Hydrochorous Seed Transport in the Lower Traisen River before and after Riverbed Restoration
Abstract
1. Introduction
- i.
- How does the transport pool in running water change due to hydrological measures?
- ii.
- Could functional species traits be used as predictors for the survival of transported diaspores and for special/temporal changes?
2. Results
2.1. Trait-Dependent Differences between Total and Viable Seed Transport (2014)
2.2. Composition of Viable Seeds in 2017
3. Discussion
4. Materials and Methods
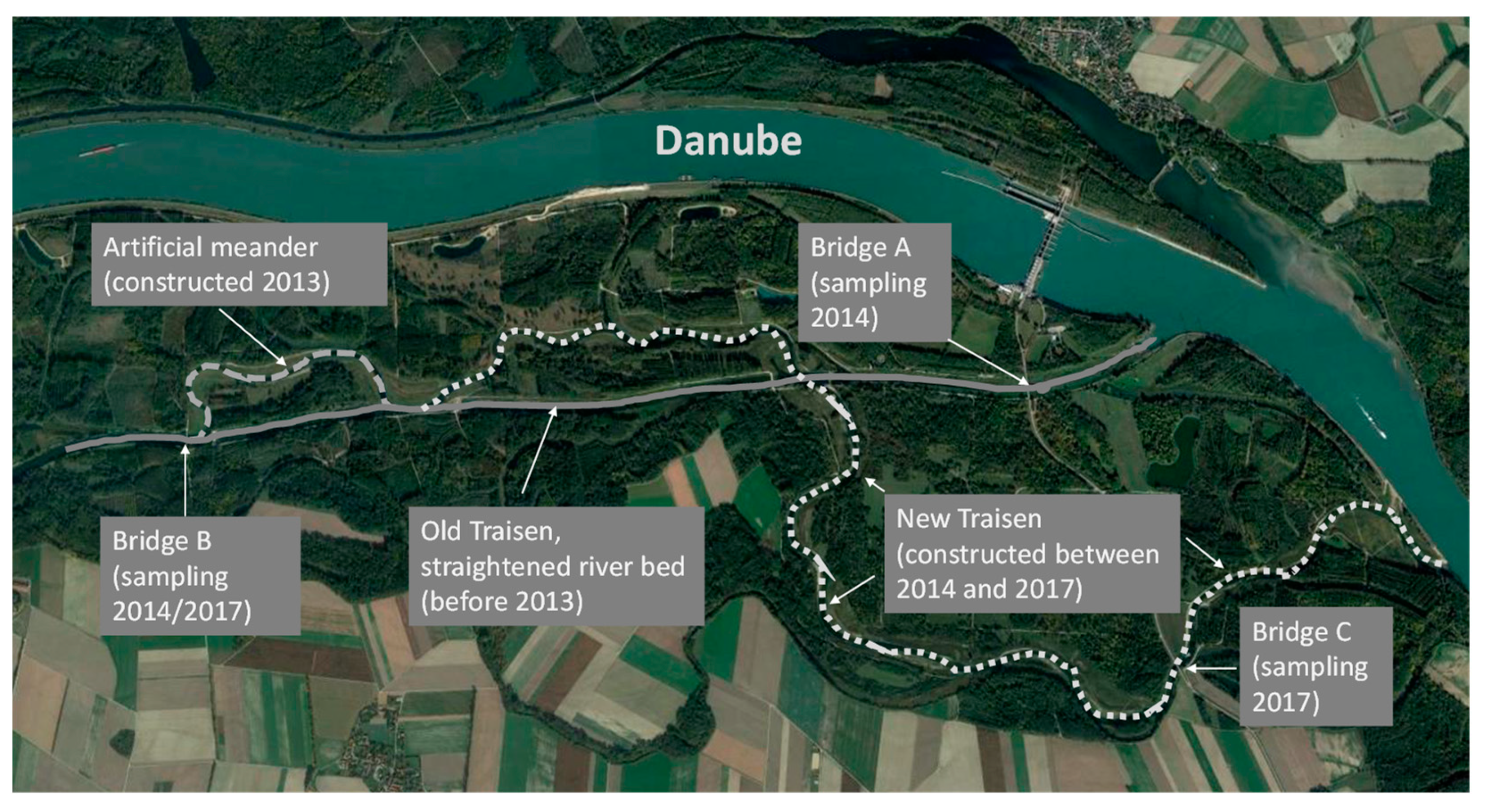
4.1. Study Area—Lower Traisen River (Danube Floodplain, Lower Austria; 48°22′ N, 15°49′ E)
4.2. Sampling
4.3. Species Traits
4.4. Data Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ward, J.V.; Tockner, K.; Schiemer, F. Biodiversity of floodplain river ecosystems: Ecotones and connectivity. Regul. River 1999, 15, 125–139. [Google Scholar] [CrossRef]
- Amoros, C. The concept of habitat diversity between and within ecosystems applied to river side-arm restoration. Environ. Manag. 2001, 28, 805–817. [Google Scholar] [CrossRef]
- Nilsson, C.; Svedmark, M. Basic principles and ecological consequences of changing water regimes: Riparian plant communities. Environ. Manag. 2002, 30, 468–480. [Google Scholar] [CrossRef] [PubMed]
- Gumiero, B.; Mant, J.; Hein, T.; Elso, J.; Boz, B. Linking the restoration of rivers and riparian zones/wetlands in Europe: Sharing knowledge through case studies. Ecol. Eng. 2013, 56, 36–50. [Google Scholar] [CrossRef]
- Koebel, J.W., Jr.; Bousquin, S.G. The Kissimmee River restoration project and evaluation program, Florida, USA. Restor. Ecol. 2014, 22, 345–352. [Google Scholar] [CrossRef]
- Nakamura, F.; Ishiyama, N.; Sueyoshi, M.; Negishi, J.N.; Akasaka, T. The significance of meander restoration for the hydrogeomorphology and recovery of wetland organisms in the Kushiro River, a lowland river in Japan. Restor. Ecol. 2014, 22, 544–554. [Google Scholar] [CrossRef]
- Gurnell, A.; Thompson, K.; Goodson, J.; Moggridge, H. Propagule Deposition along River Margins: Linking Hydrology and Ecology. J. Ecol. 2008, 96, 553–565. [Google Scholar] [CrossRef]
- Fraaije, R.G.; Moinier, S.; van Gogh, I.; Timmers, R.; van Deelen, J.J.; Verhoeven, J.T.; Soons, M.B. Spatial patterns of water-dispersed seed deposition along stream riparian gradients. PLoS ONE 2017, 12, e0185247. [Google Scholar] [CrossRef]
- Schwab, A.; Stammel, B.; Kiel, K. Seed dispersal via a new watercourse in a reconnected floodplain: Differences in species groups and seasonality. Restor. Ecol. 2018, 26, 103–113. [Google Scholar] [CrossRef]
