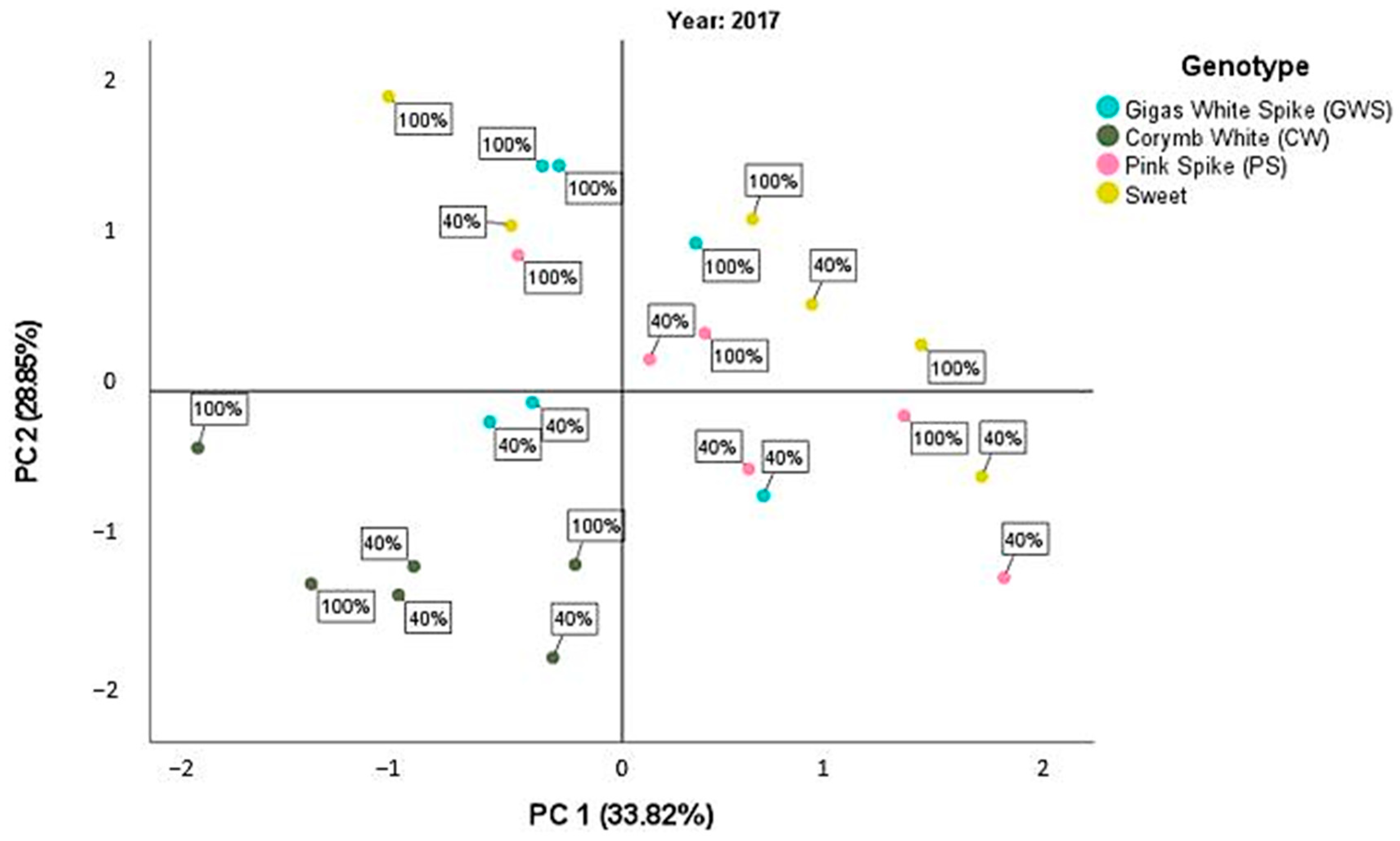
Figure 1.
Scatterplot of the PCA score for the first year, 2017, between the different basil cultivars and the two irrigation treatments d40 and d100.
Figure 1.
Scatterplot of the PCA score for the first year, 2017, between the different basil cultivars and the two irrigation treatments d40 and d100.
Figure 2.
Scatterplot of the PCA score for the second year, 2018, between the different basil cultivars and the two irrigation treatments d40 and d100.
Figure 2.
Scatterplot of the PCA score for the second year, 2018, between the different basil cultivars and the two irrigation treatments d40 and d100.
Figure 3.
Scatterplot of the PCA score for the third year, 2020, between the different basil cultivars and the two irrigation treatments d40 and d100.
Figure 3.
Scatterplot of the PCA score for the third year, 2020, between the different basil cultivars and the two irrigation treatments d40 and d100.
