Agromorphological and Physiological Performance of Ethiopian Common Bean (Phaseolus vulgaris L.) Genotypes under Different Agroecological Conditions
Abstract
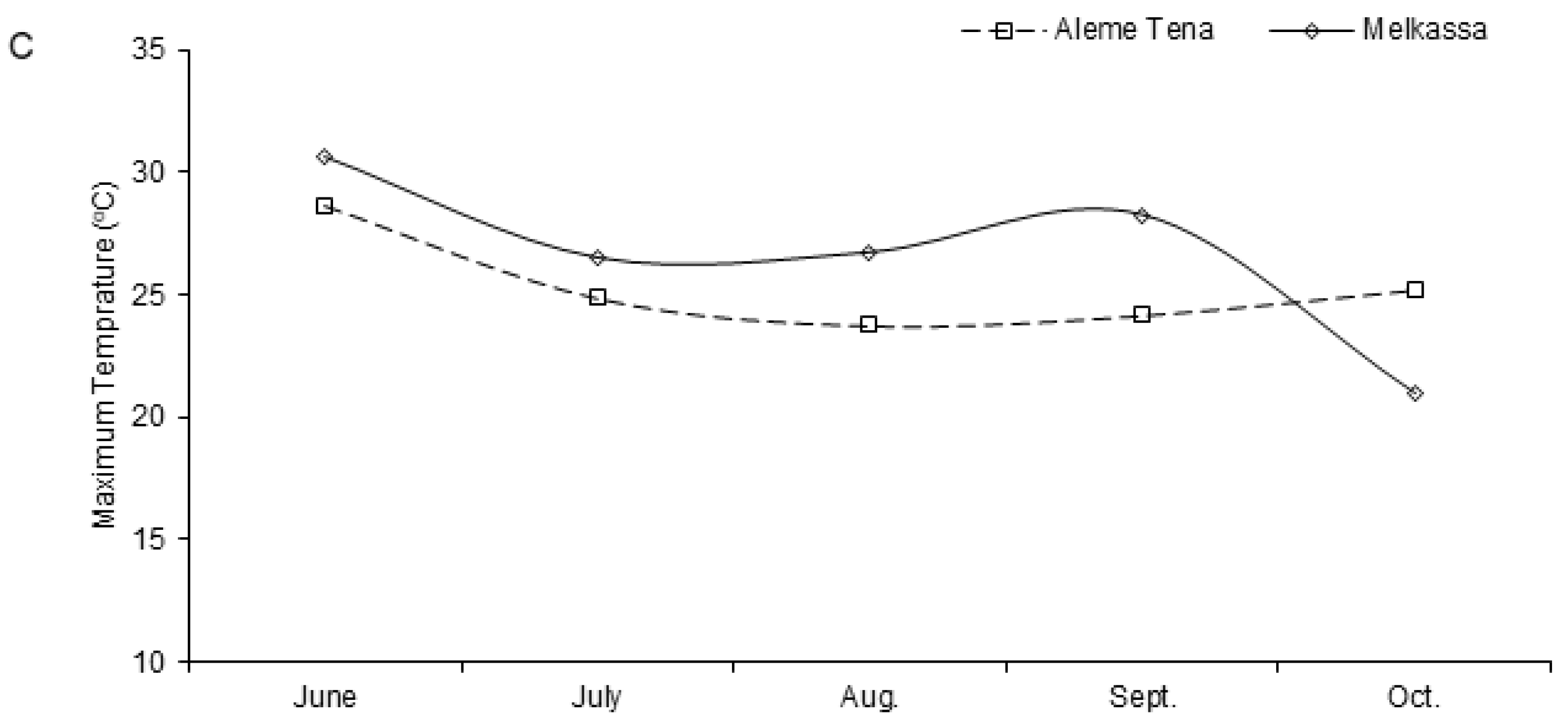
:1. Introduction
2. Results
2.1. Agronomic Performance
2.2. Principal Component Analysis (PCA)
2.3. Correlations of Yield and Its Components
2.4. Cluster Analysis
2.5. Performances of Genotypes in Different Clusters
3. Discussion
3.1. Agronomic Performance
3.2. Correlations of Yield and Its Components
3.3. Cluster Analysis
4. Materials and Methods
4.1. Description of the Study Site
4.2. Experimental Material and Experimental Design
4.3. Data Collection
4.4. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Broughton, W.J.; Hernandez, G.; Blair, M.; Beebe, S.; Gepts, P.; Vanderleyden, J. Beans (Phaseolus spp.)–Model food legumes. Plant Soil 2003, 252, 55–128. [Google Scholar] [CrossRef] [Green Version]
- Buruchara, R.; Chirwa, R.; Sperling, L.; Mukankusi, C.; Rubyogo, J.C.; Muthoni, R.; Abang, M.M. Development and delivery of bean varieties in Africa: The Pan-Africa Bean Research Alliance (PABRA) model. Afr. Crop Sci. J. 2011, 19, 227–245. [Google Scholar]
- Asfaw, A.; Blair, M.W.; Almekinders, C. Genetic diversity and population structure of common bean (Phaseolus vulgaris L.) landraces from the East African highlands. Theor. Appl. Genet. 2009, 120, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Hillocks, R.J.; Madata, C.S.; Chirwa, R.; Minja, E.M.; Msolla, S. Phaseolus bean improvement in Tanzania, 1959–2005. Euphytica 2006, 150, 215–231. [Google Scholar] [CrossRef]
- Karanja, D.; Endire, S.G.; Ruraduma, C.; Kimani, P.M.; Kweka, S.O.; Louis, B. Value-Added Bean Technologies for Enhancing Foodsecurity, Nutrition, Income and Resilience to Cope with Climate Change and Variability Challenges in Eastern Africa; International Livestock Research Institute (ILRI): Nairobi, Kenya, 2011. [Google Scholar]
- Ferris, S.; Kaganzi, E. Evaluating Marketing Opportunities for Haricot Beans in Ethiopia. IPMS (Improving Productivity and Market success) of Ethiopian Farmers Project Working Paper 7; ILRI (International Livestock Research Institute): Nairobi, Kenya, 2008. [Google Scholar]
- CSA. Report on Area and Production of Crops: Agricultural Sample Survey on Private Peasant Holdings of 2014/2015 Meher Season; Central Statistic Authority: Addis Ababa, Ethiopia, 2015. [Google Scholar]
- Legesse, D.; Kumssa, G.; Assefa, T.; Taha, M.; Gobena, J.; Alema, T.; Abebe, A.; Mohhamed, Y.; Terefe, H. Production and Marketing of White Pea Beans in the Rift Valley, Ethiopia. Natl. Bean Res. Program Ethiop. Inst. Agric. Res. 2006, 2, 88–92. [Google Scholar]
- Assefa, T.; Rubyogo, J.C.; Sperling, L.; Amsalu, B.; Deressa, A.; Reda, F.R.; Kirkby, R.; Buruchara, R. Creating partnerships for enhanced impact: Bean variety delivery in Ethiopia. J. Crop Sci. Soci. 2006, 12, 1–17. [Google Scholar]
- Fisseha, Z. Genetic Diversity and Population Structure of Common Bean (Phaseolus vulgaris L.) Germplasm from Ethiopia. Ph.D. Thesis, Addis Ababa University, Addis Ababa, Ethiopia, 2015. [Google Scholar]
