Identification of the Regulatory Role of SlWRKYs in Tomato Defense against Meloidogyne incognita
Abstract
:1. Introduction
2. Results
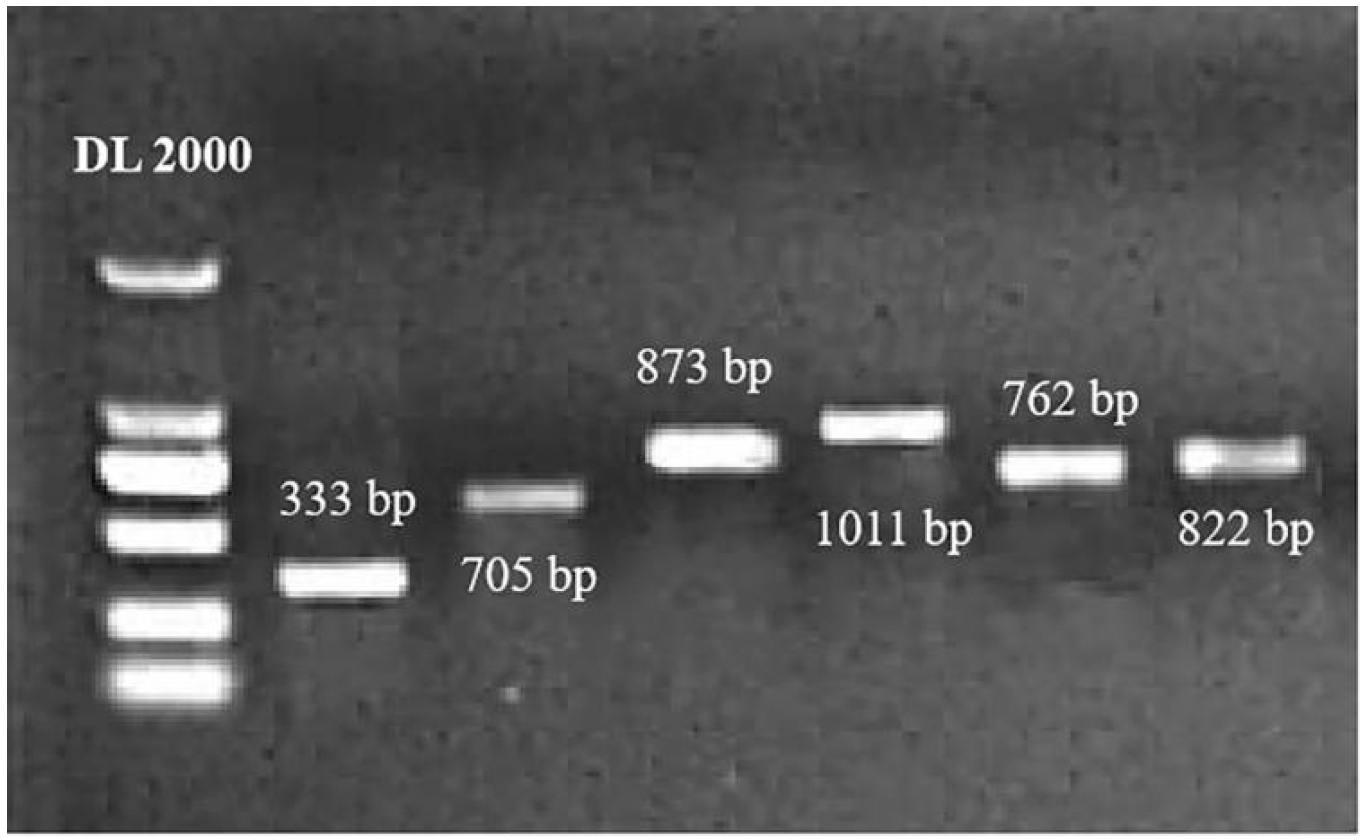
2.1. Homologous Recombinant Cloning of SlWRKYs
2.2. Screening and Identification of Conserved SlWRKYs Domains
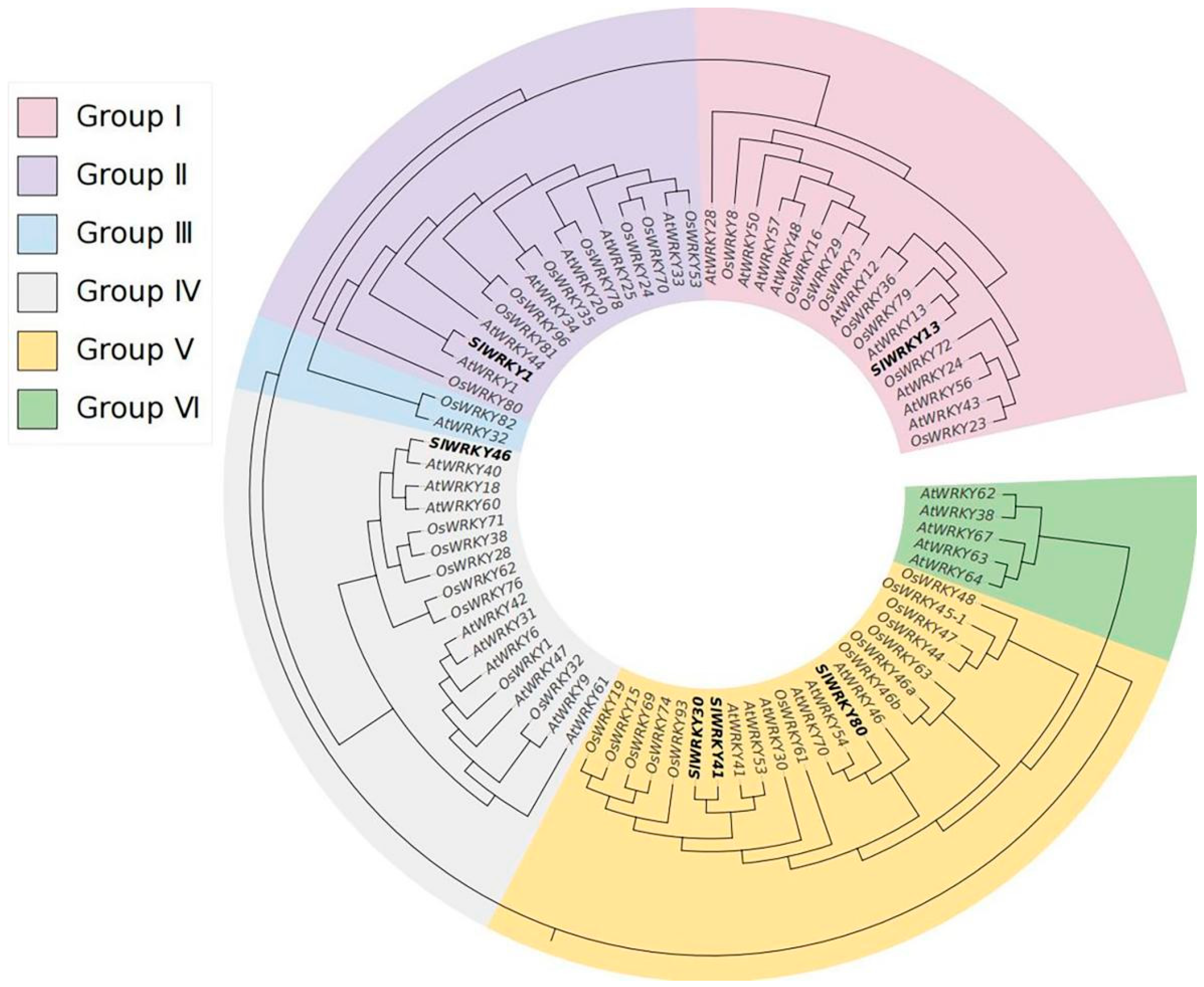
2.3. SlWRKYs Phylogenetic Tree Analysis
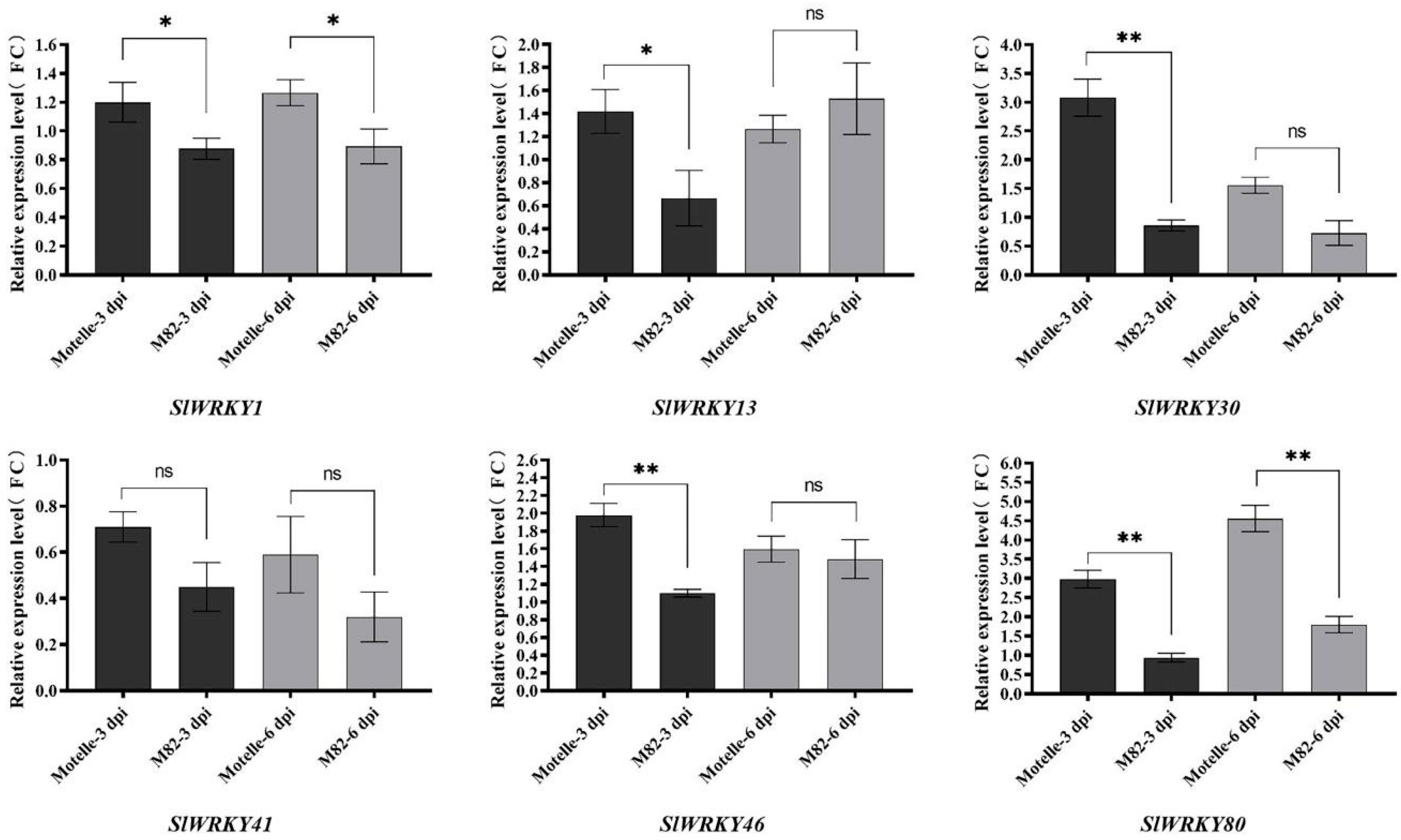
2.4. Tissue Expression Analysis and Root Expression Pattern of SlWRKYs
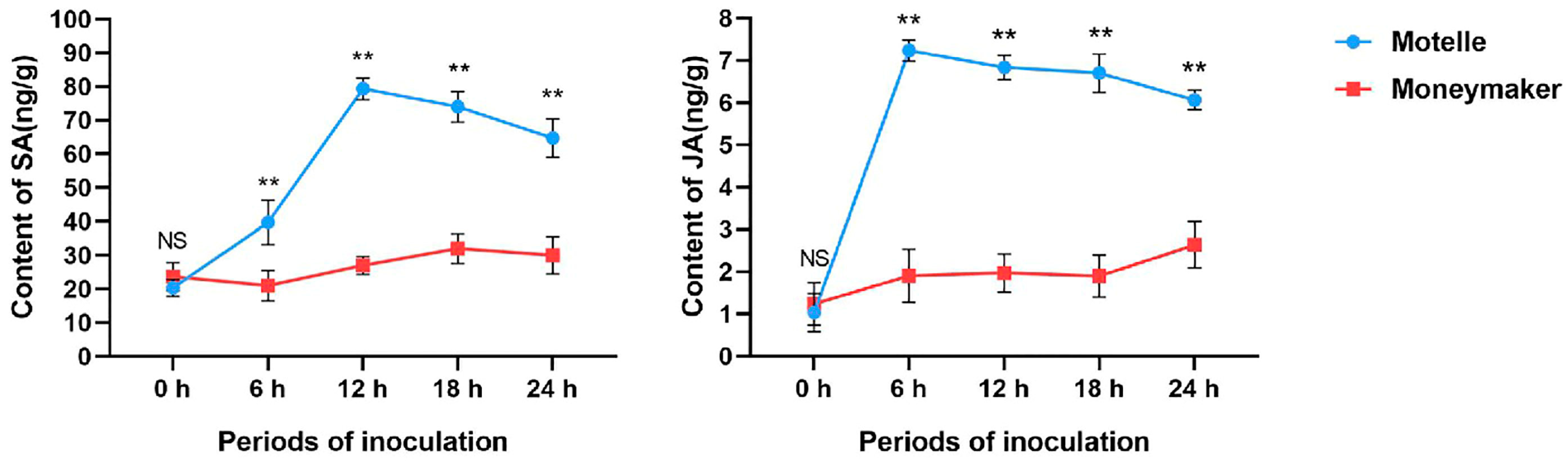
2.5. Determination of Endogenous SA and JA Contents in Motelle and M82 Tomato after Inoculation
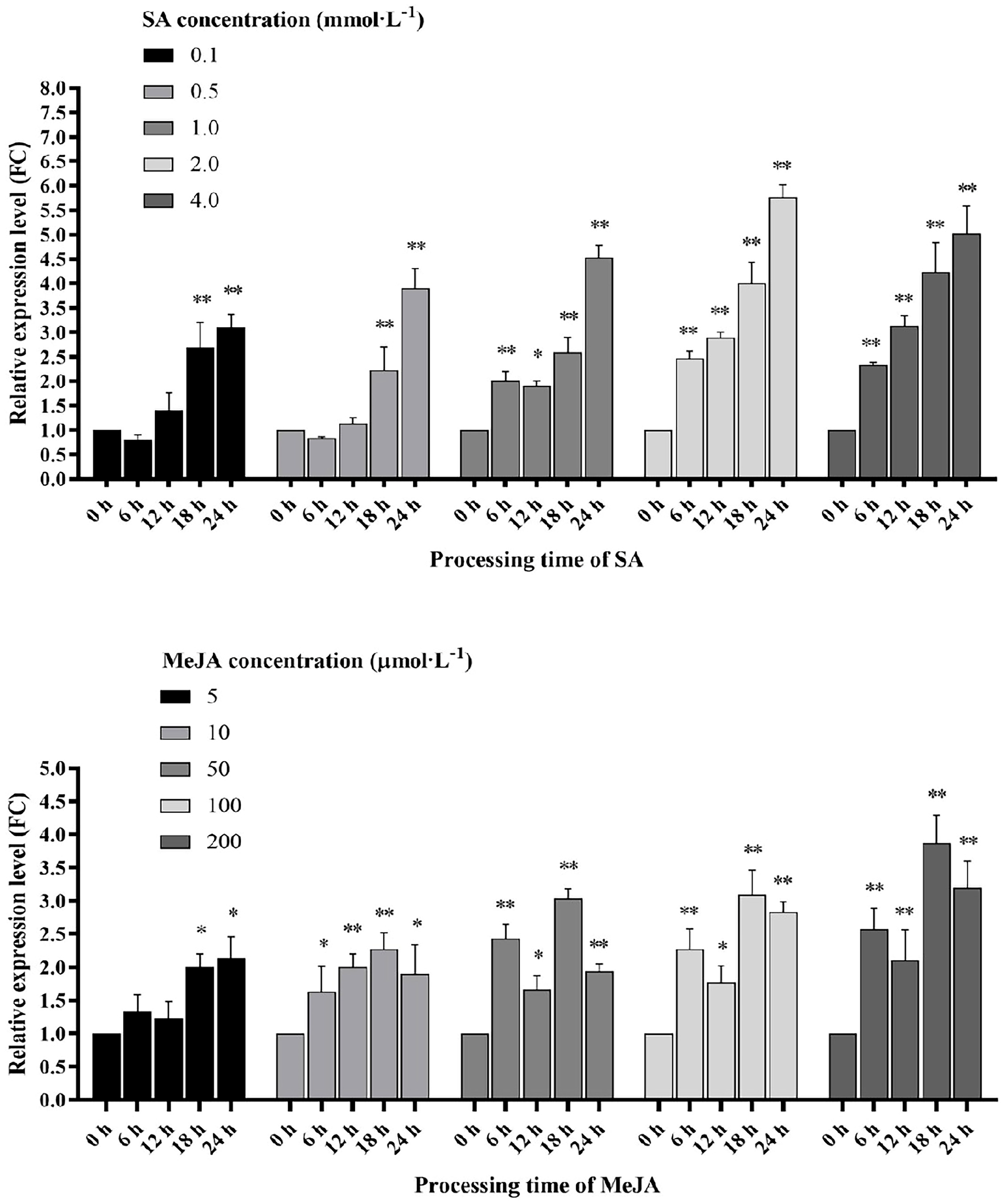
2.6. Regulation of SlWRKY80 Expression by Exogenous SA and JA
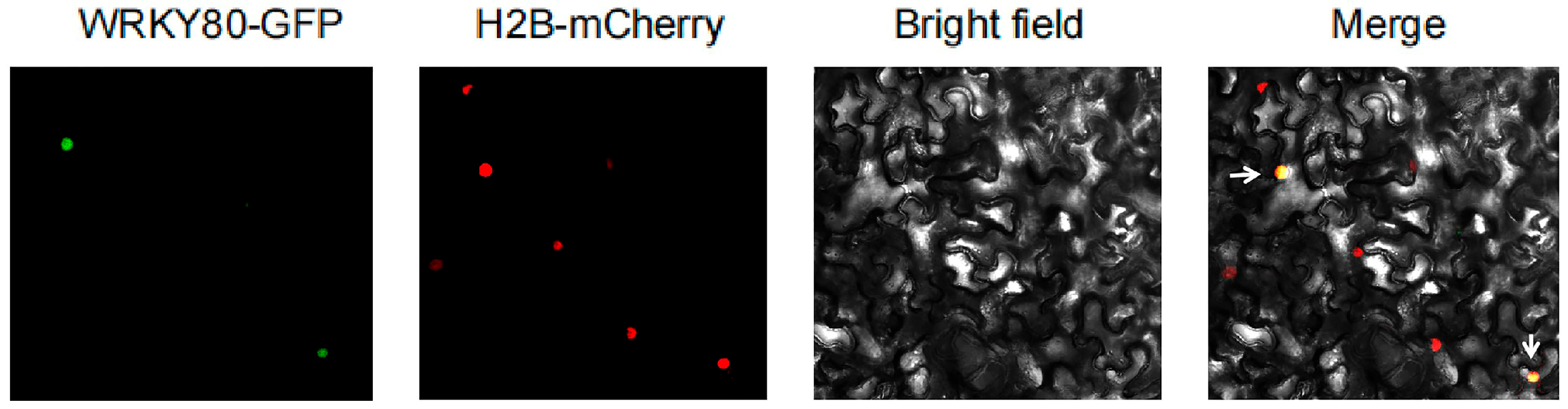
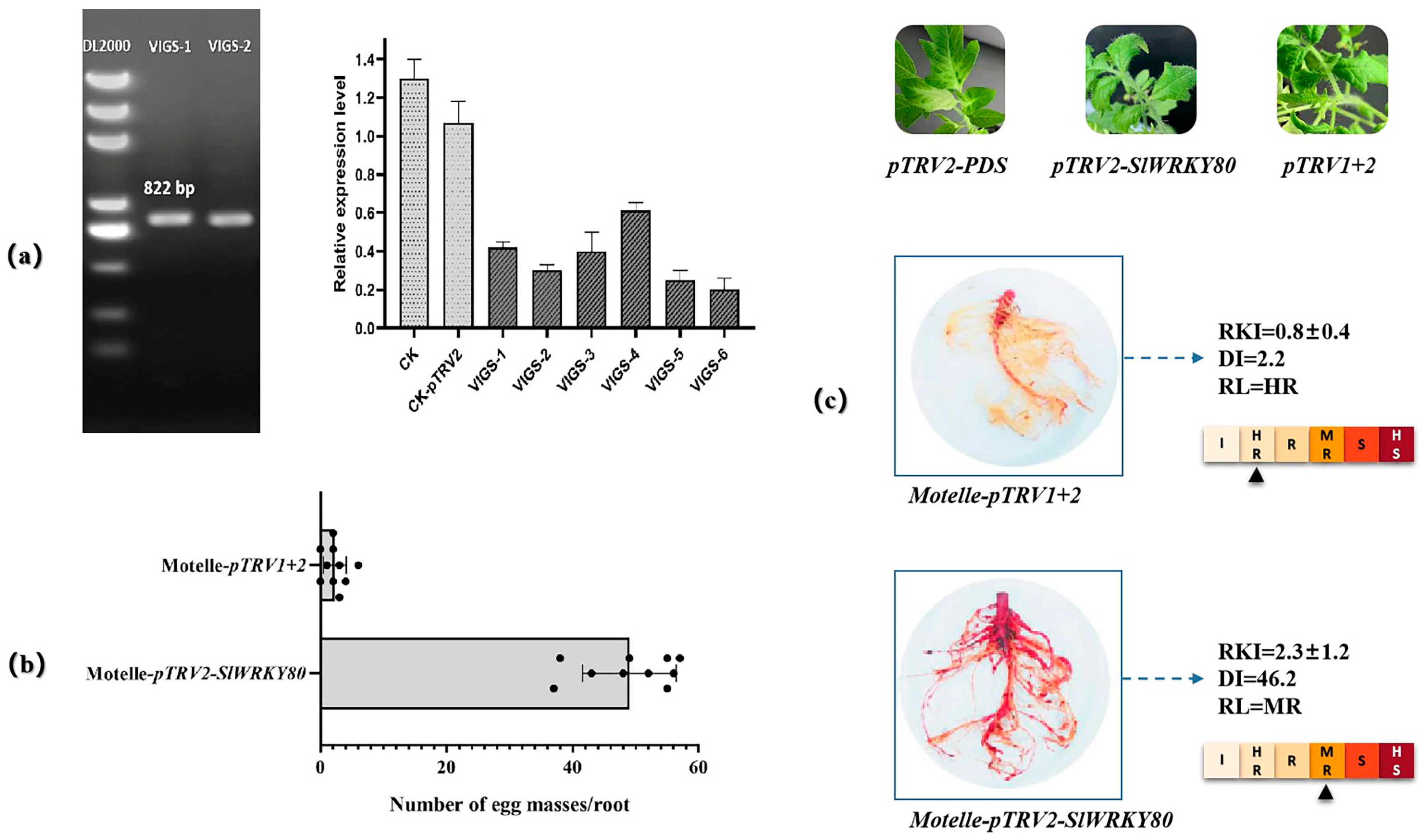
2.7. Subcellular Colocalization and Resistance Function Analysis of SlWRKY80
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Treatments
4.2. Identification of Conserved Domains and Phylogenetic Tree Construction
4.3. Nematode Assay and Disease Resistance Statistics
4.4. Exogenous Hormone SA and JA Treatment
4.5. Determination of Endogenous SA and JA
4.6. Expression Pattern Analysis
4.7. Screening and Homologous Recombinant Cloning of SlWRKYs
4.8. VIGS Experiment
4.9. Subcellular Localization of SlWRKY80
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jones, J.T.; Haegeman, A.; Danchin, E.G.J.; Gaur, H.S.; Helder, J.; Jones, M.G.K.; Kikuchi, T.; Manzanilla-Lopez, R.; Palomares-Rius, J.E.; Wesemael, W.M.L.; et al. Top 10 plant-parasitic nematodes in molecular plant pathology. Mol. Plant Pathol. 2013, 14, 946–961. [Google Scholar] [CrossRef] [Green Version]