Table 1.
Combined effect of genotype (A), irrigation (Β) and growth stages (C) on plant height at the two irrigation levels, where 40% and 100% of the net irrigation requirements (IRn) are presented as d40 and d100, respectively, for the three years of experimentation (2017, 2018, and 2020). Data presented are mean values, where LSD is the least significant difference at 0.05 significance level.
Table 1.
Combined effect of genotype (A), irrigation (Β) and growth stages (C) on plant height at the two irrigation levels, where 40% and 100% of the net irrigation requirements (IRn) are presented as d40 and d100, respectively, for the three years of experimentation (2017, 2018, and 2020). Data presented are mean values, where LSD is the least significant difference at 0.05 significance level.
| Year | | | Plant Height (cm) | |
|---|
| | | Beginning of Flowering | Full Bloom | End of Flowering |
|---|
| | | d40 | d100 | d40 | d100 | d40 | d100 |
| 2017 | Gigas white spike (GWS) | 26.3 a Ϯ | 32.8 a | 28.8 b | 37.5 a | 32.0 a | 38.9 a |
| | Corymb White (CW) | 29.2 a | 33.8 a | 34.2 a | 34.8 a | 28.6 b | 41.6 a |
| | Pink spike (PS) | 41.9 a | 47.7 a | 43.3 a | 49.8 a | 48.8 b | 56.1 a |
| | Sweet (S) | 52.9 a | 56.1 a | 55.3 a | 58.8 a | 57.7 b | 67.1 a |
| LSDA×B×C | 7.5 |
| 2018 | Athos white spike (AWS) | 46.7 a | 48.1 a | 50.5 a | 45.9 a | 51.9 a | 52.2 a |
| | Gigas white spike (GWS) | 33.2 b | 40.0 a | 34.3 b | 43.0 a | 34.8 a | 33.5 a |
| | Corymb violet (CV) | 51.2 a | 51.0 a | 51.5 a | 44.6 b | 54.1 a | 54.9 a |
| | Pink spike (PS) | 43.9 b | 51.4 a | 48.4 a | 46.2 a | 40.9 b | 50.1 a |
| | Genovese (G) | 45.6 a | 46.7 a | 49.2 a | 48.3 a | 50.7 a | 51.9 a |
| LSDA×B×C | 5.9 |
| 2020 | Athos white spike (AWS) | 30.1 b | 35.2 a | 33.1 b | 46.0 a | 36.3 b | 64.3 a |
| | Gigas white spike (GWS) | 22.5 a | 26.0 a | 24.8 b | 32.0 a | 26.8 b | 37.0 a |
| | Corymb violet (CV) | 32.6 b | 44.0 a | 35.1 b | 48.8 a | 34.7 b | 67.8 a |
| | Pink spike (PS) | 32.6 b | 41.3 a | 33.7 b | 46.6 a | 41.0 b | 64.0 a |
| | Kassandra red spike (KRS) | 31.2 b | 42.4 a | 31.8 b | 54.7 a | 37.9 b | 76.6 a |
| | Genovese (G) | 32.7 b | 39.1 a | 33.8 b | 47.4 a | 38.5 b | 63.2 a |
| LSDA×B×C | 5.0 |
Table 2.
Combined effect of genotype (A), irrigation (Β) and growth stages (C) on leaf area index (LAI) at the two irrigation levels, where 40% and 100% of the net irrigation requirements (IRn) are presented as d40 and d100, respectively, for the three years of experimentation (2017, 2018, and 2020). Data presented are mean values, where LSD is the least significant difference at 0.05 significance level.
Table 2.
Combined effect of genotype (A), irrigation (Β) and growth stages (C) on leaf area index (LAI) at the two irrigation levels, where 40% and 100% of the net irrigation requirements (IRn) are presented as d40 and d100, respectively, for the three years of experimentation (2017, 2018, and 2020). Data presented are mean values, where LSD is the least significant difference at 0.05 significance level.
