Fulvic Acid Improves Salinity Tolerance of Rice Seedlings: Evidence from Phenotypic Performance, Relevant Phenolic Acids, and Momilactones
Abstract
1. Introduction
2. Results
2.1. Phenotypic Performances
2.1.1. Injury Scores and Survival Rates
2.1.2. Shoot Height, Root Length, Fresh Weight, and Dry Weight
2.2. Chemical Performances
2.2.1. Chlorophyll Contents
2.2.2. Total Phenolic (TPC) and Flavonoid (TFC) Contents and Antioxidant Activity
2.2.3. Phenolic Profiles
2.2.4. Momilactone Contents
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Phenotypic Evaluation
4.3. Determination of Chlorophyll Contents
4.4. Extraction of Plant Samples
4.5. Determination of Total Phenolic (TPC) and Total Flavonoid (TFC) Contents
4.6. Antioxidant Activity
4.7. Identification and Quantification of Phenolics by High-Performance Liquid Chromatography
4.8. Identification and Quantification of Momilactones by Ultra-Performance Liquid Chromatography-Electrospray Ionization-Mass Spectrometry
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAO Food Outlook–Biannual Report on Global Food Markets. Available online: https://www.fao.org/documents/card/en/c/cb9427en (accessed on 20 September 2022).
- Khush, G.S. What it will take to feed 5.0 billion rice consumers in 2030. Plant Mol. Biol. 2005, 59, 1–6. [Google Scholar] [CrossRef]
- FAO. FAO Statistical Yearbook 2021—World Food and Agriculture; Food and Agriculture Organization of the United Nations: Rome, Italy, 2021. [Google Scholar]
- Shabala, S.; Munns, R. Salinity stress: Physiological constraints and adaptive mechanisms. In Plant Stress Physiology; CABI: Wallingford, UK, 2012; pp. 59–93. [Google Scholar]
- Munns, R.; Tester, M. Mechanisms of salinity tolerance. Annu. Rev. Plant Biol. 2008, 59, 651–681. [Google Scholar] [CrossRef] [PubMed]
- Huong, C.T.; Anh, T.T.T.; Dat, T.D.; Khanh, T.D.; Xuan, T.D. Uniparental inheritance of salinity tolerance and beneficial phytochemicals in rice. Agronomy 2020, 10, 1032. [Google Scholar] [CrossRef]
- Hoang, T.M.L.; Tran, T.N.; Nguyen, T.K.T.; Williams, B.; Wurm, P.; Bellairs, S.; Mundree, S. Improvement of salinity stress tolerance in rice: Challenges and opportunities. Agronomy 2016, 6, 54. [Google Scholar] [CrossRef]