- Johansson, M.E.; Nilsson, C.; Nilsson, E. Do rivers function as corridors for plant dispersal? J. Veg. Sci. 1996, 7, 593–598. [Google Scholar] [CrossRef]
- Middleton, B.A. Wetland Restoration, Flood Pulsing, and Disturbance Dynamics; Wiley: New York, NY, USA, 1999; 400p. [Google Scholar]
- Andersson, E.; Nilsson, C. Temporal variation in the drift of plant litter and propagules in a small boreal river. Freshw. Biol. 2002, 47, 1674–1684. [Google Scholar] [CrossRef]
- Boedeltje, G.; Bakker, J.P.; Bekker, R.M.; van Groenendael, J.M.; Soesbergen, M. Plant dispersal in a lowland stream in relation to occurrence and three specific life-history traits of the species in the species pool. J. Ecol. 2003, 91, 855–866. [Google Scholar] [CrossRef]
- Nilsson, C.; Brown, R.L.; Jansson, R.; Merritt, D.M. The role of hydrochory in structuring riparian and wetland vegetation. Biol. Rev. 2010, 85, 837–858. [Google Scholar] [CrossRef] [PubMed]
- Skoglund, S.J. Seed dispersing agents in two regularly flooded river sites. Can. J. Bot. 1990, 68, 754–760. [Google Scholar] [CrossRef]
- Hughes, J.W.; Cass, W.B. Pattern and process of a floodplain forest, Vermont, USA: Predicted response of vegetation to perturbation. J. Appl. Ecol. 1997, 34, 594–612. [Google Scholar] [CrossRef]
- Mitsch, W.J.; Wu, X.; Nairn, R.W.; Weihe, P.E.; Wang, N.; Deal, R.; Boucher, C.E. Creating and restoring wetlands. A wholeecosystem experiment in self-design. BioScience 1998, 48, 1019–1030. [Google Scholar] [CrossRef]
- Soomers, H.; Sarneel, J.M.; Patberg, W.; Verbeek, S.K.; Verweij, P.A.; Wassen, M.J.; van Diggelen, R. Factors influencing the seed source and sink functions of a floodplain nature reserve in the Netherlands. J. Veg. Sci. 2011, 22, 445–456. [Google Scholar] [CrossRef]
- Verhoeven, J.T.; Beltman, B.; Janssen, R.; Soons, M.B. Delineating landscape-scale processes of hydrology and plant dispersal for species-rich fen conservation: The Operational Landscape Unit approach. Wetl. Ecol. Manag. 2017, 25, 761–774. [Google Scholar] [CrossRef]
- Catford, J.A.; Jansson, R. Drowned, buried and carried away: Effects of plant traits on the distribution of native and alien species in riparian ecosystems. New Phytol. 2014, 204, 19–36. [Google Scholar] [CrossRef]
- Xiong, S.; Nilsson, C. Dynamics of leaf litter accumulation and its effects on riparian vegetation: A review. Bot. Rev. 1997, 63, 240–264. [Google Scholar] [CrossRef]
- Vogt, K.; Rasran, L.; Jensen, K. Seed deposition in drift lines: Opportunity or hazard for species establishment? Aquat. Bot. 2007, 86, 385–392. [Google Scholar] [CrossRef]