| Year | | Leaf Area Index (LAI) |
|---|
| | | Beginning of Flowering | Full Bloom | End of Flowering |
|---|
| | | d40 | d100 | d40 | d100 | d40 | d100 |
| 2017 | Gigas white spike (GWS) | 1.70 b Ϯ | 2.90 a | 2.10 b | 3.90 a | 1.70 b | 3.00 a |
| | Corymb White (CW) | 1.20 a | 1.50 a | 2.00 a | 1.60 a | 0.60 a | 1.00 a |
| | Pink spike (PS) | 1.20 b | 2.30 a | 1.30 b | 2.40 a | 2.50 b | 3.60 a |
| | Sweet (S) | 2.10 a | 2.60 a | 1.90 b | 3.00 a | 2.90 b | 4.10 a |
| LSDA×B×C | 1.00 |
| 2018 | Athos white spike (AWS) | 2.44 a | 2.71 a | 1.95 a | 1.61 a | 1.26 a | 1.30 a |
| | Gigas white spike (GWS) | 1.82 a | 1.53 a | 2.01 a | 1.48 b | 1.77 a | 2.12 a |
| | Corymb violet (CV) | 2.43 a | 2.19 a | 1.93 a | 1.77 a | 2.30 a | 2.08 a |
| | Pink spike (PS) | 0.95 a | 1.59 a | 1.63 a | 1.36 a | 1.33 a | 1.45 a |
| | Genovese (G) | 3.04 a | 2.90 a | 2.01 a | 1.77 a | 1.33 a | 1.46 a |
| LSDA×B×C | 0.57 |
| 2020 | Athos white spike (AWS) | 1.99 a | 2.52 a | 2.54 a | 3.25 a | 2.45 b | 4.03 a |
| | Gigas white spike (GWS) | 1.29 b | 2.49 a | 1.82 b | 3.05 a | 2.06 b | 3.00 a |
| | Corymb violet (CV) | 1.70 b | 2.66 a | 1.88 b | 2.93 a | 2.27 b | 3.84 a |
| | Pink spike (PS) | 2.20 a | 1.61 a | 2.15 a | 2.43 a | 2.26 a | 3.05 a |
| | Kassandra red spike (KRS) | 2.06 b | 3.09 a | 2.67 b | 3.79 a | 2.59 b | 4.80 a |
| | Genovese (G) | 2.15 a | 2.24 a | 2.20 b | 3.32 a | 2.48 b | 4.19 a |
| LSDA×B×C | 0.84 |
Table 3.
Combined effect of genotype (A), irrigation (Β) and growth stages (C) on leaf chlorophyll content at the two irrigation levels, where 40% and 100% of the net irrigation requirements (IRn) are presented as d40 and d100, respectively, for the three years of experimentation (2017, 2018, and 2020). Data presented are mean values, where LSD is the least significant difference at 0.05 significance level.
Table 3.
Combined effect of genotype (A), irrigation (Β) and growth stages (C) on leaf chlorophyll content at the two irrigation levels, where 40% and 100% of the net irrigation requirements (IRn) are presented as d40 and d100, respectively, for the three years of experimentation (2017, 2018, and 2020). Data presented are mean values, where LSD is the least significant difference at 0.05 significance level.
| Year | | Chlorophyll Content |
|---|
| | | Beginning of Flowering | Full Bloom | End of Flowering |
|---|
| | | d40 | d100 | d40 | d100 | d40 | d100 |
| 2017 | Gigas white spike (GWS) | 41.3 a Ϯ | 36.9 b | 36.9 a | 34.5 a | 34.0 a | 30.7 a |
| | Corymb White (CW) | 40.5 a | 36.6 a | 40.4 a | 32.9 b | 29.7 a | 22.4 b |
| | Pink spike (PS) | 39.4 a | 40.3 a | 43.0 a | 40.4 a | 43.6 a | 39.4 a |
| | Sweet (S) | 38.9 a | 39.5 a | 43.6 a | 39.9 a | 36.8 a | 30.0 b |
| LSDA×B×C | 5.4 |
| 2018 | Athos white spike (AWS) | 37.0 a | 36.5 a | 31.6 a | 31.6 a | 30.5 a | 34.9 a |
| | Gigas white spike (GWS) | 37.7 a | 35.6 a | 37.9 a | 36.4 a | 42.3 a | 35.0 b |
| | Corymb violet (CV) | 45.3 a | 43.6 a | 46.4 a | 41.5 a | 42.0 a | 45.3 a |
| | Pink spike (PS) | 42.8 a | 42.2 a | 38.9 a | 41.2 a | 41.3 a | 36.5 a |
| | Genovese (G) | 38.0 a | 37.8 a | 32.2 a | 31.3 a | 29.2 a | 34.3 a |
| LSDA×B×C | 5.2 |
| 2020 | Athos white spike (AWS) | 34.8 a | 37.0 a | 36.0 b | 39.0 a | 35.4 a | 37.4 a |
| | Gigas white spike (GWS) | 41.6 a | 42.1 a | 40.0 a | 41.4 a | 34.1 a | 36.8 a |
| | Corymb violet (CV) | 44.0 a | 43.4 a | 41.1 b | 44.4 a | 42.2 a | 43.3 a |
| | Pink spike (PS) | 42.6 a | 45.0 a | 44.5 a | 41.8 a | 38.4 b | 43.0 a |
| | Kassandra red spike (KRS) | 42.7 b | 47.3 a | 41.6 a | 43.6 a | 41.9 a | 44.1 a |
| | Genovese (G) | 38.7 a | 39.5 a | 37.8 b | 41.6 a | 37.3 a | 39.5 a |
| LSDA×B×C | 2.97 |
Table 4.
