Cinnamaldehyde Loaded Poly(lactide-co-glycolide) (PLGA) Microparticles for Antifungal Delivery Application against Resistant Candida albicans and Candida glabrata
Abstract
:1. Introduction
2. Results
2.1. SPME-GC-MS Analyses
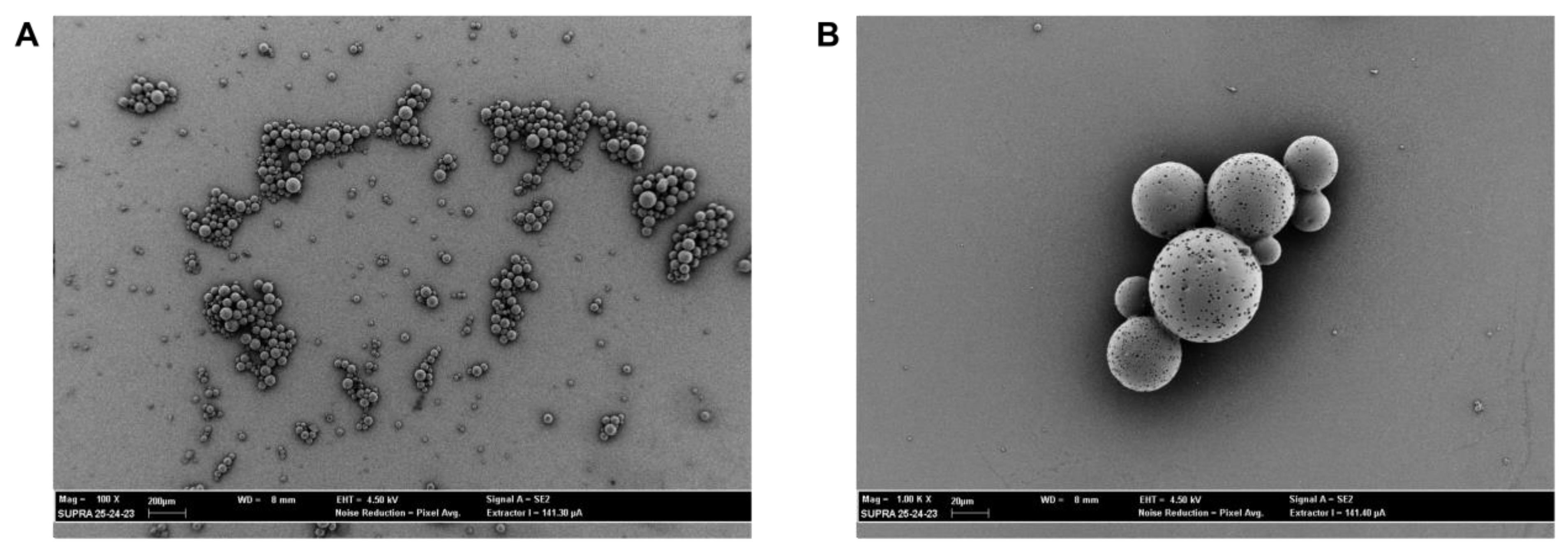
2.2. Preparation and Characterization Analysis of PLGA CIN-MPs
2.3. Cinnamaldehyde Entrapment Efficiency and In Vitro Release Studies
2.4. In Vivo Toxicity in Galleria Mellonella Model
2.5. Antifungal Activity
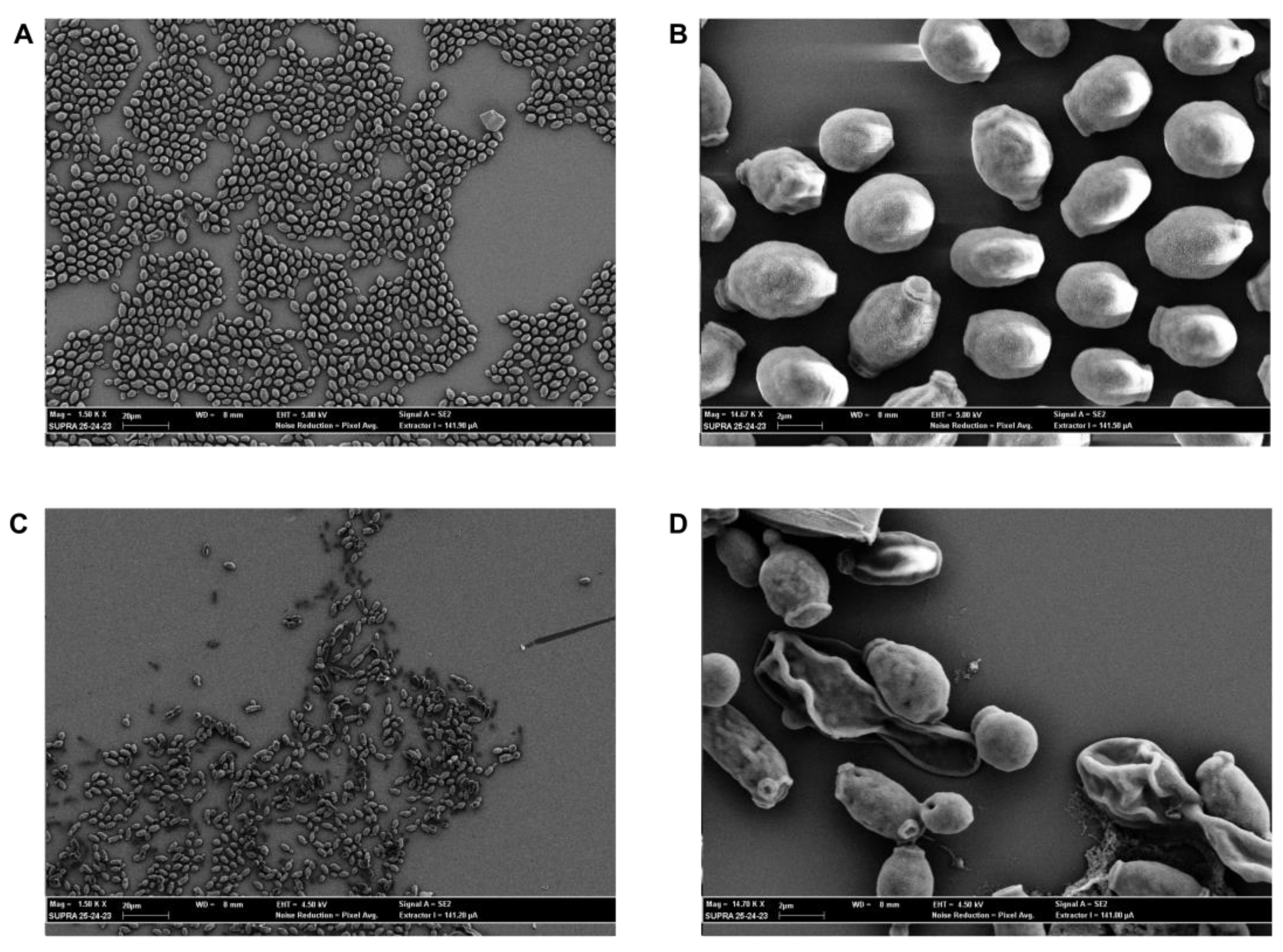
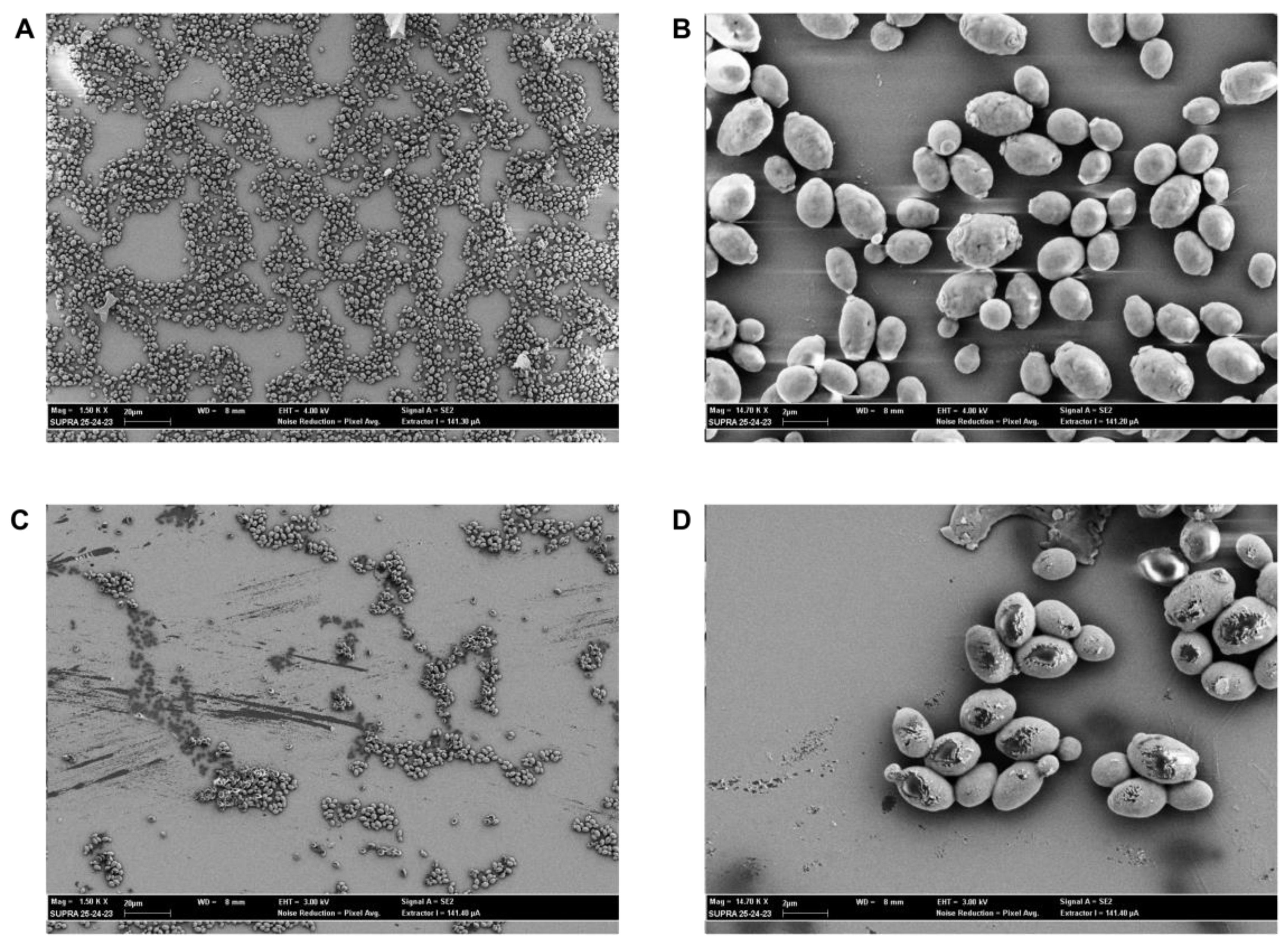
2.6. SEM Evaluation
2.7. Effect of CIN and CIN-MPs on Cell Membrane Integrity
3. Discussion
4. Materials and Methods
4.1. Essential Oil and Active Component
4.2. Solid Phase Microextraction (SPME) Sampling
4.3. GC-MS Analyses of CC-EO and CIN-MPs
4.4. Synthesis of Microparticles PLGA-CIN Microparticles and Production Efficiency
4.5. Microparticles Size and Morphology
4.6. Microparticles Entrapment Efficiency (EE) and Cinnamaldehyde Release Studies
4.7. In Vivo Toxicity Study
4.8. Clinical Strains of C. albicans and C. glabrata
4.9. Antifungal Activity
4.10. SEM Evaluation
4.11. Effect of CIN and CIN-MPs on Cell Membrane Integrity
4.12. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Edmond, M.B.; Wallace, S.E.; McClish, D.K.; Pfaller, M.A.; Jones, R.N.; Wenzel, R.P. Nosocomial Bloodstream Infections in United States Hospitals: A Three-Year Analysis. Clin. Infect. Dis. 1999, 29, 239–244. [Google Scholar] [CrossRef] [Green Version]
