Forest Ecosystem Service Trade-Offs/Synergies and System Function Optimization in Karst Desertification Control
Abstract
:1. Introduction
2. Results
2.1. Water Holding Characteristics of Forest Ecosystems
2.2. Soil Conservation Characteristics of Forest Ecosystems
2.3. Forests Ecosystem Species Diversity Characteristics
2.4. Forest Ecosystem Carbon Storage Characteristics
2.5. Trade-Offs and Synergies among Forest Ecosystem Services
3. Materials and Methods
3.1. Study Sites
3.2. Treatment Setting
3.3. Sample Collection and Index Determination
3.3.1. Sample Collection
3.3.2. Index Determination
3.4. Research Methods
- (1)
- Water conservation:
- ①
- Withered litter water holding capacity computation:
- ②
- Canopy water holding capacity computation:
- ③
- Soil water holding capacity computing:
- (2)
- Soil Conservation:
- (3)
- Species diversity:
- (4)
- Carbon storage:
- ①
- Carbon sequestration by plant computing:
- ②
- Soil carbon sequestration computation:
3.5. Data Analysis
4. Discussion
4.1. Forest Ecosystem Services for Karst Desertification Control
4.2. Trade−Offs/Synergies of Forest Ecosystem Services for Karst Desertification Control
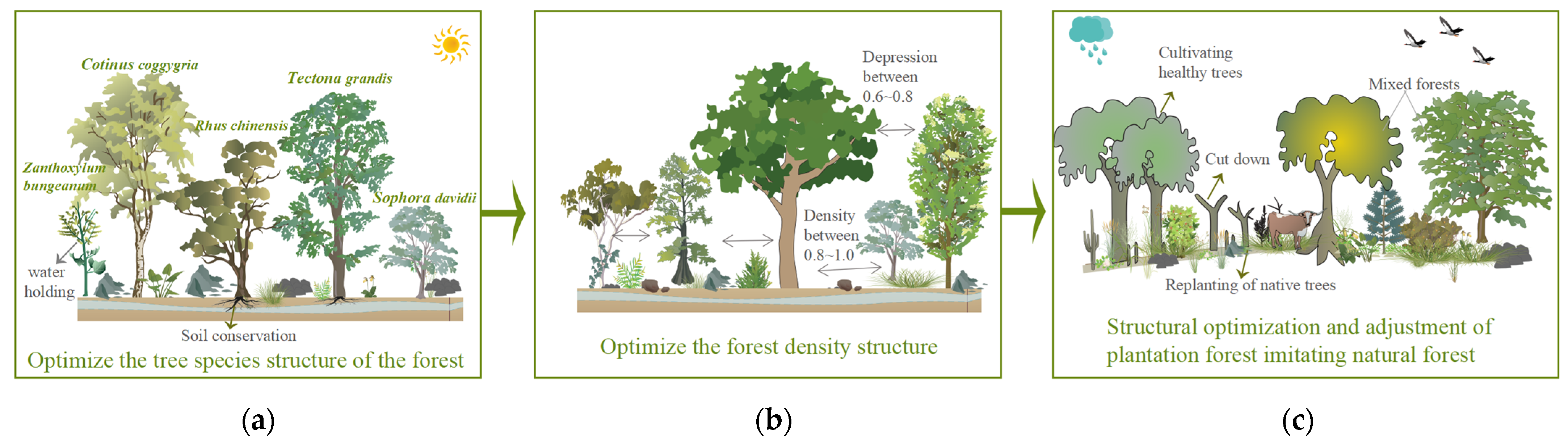
4.3. Optimization Strategy of Forest Ecosystem Service Function for Karst Desertification Control
- Optimize the structure of tree species in the stand: when selecting tree species, it is recommended to choose species with strong soil−holding and water holding capacities, whose trees are mainly Cladrastis platycarpa, Cotinus coggygria, Lindera pulcherrima, Tectona grandis, Cupressus, and Rhus chinensis. In addition, trees with waxy leaves such as Eriobotrya japonica, Yulania denudata, and other plants with high water holding capacity can be introduced [38]; shrubs are mainly selected from Zanthoxylum bungeanum, Sophora davidii, Pistacia weinmannifoli, Prunus salicina Lindl., Indigofera amblyantha, and Celtis sinensis, among other species [39].
