Comparison of Various Drought Resistance Traits in Soybean (Glycine max L.) Based on Image Analysis for Precision Agriculture
Abstract
:1. Introduction
2. Results
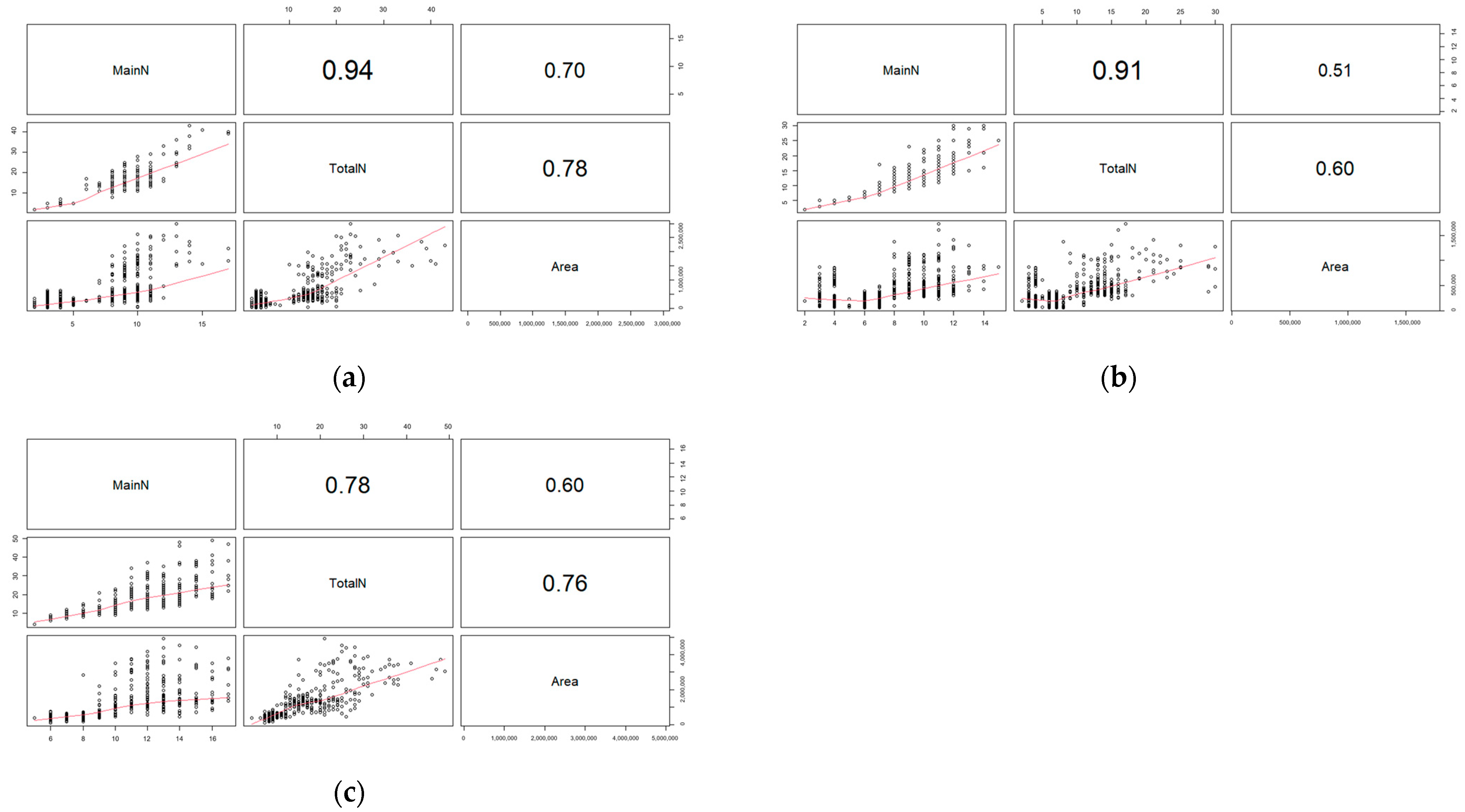
2.1. Validation with Actual Measured Data
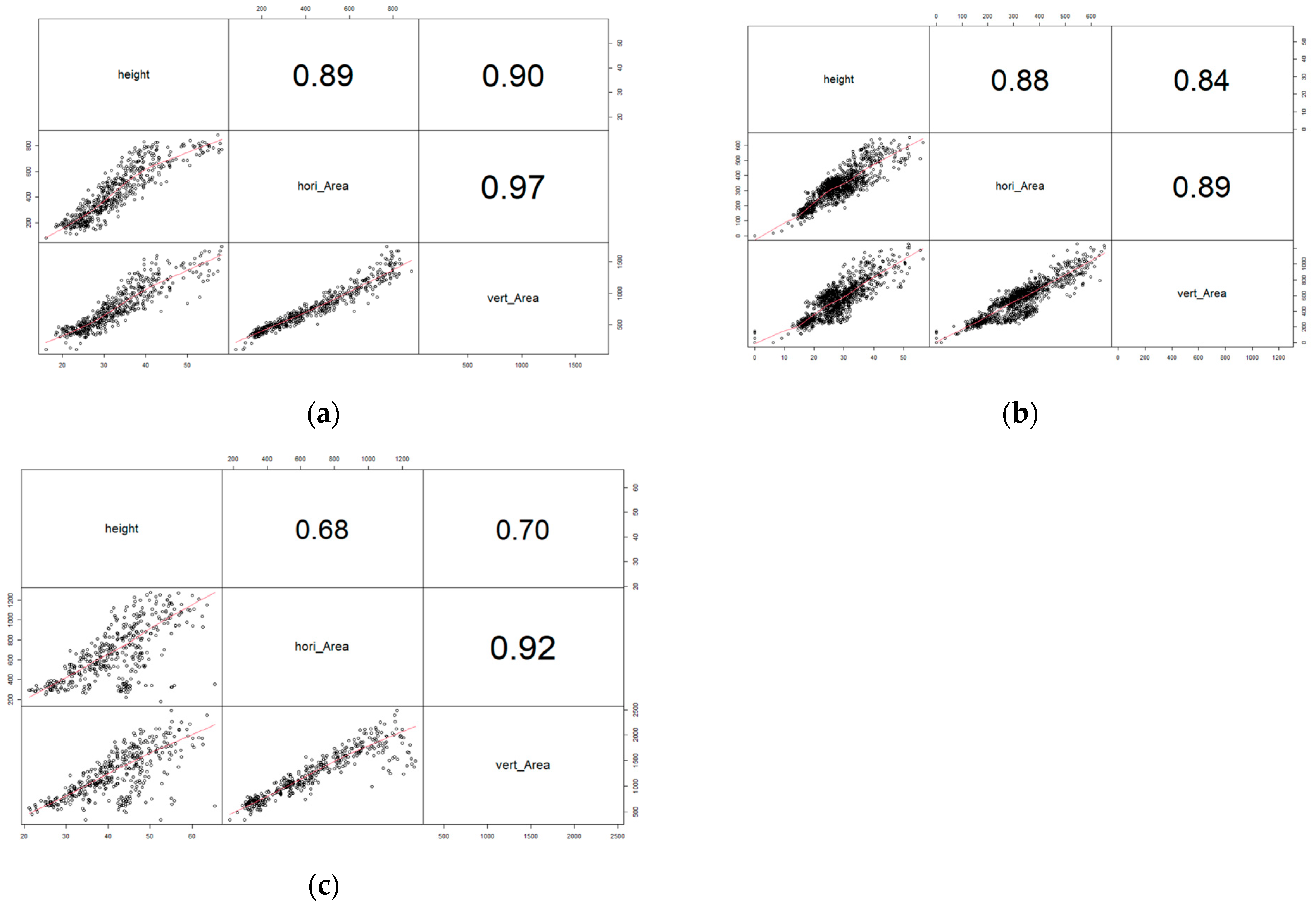
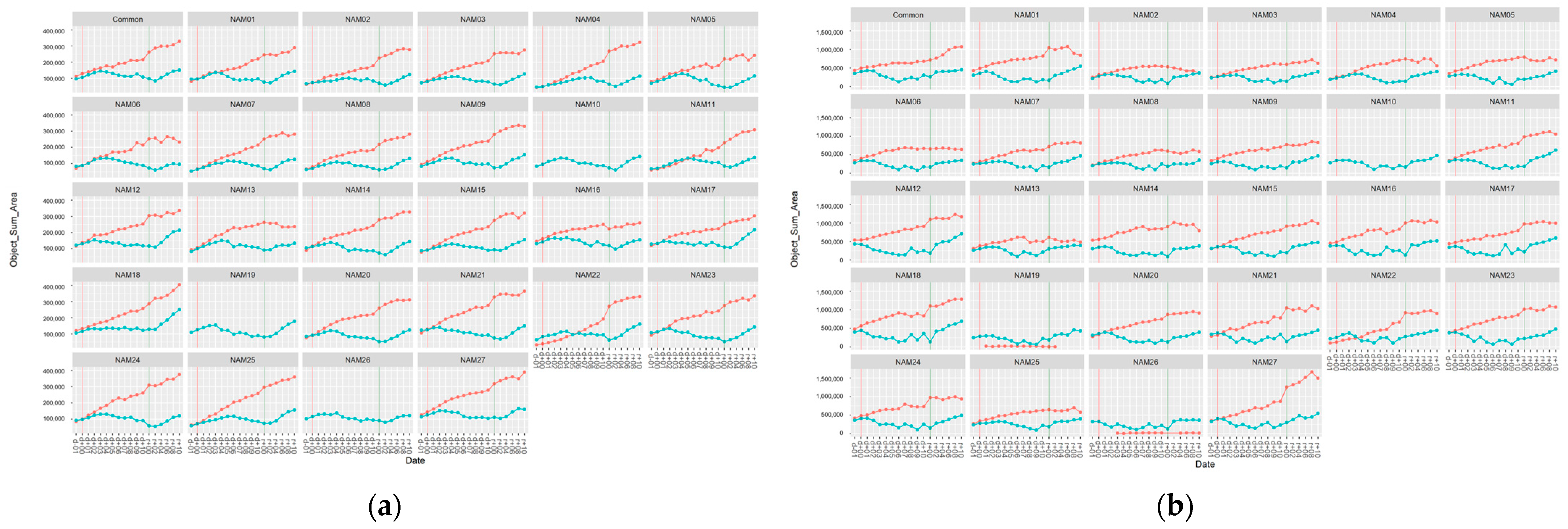
2.2. Area Related Image-Based Traits
2.3. Boundary Related Image-Based Traits
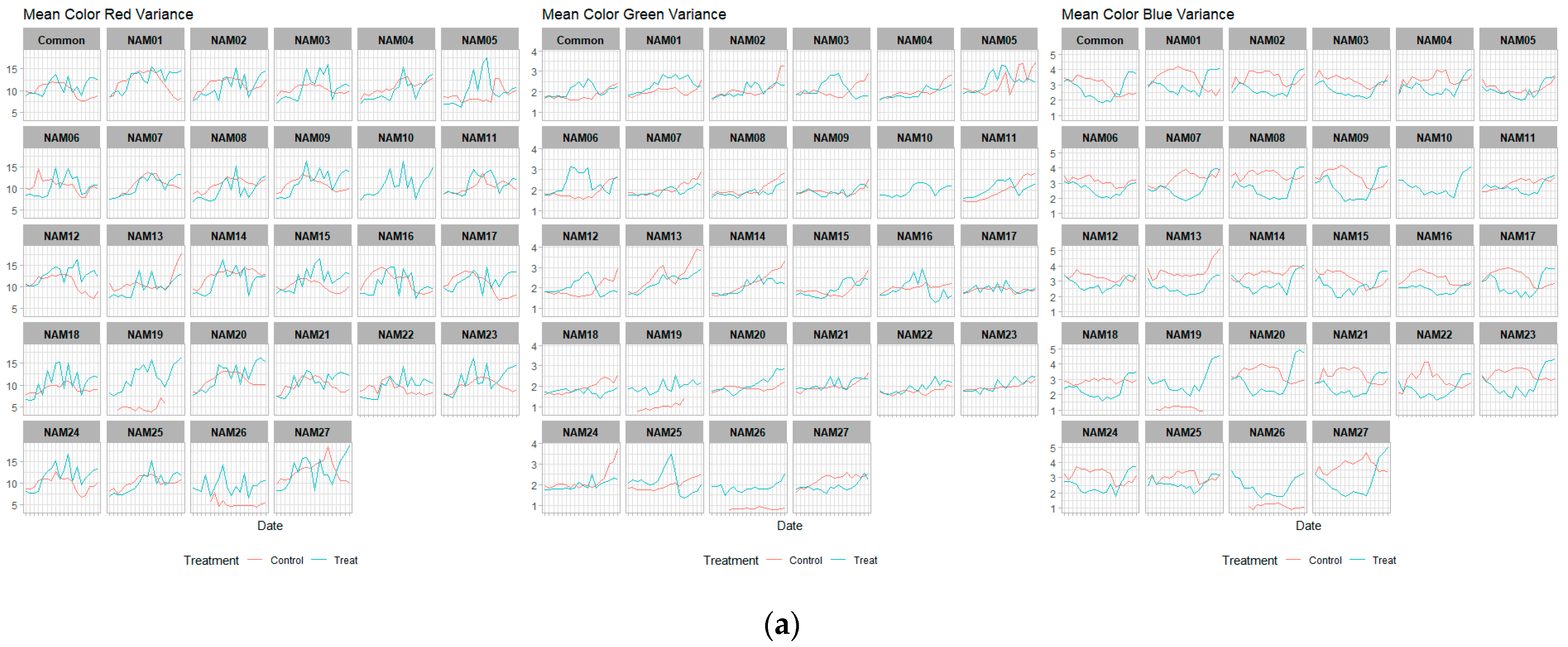
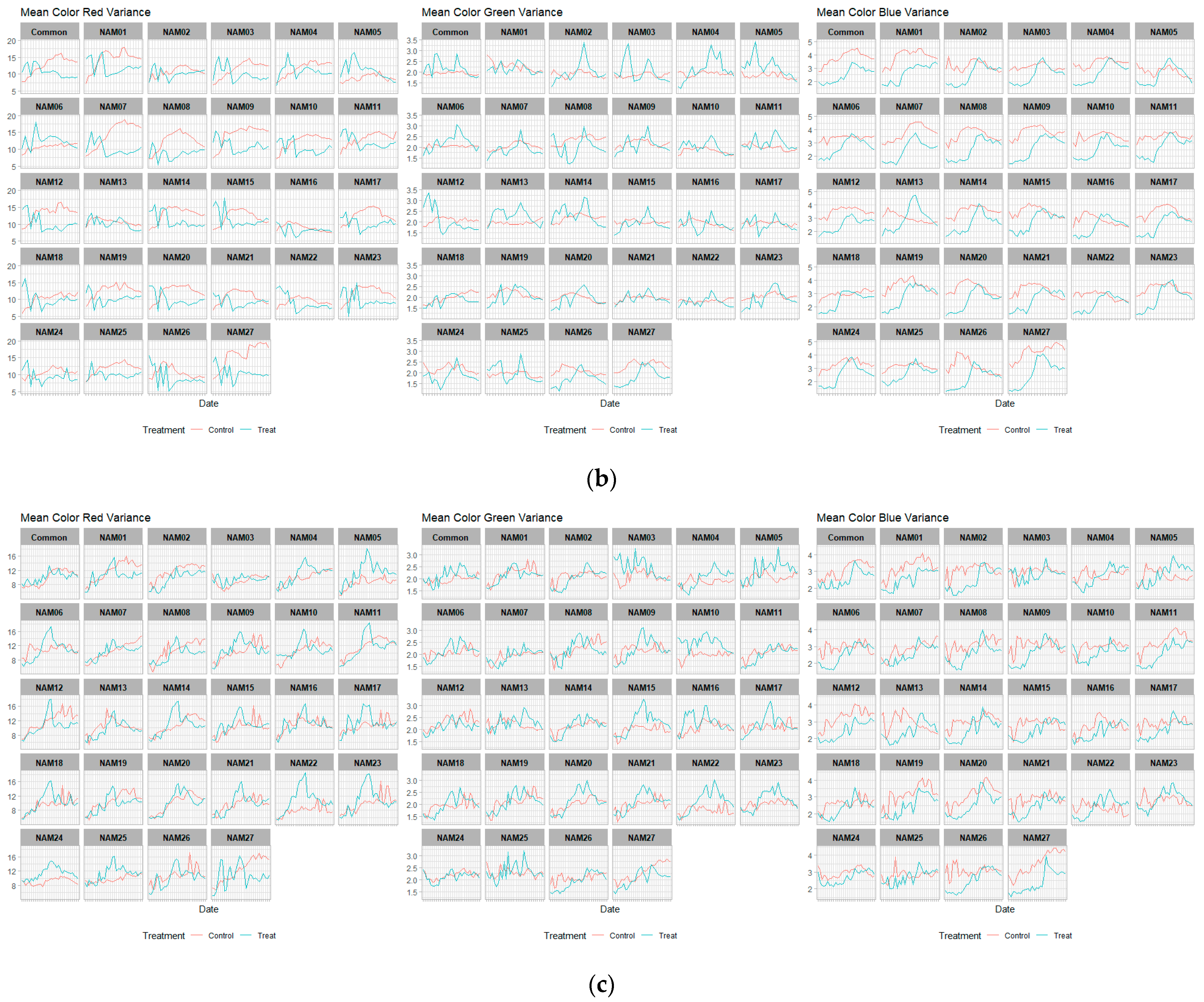
2.4. Color Related Image-Based Traits
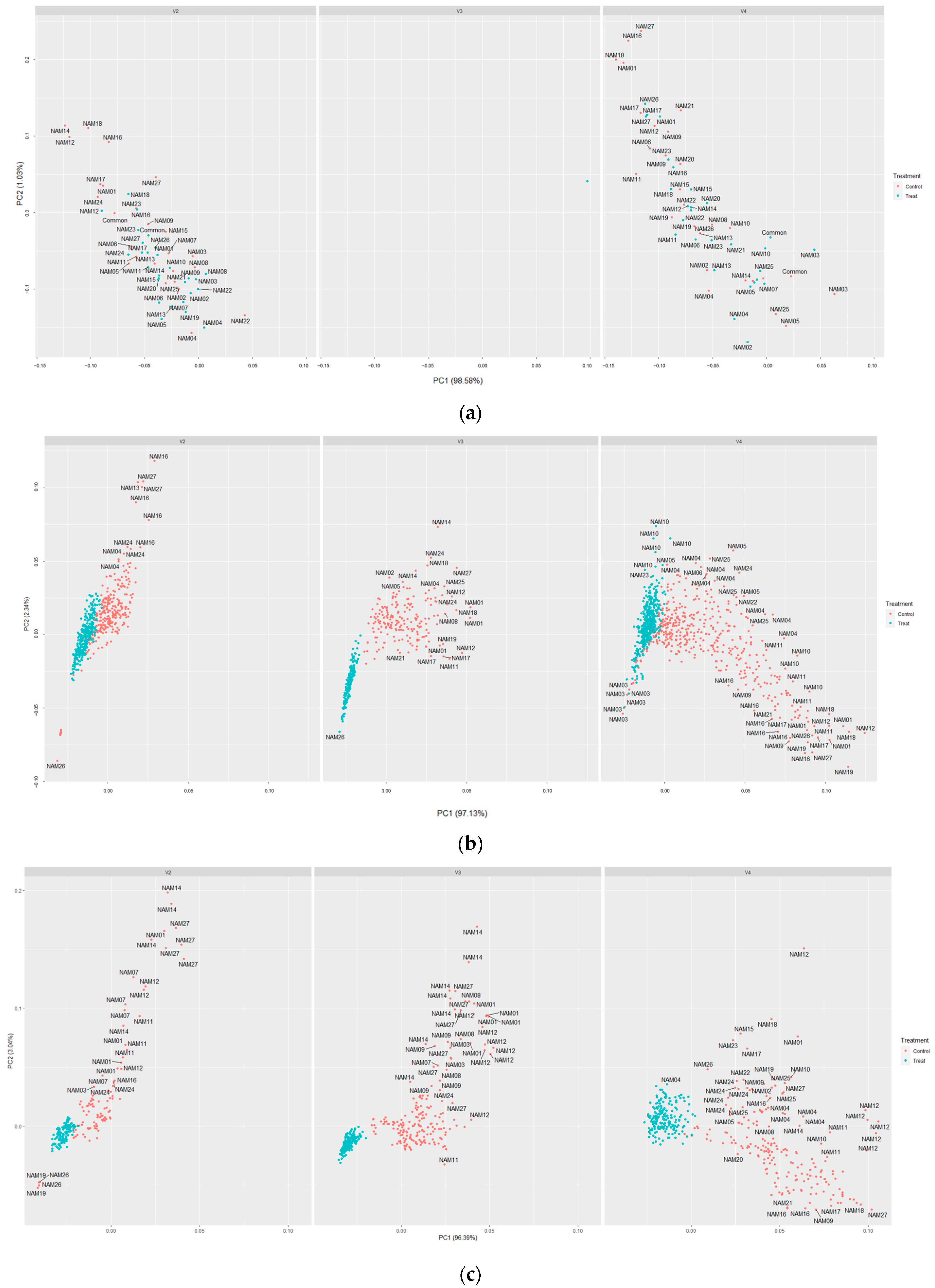
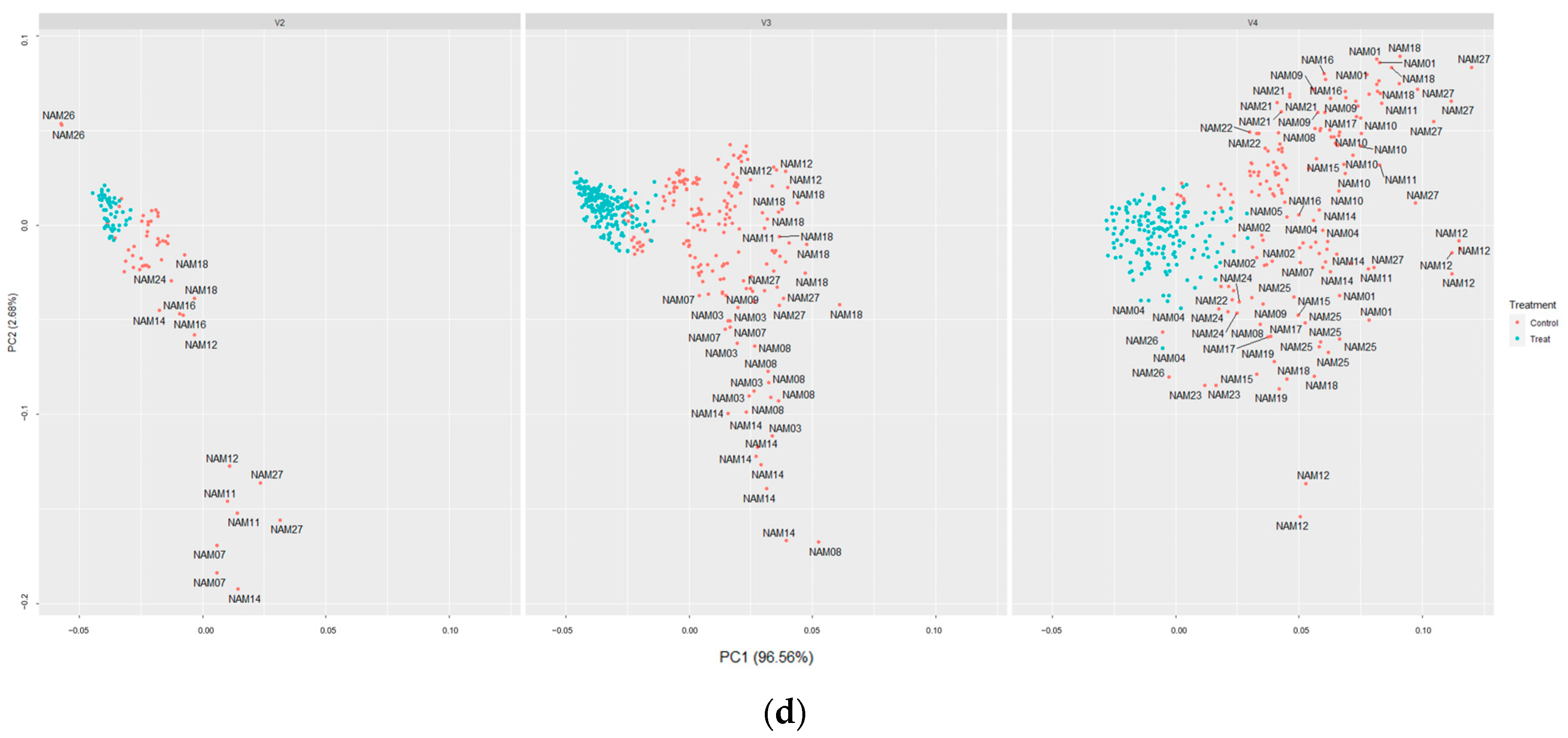
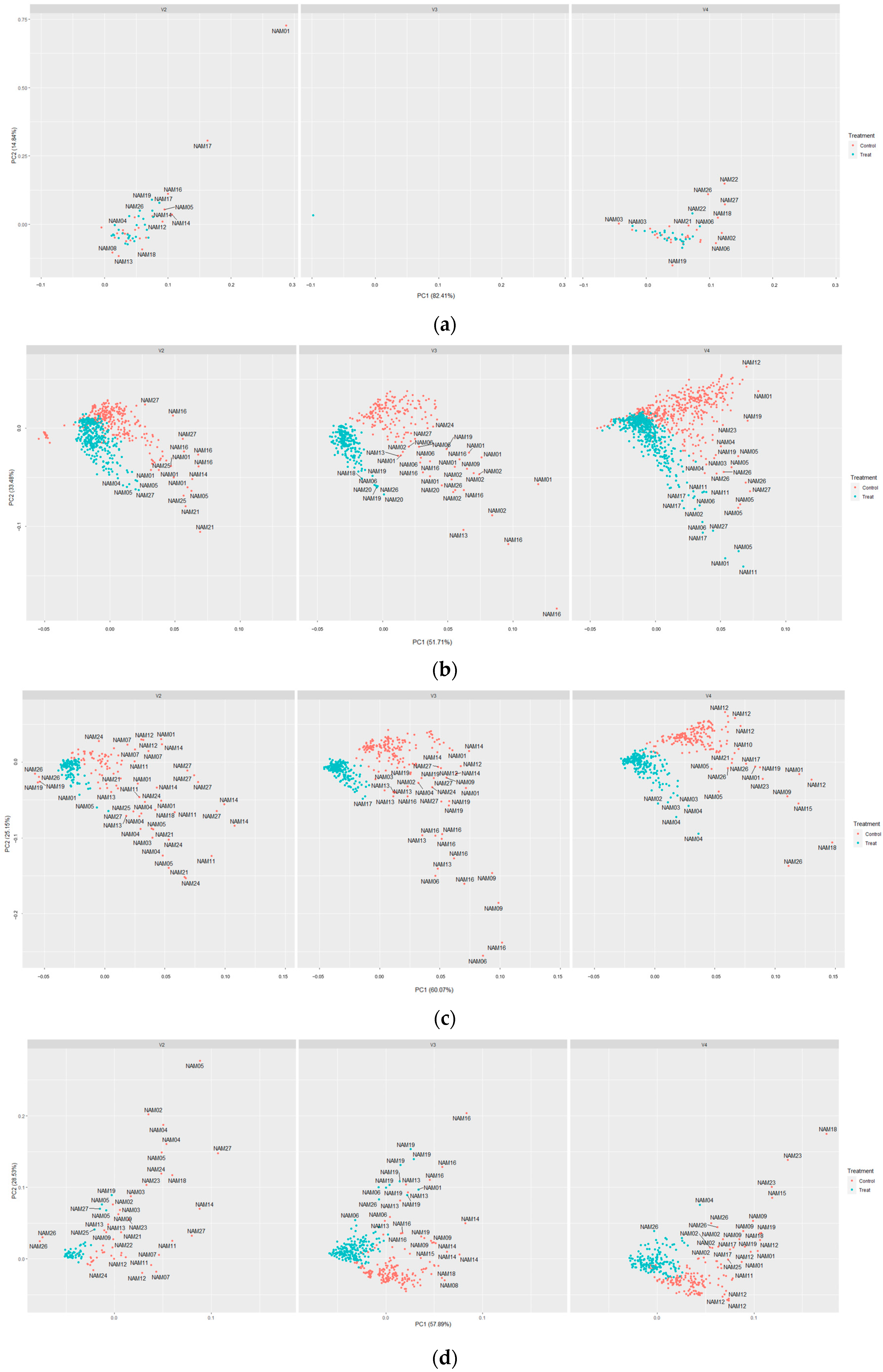
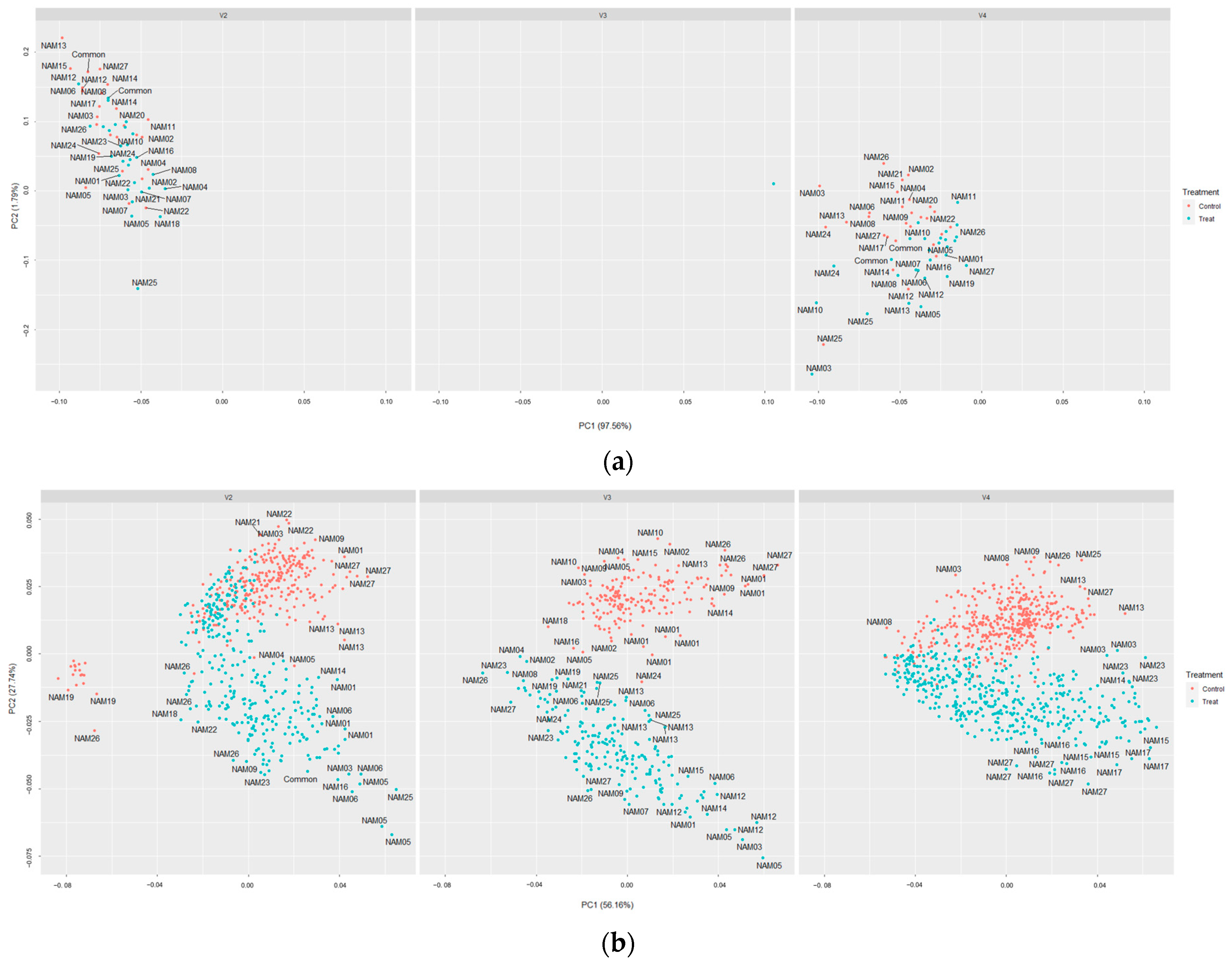
2.5. t-SNE
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Experiments
4.2. Image Acquisition
4.3. Image Processing and Analysis
4.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- El Mokhtar, M.A.; Anli, M.; Laouane, R.B.; Boutasknit, A.; Boutaj, H.; Draoui, A.; Zarik, L.; Fakhech, A. Food security and climate change. In Research Anthology on Environmental and Societal Impacts of Climate Change, 1st ed.; Information Resources Management Association, Ed.; Lgi Global: Hershey, PA, USA, 2022; pp. 44–63. [Google Scholar] [CrossRef]
- Onuşluel Gül, G.; Ali, G.; Mohamed, N. Historical evidence of climate change impact on drought outlook in river basins: Analysis of annual maximum drought severities through daily SPI definitions. Nat. Hazards 2022, 110, 1389–1404. [Google Scholar] [CrossRef]
- Zhang, R.; Qu, Y.; Zhang, X.; Wu, X.; Zhou, X.; Ren, B.; Zeng, J.; Wang, Q. Spatiotemporal variability in annual drought severity, duration, and frequency from 1901 to 2020. Clim. Res. 2022, 87, 81–97. [Google Scholar] [CrossRef]