- Masler, E.P.; Rogers, S.T.; Chitwood, D.J. Differential effects of temperature and selected phytochemicals on development and infectivity of two economically important plant parasites, the soybean cyst nematode, Heterodera glycines, and root-knot nematode, Meloidogyne incognita. Phytopathology 2014, 104, 172–173. [Google Scholar]
- Li, C.; Hua, C.; Hu, Y.; You, J.; Mao, Y.; Li, J.; Tian, Z.; Wang, C. Response of soybean genotypes to Meloidogyne incognita and M. hapla in Heilongjiang Province in China. Russ. J. Nematol. 2016, 24, 89–98. [Google Scholar]
- Collett, R.L.; Marais, M.; Daneel, M.; Rashidifard, M.; Fourie, H. Meloidogyne enterolobii, a threat to crop production with particular reference to sub-Saharan Africa: An extensive, critical and updated review. Nematology 2021, 23, 247–285. [Google Scholar] [CrossRef]
- Mishra, S.; DiGennaro, P. Root-knot nematodes demonstrate temporal variation in host penetration. J. Nematol. 2020, 52, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharif, R.; Su, L.; Chen, X.; Qi, X. Involvement of auxin in growth and stress response of cucumber. Veg. Res. 2022, 2, 1–9. [Google Scholar] [CrossRef]
- Sikandar, A.; Zhang, M.Y.; Wang, Y.Y.; Zhu, X.F.; Liu, X.Y.; Fan, H.Y.; Xuan, Y.H.; Chen, L.J.; Duan, Y.X. Review article: Meloidogyne incognita (root-knot nematode) a risk to agriculture. Appl. Ecol. Environ. Res. 2020, 18, 1679–1690. [Google Scholar] [CrossRef]