- Brandolt, T.M.; Klafke, G.B.; Gonçalves, C.V.; Bitencourt, L.R.; de Martinez, A.M.B.; Mendes, J.F.; Meireles, M.C.A.; Xavier, M.O. Prevalence of Candida Spp. in Cervical-Vaginal Samples and the in Vitro Susceptibility of Isolates. Braz. J. Microbiol. 2017, 48, 145–150. [Google Scholar] [CrossRef] [Green Version]
- Dadar, M.; Tiwari, R.; Karthik, K.; Chakraborty, S.; Shahali, Y.; Dhama, K. Candida Albicans—Biology, Molecular Characterization, Pathogenicity, and Advances in Diagnosis and Control—An Update. Microb. Pathog. 2018, 117, 128–138. [Google Scholar] [CrossRef]
- Kashem, S.W.; Igyártó, B.Z.; Gerami-Nejad, M.; Kumamoto, Y.; Mohammed, J.; Jarrett, E.; Drummond, R.A.; Zurawski, S.M.; Zurawski, G.; Berman, J.; et al. Candida Albicans Morphology and Dendritic Cell Subsets Determine T Helper Cell Differentiation. Immunity 2015, 42, 356–366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gunsalus, K.T.W.; Tornberg-Belanger, S.N.; Matthan, N.R.; Lichtenstein, A.H.; Kumamoto, C.A. Manipulation of Host Diet to Reduce Gastrointestinal Colonization by the Opportunistic Pathogen Candida Albicans. mSphere 2015, 1, e00020-15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Voss, A.; Hollis, R.J.; Pfaller, M.A.; Wenzel, R.P.; Doebbeling, B.N. Investigation of the Sequence of Colonization and Candidemia in Nonneutropenic Patients. J. Clin. Microbiol. 1994, 32, 975–980. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poulain, D. Candida Albicans, Plasticity and Pathogenesis. Crit. Rev. Microbiol. 2015, 41, 208–217. [Google Scholar] [CrossRef] [PubMed]