- Ku, Y.S.; Au-Yeung, W.K.; Yung, Y.L.; Li, M.W.; Wen, C.Q.; Liu, X.; Lam, H.M. Drought stress and tolerance in soybean. In A Comprehensive Survey of International Soybean Research—Genetics, Physiology, Agronomy and Nitrogen Relationships, 1st ed.; Board, J., Ed.; IntechOpen: London, UK, 2013; pp. 209–237. [Google Scholar] [CrossRef]
- da Silva, E.C.; Nogueira, R.J.M.C.; da Silva, M.A.; de Albuquerque, M.B. Drought stress and plant nutrition. Plant Stress 2011, 5, 32–41. [Google Scholar]
- Bernier, J.; Atlin, G.N.; Serraj, R.; Kumar, A.; Spaner, D. Breeding upland rice for drought resistance. J. Sci. Food Agric. 2008, 88, 927–939. [Google Scholar] [CrossRef]
- Cui, Z.; Liu, H.; Zhang, H.; Huang, Z.; Tian, R.; Li, L.; Fan, W.; Chen, Y.; Chen, L.; Zhang, S.; et al. The comparison of ZFNs, TALENs, and SpCas9 by GUIDE-seq in HPV-targeted gene therapy. Mol. Ther. Nucleic Acids 2021, 26, 1466–1478. [Google Scholar] [CrossRef] [PubMed]
- Rahaman, M.M.; Chen, D.; Gillani, Z.; Klukas, C.; Chen, M. Advanced phenotyping and phenotype data analysis for the study of plant growth and development. Front. Plant Sci. 2015, 6, 619. [Google Scholar] [CrossRef] [Green Version]
- Mir, R.R.; Reynolds, M.; Pinto, F.; Khan, M.A.; Bhat, M.A. High-throughput phenotyping for crop improvement in the genomics era. Plant Sci. 2019, 282, 60–72. [Google Scholar] [CrossRef]
- Schirrmann, M.; Giebel, A.; Gleiniger, F.; Pflanz, M.; Lentschke, J.; Dammer, K.H. Monitoring agronomic parameters of winter wheat crops with low-cost UAV imagery. Remote Sens. 2016, 8, 706. [Google Scholar] [CrossRef] [Green Version]
