New Reports on the Portuguese Endemic Species, Santolina impressa: Secretory Structures, Essential Oil Composition and Antiviral Activity
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization of the Secretory Structures
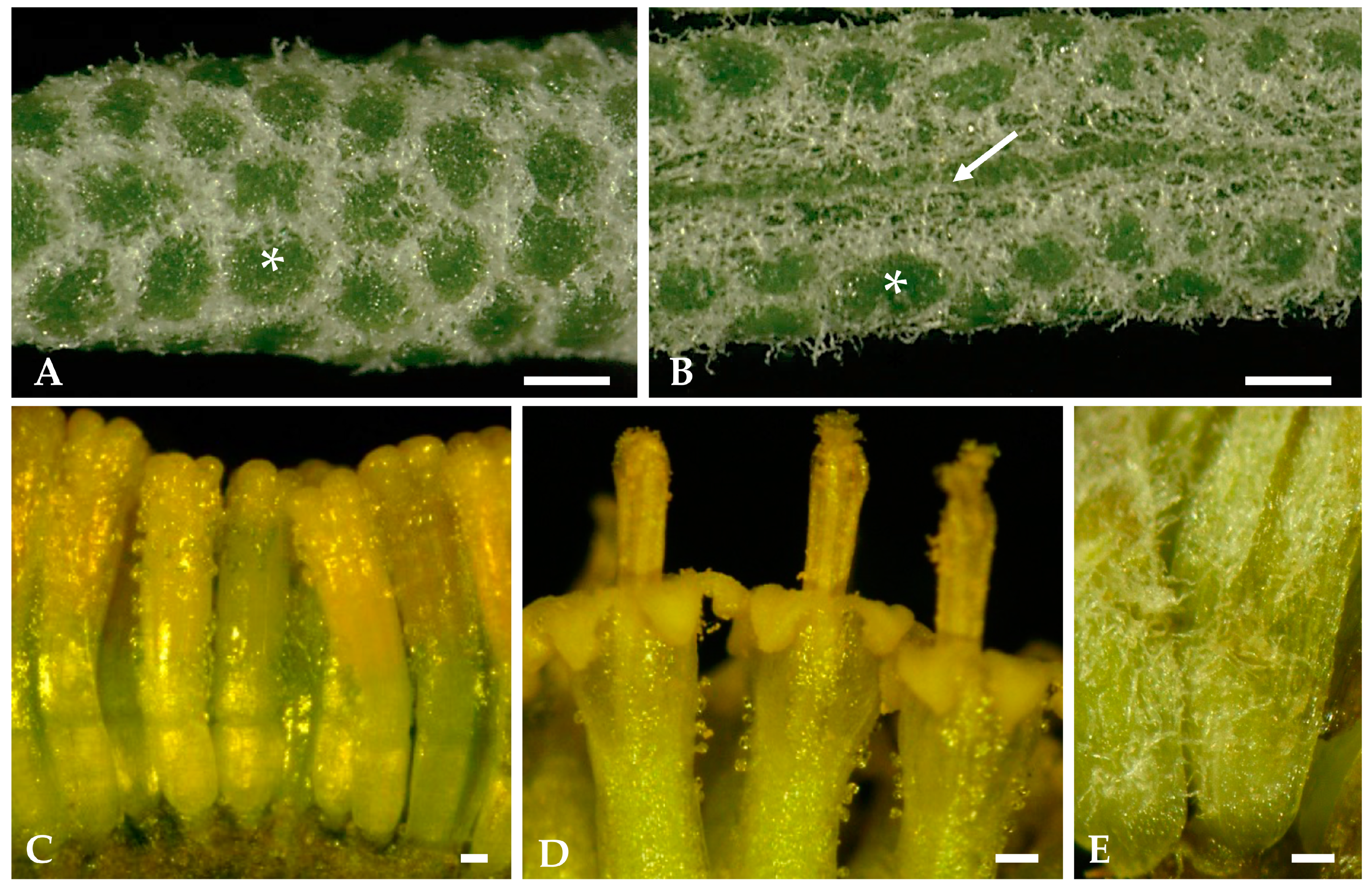
2.1.1. Surface Overview of Mature Leaves and Inflorescence Florets
2.1.2. Distribution, Micromorphology, and Anatomy of Secretory Structures
2.1.3. Histochemistry of Secreted Material
2.2. Essential Oil Composition
2.3. Cytotoxicity of Essential Oil
2.4. Essential Oil Antiherpetic Activity
2.4.1. Virucidal Effect
2.4.2. Effect on Virus Yield
2.4.3. Effect on the Virus Replication Cycle
3. Materials and Methods
3.1. Chemicals
3.2. Plant Material
3.3. Secretory Structures Characterization
3.3.1. Stereomicroscopy
3.3.2. Scanning Electron Microscopy (SEM)
3.3.3. Light Microscopy (LM)
3.4. Extraction and Chemical Analysis of the Essential Oil (EO)
3.4.1. Essential Oil Isolation
3.4.2. Essential Oil Analysis and Quantification
3.5. Antiviral Activity of the Essential Oil
3.5.1. Cells and Viruses
3.5.2. Essential Oil Stock and Work Solutions
3.5.3. Cytotoxicity Evaluation
3.5.4. Antiviral Assays
3.5.5. Data Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Piret, J.; Boivin, G. Resistance of Herpes simplex viruses to nucleoside analogues: Mechanisms, prevalence, and management. Antimicrob. Agents Chemother. 2011, 55, 459–472. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Clercq, E. Selective anti-herpesvirus agents. Antivir. Chem. Chemother. 2013, 23, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Briskin, D.P. Medicinal plants and phytomedicines. Linking plant biochemistry and physiology to human health. Plant Physiol. 2000, 124, 507–514. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gras, A.; Hidalgo, O.; Ambrosio, U.; Parada, M.; Garnatje, T.; Vallès, J. The role of botanical families in medicinal ethnobotany: A phylogenetic perspective. Plants 2021, 10, 163. [Google Scholar] [CrossRef] [PubMed]
- Carbajal, R.; Ortiz, S.; Sáez, L. Santolina L. In Flora Ibérica; Castroviejo, S.B., Benedí, C., Buira, A., Rico, E., Crespo, M.B., Quintanar, A., Aedo, C., Eds.; CSIC: Madrid, Spain, 2007; pp. 1938–1962. [Google Scholar]
- Tundis, R.; Loizzo, M.R. A review of the traditional uses, phytochemistry and biological activities of the genus Santolina. Planta Med. 2018, 84, 627–637. [Google Scholar] [CrossRef] [Green Version]