- Nilsson, C.; Ekblad, A.; Dynesius, M.; Backe, S.; Gardfjell, M.; Carlberg, B.; Hellqvist, S.; Jansson, R. A comparison of species richness and traits of riparian plants between a main river channel and its tributaries. J. Ecol. 1994, 82, 281–295. [Google Scholar] [CrossRef]
- Favre-Bac, L.; Lamberti-Raverot, B.; Puijalon, S.; Ernoult, A.; Burel, F.; Guillard, L.; Mony, C. Plant dispersal traits determine hydrochorous species tolerance to connectivity loss at the landscape scale. J. Veg. Sci. 2017, 28, 605–615. [Google Scholar] [CrossRef]
- Zhu, J.; Liu, M.; Xin, Z.; Zhao, Y.; Liu, Z. Which factors have stronger explanatory power for primary wind dispersal distance of winged diaspores: The case of Zygophyllum xanthoxylon (Zygophyllaceae)? J. Plant Ecol. 2016, 9, 346–356. [Google Scholar] [CrossRef]
- Gurnell, A.; Goodson, J.; Thompson, K.; Clifford, N.; Armitage, P. The river-bed: A dynamic store for plant propagules? Earth Surf. Process. Landf. 2007, 32, 1257–1272. [Google Scholar] [CrossRef]
- Rasran, L.; Vogt, K.; Jensen, K. Hydrochorous seed transport in a small river in Northern Germany as trait-dependent filter of plant dispersal and recruitment. Int. Rev. Hydrobiol. 2021, 106, 277–286. [Google Scholar] [CrossRef]
- Vogt, K.; Rasran, L.; Jensen, K. Water-borne seed transport and seed deposition during flooding in a small river-valley in Northern Germany. Flora 2004, 199, 377–388. [Google Scholar] [CrossRef]
- Boedeltje, G.; Bakker, J.P.; Ten Brinke, A.; van Groenendael, J.M.; Soesbergen, M. Dispersal phenology of hydrochorous plants in relation to discharge, seed release time and buoyancy of seeds: The flood pulse concept supported. J. Ecol. 2004, 92, 786–796. [Google Scholar] [CrossRef]
- Bonn, S.; Poschlod, P. Ausbreitungsbiologie der Pflanzen Mitteleuropas, Grundlagen und Kulturhistorische Aspekte; UTB: Quelle und Meyer: Wiesbaden, Germany, 1998; 404p. [Google Scholar]
- Schaumann, F.; Heinken, T. Endozoochorous seed dispersal by martens (Martes foina, M. martes) in two woodland habitats. Flora 2002, 197, 370–378. [Google Scholar] [CrossRef]
- Traveset, A.A.J.A.; Robertson, A.W.; Rodríguez-Pérez, J. A review on the role of endozoochory in seed germination. In Seed Dispersal: Theory and Its Application in a Changing World; Dennis, A.J., Green, R.A., Schupp, E.W., Westcott, D.A., Eds.; CABI: Wallingford, UK, 2007; Volume 44, pp. 78–103. [Google Scholar] [CrossRef]
- de Jager, M.; Kaphingst, B.; Janse, E.L.; Buisman, R.; Rinzema, S.G.; Soons, M.B. Seed size regulates plant dispersal distances in flowing water. J. Ecol. 2019, 107, 307–317. [Google Scholar] [CrossRef]
- VonBank, J.A.; DeBoer, J.A.; Casper, A.F.; Hagy, H.M. Ichthyochory in a temperate river system by common carp (Cyprinus carpio). J. Freshw. Ecol. 2018, 33, 83–96. [Google Scholar] [CrossRef]