- Sweet, D.D.; Tirado, S.B.; Springer, N.M.; Hirsch, C.N.; Hirsch, C.D. Opportunities and challenges in phenotyping row crops using drone-based RGB imaging. Plant Phenome J. 2022, 5, e20044. [Google Scholar] [CrossRef]
- Li, D.; Quan, C.; Song, Z.; Li, X.; Yu, G.; Li, C.; Muhammad, A. High-throughput plant phenotyping platform (HT3P) as a novel tool for estimating agronomic traits from the lab to the field. Front. Bioeng. Biotechnol. 2021, 8, 623705. [Google Scholar] [CrossRef]
- Bai, G.; Ge, Y. Crop Sensing and Its Application in Precision Agriculture and Crop Phenotyping. In Fundamentals of Agricultural and Field Robotics, 1st ed.; Karkee, M., Zhang, Q., Eds.; Springer: Berlin, Germany, 2021; pp. 137–155. [Google Scholar] [CrossRef]
- Cortes, P.M.; Sinclair, T.R. Water Relations of Field-Grown Soybean under Drought 1. Crop Sci. 1986, 26, 993–998. [Google Scholar] [CrossRef]
- Du, Y.; Zhao, Q.; Chen, L.; Yao, X.; Xie, F. Effect of drought stress at reproductive stages on growth and nitrogen metabolism in soybean. Agronomy 2020, 10, 302. [Google Scholar] [CrossRef] [Green Version]
- Tarumingkeng, R.C.; Coto, Z. Effects of drought stress on growth and yield of soybean. Kisman. Sci. Philos. 2003, 702, 798–807. [Google Scholar]
- Naik, H.S.; Zhang, J.; Lofquist, A.; Assefa, T.; Sarkar, S.; Ackerman, D.; Singh, A.; Singh, A.K.; Ganapathysubramanian, B. A real-time phenotyping framework using machine learning for plant stress severity rating in soybean. Plant Methods 2017, 13, 23. [Google Scholar] [CrossRef] [Green Version]
- Tetila, E.C.; Machado, B.B.; Belete, N.A.; Guimarães, D.A.; Pistori, H. Identification of soybean foliar diseases using unmanned aerial vehicle images. IEEE Geosci. Remote. Sens. Lett. 2017, 14, 2190–2194. [Google Scholar] [CrossRef]