- De Logu, A.; Loy, G.; Pellerano, M.L.; Bonsignore, L.; Schivo, M.L. Inactivation of HSV-1 and HSV-2 and prevention of cell-to-cell virus spread by Santolina insularis essential oil. Antivir. Res. 2000, 48, 177–185. [Google Scholar] [CrossRef]
- Demirci, B.; Özek, T.; Baser, K.H.C. Chemical composition of Santolina chamaecyparissus L. essential oil. J. Essent. Oil Res. 2000, 12, 625–627. [Google Scholar] [CrossRef]
- Liu, K.; Rossi, P.G.; Ferrari, B.; Berti, L.; Casanova, J.; Tomi, F. Composition, irregular terpenoids, chemical variability and antibacterial activity of the essential oil from Santolina corsica Jordan et Fourr. Phytochemistry 2007, 68, 1698–7105. [Google Scholar] [CrossRef] [PubMed]
- Guinoiseau, E.; Luciani, A.; Rossi, P.G.; Quilichini, Y.; Ternengo, S.; Brasedi, P.; Berti, L. Cellular effects induced by Inula graveolens and Santolina corsica essential oils on Staphylococcus aureus. Eur. J. Clin. Microbiol. Infect. Dis. 2010, 9, 873–879. [Google Scholar] [CrossRef] [Green Version]
- Djeddi, S.; Djebile, K.; Hadjbourega, G.; Achour, Z.; Argyropoulou, C.; Skaltsa, H. In vitro antimicrobial properties and chemical composition of Santolina chamaecyparissus essential oil from Algeria. Nat. Prod. Commun. 2012, 7, 937–940. [Google Scholar] [CrossRef] [Green Version]
- Gomes, A.; Pimpão, R.C.; Fortalezas, S.; Figueira, I.; Miguel, C.; Aguiar, C.; Salgueiro, L.; Cavaleiro, C.; Gonçalves, M.J.; Clemente, A.; et al. Chemical characterization and bioactivity of phytochemicals from Iberian endemic Santolina semidentata and strategies for ex situ propagation. Ind. Crop Prod. 2015, 74, 505–513. [Google Scholar] [CrossRef] [Green Version]
- Khubeiz, M.J.; Mansour, G. In vitro antifungal, antimicrobial properties and chemical composition of Santolina chamaecyparissus essential oil in Syria. Int. J. Toxicol. Pharmacol. Res. 2016, 8, 372–378. [Google Scholar]
- Alves-Silva, J.M.; Zuzarte, M.; Gonçalves, M.J.; Cruz, M.T.; Cavaleiro, C.; Salgueiro, L. Unveiling the bioactive potential of the essential oil of a Portuguese endemism, Santolina impressa. J. Ethnopharmacol. 2019, 244, 112120. [Google Scholar] [CrossRef]
- Alves-Silva, J.M.; Gonçalves, M.J.; Silva, A.; Cavaleiro, C.; Cruz, M.T.; Salgueiro, L. Chemical profile, anti-microbial and anti-inflammaging activities of Santolina rosmarinifolia L. essential oil from Portugal. Antibiotics 2023, 12, 179. [Google Scholar] [CrossRef]