- Fryirs, K.; Carthey, A. How long do seeds float? The potential role of hydrochory in passive revegetation management. River Res. Appl. 2022, 38, 1139–1153. [Google Scholar] [CrossRef]
- Berg, R.Y. Plant distribution as seen from plant dispersal: General principles and basic modes of plant dispersal. In Dispersal and Distribution—An International Symposium; Kubitzki, K., Ed.; Parey: Hamburg, Germany, 1983; pp. 13–36. [Google Scholar]
- Nathan, R.; Schurr, F.M.; Spiegel, O.; Steinitz, O.; Trakhtenbrot, A.; Tsoar, A. Mechanisms of long-distance seed dispersal. Trends Ecol. Evol. 2008, 23, 638–647. [Google Scholar] [CrossRef] [PubMed]
- Hölzel, N.; Otte, A. Inter-annual variation in the soil seed bank of flood-meadows over two years with different flooding patterns. Plant Ecol. 2004, 174, 279–291. [Google Scholar] [CrossRef]
- Pysek, P.E.T.R.; Prach, K. How important are rivers for supporting plant invasions. In Ecology and Management of Invasive Riverside Plants; De Waal, L.C., Child, L.E., Wade, P.M., Brock, J.H., Eds.; Wiley: Chichester, UK, 1994; pp. 19–26. [Google Scholar]
- Zając, A.; Tokarska-Guzik, B.; Zając, M. The role of rivers and streams in the migration of alien plants into the Polish Carpathians. Biodiv. Res. Conserv. 2011, 23, 43–56. [Google Scholar] [CrossRef]
- Nobis, A.; Rola, K.; Węgrzyn, M. Detailed study of a river corridor plant distribution pattern provides implications for river valley conservation. Ecol. Indic. 2017, 83, 314–322. [Google Scholar] [CrossRef]
- Lapin, K.; Bernhardt, K.G.; Lichtenwöhrer, P.; Roithmayr, S. Welchen Einfluss haben invasive Pflanzenarten auf die Phytodiversität von renaturierten Flusslandschaften? Gesunde Pflanz. 2015, 67, 75–82. [Google Scholar] [CrossRef]
- Bundesministerium für Land- und Forstwirtschaft, Umwelt und Wasserwirtschaft. Hydrographisches Jahrbuch von Österreich 2011; BMNT: Vienna, Austria, 2013; Volume 119. [Google Scholar]
- Cappers, R.T.J.; Bekker, R.M.; Jans, J.E.A. The Digital Seed Atlas of the Netherlands; Groningen Archaeolog Stud, 4; Barkhuis Publishing: Eelde, The Netherlands, 2006. [Google Scholar] [CrossRef]
- Hintze, C.; Heydel, F.; Hoppe, C.; Cunze, S.; König, A.; Tackenberg, O. D³: The dispersal and diaspore database–baseline data and statistics on seed dispersal. Perspect. Plant Ecol. 2013, 15, 180–192. [Google Scholar] [CrossRef]
- Bundesamt für Naturschutz Deutschland (BfN). FloraWeb—Daten und Informationen zu Wildpflanzen und zur Vegetation Deutschlands; Database: Bonn, Germany, 2017; Available online: http://www.floraweb.de (accessed on 29 January 2023).