- Ramos-Giraldo, P.; Reberg-Horton, C.; Locke, A.M.; Mirsky, S.; Lobaton, E. Drought stress detection using low-cost computer vision systems and machine learning techniques. IT Prof. 2020, 22, 27–29. [Google Scholar] [CrossRef]
- Board, J.E.; Tan, Q. Assimilatory capacity effects on soybean yield components and pod number. Crop Sci. 1995, 35, 846–851. [Google Scholar] [CrossRef]
- Sarkar, S.; Ramsey, A.F.; Cazenave, A.B.; Balota, M. Peanut leaf wilting estimation from RGB color indices and logistic models. Front. Plant Sci. 2021, 12, 713. [Google Scholar] [CrossRef]
- Riccardi, M.; Mele, G.; Pulvento, C.; Lavini, A.; d’Andria, R.; Jacobsen, S.E. Non-destructive evaluation of chlorophyll content in quinoa and amaranth leaves by simple and multiple regression analysis of RGB image components. Photosynth. Res. 2014, 120, 263–272. [Google Scholar] [CrossRef]
- Python/C API Reference Manual. Python Software Foundation. Available online: http://sephounet.free.fr/pdf/python/api.pdf (accessed on 2 November 2022).
- Schneider, C.; Rasband, W.; Eliceiri, K. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Otsu, N. A threshold selection method from gray-level histograms. IEEE Trans. Sys. Man. Cyber. 1979, 9, 62–66. [Google Scholar] [CrossRef] [Green Version]
- Van der Maaten, L.; Hinton, G. Visualizing data using t-SNE. J. Mach. Learn. Res. 2008, 9, 2579–2605. [Google Scholar]




| Days after Drought Treatment (Days) | Stages | p Value (<0.05) *, ** | ||||
|---|---|---|---|---|---|---|
| Height | Main N | Total N | Pods | |||
| Before drought treatment | (−7) | V2 | NA | NA | NA | NA |
| V3 | 0.5644 | NA | NA | NA | ||
| V4 | 0.2909 | NA | NA | NA | ||