- Qin, H.; Li, Y.; Huang, R. Advances and challenges in the breeding of salt-tolerant rice. Int. J. Mol. Sci. 2020, 21, 8385. [Google Scholar] [CrossRef] [PubMed]
- Ismail, A.M.; Heuer, S.; Thomson, M.J.; Wissuwa, M. Genetic and genomic approaches to develop rice germplasm for problem soils. Plant Mol. Biol. 2007, 65, 547–570. [Google Scholar] [CrossRef] [PubMed]
- Reddy, M.A.; Francies, R.M.; Rasool, S.N.; Reddy, V.R.P. Breeding for tolerance to stress triggered by salinity in rice. Int. J. Appl. Biol. Pharm. Technol. 2014, 5, 167–176. [Google Scholar]
- Hasanuzzaman, M.; Alam, M.; Rahman, A.; Hasanuzzaman, M.; Nahar, K.; Fujita, M. Exogenous proline and glycine betaine mediated upregulation of antioxidant defense and glyoxalase systems provides better protection against salt-induced oxidative stress in two rice (Oryza sativa L.) varieties. Biomed. Res. Int. 2014, 2014, 757219. [Google Scholar] [CrossRef]
- Rahman, A.; Nahar, K.; al Mahmud, J.; Hasanuzzaman, M.; Hossain, M.S.; Fujita, M. Salt stress tolerance in rice: Emerging role of exogenous phytoprotectants. In Advances in International Rice Research; Li, J., Ed.; InTech: London, UK, 2017. [Google Scholar]
- Varanini, Z.; Pinton, R. Direct versus indirect effects of soil humic substances on plant growth and nutrition. In The Rhizosphere, 1st ed.; Willig, S., Varanini, Z., Nannipieri, P., Eds.; CRC Press: Boca Raton, FL, USA, 2000; pp. 157–174. [Google Scholar]
- Sun, J.; Qiu, C.; Ding, Y.; Wang, Y.; Sun, L.; Sun, L.; Fan, K.; Gai, Z.; Dong, G.; Wang, J.; et al. Fulvic acid ameliorates drought stress-induced damage in tea plants by regulating the ascorbate metabolism and flavonoids biosynthesis. BMC Genom. 2020, 21, 411. [Google Scholar] [CrossRef]
- Anjum, S.A.; Wang, L.; Farooq, M.; Xue, L.; Ali, S. Fulvic acid application improves the maize performance under well-watered and drought conditions. J. Agron. Crop Sci. 2011, 197, 409–417. [Google Scholar] [CrossRef]
- Dinler, B.S.; Gunduzer, E.; Tekinay, T. Pre-treatment of fulvic acid plays a stimulant role in protection of soybean (Glycine max L.) leaves against heat and salt stress. Acta Biol. Crac. Ser. Bot. 2016, 58, 29–41. [Google Scholar] [CrossRef]