- Sperling, L. The effect of the civil war on Rwanda’s bean seed systems and unusual bean diversity. Biodiv. Conser. 2001, 10, 989–1009. [Google Scholar] [CrossRef]
- Wortmann, C.S.; Kirkby, R.A.; Eledu, C.A.; Allen, D.J. Atlas of Common Bean (Phaseolus vulgaris L.) production in Africa; CIAT-Pan-African Bean Research Alliance: Kampala, Uganda, 1998. [Google Scholar]
- Shashidhar, H.; Kanbar, A.; Toorchi, M.; Raveendra, G.M.; Kundur, P.; Vimarsha, H.S.; Soman, R.; Kumar, N.G.; Bekele, B.D.; Bhavani, P. Breeding for drought resistance using whole plant architecture: Conventional and molecular approach. In Plant Breeding from Laboratories to Fields; Andersen, S.B., Ed.; Tech Open Access Publisher: Vienna, Austria, 2013; pp. 151–166. [Google Scholar]
- Fisseha, Z.; Tesfaye, K.; Dagne, K.; Blair, M.W.; Harvey, J.; Kyallo, M.; Gepts, P. Genetic diversity and population structure of common bean (Phaseolus vulgaris L.) germplasm of Ethiopia as revealed by microsatellite markers. Afr. J. Biotechnol. 2016, 15, 2824–2847. [Google Scholar]
- Dagnew, K.; Haileselassie, T.; Feyissa, T. Genetic diversity study of common bean (Phaseolus vulgaris L.) germplasm from Ethiopia using inter simple sequence repeat (ISSR) markers. Afr. J. Biotech. 2014, 13, 3638–3649. [Google Scholar]
- Ceccarelli, S.; Acevedo, E.; Grando, S. Breeding for yield stability in unpredictable environments: Single traits, interaction between traits, and architecture of genotypes. Euphytica 1991, 56, 169–185. [Google Scholar] [CrossRef]
- Ceccarelli, S. Specific adaptation and breeding for marginal conditions. Euphytica 1994, 77, 205–219. [Google Scholar] [CrossRef]
- Teame, G.; Ephrem, S.; Getachew, B. Performance evaluation of common bean (Phaseolus vulgaris L.) varieties in Raya Valley, Northern Ethiopia. Afr. J. Plant Sci. 2017, 11, 4B6E27E62320. [Google Scholar] [CrossRef] [Green Version]
- Scarano, D.; Rubio, F.; José, J.; Rao, R.; Corrado, G. Scientia Horticulturae Morphological and genetic diversity among and within common bean (Phaseolus vulgaris L.) landraces from the Campania region. Sci. Hortic. 2014, 180, 72–78. [Google Scholar] [CrossRef]
- Boros, L.; Wawer, A.; Borucka, K. Morphological, phonological and agronomical characterization of variability among common bean (Phaseolus vulgaris L.) local population from the national center for plant genetic resources: Polish genebank. J. Hort. Res. 2014, 22, 123–130. [Google Scholar]
- Burle, M.L.; Fonseca, J.R.; del Peloso, M.J.; Melo, L.C.; Temple, S.R.; Gepts, P. Integrating phenotypic evaluations with a molecular diversity assessment of a Brazilian collection of common bean landraces. Crop Sci. 2011, 51, 2668–2680. [Google Scholar] [CrossRef]
- Stoilova, T.; Pereira, G.; De Sousa, M.T.; Carnide, V. Diversity in common bean landraces (Phaseolus vulgaris L.) from Bulgaria and Portugal. J. Cent. Eur. Agric. 2005, 6, 443–448. [Google Scholar]
- Stoilova, T.; Pereira, G.; De Sousa, M.T. Morphological characterization of a small common bean (Phaseolus vulgaris L.) collection under different environments. J. Cent. Eur. Agric. 2013, 14, 854–864. [Google Scholar] [CrossRef] [Green Version]