| 1st day of drought treatment | (0) | V2 | NA | 0.4895 | 0.5968 | NA |
| V3 | 0.3319 | NA | NA | NA | ||
| V4 | 0.6659 | NA | NA | NA | ||
| During drought treatment | (+7) | V2 | <0.05 | <0.05 | <0.05 | NA |
| V3 | <0.05 | <0.05 | <0.05 | NA | ||
| V4 | 0.2513 | <0.05 | <0.05 | NA | ||
| End of drought treatment/Recoverying started | (+14) | V2 | <0.05 | <0.05 | <0.05 | NA |
| V3 | <0.05 | <0.05 | <0.05 | NA | ||
| V4 | <0.05 | <0.05 | <0.05 | NA | ||
| During recovery | (+21) | V2 | <0.05 | <0.05 | <0.05 | NA |
| V3 | <0.05 | <0.05 | <0.05 | NA | ||
| V4 | <0.05 | 0.0522 | <0.05 | NA | ||
| End of recoverying period | (+28) | V2 | <0.05 | 0.3395 | 0.8427 | 0.01479 |
| V3 | <0.05 | 0.1934 | 0.4445 | 0.7254 | ||
| V4 | <0.05 | <0.05 | <0.05 | 0.6582 | ||
| Image-Based Traits | Angle | Vegetative Stage | p (<0.005) |
|---|---|---|---|
| Area | Top | V2 | <0.005 * |
| V3 | 0.006714 | ||
| V4 | <0.005 * | ||
| Side | V2 | <0.005 * | |
| V3 | 0.006255 | ||
| V4 | <0.005 * | ||
| Caliper length | Top | V2 | 0.007195 |
| V3 | <0.005 * | ||
| V4 | <0.005 * | ||
| Side | V2 | <0.005 * | |
| V3 | <0.005 * | ||
| V4 | <0.005 * | ||
| Convex hull area | Top | V2 | <0.005 * |
| V3 | 0.009479 | ||
| V4 | <0.005 * | ||
| Side | V2 | <0.005 * | |
| V3 | 0.0006882 | ||
| V4 | <0.005 * | ||
| Min area rectangle area | Top | V2 | <0.005 * |
| V3 | 0.007009 | ||
| V4 | <0.005 * | ||
| Side | V2 | <0.005 * | |
| V3 | <0.005 * | ||
| V4 | 0.00161 | ||
| Object sum area | Top | V2 | <0.005 * |
| V3 | 0.00297 | ||
| V4 | <0.005 * | ||
| Side | V2 | <0.005 * | |
| V3 | 0.006255 | ||
| V4 | <0.005 * |
| Image-Based Traits | Variables | Angle | Vegetative Stage | p (<0.005) |