- Kattge, J.; Diaz, S.; Lavorel, S.; Prentice, I.C.; Leadley, P.; Bönisch, G.; Cornelissen, J.H.C. TRY—A global database of plant traits. Glob. Change Biol. 2011, 17, 2905–2935. [Google Scholar] [CrossRef]
- Royal Botanic Gardens Kew. Seed Information Database (SID). Version 7.1. 2017. Available online: http://data.kew.org/sid/ (accessed on 2 November 2018).
- Grime, J.P.; Hodgson, J.P.; Hunt, R. Comparative Plant Ecology—A Functional Approach to Common British Species; Springer: Dordrecht, The Netherlands, 1988; 742p. [Google Scholar] [CrossRef]
- Ellenberg, H. Zeigerwerte von Pflanzen in Mitteleuropa, 3rd ed.; Goltze: Göttingen, Germany, 2001; 262p. [Google Scholar]
- Andersson, E.; Nilsson, C.; Johansson, M.E. Plant dispersal along boreal rivers and its relation to the riparian flora. J. Biogeogr. 2000, 27, 1095–1106. [Google Scholar] [CrossRef]
- Guppy, H.B. The river Thames as an agent in plant dispersal. J. Linn. Soc. London Bot. 1891, 29, 333–346. [Google Scholar] [CrossRef]
- Praeger, R.L. On the buoyancy of the seeds of some britannic plants. Sci. Proc. R Dublin Soc. 1913, 143, 13–62. [Google Scholar]
- Bill, H.C.; Poschlod, P.; Reich, M.; Plachter, H. Experiments and observations on seed dispersal by running water in an Alpine floodplain. Bull. Geobot. Inst. ETH 1999, 65, 13–28. [Google Scholar]
- Jäger, E.J.; Werner, K. Exkursionsflora von Deutschland. Band 4. Gefäßpflanzen: Kritischer Band, 10th ed.; Spektrum: Munich, Germany, 2005; 980p. [Google Scholar]
- Raunkiær, C. Plant Life Forms; Clarendon Press: Oxford, UK, 1937; 104p. [Google Scholar]
- Schubert, R.; Hilbig, W.; Klotz, S. Bestimmungsbuch der Pflanzengesellschaften Deutschlands; Spektrum Akademischer Verlag: Heidelberg, Germany, 2001; 472p. [Google Scholar]
- 58 Smilauer, P.; Leps, J. Multivariate Analysis of Ecological Data Using CANOCO 5, 2nd ed.; Cambridge University Press: Cambridge, UK, 2014. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022; Available online: https://www.R-project.org/ (accessed on 9 January 2023).
- Lapin, K.; Bernhardt, K.-G.; Mayer, E.; Roithmayr, S.; Neureiter, J.; Horvath, C. Monitoring River Restoration Efforts: Do Invasive Alien Plants Endanger the Success? A Case Study of the Traisen River. J. Environ. Prot. 2016, 7, 831–843. [Google Scholar] [CrossRef]
- Society for Ecological Restoration; International Network for Seed Based Restoration; Royal Botanic Gardens Kew. Seed Information Database (SID). Available online: https://ser-sid.org/ (accessed on 1 February 2023).