- Hatami, E.; Shokouhian, A.A.; Ghanbari, A.R.; Naseri, L.A. Alleviating salt stress in almond rootstocks using of humic acid. Sci. Hortic. 2018, 237, 296–302. [Google Scholar] [CrossRef]
- Bayat, H.; Shafie, F.; Aminifard, M.H.; Daghighi, S. Comparative effects of humic and fulvic acids as biostimulants on growth, antioxidant activity and nutrient content of yarrow (Achillea millefolium L.). Sci. Hortic. 2021, 279, 109912. [Google Scholar] [CrossRef]
- Gabr, S.; Abouelsaad, I.; Brengi, S.; Gouda, A. Salt stress mitigation of spinach plants as affected by silicon and fulvic acid. J. Adv. Agric. Res. 2022, 27, 26–42. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Parvin, K.; Bardhan, K.; Nahar, K.; Anee, T.I.; Masud, A.A.C.; Fotopoulos, V. Biostimulants for the regulation of reactive oxygen species metabolism in plants under abiotic stress. Cells 2021, 10, 2537. [Google Scholar] [CrossRef]
- Xuan, T.D.; Huong, C.T.; Quan, N.V.; Anh, L.H.; Khanh, T.D.; Rayee, R. Improvement of salinity tolerance in rice seedlings by exogenous magnesium sulfate application. Soil Syst. 2022, 6, 69. [Google Scholar] [CrossRef]
- Minh, L.T.; Khang, D.T.; Thu Ha, P.T.; Tuyen, P.T.; Minh, T.N.; Quan, N.V.; Xuan, T.D. Effects of salinity stress on growth and phenolics of rice (Oryza sativa L.). Int. Lett. Nat. Sci. 2016, 57, 1–10. [Google Scholar] [CrossRef]
- Kakar, N.; Jumaa, S.H.; Redoña, E.D.; Warburton, M.L.; Reddy, K.R. Evaluating rice for salinity using pot-culture provides a systematic tolerance assessment at the seedling stage. Rice 2019, 12, 57. [Google Scholar] [CrossRef]
- Anh, L.H.; Hue, H.T.; Quoc, N.K.; Nghia, L.T.; Trung, K.H.; Trung, T.N.; Trang, D.H.; Xuan, T.D.; Khanh, T.D. Effect of salt on growth of rice landraces in Vietnam. Int. Lett. Nat. Sci. 2016, 59, 72–81. [Google Scholar] [CrossRef]
- Suh, H.Y.; Yoo, K.S.; Suh, S.G. Effect of foliar application of fulvic acid on plant growth and fruit quality of tomato (Lycopersicon esculentum L.). Hortic. Environ. Biotechnol. 2014, 55, 455–461. [Google Scholar] [CrossRef]