- Rivero-Guerra, A.O. Cytogenetics, geographical distribution, pollen stainability and fecundity of Santolina impressa (Asteraceae: Anthemideae). Folia Geobot. 2010, 45, 95–109. [Google Scholar] [CrossRef]
- Rivero-Guerra, A.O. Typification and synonymy of names in Santolina (Asteraceae: Anthemideae) published by Hoffmannsegg and Link. Nord J. Bot. 2010, 28, 581–587. [Google Scholar] [CrossRef]
- Rivero-Guerra, A.O.; Laurin, M. Phylogenetic analysis of the Santolina rosmarinifolia aggregate (Asteraceae: Anthemideae: Santolininae) based on morphological characteristics. Nord J. Bot. 2012, 30, 533–545. [Google Scholar] [CrossRef]
- Proença da Cunha, A.; Ribeiro, J.A.; Roque, O.R. Plantas Aromáticas em Portugal: Caracterização e Utilizações, 1st ed.; Fundação Calouste Gulbenkian: Lisbon, Portugal, 2007; pp. 55–57. [Google Scholar]
- Proença da Cunha, A.; Roque, O.R.; Nogueira, M.T. Plantas Aromáticas e Óleos Essenciais, Composição e Aplicações; Fundação Calouste Gulbenkian: Lisboa, Portugal, 2012. [Google Scholar]
- Rivero-Guerra, A.O. Morphological variation within and between taxa of the Santolina rosmarinifolia L. (Asteraceae: Anthemideae) Aggregate. Syst. Bot. 2011, 36, 171–190. [Google Scholar] [CrossRef]
- Rodrigues, A.M.; Falé, P.L.V.; Madeira, P.; Pacheco, R.; Florêncio, M.H.; Ascensão, L.; Serralheiro, M.L.M. Phenolic profile and biological activities of decoctions from Santolina impressa, a Portuguese endemic species. J. Herb. Med. 2020, 21, 100335. [Google Scholar] [CrossRef]
- Bilz, M. Santolina Impressa. The IUCN Red List of Threatened Species. 2011. Available online: https://www.iucnredlist.org/species/162385/5583979 (accessed on 15 March 2023).
- Iglesias, I.; Feijóo, M.C.; Ortiz, S. Contribution to the conservation studies of Santolina melidensis (Rodr. -Oubiñia & S. Ortiz) Rodr.-Oubiña & S. Ortiz. Portugaliae Acta Biol. 2000, 19, 107–112. [Google Scholar]
- Casado, J.P.; Navarro, M.C.; Utrilla, M.P.; Martínez, A.; Jiménez, J. Micropropagation of Santolina canescens Lagasca and in vitro volatiles production by shoot explants. Plant Cell Tissue Org. Cult. 2002, 69, 147–153. [Google Scholar] [CrossRef]
- European Council. Council Directive 92/43/EEC of 21 May 1992 on the Conservation of Natural Habitats and of Wild Fauna and Flora—Consolidated Version 01/01/2007. Annex II: Animal and Plant Species of Community Interest Whose Conservation Requires the Designation of Special Areas of Conservation. Available online: http://ec.europa.eu/environment/index_en.htm (accessed on 27 May 2023).