- Optimize the structure of stand density: for natural forests with high stand density, the stand density should be controlled between 0.8 and 1.0 by adopting inter−felling and branching, and the degree of depression should be controlled between 0.6 and 0.8. Enlarging the distance between stands reduces the number of plants per unit area so that plants are given sufficient light conditions and growing space [40] (Figure 6).
- The structure of plantation forests imitates natural forests to optimize the regulation: Most forests in the karst stone desertification control area are artificial forests, and the spatial structure of forest stands should be adjusted according to the original forest or the spatial structure of forest stands close to the original forest [41]. The tree species composition of the stand is determined according to the stand conditions and the climate zone in which the stand is located, and the tree species composition of the stand is adjusted by the method of “cutting small and leaving big, cutting dense and leaving thin”, which preserves trees with a straight and complete stem shape and large diameter at breast height and harvests small trees with poor growth and a poor stem shape. We also adjusted the angular scale of retained trees to increase the proportion of structural units with values of 0.75 and 1 and replanted other native species in the forest gaps by harvesting some of the nearest neighboring trees. It is important to plant other suitable native species in the canopy and gaps of planted forests and promote forest regeneration to change the status quo of single species in forest stands and gradually form a mixed multi−species state to reduce the emergence of trade−off relationships (Figure 7).
4.4. Shortcomings and Progress
5. Conclusions
- (1)
- The Cladrastis platycarpa + Cotinus coggygria community (H1) and the Pistacia weinmannifolia + Lindera pulcherrima community (H2) have the strongest water holding capacity, followed by the Viburnum utile + Indigofera amblyantha community (H4) and the Tectona grandis community (H8). The Zanthoxylum bungeanum + Prunus salicina Lindl. community (H5) and the Eucalyptus robusta + Cupressus funebris community (H7) have the worst water holding capacity.
- (2)
- The variation in soil fertility coefficients in forest ecosystems ranged from 0.904 to 1.562, and the soil fertility was of average grade. The best soil fertility index was for the Zanthoxylum bungeanum + Prunus salicina Lindl. (H5) communities (1.562), and the worst was for the Tectona grandis community (H8) (0.905).
- (3)
- The variation in the species diversity index of the forest ecosystem ranged from 1 to 2.56, among which, the Cladrastis platycarpa+ Cotinus coggygria community (H1) had the highest species diversity index (2.56), followed by the Viburnum utile + Indigofera amblyantha community (H4) (2.38) and the Buddleja officinalis + Indigofera amblyantha community (H3) (2.34) communities, and the lowest species diversity index was in Tectona grandis community (H8) (1.00).
- (4)
- The distribution of soil carbon stock in forest ecosystems ranged from 0.79 to 8.63 t·hm−2, and the distribution of plant carbon stock ranged from 3.7 to 103.13 t·hm−2. The overall forest ecosystem carbon stock size was ranked as the Tectona grandis community (H8) > Cladrastis platycarpa+ Cotinus coggygria community (H1) > Pistacia weinmannifolia + Lindera pulcherrima community (H2) > Eucalyptus robusta + Cupressus funebris community (H7) > Zanthoxylum bungeanum + Prunus salicina Lindl. community (H5) > Buddleja officinalis + Indigofera amblyantha community (H3) > Viburnum utile + Indigofera amblyantha community (H4) > Zanthoxylum bungeanum + Glycine max community (H6).
- (5)
- There is a synergistic relationship between water conservation, species diversity, carbon storage, and soil conservation in forest ecosystems; carbon storage and soil conservation also show a synergistic relationship. There is a trade-off relationship between species diversity, carbon storage, and soil conservation.