- Kauffman, C.A.; Vazquez, J.A.; Sobel, J.D.; Gallis, H.A.; McKinsey, D.S.; Karchmer, A.W.; Sugar, A.M.; Sharkey, P.K.; Wise, G.J.; Mangi, R.; et al. Prospective Multicenter Surveillance Study of Funguria in Hospitalized Patients. The National Institute for Allergy and Infectious Diseases (NIAID) Mycoses Study Group. Clin. Infect. Dis. 2000, 30, 14–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruan, S.Y.; Hsueh, P.R. Invasive Candidiasis: An Overview from Taiwan. J. Formos. Med. Assoc. 2009, 108, 443–451. [Google Scholar] [CrossRef] [Green Version]
- Hajjeh, R.A.; Sofair, A.N.; Harrison, L.H.; Lyon, G.M.; Arthington-Skaggs, B.A.; Mirza, S.A.; Phelan, M.; Morgan, J.; Lee-Yang, W.; Ciblak, M.A.; et al. Incidence of Bloodstream Infections Due to Candida Species and in Vitro Susceptibilities of Isolates Collected from 1998 to 2000 in a Population-Based Active Surveillance Program. J. Clin. Microbiol. 2004, 42, 1519–1527. [Google Scholar] [CrossRef] [Green Version]
- Hammer, K.A.; Carson, C.F.; Riley, T.V. Antimicrobial Activity of Essential Oils and Other Plant Extracts. J. Appl. Microbiol. 1999, 86, 985–990. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hikmasari, I. Handbook of Herbs and Spices; Volume 1 KV Peter, CRC Press: Washington, DC, USA, 2006. [Google Scholar]
