Exploring the Anticancer Potential of Premna resinosa (Hochst.) Leaf Surface Extract: Discovering New Diterpenes as Heat Shock Protein 70 (Hsp70) Binding Agents
Abstract
:1. Introduction
2. Results
2.1. Phytochemical Investigation
2.2. Surface Plasmon Resonance (SPR)
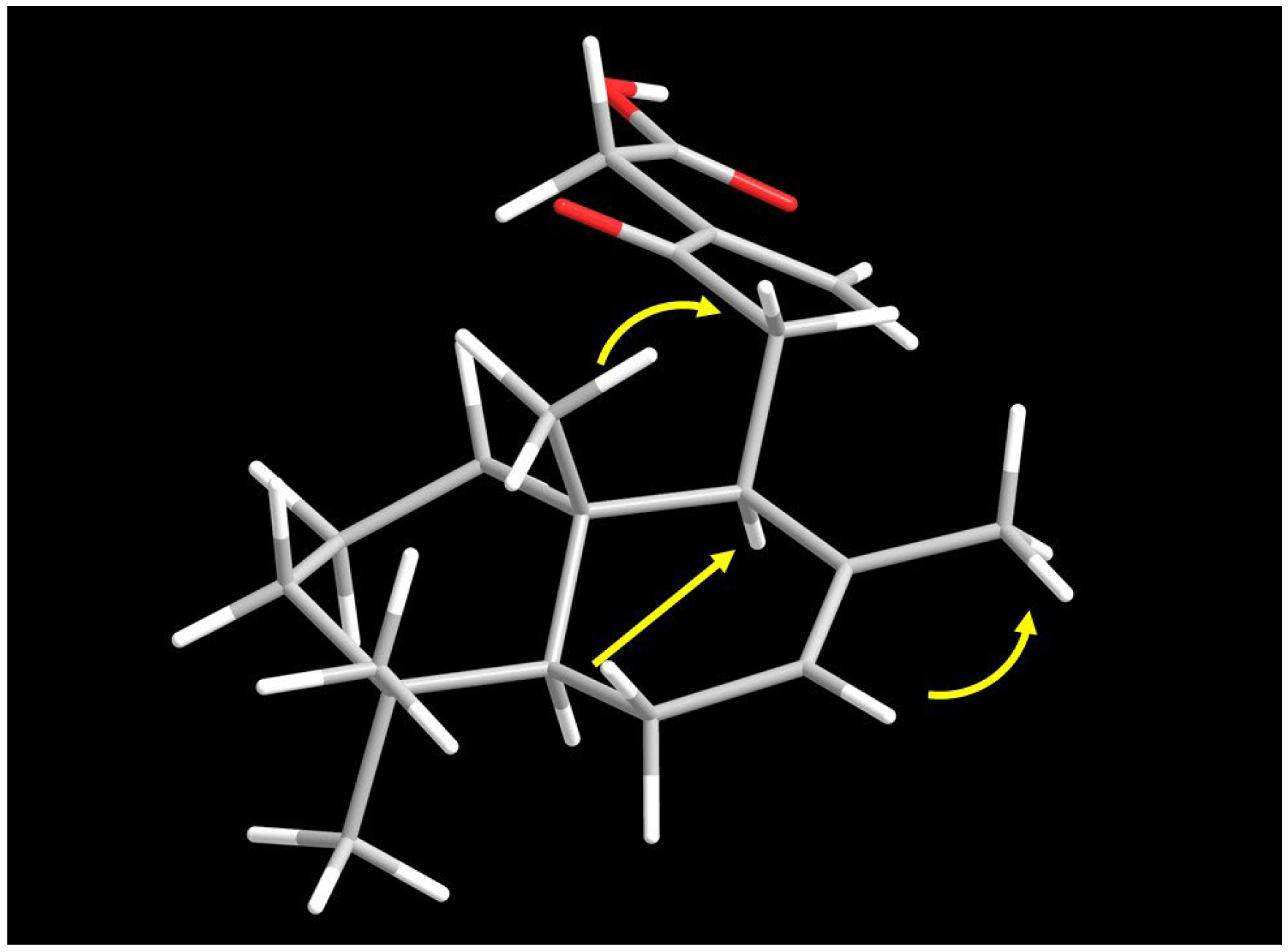
2.3. Computational Analyses
3. Discussion
4. Materials and Methods
4.1. General
4.2. Plant Material
4.3. Extraction and Isolation
4.4. Surface Plasmon Resonance Analyses (SPR)
4.5. Cell Culture and Treatment
4.6. Cell Viability
4.7. Computational Details
4.8. Molecular Docking
4.9. Molecular Dynamics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dianita, R.; Jantan, I. Ethnomedicinal uses, phytochemistry and pharmacological aspects of the genus Premna: A review. Pharm. Biol. 2017, 55, 1715–1739. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rekha, K.; Richa, P.; Babu, S.; Rao, M. A phytochemistry of the genus Premna: A review. Int. J. Pharm. Chem. Sci. 2015, 4, 317–325. [Google Scholar]
- Gushash, A.S. Plants in the Mountains of Sarat and Hejaz; Sarawat Designer and Printers: Madinah, Saudi Arabia, 2006; Volume 2, pp. 15–19. [Google Scholar]
- Albadawi, D.A.; Mothana, R.A.; Khaled, J.M.; Ashour, A.E.; Kumar, A.; Ahmad, S.F.; Al-Said, M.S.; Al-Rehaily, A.J.; Almusayeib, N.M. Antimicrobial, anticancer, and antioxidant compounds from Premna resinosa growing in Saudi Arabia. Pharm. Biol. 2017, 55, 1759–1766. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abdallah, Q.; Al-Deeb, I.; Bader, A.; Hamam, F.; Saleh, K.; Abdulmajid, A. Anti-angiogenic activity of Middle East medicinal plants of the Lamiaceae family. Mol. Med. Rep. 2018, 18, 2441–2448. [Google Scholar] [CrossRef]
- Iannuzzi, A.M.; Camero, C.M.; DʼAmbola, M.; DʼAngelo, V.; Amira, S.; Bader, A.; Braca, A.; De Tommasi, N.; Germanò, M.P. Antiangiogenic Iridoids from Stachys ocymastrum and Premna resinosa. Planta Med. 2019, 85, 1034–1039. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, R.; Alqathama, A.; Aldholmi, M.; Riaz, M.; Abdalla, A.N.; Mostafa, A.; Al-Said, H.M.; Alqarni, A.M.; Ullah, R.; Asgher, S.S. Gas chromatography-mass spectrometry (GC-MS) metabolites profiling and biological activities of various Capsicum annum cultivars. Plants 2022, 11, 1022. [Google Scholar] [CrossRef]
- Abdelhady, M.I.; Motaal, A.A. A cytotoxic C-glycosylated derivative of apigenin from the leaves of Ocimum basilicum var. thyrsiflorum. Rev. Bras. Farmacogn. 2016, 26, 763–766. [Google Scholar] [CrossRef] [Green Version]