- Martínez-Quezada, D.M.; Rivera, P.; Rojas-Leal, A.; Villaseñor, J.L.; Terrazas, T. Leaf secretor structures in Asteraceae: A synthesis of their diversity and evolution. Bot. Rev. 2023, 85, 59–90. [Google Scholar] [CrossRef]
- Fahn, A. Secretory Tissues in Plants; Academic Press, Inc.: London, England, 1979. [Google Scholar]
- Ascensão, L.; Pais, M.S. Glandular trichomes of Artemisia campestris (ssp. maritima)—Ontogeny and histochemistry of the secretory product. Bot. Gaz. 1987, 148, 221–227. [Google Scholar] [CrossRef]
- Ascensão, L.; Pais, M.S. Ultrastructure and histochemistry of secretory ducts in Artemisia campestris ssp. maritima (Compositae). Nord J. Bot. 1988, 8, 283–292. [Google Scholar] [CrossRef]
- Duke, S.O.; Paul, R.N. Development and fine structure of the glandular trichomes of Artemisia annua L. Int. J. Plant Sci. 1993, 154, 107–118. [Google Scholar] [CrossRef]
- Figueiredo, A.C.; Pais, M.S. Ultrastructural aspects of the glandular cells from the secretory trichomes and from the cell suspension cultures of Achillea millefolium L. ssp. millefolium. Ann. Bot. 1994, 74, 179–190. [Google Scholar] [CrossRef]
- Pagni, A.M.; Orlando, R.; Masini, A.; Ciccarelli, D. Secretory structures of Santolina ligustica Arrigoni (Asteraceae), an Italian endemic species. ISR J. Plant Sci. 2003, 51, 185–192. [Google Scholar] [CrossRef]
- Andreucci, A.C.; Ciccarelli, D.; Desideri, I.; Pagni, A.M. Glandular hairs and secretory ducts of Matricaria chamomilla (Asteraceae): Morphology and histochemistry. Ann. Bot. Fenn. 2008, 45, 11–18. [Google Scholar] [CrossRef]
- Afolayan, A.J.; Meyer, J.J.M. Morphology and ultrastructure of secreting and nonsecreting foliar trichomes of Helichrysum aureonitens (Asteraceae). Int. J. Plant Sci. 1995, 156, 481–487. [Google Scholar] [CrossRef]
- Ascensão, L.; Da Silva, J.A.T.; Barroso, J.G.; Figueiredo, A.C.; Pedro, L.G. Glandular trichomes and essential oils of Helichrysum stoechas. ISR J. Plant Sci. 2001, 49, 115–122. [Google Scholar] [CrossRef]
- Perrini, R.; Morone-Fortunato, I.; Lorusso, E.; Avato, P. Glands, essential oils and in vitro establishment of Helichrysum italicum (Roth) G. Don ssp. microphyllum (Willd.) Nyman. Ind. Crops Prod. 2009, 29, 395–403. [Google Scholar] [CrossRef]
- Sacchetti, G.; Romagnoli, C.; Ballero, M.; Tosi, B.; Poli, F. Internal secretory structures and preliminary phytochemical investigation on flavonoid and coumarin content in Santolina insularis (Asteraceae). Phyton-Ann. Rei. Bot. 1997, 32, 219–228. [Google Scholar]
- Pagni, A.M.; Masini, A. Morphology, distribution, and histochemistry of secretory structures in vegetative organs of Santolina leucantha bertol. (Asteraceae). ISR J. Plant Sci. 1999, 47, 257–263. [Google Scholar] [CrossRef]
- Tavares, L.; Fortalezas, S.; Tyagi, M.; Barata, D.; Serra, T.; Duarte, C.; Duarte, R.; Feliciano, R.; Bronze, M.R.; Espírito-Santo, M.D.; et al. Bioactive compounds from endemic plants of Southwest Portugal: Inhibition of acetylcholine esterase and radical scavenging activities. Pharm. Biol. 2012, 50, 239–246. [Google Scholar] [CrossRef] [PubMed]