- Negash, K. Studies on Genetic Divergence in Common Bean (Phaseolus vulgaris L.) Introductions of Ethiopia. Ph.D. Thesis, Addis Ababa University, Addis Ababa, Ethiopia, 2006. [Google Scholar]
- Prakash, J.; Ram, R.B.; Meena, M.L. Genetic variation and characters interrelationship studies for quantitative and qualitative traits in french bean (Phaseolus vulgaris L.) under Lucknow conditions. Legume Res. 2015, 38, 425. [Google Scholar] [CrossRef]
- Awan, F.K.; Khurshid, M.Y.; Afzal, O.; Ahmed, M.; Chaudhry, A.N. Agro-morphological evaluation of some exotic common bean (Phaseolus vulgaris L.) genotypes under rainfed conditions of Islamabad, Pakistan. Pakistan J. Bot. 2014, 46, 259–264. [Google Scholar]
- De Lima, M.S.; Carneiro, J.E.d.S.; Carneiro, P.C.S.; Pereira, C.S.; Vieira, R.F.; Cecon, P.R. Characterization of genetic variability among common bean genotypes by morphological descriptors. Crop Breed. Appl. Biotechnol. 2012, 12, 76–84. [Google Scholar] [CrossRef] [Green Version]
- Duran, L.A.; Blair, M.W.; Giraldo, M.C.; Macchiavelli, R.; Prophete, E.; Nin, J.C.; Beaver, J.S. Morphological and molecular characterization of common bean landraces and cultivars from the Caribbean. Crop Sci. 2005, 45, 1320–1328. [Google Scholar] [CrossRef]
- Oscar, J.G.; Blair, M.W.; Frankow-Lindberg, B.E.; Gullberg, U. Molecular and phenotypic diversity of common bean landraces from Nicaragua. Crop Sci. 2004, 44, 1412–1418. [Google Scholar]
- Okii, D.; Tukamuhabwa, P.; Odong, T.; Namayanja, A.; Mukabaranga, J.; Paparu, P.; Gepts, P.; Sciences, E. Morphological diversity of tropical common bean germplasm. Afr. Crop Sci. J. 2014, 22, 59–67. [Google Scholar]
- Marzooghian, A.; Moghaddam, M.; Valizadeh, M.; Kooshki, M.H. Genetic diversity of common bean genotypes as revealed by seed storage proteins and some agronomic traits. Plant Breed. Seed Sci. 2013, 67, 125–137. [Google Scholar] [CrossRef]
- Singh, S.P.; Gutiérrez, J.; Molina, A.; Urrea, C.; Gepts, P. Genetic diversity in cultivated common bean: II. Marker-based analysis of morphological and agronomic traits. Crop Sci. 1991, 31, 23–29. [Google Scholar] [CrossRef] [Green Version]
- Darkwa, K.; Ambachew, D.; Mohammed, H.; Asfaw, A.; Blair, M.W. Evaluation of common bean (Phaseolus vulgaris L.) genotypes for drought stress adaptation in Ethiopia. Crop J. 2016, 4, 367–376. [Google Scholar] [CrossRef] [Green Version]
- Negahi, A.; Bihamat, R.; Mohammed, I.; Negahi, Z.; Mohammed, A. Evaluation of genetic variation of some agronomical and morphological traits in Iranian and exotic common bean (Phaseolus vulgaris L.). Agric. Comm. 2014, 2, 22–26. [Google Scholar]
- Blum, A. Plant Breeding for Stress Environments; CRC Press Inc.: Boca Raton, FL, USA, 1988. [Google Scholar]
- Karasu, A.; Oz, M. A Study on coefficient analysis and association between agronomical characters in drey bean (Phaseolus vulgaris L.). Bulgarian J. Agricul. Sci. 2010, 16, 203–211. [Google Scholar]
- Mohammad, A.; Tajik, M.; Ebadi, A.G. The study of relationship between differnt traits in common bean (Phaseolus vulgaris L.) with multivariate statstical methods. Am. J. Agric. Environ. Sci. 2008, 3, 806–809. [Google Scholar]