- Ashraf, M.; Harris, P.J.C. Photosynthesis under stressful environments: An overview. Photosynthetica 2013, 51, 163–190. [Google Scholar] [CrossRef]
- Chawla, S.; Jain, S.; Jain, V. Salinity induced oxidative stress and antioxidant system in salt-tolerant and salt-sensitive cultivars of rice (Oryza sativa L.). J. Plant Biochem. Biotechnol. 2013, 22, 27–34. [Google Scholar] [CrossRef]
- Rout, N.P.; Shaw, B.P. Salt tolerance in aquatic macrophytes: Possible involvement of the antioxidative enzymes. Plant Sci. 2001, 160, 415–423. [Google Scholar] [CrossRef] [PubMed]
- Xuan, T.; Khang, D. Effects of exogenous application of protocatechuic acid and vanillic acid to chlorophylls, phenolics and antioxidant enzymes of rice (Oryza sativa L.) in submergence. Molecules 2018, 23, 620. [Google Scholar] [CrossRef]
- Soong, Y.-Y.; Barlow, P.J. Antioxidant activity and phenolic content of selected fruit seeds. Food Chem. 2004, 88, 411–417. [Google Scholar] [CrossRef]
- Quan, N.T.; Anh, L.H.; Khang, D.T.; Tuyen, P.T.; Toan, N.P.; Minh, T.N.; The Minh, L.; Bach, D.T.; Thu Ha, P.T.; Elzaawely, A.A.; et al. Involvement of secondary metabolites in response to drought stress of rice (Oryza sativa L.). Agriculture 2016, 6, 23. [Google Scholar] [CrossRef]
- Rayee, R.; Tran, H.-D.; Xuan, T.; Khanh, T. Imposed water deficit after anthesis for the improvement of macronutrients, quality, phytochemicals, and antioxidants in rice grain. Sustainability 2018, 10, 4843. [Google Scholar] [CrossRef]
- Sharma, A.; Thakur, S.; Kumar, V.; Kanwar, M.K.; Kesavan, A.K.; Thukral, A.K.; Bhardwaj, R.; Alam, P.; Ahmad, P. Pre-sowing seed treatment with 24-epibrassinolide ameliorates pesticide stress in Brassica juncea L. through the modulation of stress markers. Front. Plant Sci. 2016, 7, 1569. [Google Scholar] [CrossRef]
- Khan, M.I.R.; Fatma, M.; Per, T.S.; Anjum, N.A.; Khan, N.A. Salicylic acid-induced abiotic stress tolerance and underlying mechanisms in plants. Front. Plant Sci. 2015, 6, 462. [Google Scholar] [CrossRef]
- Miura, K.; Tada, Y. Regulation of water, salinity, and cold stress responses by salicylic acid. Front. Plant Sci. 2014, 5, 4. [Google Scholar] [CrossRef]