- Alqahtani, A.A.; Attia, G.H.; Elgamal, A.; Aleraky, M.; Youns, M.; Ibrahim, A.M.; Abdou, R.; Shaikh, I.A.; El Raey, M.A. Cytotoxic activity of zinc oxide nanoparticles mediated by Euphorbia retusa. Crystals 2022, 12, 903. [Google Scholar] [CrossRef]
- Mohammed, H.A.; Almahmoud, S.A.; Arfeen, M.; Srivastava, A.; El-Readi, M.Z.; Ragab, E.A.; Shehata, S.M.; Mohammed, S.A.; Mostafa, E.M.; El-khawaga, H.A. Phytochemical profiling, molecular docking, and in vitro anti-hepatocellular carcinoid bioactivity of Suaeda vermiculata extracts. Arab. J. Chem. 2022, 15, 103950. [Google Scholar] [CrossRef]
- ElNaggar, M.H.; Eldehna, W.M.; Abourehab, M.A.; Abdel Bar, F.M. The old world salsola as a source of valuable secondary metabolites endowed with diverse pharmacological activities: A review. J. Enzym. Inhib. Med. Chem. 2022, 37, 2036–2062. [Google Scholar] [CrossRef]
- Morad, S.A.; Schmidt, C.; Büchele, B.; Schneider, B.; Wenzler, M.; Syrovets, T.; Simmet, T. (8 R)-3β, 8-Dihydroxypolypoda-13E, 17E, 21-triene induces cell cycle arrest and apoptosis in treatment-resistant prostate cancer cells. J. Nat. Prod. 2011, 74, 1731–1736. [Google Scholar] [CrossRef] [PubMed]
- Madrid, A.; Cardile, V.; González, C.; Montenegro, I.; Villena, J.; Caggia, S.; Graziano, A.; Russo, A. Psoralea glandulosa as a potential source of anticancer agents for melanoma treatment. Int. J. Mol. Sci. 2015, 16, 7944–7959. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shen, T.; Li, G.H.; Zhong, Q.Q.; Wang, S.Q.; Ren, D.M.; Lou, H.X.; Wang, X.N. Myrrhanolide D and Myrrhasin A, new Germacrane-type sesquiterpenoids from the resin of Commiphora opobalsamum. Helv. Chim. Acta 2014, 97, 881–886. [Google Scholar] [CrossRef]
- Ahmad, S.; Khan, S.A.; Kindelin, A.; Mohseni, T.; Bhatia, K.; Hoda, M.N.; Ducruet, A.F. Acetyl-11-keto-β-boswellic acid (AKBA) attenuates oxidative stress, inflammation, complement activation and cell death in brain endothelial cells following OGD/reperfusion. Neuromolecular Med. 2019, 21, 505–516. [Google Scholar] [CrossRef]
- Bellone, M.L.; Fiengo, L.; Cerchia, C.; Cotugno, R.; Bader, A.; Lavecchia, A.; De Tommasi, N.; Piaz, F.D. Impairment of nucleolin activity and phosphorylation by a trachylobane diterpene from Psiadia punctulata in cancer cells. Int. J. Mol. Sci. 2022, 23, 11390. [Google Scholar] [CrossRef]