- Roy, S.K.; Karim, M.A.; Islam, A.K.M.; Bari, M.N.; Mian, M.A.K.; Tetsushi, H. Relationship between yield and its component characters of bush bean (Phaseolus vulgaris L.). S. Pac. Stud. 2006, 27, 2–12. [Google Scholar]
- Ahmed, S. Correlation and path analysis for agro-morphological traits in rajmash beans under Baramulla-Kashmir region. Afr. J. Agric. Res. 2013, 8, 2027–2032. [Google Scholar]
- Kumar, A.; Singh, A.; Singh, P.; Singh, S.B.; Singh, V. Relationship and path analysis for green pod yield and its contributing characters over environments in French bean (Phaseolus vulgaris L.). Legume Res. 2009, 32, 270–273. [Google Scholar]
- Hair, J.F.; Anderson, R.E.; Tatham, R.L.; Black, W.C. Multivariate Data Analysis; Macmillan Publishing Company: New York, NY, USA, 1995. [Google Scholar]
- Singh, R.K.; Chaudhary, B.D. Biometrical Methods in Quantitative Genetic Analysis; Kalyani Publishers: New Delhi, India, 1985. [Google Scholar]
- Singh, S.; Gepts, P.; Debouck, D. Races of common bean (Phaseolus vulgaris, Fabaceae). Econ. Bot. 1991, 45, 379–396. [Google Scholar] [CrossRef]
- Madakbaş, S.Y.; Ergin, N. Morphological and phe-nological characterization of Turkish bean (Phaseolus vulgaris L.) genotypes and their present variation states. Afr. J. Agric. Res. 2011, 6, 6155–6166. [Google Scholar]
- Girsil, T.S. Genetic Studies on Host Plant Resistance to Mexican Bean Weevil (Zabrotes subfasciatus Boheman) in Ethiopian Common Bean (Phaseolus vulgaris L.) Germplasms. Ph.D. Thesis, African Center for Crop Improvement School of Agricultural, Earth and Environmental Sciences College of Agriculture, Engineering and Science University of KwaZulu-Natal, Pietermaritzburg, South Africa, 2017. [Google Scholar]
- Liao, M.; Hocking, P.J.; Dong, B.; Delhaize, E.; Richardson, A.E.; Ryan, P.R. Variation in early phosphorus-uptake efficiency among wheat genotypes grown on two contrasting Australian soils Australian. J. Agricul. Res. 2008, 59, 57–166. [Google Scholar] [CrossRef]
- Assefa, T.; Beebe, S.E.; Rao, I.M.; Cuasquer, J.B.; Duque, M.C.; Rivera, M.; Battisti, A.; Lucchin, M. Pod harvest index as a selection criterion to improve drought resistance in white pea bean. Field Crop Res. 2013, 148, 24–33. [Google Scholar] [CrossRef]
- IBPGR. Descriptors for Phaseolus vulgaris; IBPGR, International Plant Genetic Resources Institute: Rome, Italy, 1982. [Google Scholar]
- Payne, R.W.; Murray, D.A.; Harding, S.A.; Baird, D.B.; Soutar, D.M. GenStat for Windows Introduction, 12th ed.; VSN International: Hemel Hempstead, UK, 2017. [Google Scholar]
- Manly, B.F.J. Multivariate Statistical Methods: A Primer; Chapman and Hall: London, UK, 1986. [Google Scholar]
- Shena, Z.; Zhang, K.; Ma, L.; Duan, J.; Ao, Y. Analysis of the genetic relationships and diversity among 11 populations of Xanthoceras sorbifolia using phenotypic and microsatellite marker data. Electro. J. Biotech. 2017, 26, 33–39. [Google Scholar] [CrossRef]
- Perrier, X.; Jacquemoud-Collet, J. DARwin Software: Dissimilarity Analysis and Representation for Windows. 2006. Available online: http://www.darwin.cirad.fr/darwin (accessed on 12 May 2021).