Combined effect of genotype (A), irrigation (Β) and growth stages (C) on chlorophyll fluorescence at the two irrigation levels, where 40% and 100% of the net irrigation requirements (IRn) are presented as d40 and d100, respectively, for the three years of experimentation (2017, 2018, and 2020). Data presented are mean values, where LSD is the least significant difference at 0.05 significance level.
Table 4.
Combined effect of genotype (A), irrigation (Β) and growth stages (C) on chlorophyll fluorescence at the two irrigation levels, where 40% and 100% of the net irrigation requirements (IRn) are presented as d40 and d100, respectively, for the three years of experimentation (2017, 2018, and 2020). Data presented are mean values, where LSD is the least significant difference at 0.05 significance level.
| Year | | Chlorophyll Fluorescence |
|---|
| | | Beginning of Flowering | Full Bloom | End of Flowering |
|---|
| | | d40 | d100 | d40 | d100 | d40 | d100 |
| 2017 | Gigas white spike (GWS) | 0.64 a Ϯ | 0.60 a | 0.62 a | 0.61 a | 0.67 a | 0.66 a |
| | Corymb White (CW) | 0.58 b | 0.64 a | 0.56 a | 0.56 a | 0.62 a | 0.57 a |
| | Pink spike (PS) | 0.74 a | 0.71 a | 0.67 a | 0.63 a | 0.66 a | 0.58 b |
| | Sweet (S) (C) | 0.71 a | 0.71 a | 0.67 a | 0.66 a | 0.62 a | 0.58 a |
| LSDA×B×C | 0.06 |
| 2018 | Athos white spike (AWS) | 0.66 a | 0.65 a | 0.68 a | 0.68 a | 0.66 a | 0.65 a |
| | Gigas white spike (GWS) | 0.72 a | 0.71 a | 0.71 a | 0.67 a | 0.69 a | 0.70 a |
| | Corymb violet (CV) | 0.68 a | 0.67 a | 0.72 a | 0.70 a | 0.72 a | 0.68 a |
| | Pink spike (PS) | 0.72 a | 0.68 a | 0.69 a | 0.66 a | 0.71 a | 0.69 a |
| | Genovese (G) | 0.63 a | 0.64 a | 0.72 a | 0.68 a | 0.67 a | 0.63 a |
| LSDA×B×C | 0.05 |
| 2020 | Athos white spike (AWS) | 0.66 b | 0.75 a | 0.65 a | 0.65 a | 0.66 b | 0.73 a |
| | Gigas white spike (GWS) | 0.66 b | 0.74 a | 0.62 b | 0.76 a | 0.70 b | 0.75 a |
| | Corymb violet (CV) | 0.69 a | 0.71 a | 0.67 a | 0.68 a | 0.71 b | 0.76 a |
| | Pink spike (PS) | 0.73 a | 0.73 a | 0.67 b | 0.72 a | 0.77 a | 0.78 a |
| | Kassandra red spike (KRS) | 0.70 a | 0.73 a | 0.64 b | 0.70 a | 0.77 a | 0.76 a |
| | Genovese (G) | 0.71 a | 0.72 a | 0.68 a | 0.69 a | 0.66 b | 0.74 a |
| LSDA×B×C | 0.04 |
Table 5.
Combined effect of genotype (A), irrigation (Β) and growth stages (C) on assimilation rate (A) of CO2 at the two irrigation levels, where 40% and 100% of the net irrigation requirements (IRn) are presented as d40 and d100, respectively, for the three years of experimentation (2017, 2018, and 2020). Data presented are mean values, where LSD is the least significant difference at 0.05 significance level.
Table 5.
Combined effect of genotype (A), irrigation (Β) and growth stages (C) on assimilation rate (A) of CO2 at the two irrigation levels, where 40% and 100% of the net irrigation requirements (IRn) are presented as d40 and d100, respectively, for the three years of experimentation (2017, 2018, and 2020). Data presented are mean values, where LSD is the least significant difference at 0.05 significance level.
