectol. 2011, 64, S17–S18. [Google Scholar]
- Mazraie, M.A.; Izadpanah, K.; Hamzehzarghani, H.; Salehi, M.; Faghihi, M.M. Spread and colonization pattern of ‘Candidatus Phytoplasma aurantifolia’in lime plants [Citrus aurantifolia (Christm.) Swingle] as revealed by real-time PCR assay. J. Plant Pathol. 2019, 101, 629–637. [Google Scholar] [CrossRef]
- Carminati, G.; Brusa, V.; Loschi, A.; Ermacora, P.; Martini, M. Spatiotemporal and quantitative Monitoring of the Fate of “Candidatus Phytoplasma Solani” in tomato plants infected by grafting. Pathogens 2021, 10, 811. [Google Scholar] [CrossRef]
- Lee, I.M.; Gundersen-Rindal, D.E.; Davis, R.E.; Bottner, K.D.; Marcone, C.; Seemüller, E. ‘Candidatus Phytoplasma asteris’, a novel phytoplasma taxon associated with aster yellows and related diseases. Int. J. Syst. Evol. Microbiol. 2004, 54, 1037–1048. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, I.M.; Hammond, R.W.; Davis, R.E.; Gundersen, D.E. Universal amplification and analysis of pathogen 16S rDNA for classification and identification of mycoplasmalike organisms. Phytopathology 1993, 83, 834–842. [Google Scholar] [CrossRef]
- Lee, I.M.; Gundersen, D.E.; Hammond, R.W.; Davis, R.E. Use of mycoplasmalike organism (MLO) group-specific oligonucleotide primers for nested-PCR assays to detect mixed-MLO infections in a single host plant. Phytopathology 1994, 84, 559–566. [Google Scholar] [CrossRef]
- Cuellar, W.J.; Mwanzia, L.; Lourido, D.; Martínez Rodriguez, R.; Garcia, C. PestDisPlace: Monitoring the Distribution of Pests and Diseases, 2018 v3.0. Available online: https://pestdisplace.org (accessed on 15 May 2023).
- Chittarath, K.; Jimenez, J.; Vongphachanh, P.; Leiva, A.M.; Sengsay, S.; Lopez-Alvarez, D.; Bounvilayvong, T.; Lourido, D.; Vorlachith, V.; Cuellar, W.J. First report of Sri Lankan cassava mosaic virus and cassava mosaic disease in Laos. Plant Dis. 2021, 105, 1861. [Google Scholar] [CrossRef]
- Wang, H.L.; Cui, X.Y.; Wang, X.W.; Liu, S.S.; Zhang, Z.H.; Zhou, X.P. First report of Sri Lankan cassava mosaic virus infecting cassava in Cambodia. Plant Dis. 2016, 100, 1029. [Google Scholar] [CrossRef]
- Malik, A.I.; Sophearith, S.; Delaquis, E.; Cuellar, W.J.; Jimenez, J.; Newby, J. Susceptibility of cassava varieties to disease caused by Sri Lankan cassava mosaic virus and impacts on yield by use of asymptomatic and virus-free planting material. Agronomy 2022, 12, 1658. [Google Scholar] [CrossRef]
- Musetti, R.; Buxa, S.V.; De Marco, F.; Loschi, A.; Polizzotto, R.; Kogel, K.H.; van Bel, A.J. Phytoplasma-triggered Ca2+ influx is involved in sieve-tube blockage. Mol. Plant. Microbe Interact. 2013, 26, 379–386. [Google Scholar] [CrossRef] [Green Version]
- Pagliari, L.; Martini, M.; Loschi, A.; Musetti, R. Looking inside phytoplasma-infected sieve elements: A combined microscopy approach using Arabidopsis thaliana as a model plant. Micron 2016, 89, 87–97. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; MacLean, A.M.; Sugio, A.; Maqbool, A.; Busscher, M.; Cho, S.T.; Kamoun, S.; Kuo, C.H.; Immink, R.G.; Hogenhout, S.A. Parasitic modulation of host development by ubiquitin-independent protein degradation. Cell 2021, 184, 5201–5214. [Google Scholar] [CrossRef]
- Zhao, Y.; Wei, W.; Lee, M.; Shao, J.; Suo, X.; Davis, R.E. The iPhyClassifier, an interactive online tool for phytoplasma classification and taxonomic assignment. In Phytoplasma; Humana Press: Totowa, NJ, USA, 2013; pp. 329–338. [Google Scholar]