| Traits | Mean Square | |||||||
|---|---|---|---|---|---|---|---|---|
| Replication (DF = 2) | Block (DF = 11) | iBlock (DF = 11) | Genotype (G) (DF = 121) | Environment (E) (DF = 2) | G × E Interaction (GEI) (DF =286) | Error (DF = 862) | R2 | |
| Days to 50% flowering (DTF) | 1.87 | 71.38 | 44.09 | 49.00 ** | 5174.08 ** | 8.99 ** | 2.41 | 0.93 |
| Plant height (PH) | 219.97 | 153.7 | 116.38 | 204.82 ** | 19,842.36 ** | 197.00 ** | 32.57 | 0.82 |
| Total chlorophyll content (TCC) | 227.23 | 386.6 | 106.05 | 111.18 ** | 2050.41 ** | 65.64 ** | 4.78 | 0.91 |
| Leaf area (LA) | 0.59 | 2.02 | 1.47 | 1.50 ** | 224.60 ** | 1.02 ** | 0.04 | 0.97 |
| Days to 90% maturity (DTM) | 50.43 | 539.4 | 124.7 | 182.58 ** | 19,915.01 ** | 54.92 ** | 4.55 | 0.96 |
| Grain filling period (GFP) | 33.71 | 351.6 | 89.16 | 137.80 ** | 6043.10 ** | 51.46 ** | 6.41 | 0.9 |
| Pods per plant (PPP) | 55.55 | 971.8 | 682.06 | 540.76 ** | 105.54 ** | 148.81 ** | 7.41 | 0.95 |
| Seeds per pod (SPP) | 0.77 | 14.49 | 8.91 | 4.24 ** | 9.17 ** | 1.80 ** | 0.21 | 0.88 |
| Hundred seed weight (HSW) | 12.83 | 2571 | 536.61 | 675.19 ** | 589.14 ** | 24.16 ** | 2.1 | 0.99 |
| Aboveground biomass (AGBM) | 30.2 | 300.9 | 88.8 | 153.36 ** | 4539.12 ** | 82.36 ** | 4.14 | 0.94 |
| Grain yield (g/plants) (GY) | 24.28 | 568.1 | 281.35 | 219.75 ** | 240.55 ** | 111.65 ** | 4.3 | 0.95 |
| Harvest index (HI) | 15.62 | 1620 | 1042.57 | 783.12 ** | 12,005.66 ** | 708.42 ** | 23.46 | 0.95 |
| Grain production efficiency (GPE) | 60.61 | 915.4 | 558.21 | 436.05 ** | 1359.47 ** | 184.89 ** | 10.54 | 0.93 |
| Biomass production rate (%) (BPR) | 17.38 | 652.6 | 75.51 | 164.86 ** | 1352.50 ** | 102.99 ** | 5.15 | 0.93 |
| Economic growth rate (%) (EGR) | 37.79 | 2672 | 832.04 | 851.69 ** | 2826.06 ** | 483.27 ** | 24.76 | 0.93 |
| Trait | Min | Max | Mean ± SE | SD | CV% |
|---|---|---|---|---|---|
| Days to 50% flowering (Days) | 37 | 51.9 | 44.17 ± 0.12 | 1.55 | 3.51 |
| Plant height (cm) | 31.7 | 56.2 | 44.20 ± 0.30 | 5.71 | 12.91 |
| Total chlorophyll content (µmol/m2) | 35 | 57.1 | 45.01 ± 0.17 | 2.19 | 4.86 |
| Leaf area (m2/plant) | 0.8 | 5.8 | 3.00 ± 0.03 | 0.19 | 6.39 |
| Days to 90% maturity (Days) | 79.1 | 103.8 | 94.41 ± 0.23 | 2.13 | 2.26 |
| Grain filling period (Days) | 37.4 | 58.6 | 50.25 ± 0.18 | 2.53 | 5.04 |
| Pods per plant (No) | 13.1 | 50.9 | 27.70 ± 0.28 | 2.72 | 9.83 |
| Seeds per pod (No) | 2.1 | 6.1 | 4.21 ± 1.06 | 0.46 | 10.84 |
| Hundred seed weight (gm) | 12.1 | 58.8 | 24.84 ± 0.27 | 1.45 | 5.84 |