| Year | | CO2 Assimilation Rate (A) |
|---|
| | | Beginning of Flowering | Full Bloom | End of Flowering |
|---|
| | | d40 | d100 | d40 | d100 | d40 | d100 |
| 2017 | Gigas white spike (GWS) | 11.2 a Ϯ | 13.6 a | 8.4 a | 11.2 a | 9.4 a | 9.2 a |
| | Corymb White (CW) | 12.9 a | 11.0 a | 8.8 a | 7.4 a | 12.5 a | 5.2 b |
| | Pink spike (PS) | 26.7 a | 23.5 a | 11.0 a | 13.8 a | 12.3 a | 12.4 a |
| | Sweet (S) | 23.1 a | 25.5 a | 7.0 b | 12.9 a | 10.1 a | 8.6 a |
| LSDA×B×C | 4.8 |
| 2018 | Athos white spike (AWS) | 9.6 a | 5.3 a | 6.9 a | 5.2 a | 8.3 a | 11.6 a |
| | Gigas white spike (GWS) | 5.9 a | 7.5 a | 9.9 a | 11.6 a | 10.5 a | 11.7 a |
| | Corymb violet (CV) | 10.8 a | 10.1 a | 5.1 a | 7.8 a | 9.3 a | 11.9 a |
| | Pink spike (PS) | 8.1 a | 9.6 a | 8.3 a | 8.6 a | 10.2 a | 10.7 a |
| | Genovese (G) | 8.2 a | 6.6 a | 3.7 a | 7.9 a | 4.3 a | 6.7 a |
| LSDA×B×C | 5.0 |
| 2020 | Athos white spike (AWS) | 4.3 a | 4.5 a | 3.8 a | 4.7 a | 5.5 a | 3.4 a |
| | Gigas white spike (GWS) | 3.9 a | 4.7 a | 4.1 a | 6.1 a | 2.7 a | 2.4 a |
| | Corymb violet (CV) | 4.6 b | 7.0 a | 4.5 a | 5.1 a | 2.4 a | 4.4 a |
| | Pink spike (PS) | 3.3 b | 5.8 a | 9.6 a | 5.0 b | 3.2 a | 3.4 a |
| | Kassandra red spike (KRS) | 5.0 a | 4.3 a | 4.6 a | 5.3 a | 4.7 a | 4.1 a |
| | Genovese (G) | 6.0 a | 6.0 a | 3.4 a | 5.2 a | 1.8 a | 3.4 a |
| LSDA×B×C | 2.3 |
Table 6.
Combined effect of genotype (A), irrigation (Β) and growth stages (C) on dry weight at the two irrigation levels, where 40% and 100% of the net irrigation requirements (IRn) are presented as d40 and d100, respectively, for the three years of experimentation (2017, 2018, and 2020). Data presented are mean values, where LSD is the least significant difference at 0.05 significance level.
Table 6.
Combined effect of genotype (A), irrigation (Β) and growth stages (C) on dry weight at the two irrigation levels, where 40% and 100% of the net irrigation requirements (IRn) are presented as d40 and d100, respectively, for the three years of experimentation (2017, 2018, and 2020). Data presented are mean values, where LSD is the least significant difference at 0.05 significance level.
| Year | | Dry Weight (g/m2) |
|---|
| | | Beginning of Flowering | Full Bloom | End of Flowering |
|---|
| | | d40 | d100 | d40 | d100 | d40 | d100 |
| 2017 | Gigas white spike (GWS) | 248 b Ϯ | 388 a | 248 b | 420 a | 275 b | 455 a |
| | Corymb White (CW) | 107 a | 155 a | 87 a | 131 a | 192 a | 277 a |
| | Pink spike (PS) | 170 a | 228 a | 203 a | 315 a | 280 a | 323 a |
| | Sweet (S) | 230 a | 260 a | 328 b | 380 a | 390 a | 468 a |
| LSDA×B×C | 124 |
| 2018 | Athos white spike (AWS) | 275 a | 233 a | 283 a | 228 a | 265 a | 238 a |
| | Gigas white spike (GWS) | 230 a | 215 a | 218 a | 270 a | 238 a | 200 a |
| | Corymb violet (CV) | 273 a | 238 a | 310 a | 223 b | 348 a | 353 a |
| | Pink spike (PS) | 170b | 258 a | 300 a | 205 b | 198 a | 223 a |
| | Genovese (G) | 270 a | 233 a | 223 a | 195 a | 213 a | 183 a |
| LSDA×B×C | 79 |
| 2020 | Athos white spike (AWS) | 222 b | 290 a | 229 a | 294 a | 372 a | 423 a |
| | Gigas white spike (GWS) | 184 a | 223 a | 220 a | 272 a | 237 b | 304 a |
| | Corymb violet (CV) | 156 b | 245 a | 270 b | 379 a | 251 b | 343 a |
| | Pink spike (PS) | 119 a | 162 a | 216 a | 206 a | 229 b | 341 a |
| | Kassandra red spike (KRS) | 241 b | 319 a | 261 b | 340 a | 281 b | 388 a |
| | Genovese (G) | 183 a | 229 a | 294 b | 379 a | 333 a | 350 a |
| LSDA×B×C | 67 |
Table 7.