- Vestergard, M. Trap crops for Meloidogyne hapla management and its integration with supplementary strategies. Appl. Soil Ecol. 2019, 134, 105–110. [Google Scholar] [CrossRef]
- Zhou, J.; Xu, X.-C.; Cao, J.-J.; Yin, L.-L.; Xia, X.-J.; Shi, K.; Zhou, Y.-H.; Yu, J.-Q. Heat Shock Factor HsfA1a Is Essential for R Gene-Mediated Nematode Resistance and Triggers H2O2 Production. Plant Physiol. 2018, 176, 2456–2471. [Google Scholar] [CrossRef] [Green Version]
- Nino Castaneda, N.E.; Costa Alves, G.S.; Almeida, R.M.; Amorim, E.P.; Ferreira, C.F.; Togawa, R.C.; Do Carmo Costa, M.M.; Grynberg, P.; Pereira Santos, J.R.; Cares, J.E.; et al. Gene expression analysis in Musa acuminata during compatible interactions with Meloidogyne incognita. Ann. Bot. 2017, 119, 915–930. [Google Scholar]
- Wang, Y.; Yang, W.; Zhang, W.; Han, Q.; Feng, M.; Shen, H. Mapping of a Heat-Stable Gene for Resistance to Southern Root-Knot Nematode in Solanum lycopersicum. Plant Mol. Biol. Report. 2013, 31, 352–362. [Google Scholar] [CrossRef]
- Wani, S.H.; Anand, S.; Singh, B.; Bohra, A.; Joshi, R. WRKY transcription factors and plant defense responses: Latest discoveries and future prospects. Plant Cell Rep. 2021, 40, 1071–1085. [Google Scholar] [CrossRef] [PubMed]
- Du, C.; Jiang, J.; Zhang, H.; Zhao, T.; Yang, H.; Zhang, D.; Zhao, Z.; Xu, X.; Li, J. Transcriptomic profiling of Solanum peruvianum LA3858 revealed a Mi-3-mediated hypersensitive response to Meloidogyne incognita. BMC Genom. 2020, 21, 250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, C.-Y.; Kieu Thi Xuan, V.; Cong Danh, N.; Jeong, D.-H.; Lee, S.-K.; Kumar, M.; Kim, S.-R.; Park, S.-H.; Kim, J.-K.; Jeon, J.-S. Functional analysis of a cold-responsive rice WRKY gene, OsWRKY71. Plant Biotechnol. Rep. 2016, 10, 13–23. [Google Scholar] [CrossRef]