- Micco, V.D.; Aronne, G. Morpho-anatomical traits for plant adaptation to drought. In Plant Responses to Drought Stress; Aroca, R., Ed.; Springer-Verlag: Berlin/Heidelberg, Germany, 2012; pp. 37–61. [Google Scholar]
- Figueiredo, A.C.; Barroso, J.G.; Pedro, L.G.; Scheffer, J.J.C. Factors affecting secondary metabolites production in plants: Volatile components and essential oils. Flavour Fragr. J. 2008, 23, 213–226. [Google Scholar] [CrossRef]
- Vernin, G. Volatile Constituents of the essential oil of Santolina chamaecyparissus L. J. Essent. Oil Res. 1991, 3, 49–53. [Google Scholar] [CrossRef]
- Tognolini, M.; Barocelli, E.; Ballabeni, V.; Bruni, R.; Bianchi, A.; Chiavarini, M.; Impicciatore, M. Comparative screening of plant essential oils: Phenylpropanoid moiety as basic core for antiplatelet activity. Life Sci. 2006, 78, 1419–1432. [Google Scholar] [CrossRef]
- Garg, S.N.; Gupta, D.; Mehta, V.K.; Kumar, S. Volatile constituents of the essential oil of Santolina chamaecyparissus Linn, from the Southern Hills of India. J. Essent. Oil Res. 2001, 13, 234–235. [Google Scholar] [CrossRef]
- Verdeguer, M.; Castañeda, L.G.; Torres-Pagan, N.; Llorens-Molina, J.A.; Carrubba, A. Control of Erigeron bonariensis with Thymbra capitata, Mentha piperita, Eucalyptus camaldulensis, and Santolina chamaecyparissus Essential Oils. Molecules 2020, 25, 562. [Google Scholar] [CrossRef] [Green Version]
- Flamini, G.; Bertoli, A.; Taglioli, V.; Cioni, P.L.; Morelli, I.; Spinelli, G. Composition of the essential oil of Santolina ligustica. J. Essent. Oil Res. 1999, 11, 6–8. [Google Scholar] [CrossRef]
- Cherchi, G.; Deidda, D.; Gioannis, B.D.; Marongiu, B.; Pompei, R.; Porcedda, S. Extraction of Santolina insularis essential oil by supercritical carbon dioxide: Influence of some process parameters and biological activity. Flavour Fragr. J. 2001, 16, 35–43. [Google Scholar] [CrossRef]
- Pérez-Alonso, M.J.; Negueruela, A.V. The essential oils of four Santolina species. Flavour Fragr. J. 1988, 3, 37–42. [Google Scholar] [CrossRef]
- Palá-Paúl, J.; Pérez-Alonso, M.J.; Velasco-Negueruela, A.; Palá-Paúl, R.; Sanz, J.; Conejero, F. Seasonal variation in chemical constituents of Santolina rosmarinifolia L. ssp. rosmarinifolia. Biochem. Syst. Ecol. 2001, 29, 663–672. [Google Scholar] [CrossRef] [PubMed]
- Djamel, S.; Hendel, N.; Hadjer, F.; Giuseppe, R.; Madani, S. Chemical composition of essential oil from the aerial parts of Santolina rosmarinifolia L. a wild Algerian medicinal plant. Nat. Volatiles Essent. Oils 2021, 8, 22–28. [Google Scholar] [CrossRef]
- Cos, P.; Vlietinck, A.J.; Berghe, D.V.; Maes, L. Antiinfective potential of natural products: How to develop a stronger in vitro ‘proof-of-concept’. J. Ethnopharmacol. 2006, 106, 290–302. [Google Scholar] [CrossRef] [PubMed]
- Schnitzler, P. Essential oils for the treatment of herpes simplex virus infections. Chemotherapy 2019, 64, 1–7. [Google Scholar] [CrossRef]
- Reichling, J. Antiviral and virucidal properties of essential oils and isolated compounds—A scientific approach. Planta Med. 2022, 88, 587–603. [Google Scholar] [CrossRef] [PubMed]