- (6)
- Karst stone desertification control forests can optimize ecosystem service relationships and enhance forest ecosystem service capacity by regulating tree species structure, density structure, and plantation imitating natural forest structures.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Costanza, R.; d’Arge, R.; De Groot, R.; Farber, S.; Grasso, M.; Hannon, B.; Limburg, K.; Naeem, S.; O’neill, R.V.; Paruelo, J. The value of the world’s ecosystem services and natural capital. Nature 1997, 387, 253–260. [Google Scholar] [CrossRef]
- Millennium Ecosystem Assessment. Ecosystems and Human Well-Being: Synthesis; Island Press: Washington, DC, USA, 2005; Volume 5, p. 563. [Google Scholar]
- Fu, B.J.; Yu, D.D. Trade-off analyses and synthetic integrated method of multiple ecosystem services. Resour. Sci. 2016, 38, 1–9. [Google Scholar]
- Power, A.G. Ecosystem services and agriculture: Tradeoffs and synergies. Philos. Trans. Biol. Sci. 2010, 365, 2959–2971. [Google Scholar] [CrossRef] [PubMed]
- Pohjanmies, T.; Trivino, M.; Le, T.E. Impacts of forestry on boreal forests: An ecosystem services perspective. Ambio 2017, 46, 743–755. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Chu, X.L.; Gou, M.M.; Li, L.; La, L.M.; Liu, C.F. Tradeoffs and synergies analysis on forest ecosystem services in the Three Gorges Reservoir Area. Ecol. Environ. Sci. 2021, 30, 475–484. [Google Scholar]
- Qin, K.Y.; Li, J.; Yang, X.N. Trade-Off and Synergy among Ecosystem Services in the Guanzhong-Tianshui Economic Region of China. Public Health 2015, 12, 14094–14113. [Google Scholar] [CrossRef] [Green Version]
- Rana, E.; Thwaites, R.; Luck, G. Trade-offs and synergies between carbon, forest diversity and forest products in Nepal community forests. Environ. Conserv. 2017, 44, 5–13. [Google Scholar] [CrossRef] [Green Version]
- Kearney, S.P.; Fonte, S.J.; García, E.; Siles, P.; Smukler, S.M. Evaluating ecosystem service trade-offs and synergies from slash-and-mulch agroforestry systems in El Salvador. Ecol. Indic. 2017, 105, 264–278. [Google Scholar] [CrossRef]
- Qi, L.; Zhang, Y.; Xu, D. Trade-offs and synergies of ecosystem services in forest barrier belt of Northeast China. Chin. J. Ecol. 2021, 40, 3401–3411. [Google Scholar]
- Zang, J.J.; Zhu, W.B.; Zhu, L.Q.; Li, Y.H. Multi-scale analysis of trade-off/synergy effects of forest ecosystem services in the Funiu Mountain Region. Acta Geogr. Sin. 2020, 75, 975–988. [Google Scholar]
- Xiong, K.N.; Cheng, Q.W. Discussion on karst rocky desert evolution trend based on ecologically comprehensive treatment. Casologica Sin. 2010, 29, 267–273. [Google Scholar]
- Xiong, K.N.; Zhu, D.Y.; Peng, T.; Yu, L.F.; Xue, J.H.; Li, P. Study on Ecological industry technology and demonstration for Karst rocky desertification control of the Karst Plateau-Gorge. Acta Ecol. Sin. 2016, 36, 7109–7113. [Google Scholar]
- Yu, Y.H.; Zhong, X.P.; Li, H. Ecological stoichiometry of Zanthoxylum planispinum var. dintanensis plantation at different altitudes in rocky desertification area of central Guizhou. Acta Ecol. Sin. 2019, 39, 5536–5545. [Google Scholar]