- Shu, P.; Zhang, S.; Li, Y.; Wang, X.; Yao, L.; Sheng, J.; Shen, L. Over-expression of SlWRKY46 in tomato plants increases susceptibility to Botrytis cinerea by modulating ROS homeostasis and SA and JA signaling pathways. Plant Physiol. Biochem. 2021, 166, 1–9. [Google Scholar] [CrossRef]
- Bhattarai, K.K.; Atamian, H.S.; Kaloshian, I.; Eulgem, T. WRKY72-type transcription factors contribute to basal immunity in tomato and Arabidopsis as well as gene-for-gene resistance mediated by the tomato R gene Mi-1. Plant J. 2010, 63, 229–240. [Google Scholar] [CrossRef]
- Chinnapandi, B.; Bucki, P.; Miyara, S.B. SlWRKY45, nematode-responsive tomato WRKY gene, enhances susceptibility to the root knot nematode; M. javanica infection. Plant Signal. Behav. 2017, 12, e1356530. [Google Scholar] [CrossRef] [Green Version]
- Huang, H.; Zhao, W.; Qiao, H.; Li, C.; Sun, L.; Yang, R.; Ma, X.; Ma, J.; Song, S.; Wang, S. SlWRKY45 interacts with jasmonate-ZIM domain proteins to negatively regulate defense against the root-knot nematode Meloidogyne incognita in tomato. Hortic. Res. 2022, 9. [Google Scholar] [CrossRef]
- Chinnapandi, B.; Bucki, P.; Fitoussi, N.; Kolomiets, M.; Borrego, E.; Miayara, S.B. Tomato SlWRKY3 acts as a positive regulator for resistance against the root-knot nematode Meloidogyne javanica by activating lipids and hormone-mediated defense-signaling pathways. Plant Signal. Behav. 2019, 14, 1601951. [Google Scholar] [CrossRef]