- Lai, W.L.; Chuang, H.S.; Lee, M.H.; Wei, C.L.; Lin, C.F.; Tsai, Y.C. Inhibition of herpes simplex virus type 1 by thymol-related monoterpenoids. Planta Med. 2012, 78, 1636–1638. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reichling, J. Antibacterial and antiviral Effects of aromatic Plant-derived essential Oils—A scientific and medicinal Approach. In Medicinal Plants—Biodiversity and Drugs; Rai, M., Cordell, G.A., Martinez, J.L., Marinoff, M., Rastrelli, L., Eds.; CRC Press: Boca Raton, FL, USA, 2012; pp. 622–640. [Google Scholar]
- Civitelli, L.; Panella, S.; Marcocci, M.E.; De Petris, A.; Garzoli, S.; Pepi, F.; Vavala, E.; Ragno, R.; Nencioni, L.; Palamara, A.T.; et al. In vitro inhibition of herpes simplex virus type 1 replication by Mentha suaveolens essential oil and its main component piperitenone oxide. Phytomedicine 2014, 21, 857–865. [Google Scholar] [CrossRef]
- Venturi, C.R.; Danielli, L.J.; Klein, F.; Apel, M.A.; Montanha, J.A.; Bordignon, S.A.L.; Roehe, P.M.; Fuentefria, A.M.; Henriques, A.T. Chemical analysis and in vitro antiviral and antifungal activities of essential oils from Glechon spathulata and Glechon marifolia. Pharm. Biol. 2015, 53, 682–688. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gatherer, D.; Depledge, D.P.; Hartley, C.A.; Szpara, M.L.; Vaz, P.K.; Benkő, M.; Brandt, C.R.; Bryant, N.A.; Dastjerdi, A.; Doszpoly, A.; et al. ICTV virus taxonomy profile: Herpesviridae. J. Gen. Virol. 2021, 102, 001673. [Google Scholar] [CrossRef]
- van de Sand, L.; Bormann, M.; Schmitz, Y.; Heilingloh, C.S.; Witzke, O.; Krawczyk, A. Antiviral active compounds derived from natural sources against herpes simplex viruses. Viruses 2021, 13, 1386. [Google Scholar] [CrossRef] [PubMed]
- Feder, N.; O’Brien, T.P. Plant microtechnique: Some principles and new methods. Am. J. Bot. 1968, 55, 123–142. [Google Scholar] [CrossRef]
- Meira, R.M.S.A.; Francino, D.M.T.; Ascensão, L. Oleoresin trichomes of Chamaecrista dentata (Leguminosae): Structure, function, and secretory products. Int. J. Plant Sci. 2014, 175, 336–345. [Google Scholar] [CrossRef]
- Council of Europe. European Directorate for the Quality of Medicines, in European Pharmacopoeia, 7th ed.; Council of Europe: Strasbourg, France, 2010; p. 241. [Google Scholar]
- ISO 7609:1985; Essential Oils—Analysis by Gas Chromatography on Capillary Columns—General Method. ISO: London, UK.
- Mosmann, T.R. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1982, 65, 55–63. [Google Scholar] [CrossRef] [PubMed]








| Target Class of Compounds | Histochemical Tests | Positive Reaction Color | Glandular Trichomes | Secretory Ducts |
|---|---|---|---|---|
| Total lipids | Sudan Black B | Dark blue to Black | ++ | ++ |
| Sudan Red IV | Red | ++ | ++ | |
| Acidic/Neutral lipids | Nile Blue A | Blue/Red | ++ (Red) | ++ (Red) |
| Unsaturated lipids | Osmium Tetroxide | Black | ++ | ++ |
| EOs and resins | Nadi Reagent | Blue (EOs)/Red (Resins) | ++ (Violet) | ++ (Red) |
| Polysaccharides | PAS | Bright rose | − | − |
| Pectins | Ruthenium Red | Red | − | − |
| Phenolic compounds | Potassium Dichromate | Brown-orange | ++ | ++ |
| Ferric Trichloride | Dark brown | ++ | ++ | |