- Rahman, A.; Alam, M.U.; Hossain, M.S.; Mahmud, J.A.; Nahar, K.; Fujita, M.; Hasanuzzaman, M. Exogenous gallic acid confers salt tolerance in rice seedlings: Modulation of ion homeostasis, osmoregulation, antioxidant defense, and methylglyoxal detoxification systems. Agronomy 2022, 13, 16. [Google Scholar] [CrossRef]
- Anh, L.H.; Quan, N.V.; Lam, V.Q.; Takami, A.; Khanh, T.D.; Xuan, T.D. Rice momilactones and phenolics: Expression of relevant biosynthetic genes in response to UV and chilling stresses. Agronomy 2022, 12, 1731. [Google Scholar]
- Quan, N.V.; Tran, H.-D.; Xuan, T.D.; Ahmad, A.; Dat, T.D.; Khanh, T.D.; Teschke, R. Momilactones A and B are α-amylase and α-glucosidase inhibitors. Molecules 2019, 24, 482. [Google Scholar] [CrossRef] [PubMed]
- Quan, N.V.; Xuan, T.D.; Tran, H.-D.; Ahmad, A.; Khanh, T.D.; Dat, T.D. Contribution of momilactones A and B to diabetes inhibitory potential of rice bran: Evidence from in vitro assays. Saudi Pharm. J. 2019, 27, 643–649. [Google Scholar] [CrossRef]
- Quan, N.V.; Thien, D.D.; Khanh, T.D.; Tran, H.-D.; Xuan, T.D. Momilactones A, B, and tricin in rice grain and by-products are potential skin aging inhibitors. Foods 2019, 8, 602. [Google Scholar] [CrossRef]
- Anh, L.H.; Lam, V.Q.; Takami, A.; Khanh, T.D.; Quan, N.V.; Xuan, T.D. Cytotoxic mechanism of momilactones A and B against acute promyelocytic leukemia and multiple myeloma cell lines. Cancers 2022, 14, 4848. [Google Scholar] [CrossRef]
- Xuan, T.D.; Minh, T.N.; Anh, L.H.; Khanh, T.D. Allelopathic momilactones A and B are implied in rice drought and salinity tolerance, not weed resistance. Agron. Sustain. Dev. 2016, 36, 52. [Google Scholar] [CrossRef]
- Anh, L.H.; Quan, N.V.; Nghia, L.T.; Xuan, T.D. Phenolic allelochemicals: Achievements, limitations, and prospective approaches in weed management. Weed Biol. Manag. 2021, 21, 37–67. [Google Scholar]
- Shimura, K.; Okada, A.; Okada, K.; Jikumaru, Y.; Ko, K.-W.; Toyomasu, T.; Sassa, T.; Hasegawa, M.; Kodama, O.; Shibuya, N.; et al. Identification of a biosynthetic gene cluster in rice for momilactones. J. Biol. Chem. 2007, 282, 34013–34018. [Google Scholar] [CrossRef]