- Huang, Y.; Li, M.-Y.; Wu, P.; Xu, Z.-S.; Que, F.; Wang, F.; Xiong, A.-S. Members of WRKY Group III transcription factors are important in TYLCV defense signaling pathway in tomato (Solanum lycopersicum). BMC Genom. 2016, 17, 788. [Google Scholar] [CrossRef] [Green Version]
- Ng, D.W.K.; Abeysinghe, J.K.; Kamali, M. Regulating the Regulators: The Control of Transcription Factors in Plant Defense Signaling. Int. J. Mol. Sci. 2018, 19, 3737. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qiao, Z.; Li, C.-L.; Zhang, W. WRKY1 regulates stomatal movement in drought-stressed Arabidopsis thaliana. Plant Mol. Biol. 2016, 91, 53–65. [Google Scholar] [CrossRef]
- Heerah, S.; Katari, M.; Penjor, R.; Coruzzi, G.; Marshall-Colon, A. WRKY1 Mediates Transcriptional Regulation of Light and Nitrogen Signaling Pathways. Plant Physiol. 2019, 181, 1371–1388. [Google Scholar] [CrossRef] [Green Version]
- Wu, X.; Xu, J.; Meng, X.; Fang, X.; Xia, M.; Zhang, J.; Cao, S.; Fan, T. Linker histone variant HIS1-3 and WRKY1 oppositely regulate salt stress tolerance in Arabidopsis. Plant Physiol. 2022, 189, 1833–1847. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Tian, Z.; Yu, D. WRKY13 acts in stem development in Arabidopsis thaliana. Plant Sci. 2015, 236, 205–213. [Google Scholar] [CrossRef]
- Ma, Z.; Li, W.; Wang, H.; Yu, D. WRKY transcription factors WRKY12 and WRKY13 interact with SPL10 to modulate age-mediated flowering. J. Integr. Plant Biol. 2020, 62, 1659–1673. [Google Scholar] [CrossRef]