| Above ground biomass (gm/plants) | 27.7 | 53.2 | 36.74 ± 0.19 | 2.04 | 5.54 |
| Grain yield (gm/plant) | 14.6 | 41.8 | 25.64 ± 0.21 | 2.07 | 8.09 |
| Harvest index | 39.76 | 92.1 | 69.99 ± 0.47 | 4.84 | 6.92 |
| Grain production efficiency (gm/plants) | 14 | 54.5 | 29.52 ± 0.28 | 3.25 | 11 |
| Biomass production rate (%) | 30.3 | 56.2 | 39.18 ± 0.20 | 2.27 | 5.79 |
| Economic growth rate (%) | 28.5 | 82 | 51.48 ± 0.43 | 4.98 | 9.67 |
| Trait | PC1 | PC2 | PC3 | PC4 | PC5 | PC6 |
|---|---|---|---|---|---|---|
| AGBM | 0.41 | 0.00 | −0.03 | 0.17 | −0.22 | 0.19 |
| BPR | 0.38 | −0.23 | 0.06 | 0.15 | −0.24 | 0.15 |
| DTF | −0.12 | 0.31 | 0.02 | 0.51 | −0.26 | −0.30 |
| DTM | 0.02 | 0.55 | −0.21 | 0.06 | 0.10 | 0.05 |
| EGR | 0.42 | −0.14 | 0.13 | 0.11 | −0.04 | −0.16 |
| GFP | 0.10 | 0.46 | −0.25 | −0.23 | 0.26 | 0.23 |
| GPE | 0.43 | 0.12 | −0.08 | −0.18 | 0.19 | 0.08 |
| GY | 0.45 | 0.04 | 0.02 | 0.01 | 0.05 | −0.06 |
| HI | 0.19 | 0.11 | 0.05 | −0.27 | 0.22 | −0.64 |
| HSW | 0.10 | −0.21 | −0.63 | −0.02 | 0.04 | 0.05 |
| LA | 0.09 | 0.27 | 0.16 | 0.07 | −0.22 | 0.44 |
| PH | 0.09 | 0.13 | −0.38 | 0.50 | −0.04 | −0.27 |
| PPP | 0.10 | 0.26 | 0.52 | 0.21 | 0.31 | 0.01 |
| TCC | 0.09 | −0.27 | 0.08 | 0.22 | 0.48 | −0.02 |
| SPP | 0.11 | 0.16 | 0.08 | −0.42 | −0.53 | −0.28 |
| Eigenvalue | 4.45 | 2.91 | 1.68 | 1.36 | 1.15 | 1.00 |
| % total variation | 29.67 | 19.37 | 11.23 | 9.09 | 7.65 | 6.66 |
| % cumulative variation | 29.67 | 49.04 | 60.27 | 69.36 | 77.01 | 83.67 |
| Genotype | SC | DTF | PH | TCC | LA | DTM | PPP | SPP | HSW | AGBM | GY | HI | GPE | BPR | EGR | GFP |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Top ten small-seeded genotypes | ||||||||||||||||
| Nasir | Red | 41.6 | 53.3 | 48.0 | 2.5 | 95.4 | 36.0 | 5.1 | 24.6 | 46.1 | 41.8 | 63.5 | 54.5 | 48.3 | 77.5 | 53.9 |
| SER-125 | Red | 41.8 | 41.7 | 47.1 | 2.6 | 90.7 | 26.0 | 3.5 | 25.6 | 39.7 | 36.5 | 77.3 | 42.6 | 44.3 | 75.9 | 48.9 |
| Awash Melka | White | 46.6 | 52.8 | 49.1 | 3.0 | 93.9 | 33.7 | 4.5 | 21.7 | 46.6 | 34.6 | 63.5 | 34.0 | 52.6 | 76.9 | 47.3 |
| RAZ-36 | White | 42.7 | 45.0 | 53.4 | 3.2 | 96.2 | 46.3 | 3.1 | 18.1 | 45.5 | 33.1 | 66.9 | 41.3 | 47.1 | 63.3 | 53.6 |
| 241757 | Red | 47.0 | 47.2 | 41.5 | 2.9 | 95.7 | 29.8 | 4.4 | 22.7 | 43.7 | 32.9 | 76.3 | 34.0 | 45.6 | 68.7 | 48.7 |
| 230526 | Red | 42.9 | 41.1 | 40.4 | 3.4 | 96.6 | 27.0 | 5.0 | 23.6 | 37.0 | 32.2 | 86.9 | 40.3 | 38.3 | 59.8 | 53.7 |
| RAZ-44 | White | 42.8 | 48.3 | 50.1 | 2.9 | 96.2 | 31.2 | 4.1 | 18.1 | 42.1 | 31.4 | 82.5 | 39.2 | 43.8 | 60.7 | 53.4 |