- Singh, R.K.; Kota, S.; Flowers, T.J. Salt tolerance in rice: Seedling and reproductive stage QTL mapping come of age. Theor. Appl. Genet. 2021, 134, 3495–3533. [Google Scholar] [CrossRef] [PubMed]
- IRRI. Phenotyping Protocol for Abiotic Stress Tolerance in Rice; International Rice Research Institute: Los Banos, Philippines, 2021; p. 9. [Google Scholar]
- Quy, T.N.; Xuan, T.D.; Andriana, Y.; Tran, H.D.; Khanh, T.D.; Teschke, R. Cordycepin isolated from Cordyceps militaris: Its newly discovered herbicidal property and potential plant-based novel alternative to glyphosate. Molecules 2019, 24, 2901. [Google Scholar] [CrossRef] [PubMed]
- Anh, L.H.; Quan, N.V.; Lam, V.Q.; Iuchi, Y.; Takami, A.; Teschke, R.; Xuan, T.D. Antioxidant, anti-tyrosinase, anti-α-amylase, and cytotoxic potentials of the invasive weed Andropogon virginicus. Plants 2020, 10, 69. [Google Scholar] [CrossRef] [PubMed]
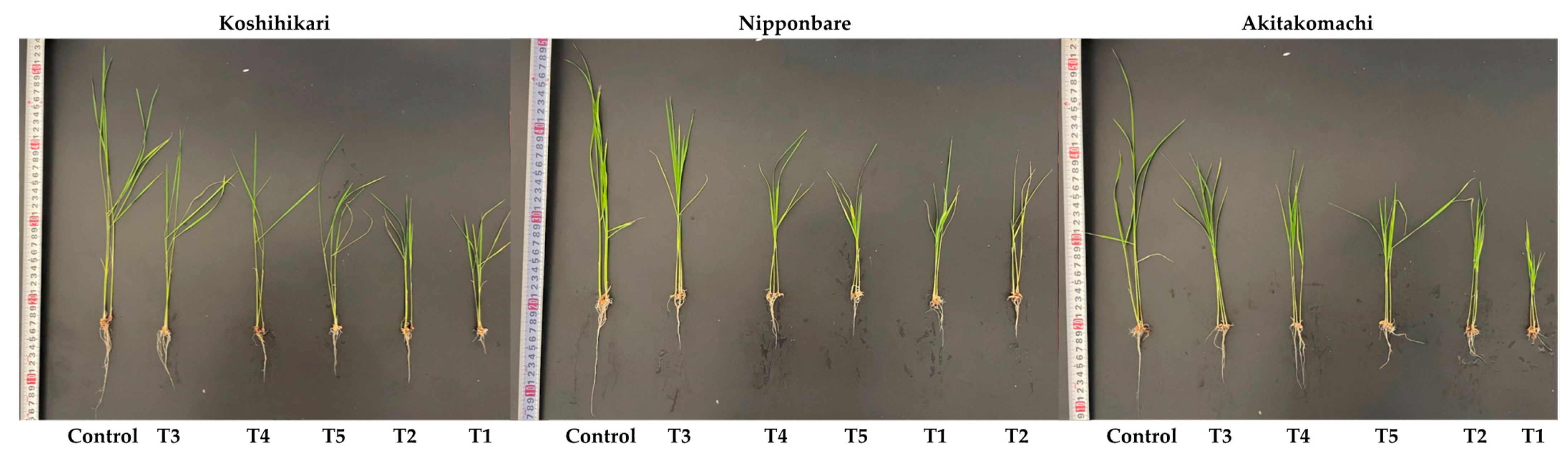
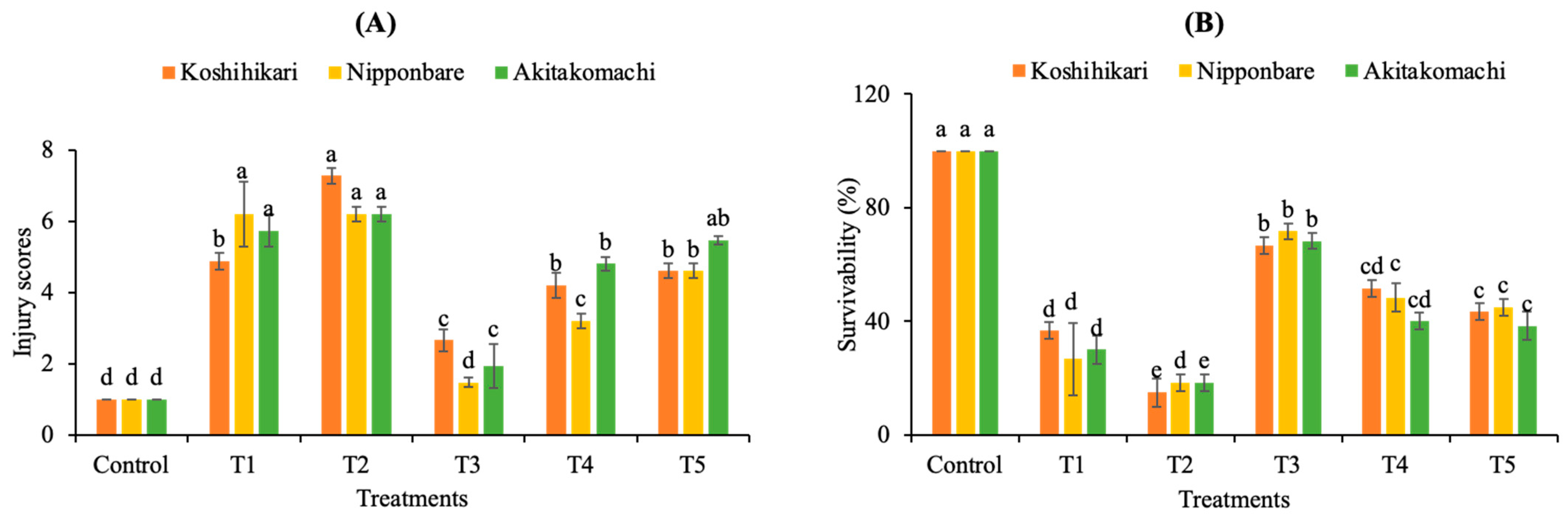
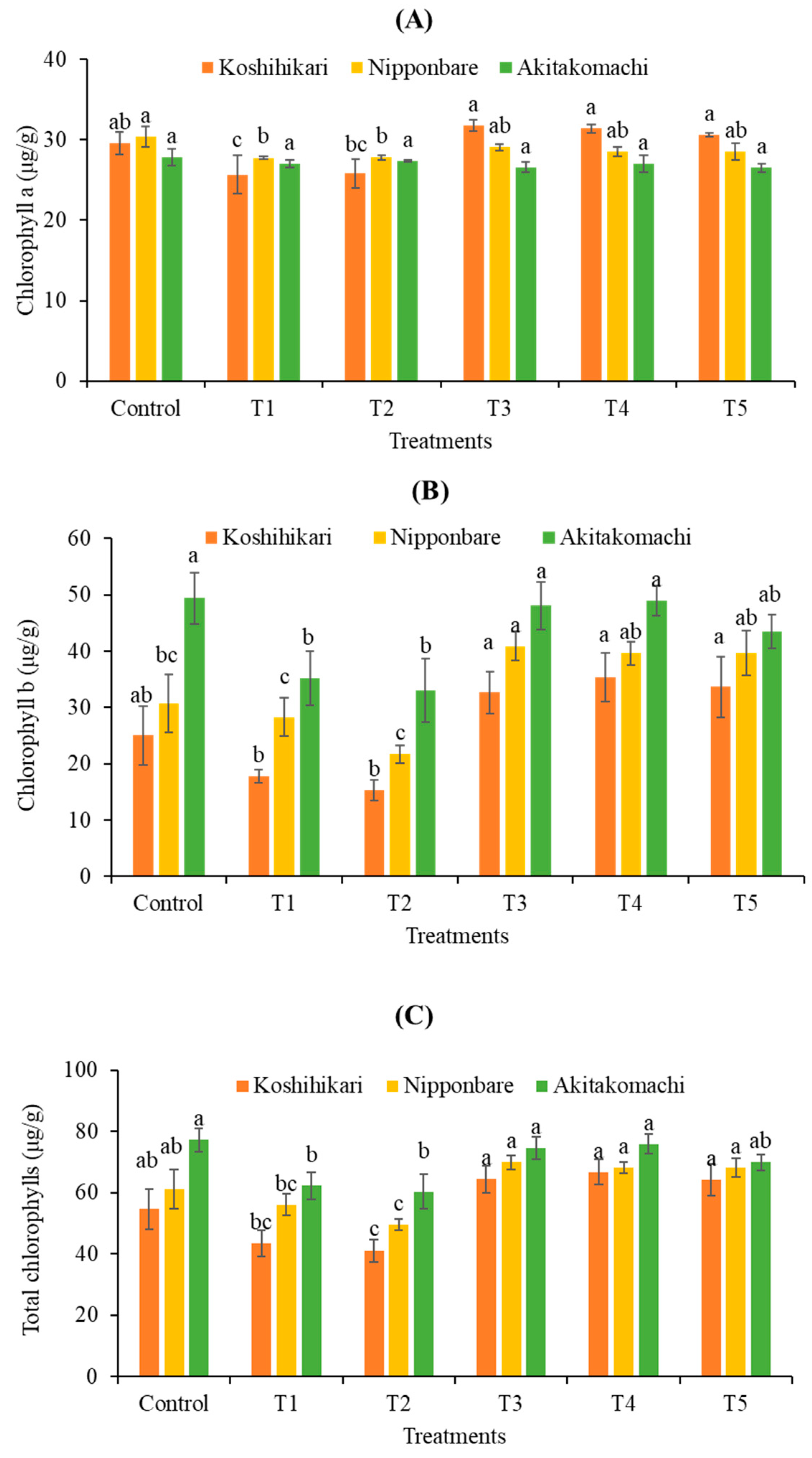
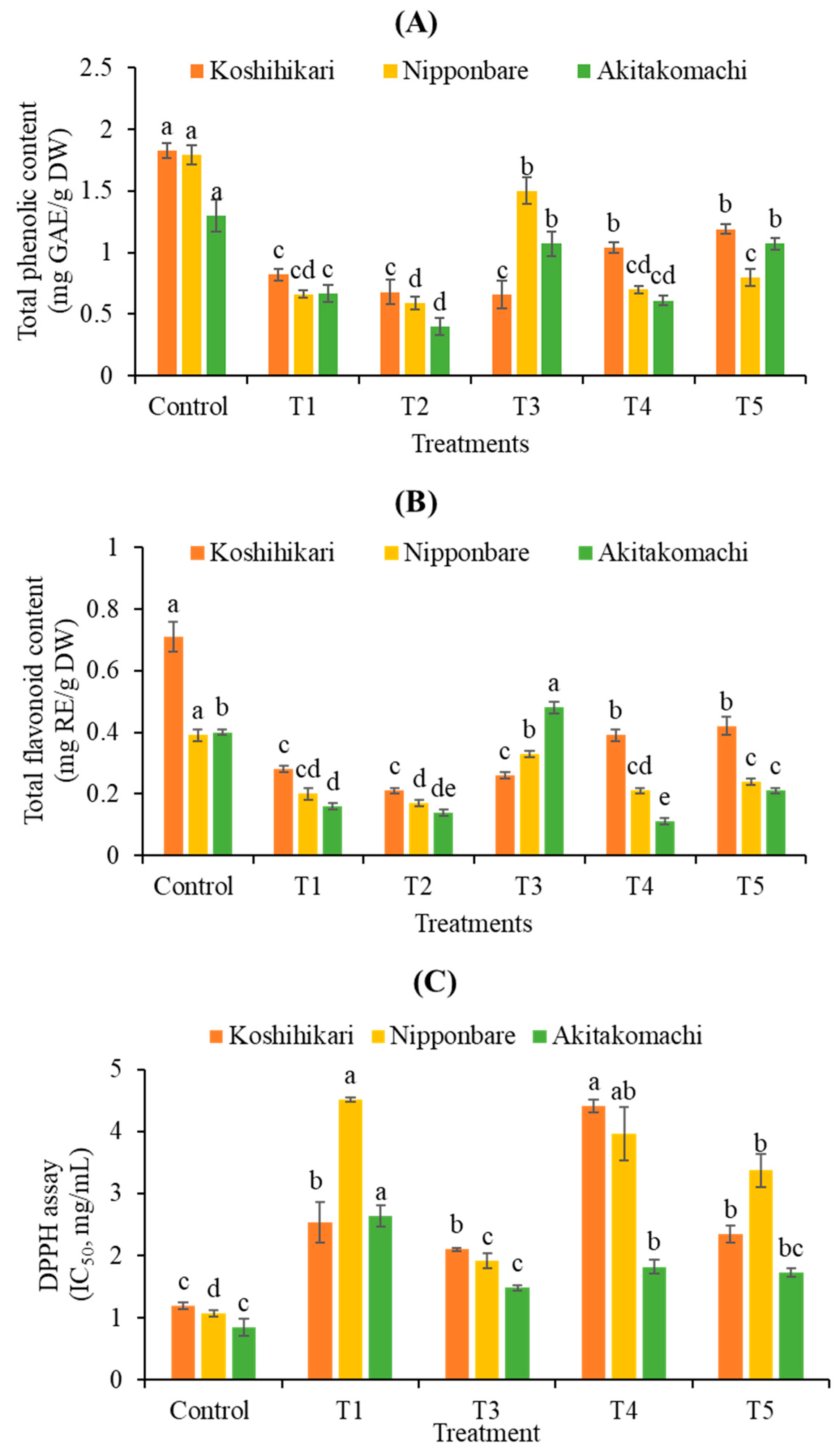
| Varieties | Treatments | Shoot Height (cm) | Root Length (cm) | Fresh Weight (mg) | Dry Weight (mg) |
|---|---|---|---|---|---|
| Koshihikari | Control | 26.11 ± 0.47 a | 5.92 ± 0.36 a | 596.70 ± 47.23 a | 96.67 ± 11.55 ab |
| T1 | 4.82 ± 0.60 c | 4.69 ± 0.42 b | 410.00 ± 10.00 cd | 66.67 ± 5.77 c | |
| T2 | 12.18 ± 3.29 c | 3.27 ± 0.76 c | 343.33 ± 66.58 d | 6.67 ± 5.77 bc | |
| T3 | 23.90 ± 0.20 ab | 6.23 ± 0.08 a | 540.00 ± 26.46 ab | 113.33 ± 11.55 a | |
| T4 | 22.05 ± 0.31 b | 6.50 ± 0.14 a | 470.00 ± 10.00 bc | 96.67 ± 11.55 ab | |
| T5 | 22.30 ± 1.10 ab | 6.19 ± 0.05 a | 493.33 ± 20.82 bc | 93.33 ± 11.55 abc | |
| Nipponbare | Control | 20.39 ± 0.70 a | 5.64 ± 0.32 ab | 666.67 ± 20.82 a | 90.00 ± 10.00 ab |
| T1 | 14.22 ± 0.50 c | 3.76 ± 0.32 b | 400.00 ± 10.00 c | 66.67 ± 5.77 b | |
| T2 | 15.59 ± 1.39 bc | 6.32 ± 0.96 a | 423.33 ± 76.38 c | 80.00 ± 10.00 ab | |
| T3 | 20.65 ± 0.31 a | 5.74 ± 0.47 a | 606.67 ± 25.17 ab | 106.67 ± 11.55 a | |
| T4 | 17.79 ± 1.89 ab | 5.27 ± 0.32 ab | 420.00 ± 30.00 c | 83.33 ± 11.55 ab | |