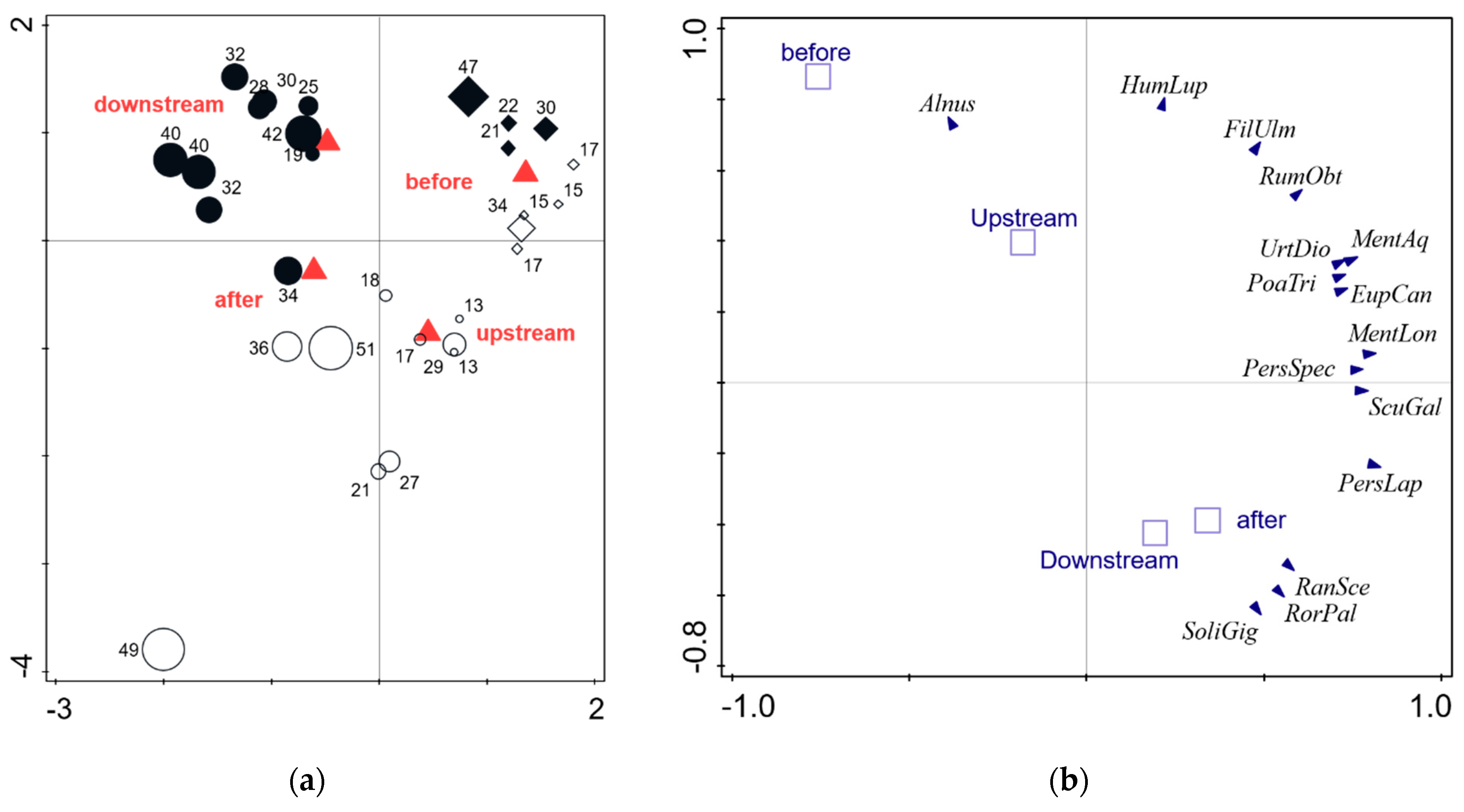
| Partial RDA (Results of Permutation Test) | Axis | Eigenvalue | Explained Variation | Pseudo-F | p |
|---|---|---|---|---|---|
| Ordination following species composition | |||||
| Explanatory variable, method (seeds vs. seedlings); co-variables, bridge and week | 1 | 0.1655 | 20.26% | 4.3 | 0.001 |
| Ordination following CWMs of functional traits | |||||
| Explanatory variable, method (seeds vs. seedlings); co-variables, bridge and week | 1 | 0.346 | 37.85% | 10.4 | 0.002 |
| Ordination following Axis 1 scores out of previous ordination (CWM), Interactive-forward-selection | 1 | 0.1409 | 14.09% | 0.7 | 0.761 |
| Relevant traits | |||||
| Dispersal mode: endozoochory | 7% | 2.8 | 0.092 | ||
| CS-Strategy | 7.10% | 3.1 | 0.099 |
| Functional Trait | Time | Location | Interaction |
|---|---|---|---|
| Seedweight | 25.06 *** | 1.52 | 0.72 |
| Seed size_length | 11.11 ** | 2.33 | 0.99 |
| Seed size_width | 16.56 *** | 4.4 * | 3.66 |
| Seed size_thickness | 1.1 | 0.63 | 0.31 |
| Reprod. strategy_only by seed | 6.79 * | 3.63 | 3.02 |
| Reprod. strategy_mainly by seed | 27.97 *** | 0.19 | 0.02 |
| Reprod. strategy_seeds and vegetative | 42.12 *** | 0.91 | 1.17 |
| Reprod. strategy_mainly vegetative | 0.25 | 0.54 | 2.59 |
| Mid_height | 34.24 *** | 0.24 | 0.65 |
| Buoyancy_day | 19.13 *** | 1.09 | 4.68 * |
| Dispersal_wind_hairs | 10.2 ** | 1.67 | 2.97 |
| Dispersal_wind_wings | 11.11 ** | 1.00 | 1.92 |
| Dispersal_wind_dust | 11.24 ** | 15.67 *** | 8.3 ** |
| Dispersal_hydrochory | 3.38 | 4.85 * | 0.01 |
| Disp_myrmechory | 7.77 ** | 12.18 ** | 7.1 * |
| Disp_epizoochory | 5.92 * | 40.95 *** | 26.6 *** |
| Disp_endozoochory | 0.37 | 1.84 | 1.61 |
| Coniferous and deciduous forests outside the floodplain (LNW) | 0.08 | 37.59 *** | 2.83 |
| Floodplain forests and alder carrs (BAW) | 41.15 *** | 13.19 ** | 8.59 ** |
| Pioneer vegetation of mudbanks (BI) | 0.05 | 0.14 | 1.4 |
| Aquatic macrophytes (H) | 2.57 | 0.01 | 0.76 |
| Agricultural grasslands (MA) | 0.25 | 0.01 | 1.93 |
| Wet meadows (MO) | 0.77 | 3.77 | 0.34 |