Combined effect of genotype (A), irrigation (Β) and growth stages (C) on essential oil content during 2017, 2018, and 2020, at the two irrigation levels, where 40% and 100% of the net irrigation requirements (IRn) are presented as d40 and d100, respectively, for the three years of experimentation (2017, 2018, and 2020). Data presented are mean values, where LSD is the least significant difference at 0.05 significance level.
Table 7.
Combined effect of genotype (A), irrigation (Β) and growth stages (C) on essential oil content during 2017, 2018, and 2020, at the two irrigation levels, where 40% and 100% of the net irrigation requirements (IRn) are presented as d40 and d100, respectively, for the three years of experimentation (2017, 2018, and 2020). Data presented are mean values, where LSD is the least significant difference at 0.05 significance level.
| Year | | Essential Oil Content (mL/100 g Dry Weight) |
|---|
| | | Beginning of Flowering | Full Bloom | End of Flowering |
|---|
| | | d40 | d100 | d40 | d100 | d40 | d100 |
| 2017 | Gigas white spike (GWS) | 1.44 a Ϯ | 1.63 a | 1.28 a | 1.22 a | 1.16 a | 1.35 a |
| | Corymb White (CW) | 1.50 b | 1.94 a | 1.53 a | 1.60 a | 1.31 a | 1.19 a |
| | Pink spike (PS) | 1.47 a | 1.66 a | 1.31 a | 1.56 a | 1.16 a | 1.22 a |
| | Sweet (S) (C) | 1.31 a | 1.38 a | 1.06 a | 1.28 a | 0.85 a | 0.88 a |
| LSDA×B×C | 0.38 |
| 2018 | Athos white spike (AWS) | 0.75 a | 0.69 a | 0.63 a | 0.50 a | 0.56 a | 0.75 a |
| | Gigas white spike (GWS) | 0.50 a | 0.56 a | 0.94 a | 0.44 b | 0.75 a | 0.69 a |
| | Corymb violet (CV) | 0.56 a | 0.56 a | 0.44 a | 0.69 a | 0.75 a | 0.56 a |
| | Pink spike (PS) | 0.75 a | 1.00 a | 0.81 a | 0.88 a | 0.94 a | 1.19 a |
| | Genovese (G) | 0.75 a | 0.56 a | 0.69 a | 0.88 a | 0.69 a | 0.69 a |
| LSDA×B×C | 0.39 |
| 2020 | Athos white spike (AWS) | 1.31 a | 1.31 a | 1.03 a | 1.09 a | 1.28 a | 1.12 a |
| | Gigas white spike (GWS) | 1.40 a | 1.18 b | 1.31 a | 1.18 a | 1.43 a | 1.25 a |
| | Corymb violet (CV) | 1.06 a | 1.00 a | 1.00 a | 0.96 a | 0.81 a | 1.09 a |
| | Pink spike (PS) | 1.18 a | 0.93 a | 0.93 a | 1.15 a | 1.34 b | 1.81 a |
| | Kassandra red spike (KRS) | 1.43 a | 1.53 a | 1.28 a | 1.06 a | 1.40 a | 1.46 a |
| | Genovese (G) | 1.00 a | 1.18 a | 1.15 a | 1.03 a | 1.15 a | 1.15 a |
| LSDA×B×C | 0.28 |
Table 8.
Combined effect of genotype (A), irrigation (Β) and growth stages (C) on essential oil yield at the two irrigation levels, where 40% and 100% of the net irrigation requirements (IRn) are presented as d40 and d100, respectively, for the three years of experimentation (2017, 2018, and 2020). Data presented are mean values, where LSD is the least significant difference at 0.05 significance level.
Table 8.
Combined effect of genotype (A), irrigation (Β) and growth stages (C) on essential oil yield at the two irrigation levels, where 40% and 100% of the net irrigation requirements (IRn) are presented as d40 and d100, respectively, for the three years of experimentation (2017, 2018, and 2020). Data presented are mean values, where LSD is the least significant difference at 0.05 significance level.