- Wu, W.; Xiang, W.H.; Gou, M.M.; Xu, C.H.; Ouyang, S.; Fang, X. Trade-off and synergy between ecosystem services in three secondary forests in the mid-subtropical area of Southern China. J. For. Environ. 2019, 39, 256–264. [Google Scholar]
- Bao, S.D. Soil Agrochemical Analysis, 3rd ed.; China Agriculture Press: Beijing, China, 2000. [Google Scholar]
- Kan, W.J.; Wu, Q.T. A Preliminary Study on a Method for Quantitative and Comprehensive Evaluation of Soil Fertility. Chin. J. Soil Sci. 1994, 25, 245–247. [Google Scholar]
- Zhou, W.; Wang, W.J.; Zhang, B.; Xiao, L.; Lv, H.L.; He, X.Y. Soil fertility evaluation for urban forests and green spaces in Changchun City. Acta Ecol. Sin. 2017, 37, 1211–1220. [Google Scholar]
- Tuo, B.N.; Chen, H.L.; Yang, Y.; Ye, X.Q.; Huang, X. Evaluation of Soil Fertility in the Mitika Wetland National Nature Reserve, Tibet, China. J. Soil Water Conserv. 2022, 36, 287–295. [Google Scholar]
- Ji, C.Z. Monitoring and Evaluation of Eco-Benefits of Forest-Shrub-Grass Restoration and Superior Characteristic Forest Industry in the Karst Rocky Desertification Control; Guizhou Normal University: Guiyang, China, 2020. [Google Scholar]
- GB/T 38582-2020; Specification for Assessment of Forest Ecosystem Services. State Administration for Market Regulation: Beijing, China, 2020. Available online: www.nssi.org.cn (accessed on 6 March 2020).
- Guo, J.; Wei, B.; Liu, J.; Eissenstat, D.M.; Yu, S.; Gong, X.; Wu, J.; He, X.; Yu, M. Linkages between Plant Community Composition and Soil Microbial Diversity in Masson Pine Forests. Plants 2023, 12, 1750. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.L.; Dai, E.F.; Zhu, J.J. Spatial Patterns of Forest Ecosystem Services and Influencing Factors in the Ganjiang River Basin. J. Resour. Ecol. 7, 439–452.
- Yageta, Y.; Osbahr, H.; Morimoto, Y.; Clark, J. Comparing farmers’ qualitative evaluation of soil fertility with quantitative soil fertility indicators in Kitui County, Kenya. Geoderma 2019, 344, 153–163. [Google Scholar] [CrossRef]
- Yu, Y.H.; Wei, Z.; Zhong, X.P.; Ying, B. Stoichiometric characteristics in Zanthoxylum planispinum var. dintanensis plantation of different ages. Agron. J. 2021, 113, 685–695. [Google Scholar]
- Xu, S.; Eisenhauer, N.; Ferlian, O.; Zhang, J.; Zhou, G.; Lu, X.; Liu, C.; Zhang, D. Species richness promotes ecosystem carbon storage: Evidence from biodiversity-ecosystem functioning experiments. Proc. R. Soc. 2020, 287, 2020–2063. [Google Scholar] [CrossRef] [PubMed]
- Strengbom, J.; Nordin, A. Commercial forest fertilization causes long-term residual effects in ground vegetation of boreal forests. For. Ecol. Manag. 2008, 256, 2175–2181. [Google Scholar] [CrossRef]
- Haywood, J.D.; Goelz, J.C.; Sayer, M.A.S.; Tiarks, A.E. Influence of fertilization, weed control, and pine litter on loblolly pine growth and productivity and understory plant development through 12 growing seasons. Can. J. For. Res. 2003, 33, 1974–1982. [Google Scholar] [CrossRef]