- Yossa, N.; Patel, J.; Millner, P.; Lo, Y.M. Essential Oils Reduce Escherichia Coli O157:H7 and Salmonella on Spinach Leaves. J. Food Prot. 2012, 75, 488–496. [Google Scholar] [CrossRef] [PubMed]
- Di Pasqua, R.; Betts, G.; Hoskins, N.; Edwards, M.; Ercolini, D.; Mauriello, G. Membrane Toxicity of Antimicrobial Compounds from Essential Oils. J. Agric. Food Chem. 2007, 55, 4863–4870. [Google Scholar] [CrossRef] [PubMed]
- Natan, M.; Banin, E. From Nano to Micro: Using Nanotechnology to Combat Microorganisms and Their Multidrug Resistance. FEMS Microbiol. Rev. 2017, 41, 302–322. [Google Scholar] [CrossRef] [Green Version]
- Xiong, M.H.; Bao, Y.; Yang, X.Z.; Zhu, Y.H.; Wang, J. Delivery of Antibiotics with Polymeric Particles. Adv. Drug. Deliv. Rev. 2014, 78, 63–76. [Google Scholar] [CrossRef] [PubMed]
- Lü, J.M.; Wang, X.; Marin-Muller, C.; Wang, H.; Lin, P.H.; Yao, Q.; Chen, C. Current Advances in Research and Clinical Applications of PLGA-Based Nanotechnology. Expert. Rev. Mol. Diagn. 2009, 9, 325–341. [Google Scholar] [CrossRef] [Green Version]
- Di Vito, M.; Garzoli, S.; Rosato, R.; Mariotti, M.; Gervasoni, J.; Santucci, L.; Ovidi, E.; Cacaci, M.; Lombarini, G.; Torelli, R.; et al. A New Potential Resource in the Fight against Candida Auris: The Cinnamomum Zeylanicum Essential Oil in Synergy with Antifungal Drug. Microbiol. Spectr. 2023, 11. [Google Scholar] [CrossRef]