| Flavonoids | Aluminum Chloride (UV) | Green yellowish | ++ | ++ |
| Components | RI | Santolina impressa |
|---|---|---|
| Tricyclene | 921 | 0.1 |
| α-Thujene | 924 | 0.4 |
| α-Pinene | 930 | 1.7 |
| Camphene | 938 | 1.5 |
| Thuja-2,4(10)-diene * | 940 | 0.2 |
| Sabinene | 958 | 1.2 |
| β-Pinene | 963 | 12.6 |
| Dehydro-1,8-cineole | 973 | 0.1 |
| β-Myrcene | 975 | 6.0 |
| Yomogi alcohol | 978 | 8.8 |
| α-Phellandrene | 995 | 0.3 |
| α-Terpinene | 1002 | 0.8 |
| p-Cymene | 1003 | 0.2 |
| 1,8-Cineole | 1005 | 6.2 |
| β-Phellandrene | 1005 | 10.4 |
| Limonene | 1009 | 8.1 |
| cis-β-Ocimene | 1017 | 1.3 |
| trans-β-Ocimene | 1027 | 1.1 |
| γ-Terpinene | 1035 | 1.2 |
| Artemisia alcohol | 1055 | 2.1 |
| Terpinolene | 1064 | 0.7 |
| Dehydro sabina ketone * | 1066 * | 0.1 |
| 2-Methyl butyric acid isoamyl ester | 1074 | 0.2 |
| Isopentyl isovalerate | 1084 | 0.1 |
| trans-p-2-Menthen-1-ol | 1099 | 0.3 |
| Camphor | 1102 | 7.1 |
| trans-Pinocarveol | 1106 | 0.3 |
| cis-p-2-menthen-1-ol | 1110 | 0.2 |
| Pinocarvone | 1121 | 0.3 |
| α-Phellandrol * | 1134 | 1.1 |
| Borneol | 1134 | 1.1 |
| Terpinen-4-ol | 1148 | 2.8 |
| α-Terpineol | 1159 | 1.0 |
| Verbenone | 1164 | 1.9 |
| Piperitone | 1211 | 0.4 |
| Bornyl acetate | 1265 | 0.5 |
| Lavandulyl acetate | 1278 | t |
| Geranyl acetate | 1370 | t |
| β-Caryophyllene | 1414 | 0.2 |
| allo-Aromadendrene | 1456 | 0.1 |
| ar-Curcumene | 1474 | 0.8 |
| γ-Muurolene | 1469 | 0.2 |
| Germacrene D | 1474 | 0.4 |
| Bicyclogermacrene | 1487 | 0.5 |
| δ-Cadinene | 1505 | 0.2 |
| Spathulenol | 1551 | 0.7 |
| β-Caryophyllene oxide | 1561 | 0.2 |
| Anhydrooplopanone | 1576 | 0.4 |
| T-Cadinol | 1616 | 0.2 |
| α-Cadinol | 1626 | 0.2 |
| % Identification | 86.5 | |
| Grouped components | ||
| Monoterpene hydrocarbons | 47.8 | |
| Oxygen-containing monoterpenes | 34.3 | |
| Sesquiterpene hydrocarbons | 2.4 | |
| Oxygen-containing sesquiterpenes | 1.7 | |
| Others | 0.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodrigues, A.M.; Mendes, A.R.; Caeiro, M.F.; Figueiredo, A.C.; Ascensão, L. New Reports on the Portuguese Endemic Species, Santolina impressa: Secretory Structures, Essential Oil Composition and Antiviral Activity. Plants 2023, 12, 2391. https://doi.org/10.3390/plants12132391
Rodrigues AM, Mendes AR, Caeiro MF, Figueiredo AC, Ascensão L. New Reports on the Portuguese Endemic Species, Santolina impressa: Secretory Structures, Essential Oil Composition and Antiviral Activity. Plants. 2023; 12(13):2391. https://doi.org/10.3390/plants12132391
Chicago/Turabian StyleRodrigues, Ana Margarida, Ana Rita Mendes, Maria Filomena Caeiro, Ana Cristina Figueiredo, and Lia Ascensão. 2023. "New Reports on the Portuguese Endemic Species, Santolina impressa: Secretory Structures, Essential Oil Composition and Antiviral Activity" Plants 12, no. 13: 2391. https://doi.org/10.3390/plants12132391
APA StyleRodrigues, A. M., Mendes, A. R., Caeiro, M. F., Figueiredo, A. C., & Ascensão, L. (2023). New Reports on the Portuguese Endemic Species, Santolina impressa: Secretory Structures, Essential Oil Composition and Antiviral Activity. Plants, 12(13), 2391. https://doi.org/10.3390/plants12132391