- Bader, A.; Abdallah, Q.M.; Abdelhady, M.; De Tommasi, N.; Malafronte, N.; Shaheen, U.; Bkhaitan, M.; Cotugno, R. Cytotoxicity of some plants of the asteraceae family: Antiproliferative activity of Psiadia punctulata root sesquiterpenes. Rec. Nat. Prod. 2019, 13, 10–25135. [Google Scholar] [CrossRef]
- Talib, W.H.; Awajan, D.; Hamed, R.A.; Azzam, A.O.; Mahmod, A.I.; Al-Yasari, I.H. Combination anticancer therapies using selected phytochemicals. Molecules 2022, 27, 5452. [Google Scholar] [CrossRef] [PubMed]
- Lauro, G.; Bifulco, G. Elucidating the relative and absolute configuration of organic compounds by quantum mechanical approaches. Eur. J. Org. Chem. 2020, 2020, 3929–3941. [Google Scholar] [CrossRef]
- De Vita, S.; Terracciano, S.; Bruno, I.; Chini, M.G. From natural compounds to bioactive molecules through NMR and in silico methodologies. Eur. J. Org. Chem. 2020, 2020, 6297–6317. [Google Scholar] [CrossRef]
- Rosenzweig, R.; Nillegoda, N.B.; Mayer, M.P.; Bukau, B. The Hsp70 chaperone network. Nat. Rev. Mol. Cell Biol. 2019, 20, 665–680. [Google Scholar] [CrossRef]
- Ambrose, A.J.; Chapman, E. Function, therapeutic potential, and inhibition of Hsp70 chaperones. J. Med. Chem. 2021, 64, 7060–7082. [Google Scholar] [CrossRef] [PubMed]
- Park, S.-H.; Baek, K.-H.; Shin, I.; Shin, I. Subcellular Hsp70 inhibitors promote cancer cell death via different mechanisms. Cell Chem. Biol. 2018, 25, 1242–1254. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fiengo, L.; Lauro, G.; Bellone, M.; Bifulco, G.; Dal Piaz, F.; De Tommasi, N. The plant diterpene epoxysiderol targets Hsp70 in cancer cells, affecting its ATPase activity and reducing its translocation to plasma membrane. Int. J. Biol. Macromol. 2021, 189, 262–270. [Google Scholar] [CrossRef] [PubMed]
- Alizadeh, Z.; Farimani, M.M.; Parisi, V.; Marzocco, S.; Ebrahimi, S.N.; De Tommasi, N. Nor-abietane diterpenoids from Perovskia abrotanoides roots with anti-inflammatory potential. J. Nat. Prod. 2021, 84, 1185–1197. [Google Scholar] [CrossRef] [PubMed]
- Bohlmann, F.; Ahmed, M.; King, R.M.; Robinson, H. Labdane and eudesmane derivatives from Ageratum fastigiatum. Phytochemistry 1981, 20, 1434–1435. [Google Scholar] [CrossRef]
- Soltana, H.; De Rosso, M.; Lazreg, H.; Vedova, A.D.; Hammami, M.; Flamini, R. LC-QTOF characterization of non-anthocyanic flavonoids in four Tunisian fig varieties. J. Mass Spectrom. 2018, 53, 817–823. [Google Scholar] [CrossRef]