- Sheng, Y.; Yan, X.; Huang, Y.; Han, Y.; Zhang, C.; Ren, Y.; Fan, T.; Xiao, F.; Liu, Y.; Cao, S. The WRKY transcription factor, WRKY13, activates PDR8 expression to positively regulate cadmium tolerance in Arabidopsis . Plant Cell Environ. 2019, 42, 891–903. [Google Scholar] [CrossRef]
- Mirabella, R.; Rauwerda, H.; Allmann, S.; Scala, A.; Spyropoulou, E.A.; de Vries, M.; Boersma, M.R.; Breit, T.M.; Haring, M.A.; Schuurink, R.C. WRKY40 and WRKY6 act downstream of the green leaf volatile E-2-hexenal in Arabidopsis. Plant J. 2015, 83, 1082–1096. [Google Scholar] [CrossRef]
- Ahmad, R.; Liu, Y.; Wang, T.-J.; Meng, Q.; Yin, H.; Wang, X.; Wu, Y.; Nan, N.; Liu, B.; Xu, Z.-Y. GOLDEN2-LIKE Transcription Factors Regulate WRKY40 Expression in Response to Abscisic Acid. Plant Physiol. 2019, 179, 1844–1860. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Yang, Y.; Fang, B.; Gannon, P.; Ding, P.; Li, X.; Zhang, Y. Arabidopsis snc2-1D Activates Receptor-Like Protein-Mediated Immunity Transduced through WRKY70. Plant Cell 2010, 22, 3153–3163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, H.; Liu, B.; Lou, S.; Bi, H.; Tang, H.; Tong, S.; Song, Y.; Chen, N.; Zhang, H.; Jiang, Y.; et al. CHYR1 ubiquitinates the phosphorylated WRKY70 for degradation to balance immunity in Arabidopsis thaliana. New Phytol. 2021, 230, 1095–1109. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Dong, Q.; Yu, D. Arabidopsis WRKY46 coordinates with WRKY70 and WRKY53 in basal resistance against pathogen Pseudomonas syringae . Plant Sci. 2012, 185, 288–297. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.-H.; Huang, Z.-Y.; Xie, P.; Gu, C.; Li, K.; Wang, D.-C.; Yu, Y.-Y.; Fan, Z.-H.; Wang, C.