| Reeds and tall herb fen communities (PH) | 0.36 | 1.98 | 1.71 |
| Communities of perennial ruderals and edges (AR) | 15.46 *** | 6.35 * | 3.14 |
| Small sedge communities of nutrient-poor mires (SC) | 0.43 | 0.9 | 0.81 |
| Annual ruderals and arable weeds (SO) | 0.04 | 1.89 | 0.64 |
| Life span_polycarpic perennials | 8.69 ** | 15.09 *** | 16.61 *** |
| Life span_monocarpic perennials | 0.07 | 1.04 | 1.44 |
| Life span_annuals | 5.99 * | 2.89 | 0.71 |
| Phanerophytes | 55.57 *** | 0.65 | 0.24 |
| Hemicryptophytes | 14.38 *** | 6.93 * | 9.69 ** |
| Kryptophytes | 9.67 ** | 18.59 *** | 5.71 * |
| Therophytes | 0.08 | 0.47 | 1.77 |
| Strategy Grime_C | 3.84 | 32.44 *** | 18.67 *** |
| Strategy Grime_CS_S | 23.14 *** | 0.55 | 0.23 |
| Strategy Grime_CR | 4.97 * | 2.36 | 0.64 |
| Strategy Grime_CSR | 0.13 | 0.9 | 0.01 |
| Strategy Grime_R_SR | 2.13 | 2.02 | 0.24 |
| Ellenberg_moisture | 8.58 ** | 25.84 *** | 4.89 * |
| Ellenberg_nitrogen | 24.83 *** | 6.05 * | 2.23 |
| Ellenberg_light | 8.58 ** | 25.84 *** | 4.89 * |
| Neophyte | 6.9 * | 11.83 ** | 4.46 * |
| Invasive neophyte | 7.49 * | 12.03 ** | 6.61 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rasran, L.; Vogt, K.; Trattnig, M.; Bernhardt, K.-G. Hydrochorous Seed Transport in the Lower Traisen River before and after Riverbed Restoration. Plants 2023, 12, 2409. https://doi.org/10.3390/plants12132409
Rasran L, Vogt K, Trattnig M, Bernhardt K-G. Hydrochorous Seed Transport in the Lower Traisen River before and after Riverbed Restoration. Plants. 2023; 12(13):2409. https://doi.org/10.3390/plants12132409
Chicago/Turabian StyleRasran, Leonid, Kati Vogt, Marc Trattnig, and Karl-Georg Bernhardt. 2023. "Hydrochorous Seed Transport in the Lower Traisen River before and after Riverbed Restoration" Plants 12, no. 13: 2409. https://doi.org/10.3390/plants12132409
APA StyleRasran, L., Vogt, K., Trattnig, M., & Bernhardt, K.-G. (2023). Hydrochorous Seed Transport in the Lower Traisen River before and after Riverbed Restoration. Plants, 12(13), 2409. https://doi.org/10.3390/plants12132409