- Stout, G.H.; Stout, V.F. The structure and synthesis of xanthomicrol. Tetrahedron 1961, 14, 296–303. [Google Scholar] [CrossRef]
- Habtemariam, S.; Gray, A.I.; Halbert, G.W.; Waterman, P.G. A novel antibacterial diterpene from Premna schimperi. Planta Med. 1990, 56, 187–189. [Google Scholar] [CrossRef]
- Bifulco, G.; Dambruoso, P.; Gomez-Paloma, L.; Riccio, R. Determination of relative configuration in organic compounds by NMR spectroscopy and computational methods. Chem. Rev. 2007, 107, 3744–3779. [Google Scholar] [CrossRef]
- Boudermine, S.; Parisi, V.; Lemoui, R.; Boudiar, T.; Chini, M.G.; Franceschelli, S.; Pecoraro, M.; Pascale, M.; Bifulco, G.; Braca, A. Cytotoxic sesquiterpenoids from Ammoides atlantica aerial parts. J. Nat. Prod. 2022, 85, 647–656. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09, Revision A.02; Gaussian. Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Grimblat, N.; Zanardi, M.M.; Sarotti, A.M. Beyond DP4: An improved probability for the stereochemical assignment of isomeric compounds using quantum chemical calculations of NMR shifts. J. Org. Chem. 2015, 80, 12526–12534. [Google Scholar] [CrossRef] [PubMed]
- Bohlmann, F.; Zdero, C.; Gupta, R.K.; King, R.M.; Robinson, H. Diterpenes and tetranorditerpenes from Acritopappus species. Phytochemistry 1980, 19, 2695–2705. [Google Scholar] [CrossRef]
- De la Torre, M.C.; García, I.; Sierra, M.A. An approach to furolabdanes and their photooxidation derivatives from r-(+)-sclareolide. J. Nat. Prod. 2002, 65, 661–668. [Google Scholar] [CrossRef] [PubMed]
- Scio, E.; Ribeiro, A.; Alves, T.M.; Romanha, A.J.; de Souza Filho, J.D.; Cordell, G.A.; Zani, C.L. Diterpenes from Alomia myriadenia (Asteraceae) with cytotoxic and trypanocidal activity. Phytochemistry 2003, 64, 1125–1131. [Google Scholar] [CrossRef] [PubMed]
- Vasaturo, M.; Cotugno, R.; Fiengo, L.; Vinegoni, C.; Dal Piaz, F.; De Tommasi, N. The anti-tumor diterpene oridonin is a direct inhibitor of Nucleolin in cancer cells. Sci. Rep. 2018, 8, 16735. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cooper, M.A. Label-free screening of bio-molecular interactions. Anal. Bioanal. Chem. 2003, 377, 834–842. [Google Scholar] [CrossRef]
- Dal Piaz, F.; Ferro, P.; Vassallo, A.; Vasaturo, M.; Forte, G.; Chini, M.G.; Bifulco, G.; Tosco, A.; De Tommasi, N. Identification and mechanism of action analysis of the new PARP-1 inhibitor 2″-hydroxygenkwanol A. Biochim. Biophys. Acta Gen. Subj. 2015, 1850, 1806–1814. [Google Scholar] [CrossRef]