- Rosato, R.; Napoli, E.; Granata, G.; Di Vito, M.; Garzoli, S.; Geraci, C.; Rizzo, S.; Torelli, R.; Sanguinetti, M.; Bugli, F. Study of the Chemical Profile and Anti-Fungal Activity against Candida Auris of Cinnamomum Cassia Essential Oil and of Its Nano-Formulations Based on Polycaprolactone. Plants 2023, 12, 358. [Google Scholar] [CrossRef]
- Khan, S.N.; Khan, S.; Iqbal, J.; Khan, R.; Khan, A.U. Enhanced Killing and Antibiofilm Activity of Encapsulated Cinnamaldehyde against Candida Albicans. Front. Microbiol. 2017, 8, 1641. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marchese, A.; Orhan, I.E.; Daglia, M.; Barbieri, R.; Di Lorenzo, A.; Nabavi, S.F.; Gortzi, O.; Izadi, M.; Nabavi, S.M. Antibacterial and Antifungal Activities of Thymol: A Brief Review of the Literature. Food Chem. 2016, 210, 402–414. [Google Scholar] [CrossRef]
- Sharifi-Rad, M.; Varoni, E.M.; Iriti, M.; Martorell, M.; Setzer, W.N.; del Mar Contreras, M.; Salehi, B.; Soltani-Nejad, A.; Rajabi, S.; Tajbakhsh, M.; et al. Carvacrol and Human Health: A Comprehensive Review. Phytother. Res. 2018, 32, 1675–1687. [Google Scholar] [CrossRef]
- Ren, S.; Wang, C.; Zhao, X.; Guo, L.; Xu, C.; Wang, Y.; Sun, C.; Cui, H. Preparation and Sustained-Release Performance of PLGA Microcapsule Carrier System. Nanomaterials 2021, 11, 1758. [Google Scholar] [CrossRef] [PubMed]
- Gursu, B.Y.; Dag, İ.; Dikmen, G. Antifungal and Antibiofilm Efficacy of Cinnamaldehyde-Loaded Poly(DL-Lactide-Co-Glycolide) (PLGA) Nanoparticles against Candida Albicans. Int. Microbiol. 2022, 25, 245–258. [Google Scholar] [CrossRef]