-J.; Wang, Y.-P.; et al. Transcription factors WRKY70 and WRKY11 served as regulators in rhizobacterium Bacillus cereus AR156-induced systemic resistance to Pseudomonas syringae pv. tomato DC3000 in Arabidopsis. J. Exp. Bot. 2016, 67, 157–174. [Google Scholar] [CrossRef] [Green Version]
- Chen, S.; Ding, Y.; Tian, H.; Wang, S.; Zhang, Y. WRKY54 and WRKY70 positively regulate SARD1 and CBP60g expression in plant immunity. Plant Signal. Behav. 2021, 16, 1932142. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Pang, S.; Lu, Z.; Jin, B. Function and Mechanism of WRKY Transcription Factors in Abiotic Stress Responses of Plants. Plants 2020, 9, 1515. [Google Scholar] [CrossRef] [PubMed]
- Dang, F.; Wang, Y.; She, J.; Lei, Y.; Liu, Z.; Eulgem, T.; Lai, Y.; Lin, J.; Yu, L.; Lei, D.; et al. Overexpression of CaWRKY27, a subgroup IIe WRKY transcription factor of Capsicum annuum, positively regulates tobacco resistance to Ralstonia solanacearum infection. Physiol. Plant. 2014, 150, 397–411. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Guo, R.; Tu, M.; Wang, D.; Guo, C.; Wan, R.; Li, Z.; Wang, X. Ectopic Expression of the Wild Grape WRKY Transcription Factor VqWRKY52 in Arabidopsis thaliana Enhances Resistance to the Biotrophic Pathogen Powdery Mildew but not to the Necrotrophic Pathogen Botrytis cinerea. Front. Plant Sci. 2017, 8, 97. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, H.; Gong, W.; Wang, Y.; Ma, Q. Contribution of a WRKY Transcription Factor, ShWRKY81, to Powdery Mildew Resistance in Wild Tomato. Int. J. Mol. Sci. 2023, 24, 2583. [Google Scholar] [CrossRef]