|---|---|---|---|---|
| Boundary point roundness | Drought treatment | Top | V2 | 0.4897 |
| V3 | 0.02647 | |||
| V4 | 0.3807 | |||
| Side | V2 | <0.005 * | ||
| V3 | <0.005 * | |||
| V4 | <0.005 * | |||
| Circumference | Varieties | Top | V2 | <0.005 * |
| V3 | <0.005 * | |||
| V4 | <0.005 * | |||
| Side | V2 | <0.005 * | ||
| V3 | <0.005 * | |||
| V4 | <0.005 * | |||
| Convex hull circumference | Varieties | Top | V2 | <0.005 * |
| V3 | 0.008018 | |||
| V4 | <0.005 * | |||
| Side | V2 | <0.005 * | ||
| V3 | <0.005 * | |||
| V4 | <0.005 * | |||
| Min enclosing circle diameter | Varieties | Top | V2 | <0.005 * |
| V3 | 0.005517 | |||
| V4 | <0.005 * | |||
| Side | V2 | <0.005 * | ||
| V3 | <0.005 * | |||
| V4 | <0.005 * | |||
| Roundness | Varieties | Top | V2 | 0.4303 |
| V3 | 0.001177 | |||
| V4 | <0.005 * | |||
| Side | V2 | 0.007898 | ||
| V3 | <0.005 * | |||
| V4 | <0.005 * |
| Image-Based Traits | Angle | Vegetative Stage | p (<0.005) |
|---|---|---|---|
| Mean color red variance | Top | V2 | <0.005 * |
| V3 | <0.005 * | ||
| V4 | 0.1753 | ||
| Side | V2 | 0.2799 | |
| V3 | <0.005 * | ||
| V4 | <0.005 * | ||
| Mean color green variance | Top | V2 | 0.1009 |
| V3 | <0.005 * | ||
| V4 | <0.005 * | ||
| Side | V2 | <0.005 * | |
| V3 | 0.308 | ||
| V4 | <0.005 * | ||
| Mean color blue variance | Top | V2 | <0.005 * |
| V3 | <0.005 * | ||
| V4 | <0.005 * | ||
| Side | V2 | <0.005 * | |
| V3 | <0.005 * | ||
| V4 | <0.005 * |
| Numbering | Variety |
|---|---|
| Common | Daepung |
| NAM 01 | Bangsa |
| NAM 02 | Pungwon |
| NAM 03 | Hannam |
| NAM 04 | Sowon |
| NAM 05 | Galche |
| NAM 06 | Somyeong |
| NAM 07 | Sinhwa |
| NAM 08 | Pureun |
| NAM 09 | Taegwang |