- Mosman, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Bellone, M.L.; Muñoz Camero, C.; Chini, M.G.; Dal Piaz, F.; Hernandez, V.; Bifulco, G.; De Tommasi, N.; Braca, A. Limonoids from Guarea guidonia and Cedrela odorata: Heat Shock Protein 90 (Hsp90) modulator properties of Chisomicine D. J. Nat. Prod. 2021, 84, 724–737. [Google Scholar] [CrossRef]
- Berman, H.M.; Westbrook, J.; Feng, Z.; Gilliland, G.; Bhat, T. Weissing & Shindyalov Bourne PE. The protein data bank. Nucleic Acids Res. 2000, 28, 235–242. [Google Scholar]
- Jones, A.M.; Westwood, I.M.; Osborne, J.D.; Matthews, T.P.; Cheeseman, M.D.; Rowlands, M.G.; Jeganathan, F.; Burke, R.; Lee, D.; Kadi, N. A fragment-based approach applied to a highly flexible target: Insights and challenges towards the inhibition of HSP70 isoforms. Sci. Rep. 2016, 6, 34701. [Google Scholar] [CrossRef] [Green Version]
- Schrödinger. Schrödinger Release 2020-4: Maestro Version 12.6.144, Glide, Ligprep, Prime; Schrödinger LLC: New York, NY, USA, 2021. [Google Scholar]
- Hospital, A.; Goñi, J.R.; Orozco, M.; Gelpí, J.L. Molecular dynamics simulations: Advances and applications. Adv. Appl. Bioinforma. Chem. 2015, 8, 37–47. [Google Scholar]
- Terracciano, S.; Lauro, G.; Russo, A.; Vaccaro, M.C.; Vassallo, A.; De Marco, M.; Ranieri, B.; Rosati, A.; Turco, M.C.; Riccio, R. Discovery and synthesis of the first selective BAG domain modulator of BAG3 as an attractive candidate for the development of a new class of chemotherapeutics. Chem. Comm. 2018, 54, 7613–7616. [Google Scholar] [CrossRef]
- Hu, C.; Zou, F.; Wang, A.; Miao, W.; Liang, Q.; Weisberg, E.L.; Wang, Y.; Liu, J.; Wang, W.; Liu, Q. Targeting chaperon protein HSP70 as a novel therapeutic strategy for FLT3-ITD-positive acute myeloid leukemia. Signal Transduct. Target. Ther. 2021, 6, 334. [Google Scholar] [CrossRef] [PubMed]
- Dal Piaz, F.; Cotugno, R.; Lepore, L.; Vassallo, A.; Malafronte, N.; Lauro, G.; Bifulco, G.; Belisario, M.A.; De Tommasi, N. Chemical proteomics reveals HSP70 1A as a target for the anticancer diterpene oridonin in Jurkat cells. J. Proteom. 2013, 82, 14–26. [Google Scholar] [CrossRef] [PubMed]
- Moradi-Marjaneh, R.; Paseban, M.; Moradi Marjaneh, M. Hsp70 inhibitors: Implications for the treatment of colorectal cancer. IUBMB Life 2019, 71, 1834–1845. [Google Scholar] [CrossRef] [PubMed]