| 241734 | Red | 43.4 | 46.1 | 45.6 | 4.0 | 101.1 | 30.0 | 4.6 | 22.1 | 44.4 | 31.3 | 72.1 | 41.6 | 44.0 | 54.5 | 57.7 |
| 214665 | Red | 43.1 | 44.4 | 43.7 | 3.4 | 99.3 | 27.4 | 5.4 | 22.8 | 41.1 | 30.1 | 74.6 | 39.5 | 41.4 | 53.7 | 56.2 |
| NC-51 | Red | 42.1 | 41.1 | 42.9 | 2.6 | 95.1 | 26.6 | 3.8 | 24.1 | 38.2 | 29.2 | 74.9 | 37.1 | 40.0 | 54.9 | 53.0 |
| Top ten medium-seeded genotypes | ||||||||||||||||
| 207935 | Carioca | 44.9 | 51.1 | 49.6 | 3.2 | 95.6 | 24.2 | 5.7 | 29.4 | 53.2 | 41.2 | 80.7 | 46.8 | 56.2 | 82.0 | 50.7 |
| SCR-11 | Red | 42.0 | 45.0 | 49.9 | 2.7 | 92.3 | 25.4 | 3.9 | 29.2 | 44.9 | 36.9 | 56.6 | 44.2 | 48.8 | 74.3 | 50.3 |
| RAZ-40 | White | 41.4 | 37.8 | 49.5 | 3.1 | 89.6 | 20.3 | 3.7 | 36.7 | 35.8 | 32.6 | 62.4 | 32.2 | 40.6 | 60.7 | 48.1 |
| NC-28 | Cream | 40.9 | 45.0 | 47.3 | 3.1 | 99.4 | 32.0 | 3.1 | 28.9 | 42.3 | 31.8 | 75.8 | 45.0 | 42.6 | 55.0 | 58.6 |
| 211302 | Brown | 39.8 | 38.3 | 47.8 | 2.8 | 89.0 | 21.6 | 4.2 | 36.5 | 42.3 | 31.7 | 77.7 | 39.0 | 47.2 | 66.1 | 49.2 |
| SCR-15 | Red | 43.3 | 38.9 | 47.6 | 2.8 | 94.0 | 27.1 | 3.7 | 38.3 | 41.5 | 31.3 | 89.0 | 36.5 | 43.8 | 62.1 | 50.7 |
| SCR-26 | Red | 43.6 | 49.4 | 47.2 | 3.0 | 92.6 | 23.9 | 4.2 | 27.7 | 42.9 | 29.2 | 67.5 | 31.8 | 46.1 | 57.8 | 49.0 |
| 228077 | Red | 42.9 | 43.3 | 37.5 | 3.4 | 100.7 | 26.3 | 5.7 | 25.9 | 38.4 | 28.4 | 75.8 | 39.3 | 38.1 | 48.8 | 57.8 |
| KK25/MAIAWA/19 | Red | 43.6 | 47.2 | 42.0 | 2.8 | 95.4 | 20.8 | 5.6 | 36.9 | 33.1 | 28.2 | 77.3 | 33.7 | 34.9 | 54.7 | 51.9 |
| RAZ-120 | White | 45.7 | 45.0 | 50.3 | 2.8 | 90.7 | 28.6 | 3.7 | 26.4 | 38.3 | 27.8 | 75.1 | 27.4 | 42.6 | 63.1 | 45.0 |
| Trait | DTF | PH | TCC | LA | DTM | GFP | PPP | SPP | HSW | AGBM | HI | GPE | BPR | EGR | GY |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DTF | 1.00 | ||||||||||||||
| PH | 0.31 *** | 1.00 | |||||||||||||
| TCC | −0.26 ** | 0.05 | 1.00 | ||||||||||||
| LA | 0.14 | 0.03 | −0.12 | 1.00 | |||||||||||
| DTM | 0.52 *** | 0.29 *** | −0.33 *** | 0.31 *** | 1.00 | ||||||||||
| GFP | 0.03 | 0.16 | −0.23 ** | 0.28 *** | 0.87 *** | 1.00 | |||||||||
| PPP | 0.21 * | −0.04 | 0.04 | 0.24 ** | 0.27 *** | 0.20 * | 1.00 | ||||||||
| SPP | −0.02 | −0.04 | −0.24 ** | 0.14 | 0.14 | 0.17 | −0.04 | 1.00 | |||||||
| HSW | −0.24 ** | 0.25 ** | 0.11 | −0.22 ** | −0.10 | 0.01 | −0.61 *** | −0.18 * | 1.00 | ||||||
| AGBM | −0.09 | 0.22 ** | 0.15 | 0.24 ** | 0.09 | 0.15 | 0.10 | 0.22 ** | 0.18 * | 1.00 | |||||
| HI | −0.07 | 0.06 | 0.08 | 0.07 | 0.12 | 0.18 * | 0.13 | 0.26 ** | −0.01 | 0.14 | 1.00 | ||||