- Zhou, Y.; She, J.; Zhu, X. Dynamic analysis of biodiversity, carbon storage and environmental factors of coniferous forest in Loudi City, Hunan Province. Int. J. Low-Carbon Technol. 2022, 17, 831–840. [Google Scholar] [CrossRef]
- Deng, C.; Liu, J.; Nie, X.; Li, Z.; Xiao, L. How trade-offs between ecological construction and urbanization expansion affect ecosystem services. Ecol. Indic. 2021, 122, 107–253. [Google Scholar] [CrossRef]
- Lafond, V.; Cordonnier, T.; Mao, Z.; Courbaud, B. Trade-offs and synergies between ecosystem services in uneven-aged mountain forests: Evidences using Pareto fronts. Eur. J. For. Res. 2017, 136, 997–1012. [Google Scholar] [CrossRef]
- Wang, S.; Huang, Y. Determinants of soil organic carbon sequestration and its contribution to ecosystem carbon sinks of planted forests. Glob. Change Biol. 2020, 26, 3163–3173. [Google Scholar] [CrossRef]
- Fornara, D.A.; Tilman, D. Plant functional composition influences rates of soil carbon and nitrogen accumulation. J. Ecol. 2008, 96, 314–322. [Google Scholar] [CrossRef]
- Qin, Y.; Xiong, K.N.; Chen, Q.W.; Zhang, S.H.; Yang, S.; Li, T.L. Ecosystem Service Changes and Trade-off Synergistic Relationships in Karst Areas. Environ. Sci. Technol. 2021, 44, 228–236. [Google Scholar]
- Widenfalk, O.; Weslien, J. Plant species richness in managed boreal forests—Effects of stand succession and thinning. For. Ecol. Manag. 2009, 257, 1386–1394. [Google Scholar] [CrossRef]
- Zhang, S.H.; Xiong, K.L.; Qin, Y.; Min, X.Y.; Xiao, J. Evolution and determinants of ecosystem services: Insights from South China Karst. Ecol. Indic. 2021, 133, 108437. [Google Scholar] [CrossRef]
- Zhang, S.H.; Xiong, K.L.; Deng, X.H.; Kong, L.W.; Min, X.Y. The impact of ecological restoration on ecosystem service trade offs: Insight from the karst desertification control. Land Degrad. Dev. 2023, 34, 2693–2706. [Google Scholar] [CrossRef]
- Deng, X.H.; Xiong, K.N.; Yu, Y.H.; Zhang, S.H.; Kong, L.W.; Zhang, Y. A Review of Ecosystem Service Trade-Offs/Synergies: Enlightenment for the Optimization of Forest Ecosystem Functions in Karst Desertification Control. Forests 2023, 14, 88. [Google Scholar] [CrossRef]
- Kong, L.W.; Xiong, K.N.; Zhang, S.H.; Zhang, Y.; Deng, X.H. Review on Driving Factors of Ecosystem Services: Its Enlightenment for the Improvement of Forest Ecosystem Functions in Karst Desertification Control. Forests 2023, 14, 582. [Google Scholar] [CrossRef]
- Xiao, J.; Xiong, K.N. A review of agroforestry ecosystem services and its enlightenment on the ecosystem improvement of rocky desertification control. Sci. Total Environ. 2022, 856, 158538. [Google Scholar] [CrossRef]
- Jiang, S.L.; Xiong, K.N.; Xiao, J. Structure and Stability of Agroforestry Ecosystems: Insights into the Improvement of Service Supply Capacity of Agroforestry Ecosystems under the Karst Rocky Desertification Control. Forests 2022, 13, 878. [Google Scholar] [CrossRef]






| Sample Number | Soil Fraction Fertility Factor | Integrated Fertility Factor F | Fertility Grade | ||||||