- Du, S.; Liu, Y.; Yuan, Y.; Wang, Y.; Chen, Y.; Wang, S.; Chi, Y. Advances in the study of HSP70 inhibitors to enhance the sensitivity of tumor cells to radiotherapy. Tumor Microenv. Cancer Ther. 2023, 326. [Google Scholar] [CrossRef] [PubMed]
- Sevin, M.; Girodon, F.; Garrido, C.; De Thonel, A. HSP90 and HSP70: Implication in inflammation processes and therapeutic approaches for myeloproliferative neoplasms. Mediat. Inflamm. 2015, 2015. [Google Scholar] [CrossRef] [Green Version]
- Vassallo, A.; Vaccaro, M.C.; De Tommasi, N.; Dal Piaz, F.; Leone, A. Identification of the plant compound geraniin as a novel Hsp90 inhibitor. PLoS ONE 2013, 8, e74266. [Google Scholar] [CrossRef] [Green Version]
- Pratt, W.B.; Toft, D.O. Regulation of signaling protein function and trafficking by the hsp90/hsp70-based chaperone machinery. Adv. Exp. Med. 2003, 228, 111–133. [Google Scholar] [CrossRef] [PubMed]
- Piaz, F.D.; Vassallo, A.; Temraz, A.; Cotugno, R.; Belisario, M.A.; Bifulco, G.; Chini, M.G.; Pisano, C.; De Tommasi, N.; Braca, A. A chemical–biological study reveals C9-type iridoids as novel Heat shock protein 90 (Hsp90) inhibitors. J. Med. Chem. 2013, 56, 1583–1595. [Google Scholar] [CrossRef]
- Zhang, C.-T.; Li, X.; Ma, W.; Xie, X.; Huang, Q. Oridonin: A review of its pharmacology, pharmacokinetics and toxicity. Front. Pharmacol. 2021, 12, 645824. [Google Scholar]
- Yan-Hua, Y.; Jia-Wang, M.; Xiao-Li, T. Research progress on the source, production, and anti-cancer mechanisms of paclitaxel. Chin. J. Nat. Med. 2020, 18, 890–897. [Google Scholar]
- Chen, Y.; Xu, S.-S.; Chen, J.-W.; Wang, Y.; Xu, H.-Q.; Fan, N.-B.; Li, X. Anti-tumor activity of Annona squamosa seeds extract containing annonaceous acetogenin compounds. J. Ethnopharmacol. 2012, 142, 462–466. [Google Scholar] [CrossRef] [PubMed]
- Parveen, N.; Abourehab, M.A.; Shukla, R.; Thanikachalam, P.V.; Jain, G.K.; Kesharwani, P. Immunoliposomes as an emerging nanocarrier for breast cancer therapy. Eur. Polym. J. 2022, 111781. [Google Scholar] [CrossRef]
- Alhajri, H.M.; Aloqaili, S.S.; Alterary, S.S.; Alqathama, A.; Abdalla, A.N.; Alzhrani, R.M.; Alotaibi, B.S.; Alsaab, H.O. Olive leaf extracts for a green synthesis of silver-functionalized multi-walled carbon nanotubes. J. Funct. Biomater. 2022, 13, 224. [Google Scholar] [CrossRef]
- Siciliano, T.; Bader, A.; Vassallo, A.; Braca, A.; Morelli, I.; Pizza, C.; De Tommasi, N. Secondary metabolites from Ballota undulata (Lamiaceae). Biochem. Syst. Ecol. 2005, 33, 341–351. [Google Scholar] [CrossRef]