| GPE | −0.34 *** | 0.12 | 0.05 | 0.15 | 0.26 ** | 0.50 *** | 0.23 ** | 0.21 * | 0.20 * | 0.69 *** | 0.40 *** | 1.00 | |||
| BPR | −0.30 *** | 0.11 | 0.27 ** | 0.09 | −0.34 *** | −0.22 ** | −0.01 | 0.12 | 0.19 * | 0.88 *** | 0.08 | 0.55 *** | 1.00 | ||
| EGR | −0.16 | 0.09 | 0.19 * | 0.01 | −0.22 ** | −0.16 | 0.21 * | 0.12 | 0.15 | 0.70 *** | 0.31 *** | 0.72 *** | 0.77 *** | 1.00 | |
| GY | −0.16 | 0.15 | 0.09 | 0.12 | 0.12 | 0.23 ** | 0.26 ** | 0.18 * | 0.18 * | 0.75 *** | 0.39 *** | 0.92 *** | 0.68 *** | 0.92 *** | 1.00 |
| Trait | Cluster Means | ||||||
|---|---|---|---|---|---|---|---|
| C-I (n = 36) | C-II (n = 71) | C-III (n = 37) | |||||
| SC-Ia (n = 18) | SC-Ib (n = 18) | SC-IIa (n = 26) | SC-IIb (n = 22) | SC-IIc (n = 23) | SC-IIIa (n = 20) | SC-IIIb (n = 17) | |
| PH | 43.4 | 47.2 | 43.0 | 40.8 | 44.2 | 47.1 | 44.9 |
| LA | 3.41 | 3.13 | 3.15 | 2.82 | 3.01 | 2.91 | 2.87 |
| TCC | 44.3 | 45.7 | 43.3 | 45.9 | 44.2 | 44.7 | 48.0 |
| DTF | 43.2 | 45.4 | 44.3 | 43.9 | 45.6 | 44.1 | 42.2 |
| DTM | 95.7 | 96.2 | 94.6 | 87.4 | 99.1 | 94.7 | 93.1 |
| GFP | 52.4 | 50.9 | 50.4 | 43.6 | 53.6 | 50.6 | 50.9 |
| PPP | 27.0 | 37.8 | 25.5 | 26.0 | 34.3 | 17.9 | 26.1 |
| SPP | 4.8 | 4.3 | 4.4 | 4.0 | 4.2 | 3.6 | 4.2 |
| HSW | 23.2 | 19.7 | 21.4 | 21.1 | 16.9 | 42.2 | 32.4 |
| AGBM | 38.8 | 41.3 | 36.2 | 33.6 | 32.8 | 36.1 | 40.8 |
| GY | 28.2 | 32.2 | 22.8 | 21.9 | 22.0 | 25.3 | 30.7 |
| HI | 75.8 | 75.6 | 64.6 | 65.9 | 68.7 | 67.2 | 76.6 |
| GPE | 34.5 | 36.3 | 26.1 | 21.8 | 25.9 | 29.5 | 37.1 |
| BPR | 40.5 | 43.2 | 38.7 | 38.7 | 33.3 | 38.2 | 44.0 |
| EGR | 53.9 | 64.2 | 45.6 | 50.8 | 41.6 | 49.6 | 61.0 |
| Genetic distance | 0.45 | 0.53 | 0.51 | 0.59 | 0.56 | 0.60 | 0.58 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tigist, S.G.; Sibiya, J.; Amelework, A.; Keneni, G. Agromorphological and Physiological Performance of Ethiopian Common Bean (Phaseolus vulgaris L.) Genotypes under Different Agroecological Conditions. Plants 2023, 12, 2342. https://doi.org/10.3390/plants12122342
Tigist SG, Sibiya J, Amelework A, Keneni G. Agromorphological and Physiological Performance of Ethiopian Common Bean (Phaseolus vulgaris L.) Genotypes under Different Agroecological Conditions. Plants. 2023; 12(12):2342. https://doi.org/10.3390/plants12122342
Chicago/Turabian StyleTigist, Shiferaw Girsil, Julia Sibiya, Assefa Amelework, and Gemechu Keneni. 2023. "Agromorphological and Physiological Performance of Ethiopian Common Bean (Phaseolus vulgaris L.) Genotypes under Different Agroecological Conditions" Plants 12, no. 12: 2342. https://doi.org/10.3390/plants12122342