| Year | | Essential Oil Yield (mL/m2) |
|---|
| | | Beginning of Flowering | Full Bloom | End of Flowering |
|---|
| | | d40 | d100 | d40 | d100 | d40 | d100 |
| 2017 | Gigas white spike (GWS) | 2.02 b Ϯ | 4.35 a | 2.17 b | 3.55 a | 2.17 b | 4.31 a |
| | Corymb White (CW) | 1.04 a | 1.97 a | 0.75 a | 1.10 a | 1.30 a | 1.85 a |
| | Pink spike (PS) | 1.40 a | 2.06 a | 1.53 b | 2.74 a | 1.53 a | 2.27 a |
| | Sweet (S) (C) | 1.50 a | 1.88 a | 1.83 a | 2.68 a | 1.81 a | 2.30 a |
| LSDA×B×C | 1.05 |
| 2018 | Athos white spike (AWS) | 1.06 a | 0.83 a | 0.91 a | 0.53 a | 0.70 a | 0.75 a |
| | Gigas white spike (GWS) | 0.65 a | 0.83 a | 1.20 a | 0.79 a | 1.60 a | 0.97 a |
| | Corymb violet (CV) | 0.65 a | 0.72 a | 0.66 a | 0.85 a | 1.32 a | 0.98 a |
| | Pink spike (PS) | 0.73 b | 1.34 a | 1.25 a | 0.87 a | 1.08 a | 1.44 a |
| | Genovese (G) | 1.12 a | 0.70 a | 0.79 a | 0.82 a | 0.65 a | 0.59 a |
| LSDA×B×C | 0.61 |
| 2020 | Athos white spike (AWS) | 1.05 a | 1.21 a | 0.92 a | 1.04 a | 1.58 a | 1.61 a |
| | Gigas white spike (GWS) | 0.86 a | 0.95 a | 1.04 a | 1.20 a | 1.35 a | 1.65 a |
| | Corymb violet (CV) | 0.46 b | 0.82 a | 0.76 b | 1.15 a | 0.64 b | 1.23 a |
| | Pink spike (PS) | 0.52 a | 0.47 a | 0.50 a | 0.81 a | 0.96 a | 2.03 a |
| | Kassandra red spike (KRS) | 1.16 a | 1.55 a | 1.21 a | 1.00 a | 1.21 b | 1.92 a |
| | Genovese (G) | 0.68 a | 0.94 a | 1.31 a | 1.41 a | 1.40 a | 1.51 a |
| LSDA×B×C | 0.32 |
Table 9.
Combined effect of genotype (A), irrigation (Β) and growth stages (C) on water use efficiency at the two irrigation levels, where 40% and 100% of the net irrigation requirements (IRn) are presented as d40 and d100, respectively, for the three years of experimentation (2017, 2018, and 2020). Data presented are mean values, where LSD is the least significant difference at 0.05 significance level.
Table 9.
Combined effect of genotype (A), irrigation (Β) and growth stages (C) on water use efficiency at the two irrigation levels, where 40% and 100% of the net irrigation requirements (IRn) are presented as d40 and d100, respectively, for the three years of experimentation (2017, 2018, and 2020). Data presented are mean values, where LSD is the least significant difference at 0.05 significance level.
| Year | | Water Use Efficiency |
|---|
| | | Beginning of Flowering | Full Bloom | End of Flowering |
|---|
| | | d40 | d100 | d40 | d100 | d40 | d100 |
| 2017 | Gigas white spike (GWS) | 14.6 a Ϯ | 11.1 a | 2.4 a | 3.3 a | 2.5 a | 3.4 a |
| | Corymb White (CW) | 6.3 a | 4.4 a | 0.8 a | 1.0 a | 1.7 a | 2.1 a |
| | Pink spike (PS) | 12.2 a | 8.5 a | 12.0 a | 9.0 a | 2.7 a | 2.6 a |
| | Sweet (S) (C) | 16.5 a | 9.7 b | 19.4 a | 10.9 b | 3.8 a | 3.7 a |
| LSDA×B×C | 4.1 |
| 2018 | Athos white spike (AWS) | 21.8 a | 12.8 b | 20.9 a | 11.0 b | 17.3 a | 10.5 b |
| | Gigas white spike (GWS) | 17.0 a | 10.4 b | 10.4 a | 12.0 a | 11.8 a | 7.1 a |
| | Corymb violet (CV) | 21.6 a | 13.1 b | 22.9 a | 10.7 b | 22.6 a | 15.6 b |
| | Pink spike (PS) | 12.6 a | 12.4 a | 14.3 a | 9.1 b | 9.8 a | 7.9 a |
| | Genovese (G) | 21.4 a | 12.8 b | 16.5 a | 9.4 b | 13.8 a | 8.1 b |
| LSDA×B×C | 4.7 |
| 2020 | Athos white spike (AWS) | 29.2 a | 23.2 b | 27.3 a | 18.5 b | 36.0 a | 21.0 b |
| | Gigas white spike (GWS) | 24.3 a | 17.9 b | 26.2 a | 17.2 b | 22.9 a | 15.1 b |
| | Corymb violet (CV) | 20.6 a | 19.6 a | 32.2 a | 23.9 b | 24.3 a | 17.0 b |
| | Pink spike (PS) | 15.6 a | 12.9 a | 25.7 a | 13.0 b | 22.1 a | 16.9 b |
| | Kassandra red spike (KRS) | 31.7 a | 25.6 b | 31.1 a | 21.5 b | 27.1 a | 19.3 b |
| | Genovese (G) | 24.1 a | 18.3 a | 35.0 a | 23.9 b | 32.2 a | 17.4 b |
| LSDA×B×C | 6.0 |
Table 10.