| NAM 10 | Wuram |
| NAM 11 | Danbek |
| NAM 12 | PI96983 |
| NAM 13 | Haman |
| NAM 14 | Willians82 |
| NAM 15 | Saedanbek |
| NAM 16 | Daewon |
| NAM 17 | Hwanggeum |
| NAM 18 | Chungja |
| NAM 19 | Chungja 3ho |
| NAM 20 | Sochung 2ho |
| NAM 21 | Ilpumgeomjung |
| NAM 22 | Daeheuk |
| NAM 23 | Josangseori |
| NAM 24 | Yeunpung |
| NAM 25 | Chunal |
| NAM 26 | Heukchung |
| NAM 27 | Seoritae |
| Process * | Features | Materials |
|---|---|---|
| Data acquisition | V2 drought treatment V3 drought treatment V4 drought treatment | Imaging chamber |
| Preprocessing | Image crop Object area selection Scale settings | Python |
| Data processing | Noise removal Channel separation Binary image creation Region of Interest Shape measurement Color measurement | Image J |
| Data analysis | Outlier detection and removal Data validation Data analysis | R programming |
| Types | Image-Based Traits | Features |
|---|---|---|
| Area | Area | Number of pixels in projected area. |
| Caliper Length | The longest distance in the object. | |
| Convex Hull Area | Number of pixels in convex hull area. | |
| Min Area Rectangle Area | Number of pixels in an area of the smallest rectangle that can contain the projected object. | |
| Object Sum Area | The sum of the numbers of the pixel of all projected objects in the image. | |
| Boundary | Boundary Point Roundness | The ratio of the boundary points of the object to the area of the circle that diameter is equal to the maximum diameter of the object. |
| Circumference | Circumference of the smallest circle that can contain the projected object. | |
| Convex Hull Circumference | Circumference of the smallest circle that can contain a convex hull. | |