| T5 | 15.62 ± 1.19 bc | 4.74 ± 1.22 ab | 533.33 ± 20.82 b | 90.00 ± 10.00 ab | |
| Akitakomachi | Control | 19.65 ± 1.08 a | 6.22 ± 0.14 ab | 646.67 ± 57.70 a | 93.33 ± 5.77 ab |
| T1 | 14.26 ± 0.40 c | 4.91 ± 0.26 b | 410.00 ± 20.00 b | 66.67 ± 5.77 c | |
| T2 | 14.75 ± 1.12 c | 6.31 ± 0.67 ab | 480.00 ± 55.68 b | 80.00 ± 10.00 bc | |
| T3 | 19.23 ± 0.58 ab | 6.87 ± 0.51 a | 643.33 ± 20.82 a | 103.33 ± 5.77 a | |
| T4 | 17.14 ± 0.41 b | 4.83 ± 0.43 b | 416.67 ± 25.17 b | 76.67 ± 5.77 bc | |
| T5 | 11.66 ± 0.67 d | 2.30 ± 0.91 c | 423.33 ± 5.70 b | 80.00 ± 10.00 bc | |
| ANOVA | |||||
| Variety (V) | *** | ** | ** | *** | |
| Treatment (T) | *** | *** | *** | *** | |
| V × T | *** | * | ns | ns | |
| Varieties | Treatments | GA | PCA | VA | SA | CnA | CA |
|---|---|---|---|---|---|---|---|
| Koshihikari | Control | 0.12 ± 0.01 a | 0.07 ± 0.00 a | 0.23 ± 0.00 a | 0.08 ± 0.00 a | 0.09 ± 0.01 b | nd |
| T1 | 0.03 ± 0.00 b | nd | 0.13 ± 0.00 c | 0.04 ± 0.00 b | nd | nd | |
| T3 | 0.03 ± 0.00 b | 0.04 ± 0.00 b | 0.17 ± 0.02 b | 0.04 ± 0.01 b | 0.16 ± 0.00 a | nd | |
| Nipponbare | Control | 0.11 ± 0.01 a | 0.05 ± 0.00 b | 0.19 ± 0.01 a | 0.08 ± 0.00 b | 0.08 ± 0.01 ab | nd |
| T1 | 0.03 ± 0.00 c | 0.03 ± 0.00 c | 0.12 ± 0.00 b | 0.03 ± 0.00 c | 0.07 ± 0.00 b | nd | |
| T3 | 0.06 ± 0.00 b | 0.06 ± 0.00 a | 0.12 ± 0.02 b | 0.25 ± 0.01 a | 0.09 ± 0.01 a | 0.14 ± 0.01 | |
| Akitakomachi | Control | 0.10 ± 0.01 a | 0.09 ± 0.00 a | 0.22 ± 0.01 a | 0.10 ± 0.01 a | 0.05 ± 0.00 a | nd |
| T1 | 0.04 ± 0.00 b | 0.05 ± 0.00 b | 0.13 ± 0.02 b | 0.02 ± 0.00 c | 0.04 ± 0.01 a | nd | |
| T3 | 0.04 ± 0.00 b | 0.04 ± 0.00 c | 0.09 ± 0.02 b | 0.05 ± 0.01 b | 0.04 ± 0.01 a | 0.07 ± 0.01 | |
| Variety (V) | *** | *** | *** | *** | ** | *** | |
| Treatment (T) | *** | *** | ns | *** | *** | *** | |
| V × T | *** | *** | *** | *** | *** | *** | |
| Treatments | Momilactone A (μg/g) | Momilactone B (μg/g) | ||||
|---|---|---|---|---|---|---|
| Koshihikari | Nipponbare | Akitakomachi | Koshihikari | Nipponbare | Akitakomachi | |
| Control | 15.47 ± 0.40 a | 12.95 ± 0.34 a | 7.15 ± 0.38 a | 7.42 ± 0.17 a | 12.31 ± 0.34 a | 7.20 ± 0.10 a |