- Zhao, W.; Zhou, X.; Lei, H.; Fan, J.; Yang, R.; Li, Z.; Hu, C.; Li, M.; Zhao, F.; Wang, S. Transcriptional evidence for cross talk between JA and ET or SA during root-knot nematode invasion in tomato. Physiol. Genom. 2018, 50, 197–207. [Google Scholar] [CrossRef]
- Janati, S.; Houari, A.; Wifaya, A.; Essarioui, A.; Mimouni, A.; Hormatallah, A.; Sbaghi, M.; Dababat, A.A.; Mokrini, F. Occurrence of the Root-Knot Nematode species in Vegetable Crops in Souss Region of Morocco. Plant Pathol. J. 2018, 34, 308–315. [Google Scholar] [CrossRef]
- Koeppel, R.; Bucher, T.B. Duplex real-time PCR for the determination of wasabi (Eutrema wasabi) contents in horseradish (Armoracia rusticana) products applying the Delta Delta ct-method. Eur. Food Res. Technol. 2016, 242, 1111–1115. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nie, W.; Liu, L.; Chen, Y.; Luo, M.; Feng, C.; Wang, C.; Yang, Z.; Du, C. Identification of the Regulatory Role of SlWRKYs in Tomato Defense against Meloidogyne incognita. Plants 2023, 12, 2416. https://doi.org/10.3390/plants12132416
Nie W, Liu L, Chen Y, Luo M, Feng C, Wang C, Yang Z, Du C. Identification of the Regulatory Role of SlWRKYs in Tomato Defense against Meloidogyne incognita. Plants. 2023; 12(13):2416. https://doi.org/10.3390/plants12132416
Chicago/Turabian StyleNie, Weidan, Lili Liu, Yinxia Chen, Mingyin Luo, Chenghao Feng, Chaonan Wang, Zhongmin Yang, and Chong Du. 2023. "Identification of the Regulatory Role of SlWRKYs in Tomato Defense against Meloidogyne incognita" Plants 12, no. 13: 2416. https://doi.org/10.3390/plants12132416