| Min Enclosing Circle Diameter | Diameter of the smallest circle that can contain the projected object. | |
| Roundness | The ratio of the object to the area of the circle that diameter is equal to the maximum diameter of the object. | |
| Color | Mean Color Red Variance | Variance in mean R values in projected object. |
| Mean Color Green Variance | Variance in mean G values in projected object. | |
| Mean Color Blue Variance | Variance in mean B values in projected object. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.; Lee, C.; Park, J.; Kim, N.; Kim, S.-L.; Baek, J.; Chung, Y.-S.; Kim, K. Comparison of Various Drought Resistance Traits in Soybean (Glycine max L.) Based on Image Analysis for Precision Agriculture. Plants 2023, 12, 2331. https://doi.org/10.3390/plants12122331
Kim J, Lee C, Park J, Kim N, Kim S-L, Baek J, Chung Y-S, Kim K. Comparison of Various Drought Resistance Traits in Soybean (Glycine max L.) Based on Image Analysis for Precision Agriculture. Plants. 2023; 12(12):2331. https://doi.org/10.3390/plants12122331
Chicago/Turabian StyleKim, JaeYoung, Chaewon Lee, JiEun Park, Nyunhee Kim, Song-Lim Kim, JeongHo Baek, Yong-Suk Chung, and Kyunghwan Kim. 2023. "Comparison of Various Drought Resistance Traits in Soybean (Glycine max L.) Based on Image Analysis for Precision Agriculture" Plants 12, no. 12: 2331. https://doi.org/10.3390/plants12122331
APA StyleKim, J., Lee, C., Park, J., Kim, N., Kim, S.-L., Baek, J., Chung, Y.-S., & Kim, K. (2023). Comparison of Various Drought Resistance Traits in Soybean (Glycine max L.) Based on Image Analysis for Precision Agriculture. Plants, 12(12), 2331. https://doi.org/10.3390/plants12122331