- Pesca, M.S.; Dal Piaz, F.; Sanogo, R.; Vassallo, A.; Bruzual de Abreu, M.; Rapisarda, A.; Germanò, M.P.; Certo, G.; De Falco, S.; De Tommasi, N. Bioassay-guided isolation of proanthocyanidins with antiangiogenic activities. J. Nat. Prod. 2013, 76, 29–35. [Google Scholar] [CrossRef]
- Braca, A.; Bader, A.; Morelli, I.; Scarpato, R.; Turchi, G.; Pizza, C.; De Tommasi, N. New pregnane glycosides from Caralluma negevensis. Tetrahedron 2002, 58, 5837–5848. [Google Scholar] [CrossRef]
- Cimino, P.; Gomez-Paloma, L.; Duca, D.; Riccio, R.; Bifulco, G. Comparison of different theory models and basis sets in the calculation of 13C NMR chemical shifts of natural products. Magn. Reson. Chem. 2004, 42, S26–S33. [Google Scholar] [CrossRef]
- Tomasi, J.; Mennucci, B.; Cammi, R. Quantum mechanical continuum solvation models. Chem. Rev. 2005, 105, 2999–3094. [Google Scholar] [CrossRef]
- Sarotti, A.M.; Pellegrinet, S.C. A multi-standard approach for GIAO 13C NMR calculations. J. Org. Chem. 2009, 74, 7254–7260. [Google Scholar] [CrossRef] [PubMed]

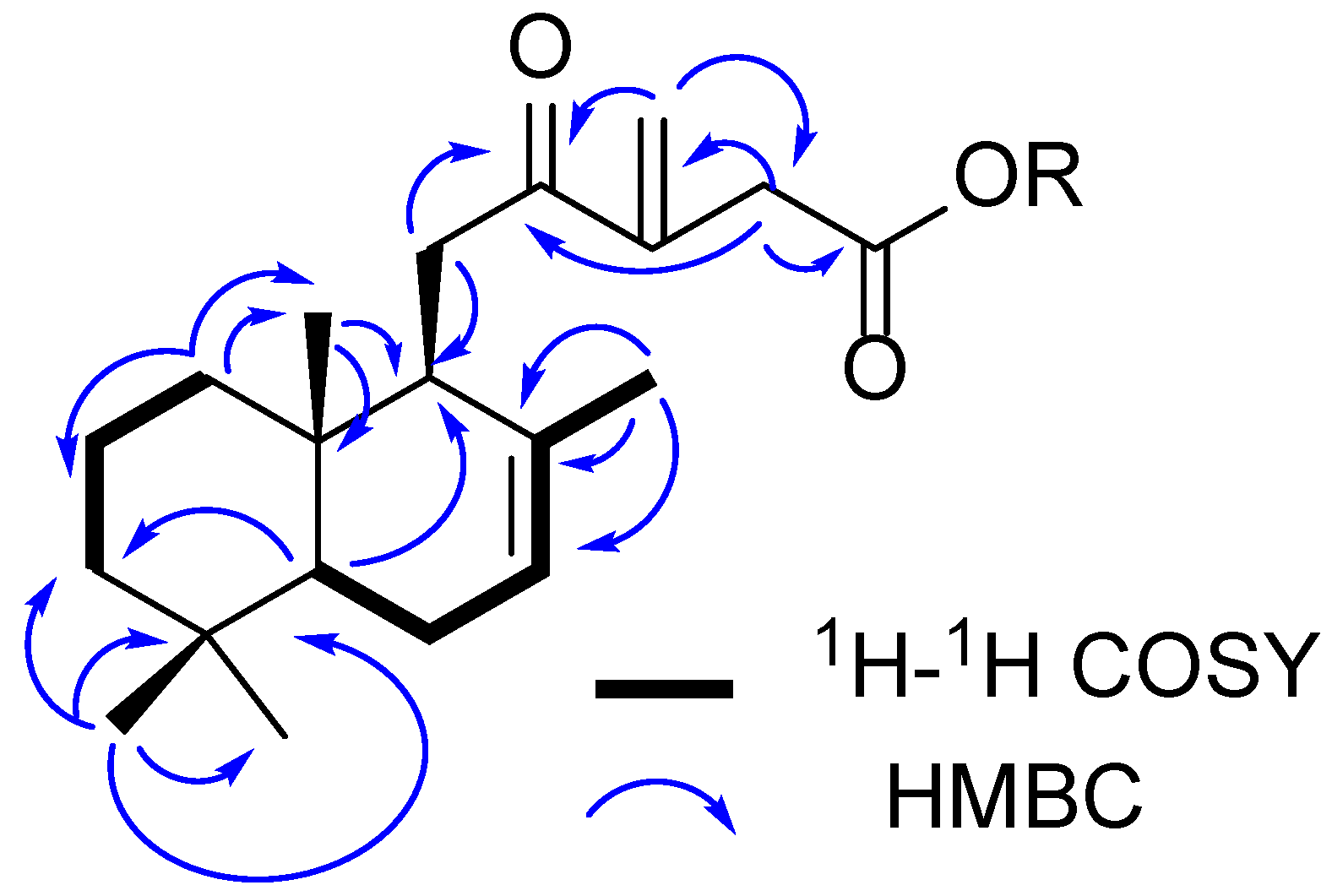

| 1 | 2 | |||||
|---|---|---|---|---|---|---|
| Position | δC, Type | δH | HMBC | δC, Type | δH | HMBC |
| 1 | 38.7, CH2 | 1.74, brd (14.0) 1.01, ddd (16.0, 13.0, 3.0) | 2, 5, 10, 20 | 38.7, CH2 | 1.74, brd (14.0) 1.01, ddd (16.0, 13.0, 3.0) | 2, 5, 10, 20 |
| 2 | 18.0, CH2 | 1.50, m 1.46 a | 1, 10 | 18.0, CH2 | 1.50, m LAKI 1.46 a | 1, 10 |
| 3 | 41.7, CH2 | 1.46 a 1.24, ddd (17.3, 14.0, 4.0) | 4, 5, 19 | 41.7, CH2 | 1.46 a 1.24, ddd (17.3, 14.0, 4.0) | 4, 5, 19 |
| 4 | 33.0, C | - | - | 33.0, C | - | - |
| 5 | 50.1, CH | 1.33, dd (12.0, 5.0) | 1, 4, 6, 9, 18, 19, 20 | 50.1, CH | 1.33, dd (12.0, 5.0) | 1, 4, 6, 9, 18, 19, 20 |
| 6 | 23.5, CH2 | 2.05, brd (16.0) 1.96, brt (16.0) | 8 | 23.5, CH2 | 2.05, brd (16.0) 1.96, brt (16.0) | 8 |
| 7 | 122.0, CH | 5.43, m | 5 | 122.0, CH | 5.43, m | 5 |
| 8 | 134.0, C | - | - | 134.0, C | - | - |
| 9 | 48.6, CH | 2.71, brd (8.5) | 8 | 48.6, CH | 2.71, brd (8.5) | 8 |
| 10 | 36.0, C | - | - | 36.0, C | - | - |
| 11 | 35.0, CH2 | 2.94, dd (18.3, 8.7) 2.63, brd (18.3) | 8, 9, 12 | 35.0, CH2 | 2.94, dd (18.3, 8.7) 2.63, brd (18.3) | 8, 9, 12 |
| 12 | 203.0, C | - | - | 203.0, C | - | - |
| 13 | 144.0, C | - | - | 144.0, C | - | - |
| 14 | 37.2, CH2 | 3.30, d. (14.0) 3.30, d. (14.0) | 12, 13, 15, 16 | 37.2, CH2 | 3.30, d. (17.5) 3.30, d. (17.5) | 12, 13, 15, 16 |