| T1 | 6.93 ± 0.35 b | 1.53 ± 0.10 c | 1.77 ± 0.08 c | 6.37 ± 0.44 b | 1.79 ± 0.12 c | 1.93 ± 0.07 c |
| T3 | 3.06 ± 0.22 c | 3.09 ± 0.13 b | 5.49 ± 0.18 b | 2.48 ± 0.09 c | 2.64 ± 0.07 b | 3.66 ± 0.20 b |
| Variety (V) | *** | *** | ||||
| Treatment (T) | *** | *** | ||||
| V × T | *** | *** | ||||
| Treatments | Description |
|---|---|
| Control | Yoshida solution |
| T1 | NaCl (10 dS/m) |
| T2 | NaCl (10 dS/m) and FA 0.125 mL/L |
| T3 | NaCl (10 dS/m) and FA 0.25 mL/L |
| T4 | NaCl (10 dS/m) and FA 0.5 mL/L |
| T5 | NaCl (10 dS/m) and FA 1.0 mL/L |
| Score | Observations | Response Category |
|---|---|---|
| 1 | Normal growth, no leaf symptoms | Highly tolerant |
| 3 | Nearly normal growth, but leaf tips of a few leaves whitish and rolled | Tolerant |
| 5 | Growth severely retarded, most leaves rolled; only a few elongating | Moderately tolerant |
| 7 | Complete cessation of growth; most leaves dry; some plants dying | Susceptible |
| 9 | Almost all plants dead or dying | Highly susceptible |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jesmin, A.; Anh, L.H.; Mai, N.P.; Khanh, T.D.; Xuan, T.D. Fulvic Acid Improves Salinity Tolerance of Rice Seedlings: Evidence from Phenotypic Performance, Relevant Phenolic Acids, and Momilactones. Plants 2023, 12, 2359. https://doi.org/10.3390/plants12122359
Jesmin A, Anh LH, Mai NP, Khanh TD, Xuan TD. Fulvic Acid Improves Salinity Tolerance of Rice Seedlings: Evidence from Phenotypic Performance, Relevant Phenolic Acids, and Momilactones. Plants. 2023; 12(12):2359. https://doi.org/10.3390/plants12122359
Chicago/Turabian StyleJesmin, Akter, La Hoang Anh, Nguyen Phuong Mai, Tran Dang Khanh, and Tran Dang Xuan. 2023. "Fulvic Acid Improves Salinity Tolerance of Rice Seedlings: Evidence from Phenotypic Performance, Relevant Phenolic Acids, and Momilactones" Plants 12, no. 12: 2359. https://doi.org/10.3390/plants12122359
APA StyleJesmin, A., Anh, L. H., Mai, N. P., Khanh, T. D., & Xuan, T. D. (2023). Fulvic Acid Improves Salinity Tolerance of Rice Seedlings: Evidence from Phenotypic Performance, Relevant Phenolic Acids, and Momilactones. Plants, 12(12), 2359. https://doi.org/10.3390/plants12122359