- Arocha, Y.; Echodu, R.; Talengera, D.; Muhangi, J.; Rockefeller, E.; Asher, O.; Nakacwa, R.; Serugga, R.; Gumisiriza, G.; Tripathi, J.; et al. Occurrence of ‘Candidatus Phytoplasma aurantifolia’ (16SrII group) in cassava and four other species in Uganda. Plant Pathol. 2009, 58, 390. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Peterson, D.; Filipski, A.; Kumar, S. MEGA6: Molecular evolutionary genetics analysis version 6.0. Mol. Bio. Evol. 2013, 30, 2725–2729. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gracen, V.E.; Kongsil, P.; Napasintuwong, O.; Duangjit, J.; Phumichai, C. The Story of Kasetsart 50: The Most Important Cassava Variety in the World; Center for Agricultural Biotechnology, Ed.; Kasetsart University: Bangkok, Thailand, 2018. [Google Scholar]
- Eckstein, B.; Barbosa, J.C.; Kreyci, P.F.; Canale, M.C.; Brunelli, K.R.; Bedendo, I.P. Broccoli stunt, a new disease in broccoli plants is associated with three distinct phytoplasma groups in Brazil. J. Phytopathol. 2013, 161, 442–444. [Google Scholar] [CrossRef]
- Horsáková, J.; Nečasová, J.; Nečas, T. Determination of synergistic interactions between Plum pox virus and ‘Candidatus Phytoplasma prunorum’ in infected peach trees. Acta Hortic. 2017, 1163, 45–52. [Google Scholar] [CrossRef]


| Variety | Seed Type 1 | 22 d.a.p. | 66 d.a.p. | 158 d.a.p. | |||
|---|---|---|---|---|---|---|---|
| CMD | CWBD | CMD | CWBD | CMD | CWBD | ||
| KU50 | +selection | 2.1 (2.1) | 0 | 6.3 (3.9) | 8.3 (8.3) | 12.4 (4.0) | 24.5 (8.3) |
| CMD | 100 (0) | 0 | 75 (25) | 2.1 (2.1) | 100 (0) | 27.1 (2.1) | |
| CWBD | 20.8 (2.4) | 0 | 31.2 (7.1) | 12.5 (7.2) | 40.8 (4) | 29.2 (7.2) | |
| Rayong 5 | +selection | 16.7 (0) | 0 | 27.1 (3.9) | 0 | 39.6 (4) | 37.5 (0) |
| CMD | 97.9 (2.1) | 0 | 100 (0) | 0 | 100 (0) | 8.3 (0) | |
| CWBD | 56.3 (3.9) | 0 | 66.7 (14.2) | 4.2 (2.4) | 77.1 (5.9) | 45.8 (2.4) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pardo, J.M.; Chittarath, K.; Vongphachanh, P.; Hang, L.T.; Oeurn, S.; Arinaitwe, W.; Rodriguez, R.; Sophearith, S.; Malik, A.I.; Cuellar, W.J. Cassava Witches’ Broom Disease in Southeast Asia: A Review of Its Distribution and Associated Symptoms. Plants 2023, 12, 2217. https://doi.org/10.3390/plants12112217
Pardo JM, Chittarath K, Vongphachanh P, Hang LT, Oeurn S, Arinaitwe W, Rodriguez R, Sophearith S, Malik AI, Cuellar WJ. Cassava Witches’ Broom Disease in Southeast Asia: A Review of Its Distribution and Associated Symptoms. Plants. 2023; 12(11):2217. https://doi.org/10.3390/plants12112217
Chicago/Turabian StylePardo, Juan M., Khonesavanh Chittarath, Pinkham Vongphachanh, Le Thi Hang, Samoul Oeurn, Warren Arinaitwe, Rafael Rodriguez, Sok Sophearith, Al Imran Malik, and Wilmer J. Cuellar. 2023. "Cassava Witches’ Broom Disease in Southeast Asia: A Review of Its Distribution and Associated Symptoms" Plants 12, no. 11: 2217. https://doi.org/10.3390/plants12112217
APA StylePardo, J. M., Chittarath, K., Vongphachanh, P., Hang, L. T., Oeurn, S., Arinaitwe, W., Rodriguez, R., Sophearith, S., Malik, A. I., & Cuellar, W. J. (2023). Cassava Witches’ Broom Disease in Southeast Asia: A Review of Its Distribution and Associated Symptoms. Plants, 12(11), 2217. https://doi.org/10.3390/plants12112217