|---|---|---|---|---|---|---|---|---|---|
| TP/ (g·kg−1) | TK/ (g·kg−1) | TN/ (g·kg−1) | SOM/ (g·kg−1) | AP/ (mg·kg−1) | ρb/ (g·cm−3) | pH | |||
| H1 | 2.82 | 0.22 | 3.00 | 3.00 | 0.28 | 1.81 | 2.09 | 1.152 | III |
| H2 | 3.00 | 0.22 | 3.00 | 3.00 | 0.17 | 3.00 | 3.00 | 1.336 | III |
| H3 | 3.00 | 0.82 | 3.00 | 3.00 | 0.08 | 0.99 | 3.00 | 1.204 | III |
| H4 | 3.00 | 4.02 | 3.00 | 3.00 | 0.10 | 1.88 | 3.00 | 1.561 | III |
| H5 | 3.00 | 0.99 | 3.00 | 3.00 | 0.59 | 0.98 | 6.00 | 1.562 | III |
| H6 | 3.00 | 1.03 | 3.00 | 3.00 | 0.39 | 0.82 | 3.00 | 1.254 | III |
| H7 | 2.02 | 0.81 | 3.00 | 3.00 | 0.25 | 0.98 | 3.00 | 1.142 | III |
| H8 | 0.72 | 1.07 | 1.53 | 1.70 | 0.34 | 1.87 | 2.95 | 0.905 | III |
| Service Category | Water Holding Capacity | Species Diversity | Carbon Storage | Soil Conservation |
|---|---|---|---|---|
| water holding capacity | 1 | |||
| Species diversity | 0.24 | 1 | ||
| Carbon storage | 0.513 * | −0.298 | 1 | |
| Soil conservation | 0.261 | −0.05 | 0.209 | 1 |
| Site | Slope/° | Aspect/° | Mean Crown/m | Mean Tree Height/m |
|---|---|---|---|---|
| H1 | 20 | Southeast 145° | 3.2 × 3.1 | 7.45 |
| H2 | 26 | Southeast 101° | 3.1 × 2.9 | 4.63 |
| H3 | 30 | East 102° | 0.8 × 1.1 | 1.04 |
| H4 | 28 | Northwest 280° | 1.20 × 1.1 | 1.47 |
| H5 | 10 | Southwest 232° | 1.35 × 1.33 | 1.43 |
| H6 | 18 | North 22° | 1.12 × 0.92 | 3.45 |
| H7 | 30 | Southwest 220° | 3.0 × 2.8 | 8.03 |
| H8 | 19 | Southeast 149° | 3.3 × 2.9 | 8.35 |
| Soil Properties | Xa | Xb | Xc |
|---|---|---|---|
| TN (g·kg−1) | 0.75 | 1.50 | 2.00 |
| TP (g·kg−1) | 0.40 | 0.60 | 1.00 |
| TK (g·kg−1) | 5.00 | 20.00 | 25.00 |
| SOM (g·kg−1) | 10.00 | 20.00 | 30.00 |
| AP (mg·kg−1) | 5.00 | 10.00 | 20.00 |
| ρb | 1.45 | 1.35 | 1.25 |
| pH (≤7.0) | 4.5 | 5.5 | 6.5 |
| pH (>7.0) | 9.0 | 8.0 | 7.0 |
| Soil Fertility Grade | Grade I (Very Fertile) | Grade II (Fertile) | Grade III (General) | Grade IV (Barren) |
|---|---|---|---|---|
| Fertility coefficient range | ≥2.70 | 2.70~1.80 | 1.80~0.90 | <0.90 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiong, K.; Deng, X.; Zhang, S.; Zhang, Y.; Kong, L. Forest Ecosystem Service Trade-Offs/Synergies and System Function Optimization in Karst Desertification Control. Plants 2023, 12, 2376. https://doi.org/10.3390/plants12122376
Xiong K, Deng X, Zhang S, Zhang Y, Kong L. Forest Ecosystem Service Trade-Offs/Synergies and System Function Optimization in Karst Desertification Control. Plants. 2023; 12(12):2376. https://doi.org/10.3390/plants12122376
Chicago/Turabian StyleXiong, Kangning, Xuehua Deng, Shihao Zhang, Yu Zhang, and Lingwei Kong. 2023. "Forest Ecosystem Service Trade-Offs/Synergies and System Function Optimization in Karst Desertification Control" Plants 12, no. 12: 2376. https://doi.org/10.3390/plants12122376
APA StyleXiong, K., Deng, X., Zhang, S., Zhang, Y., & Kong, L. (2023). Forest Ecosystem Service Trade-Offs/Synergies and System Function Optimization in Karst Desertification Control. Plants, 12(12), 2376. https://doi.org/10.3390/plants12122376