Component factor loadings for measured plant parameters in the PCA, which was performed separately for the two irrigation treatments during three growing seasons (2017, 2018, 2020).
Table 10.
Component factor loadings for measured plant parameters in the PCA, which was performed separately for the two irrigation treatments during three growing seasons (2017, 2018, 2020).
| | 2017 | 2018 | 2020 |
|---|
| Plant Parameters | Component 1 | Component 2 | Component 1 | Component 2 | Component 1 | Component 2 |
|---|
| Plant Height | 0.679 | −0.200 | 0.387 | −0.256 | 0.429 | 0.807 |
| Leaf Area Index | 0.871 | 0.011 | 0.636 | −0.025 | 0.554 | 0.718 |
| Chlorophyl Content | −0.129 | 0.680 | 0.445 | 0.547 | −0.437 | 0.587 |
| Chlorophyl Fluorescence | −0.006 | 0.867 | −0.052 | 0.302 | 0.127 | 0.706 |
| Assimilation Rate CO2 | −0.153 | 0.799 | 0.006 | 0.740 | −0.584 | 0.200 |
| Dry Weight | 0.971 | −0.025 | 0.880 | 0.202 | 0.775 | 0.366 |
| Essential Oil Content | −0.552 | 0.198 | −0.434 | 0.629 | 0.566 | −0.024 |
| Essential Oil Yield | 0.733 | 0.055 | 0.034 | 0.879 | 0.933 | 0.225 |
| Water Use Efficiency | 0.008 | 0.813 | 0.872 | −0.132 | 0.278 | −0.721 |
Table 11.
The main weather parameters (mean relative humidity (RHmean), rainfall, maximum (Tmax), minimum (Tmin), and mean (Tmean) temperature) and reference evapotranspiration (ETo), for the three years and their comparison with 30-year averages. The weather data were recorded with an automatic weather station close to the experimental site.
Table 11.
The main weather parameters (mean relative humidity (RHmean), rainfall, maximum (Tmax), minimum (Tmin), and mean (Tmean) temperature) and reference evapotranspiration (ETo), for the three years and their comparison with 30-year averages. The weather data were recorded with an automatic weather station close to the experimental site.
| | Year 2017 | Year 2018 | Year 2020 | 30-Year Average |
|---|
| | June | July | August | June | July | August | June | July | August | June | July | August |
|---|
| Tmax (°C) | 29.8 | 34.3 | 33.8 | 32.4 | 34.5 | 33.8 | 29.5 | 34.0 | 34.1 | 30.2 | 32.5 | 32.2 |
| Tmin (°C) | 17.1 | 20.5 | 20.4 | 18.7 | 21.2 | 20.4 | 18.9 | 20.8 | 19.1 | 15.9 | 18.2 | 18.0 |
| Tmean (°C) | 23.2 | 27.5 | 27.1 | 25.9 | 27.8 | 27.1 | 24.2 | 27.4 | 26.6 | 24.5 | 26.7 | 26.0 |
| RHmean (%) | 66.7 | 62.7 | 63.9 | 62.3 | 58.9 | 62.1 | 55.4 | 52.7 | 55.0 | 60 | 58 | 62 |
| Rainfall (mm) | 96.2 | 8.2 | 1.1 | 15.2 | 1.2 | 0.8 | 25.6 | 13 | 74.8 | 32 | 31 | 24 |
| ETο (mm/d) | 4.5 | 5 | 4.5 | 4.8 | 5 | 5 | 4.5 | 4.9 | 5 | 4 | 5 | 5 |
Table 12.
The different landraces and cultivars that were used in the field experiments during the three growing seasons.
Table 12.
The different landraces and cultivars that were used in the field experiments during the three growing seasons.
| 2017 | 2018 | 2020 |
|---|
| Gigas white spike (GWS) (L) Ϯ | Athos white spike (AWS) (L) | Athos white spike (AWS) (L) |
| Corymb White (CW) (L) | Gigas white spike (GWS) (L) | Gigas white spike (GWS) (L) |
| Pink spike (PS) (L) | Corymb violet (CV) (L) | Corymb violet (CV) (L) |
| Sweet (S) (C) | Pink spike (PS) (L) | Pink spike (PS) (L) |
| | | Kassandra red spike (KRS) (L) |
| | Genovese (G) (C) | Genovese (G) (C) |