| 15 | 174.5, C | - | - | 173.4, C | - | - |
| 16 | 125.5, CH2 | 6.30, brs 5.94, brs | 12, 13, 14 | 125.5, CH2 | 6.30, brs 5.94, brs | 12, 13, 14 |
| 17 | 21.1, CH3 | 1.46, s | 7, 8, 9 | 21.1, CH3 | 1.46, s | 7, 8, 9 |
| 18 | 32.0, CH3 | 0.93, s | 3, 4, 5, 18 | 32.0, CH3 | 0.93, s | 3, 4, 5, 18 |
| 19 | 21.0, CH3 | 0.96, s | 3, 4, 5, 19 | 21.0, CH3 | 0.96, s | 3, 4, 5, 19 |
| 20 | 13.0, CH3 | 0.87, s | 1, 9, 10 | 13.0, CH3 | 0.87, s | 1, 9, 10 |
| MeO | - | - | - | 51.0, CH3 | 3.68, s | |
| Compound | KD (nM) a |
|---|---|
| 1 | No binding |
| 2 | No binding |
| 3 | 90.8 ± 3.5 |
| 4 | 1000 ± 25 |
| 5 | No binding |
| Oridonin | 81.4 ± 12.4 |
| Compound | Jurkat b | HeLa c |
|---|---|---|
| 1 | 25.1 ± 1.0 | 24.5 ± 1.7 |
| 2 | 20.8 ± 1.2 | >50 |
| 3 | 15.2 ± 1.0 | >50 |
| 4 | 42.5 ± 1.4 | >50 |
| 5 | >50 | >50 |
| Etoposide | 2.5 ± 0.4 | 4.0 ± 0.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parisi, V.; Donadio, G.; Bellone, M.L.; Belaabed, S.; Bader, A.; Bisio, A.; Iobbi, V.; Gazzillo, E.; Chini, M.G.; Bifulco, G.; et al. Exploring the Anticancer Potential of Premna resinosa (Hochst.) Leaf Surface Extract: Discovering New Diterpenes as Heat Shock Protein 70 (Hsp70) Binding Agents. Plants 2023, 12, 2421. https://doi.org/10.3390/plants12132421
Parisi V, Donadio G, Bellone ML, Belaabed S, Bader A, Bisio A, Iobbi V, Gazzillo E, Chini MG, Bifulco G, et al. Exploring the Anticancer Potential of Premna resinosa (Hochst.) Leaf Surface Extract: Discovering New Diterpenes as Heat Shock Protein 70 (Hsp70) Binding Agents. Plants. 2023; 12(13):2421. https://doi.org/10.3390/plants12132421
Chicago/Turabian StyleParisi, Valentina, Giuliana Donadio, Maria Laura Bellone, Soumia Belaabed, Ammar Bader, Angela Bisio, Valeria Iobbi, Erica Gazzillo, Maria Giovanna Chini, Giuseppe Bifulco, and et al. 2023. "Exploring the Anticancer Potential of Premna resinosa (Hochst.) Leaf Surface Extract: Discovering New Diterpenes as Heat Shock Protein 70 (Hsp70) Binding Agents" Plants 12, no. 13: 2421. https://doi.org/10.3390/plants12132421
APA StyleParisi, V., Donadio, G., Bellone, M. L., Belaabed, S., Bader, A., Bisio, A., Iobbi, V., Gazzillo, E., Chini, M. G., Bifulco, G., Faraone, I., & Vassallo, A. (2023). Exploring the Anticancer Potential of Premna resinosa (Hochst.) Leaf Surface Extract: Discovering New Diterpenes as Heat Shock Protein 70 (Hsp70) Binding Agents. Plants, 12(13), 2421. https://doi.org/10.3390/plants12132421