- Chotchindakun, K.; Pekkoh, J.; Ruangsuriya, J.; Zheng, K.; Unalan, I.; Boccaccini, A.R. Fabrication and Characterization of Cinnamaldehyde-Loaded Mesoporous Bioactive Glass Nanoparticles/PHBV-Based Microspheres for Preventing Bacterial Infection and Promoting Bone Tissue Regeneration. Polymers 2021, 13, 1794. [Google Scholar] [CrossRef] [PubMed]
- Gomes, C.; Moreira, R.G.; Castell-Perez, E. Poly (DL-Lactide-Co-Glycolide) (PLGA) Nanoparticles with Entrapped Trans-Cinnamaldehyde and Eugenol for Antimicrobial Delivery Applications. J. Food Sci. 2011, 76, N16–N24. [Google Scholar] [CrossRef] [PubMed]
- Lagreca, E.; Onesto, V.; Di Natale, C.; La Manna, S.; Netti, P.A.; Vecchione, R. Recent Advances in the Formulation of PLGA Microparticles for Controlled Drug Delivery. Prog. Biomater. 2020, 9, 153–174. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Shen, Y.; Thakur, K.; Han, J.; Zhang, J.G.; Hu, F.; Wei, Z.J. Antibacterial Activity and Mechanism of Ginger Essential Oil against Escherichia Coli and Staphylococcus Aureus. Molecules 2020, 25, 3955. [Google Scholar] [CrossRef]
- Vitalini, S.; Iriti, M.; Vinciguerra, V.; Garzoli, S. A Comparative Study of the Chemical Composition by SPME-GC/MS and Antiradical Activity of Less Common Citrus Species. Molecules 2021, 26, 5378. [Google Scholar] [CrossRef]
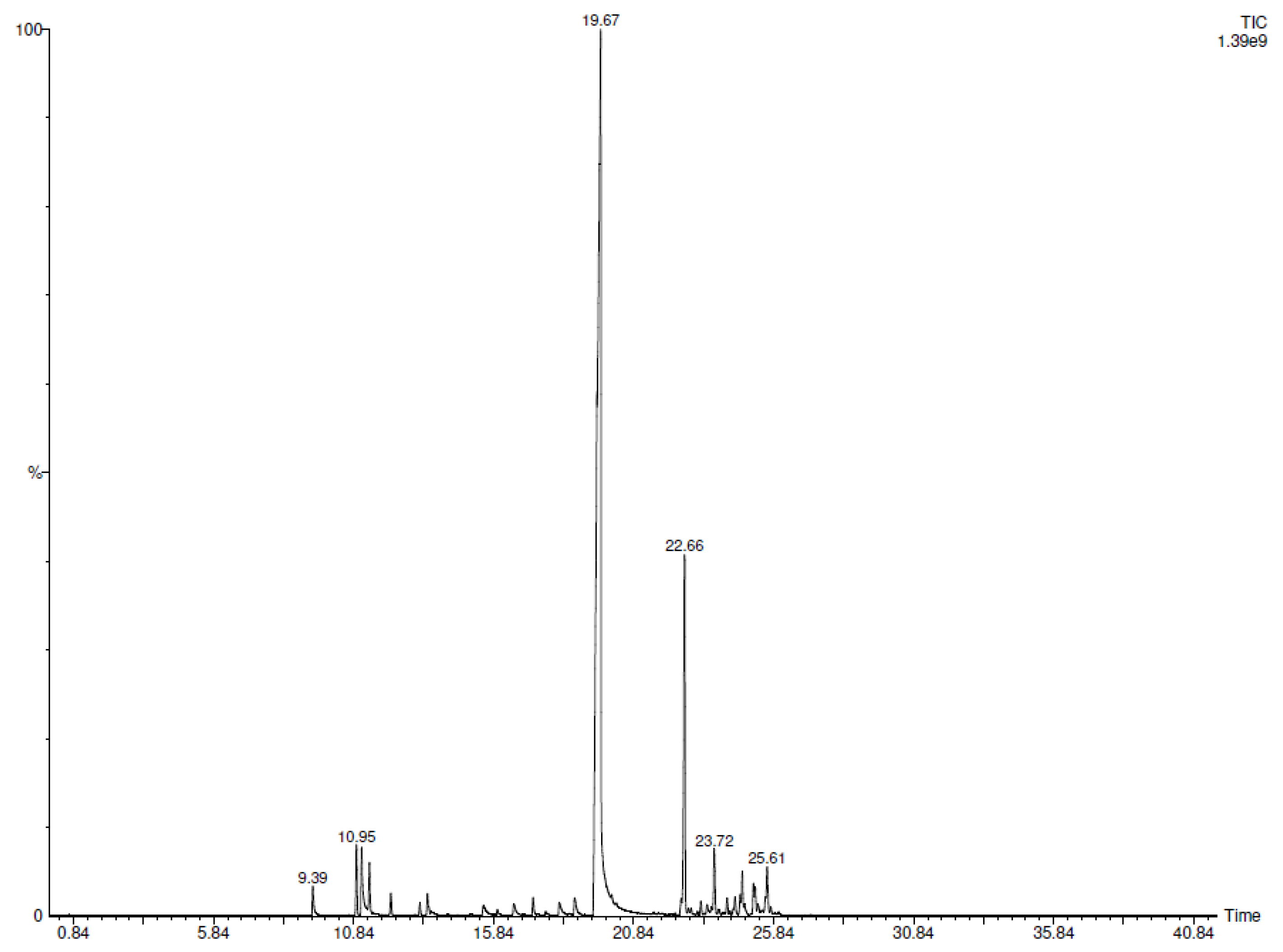

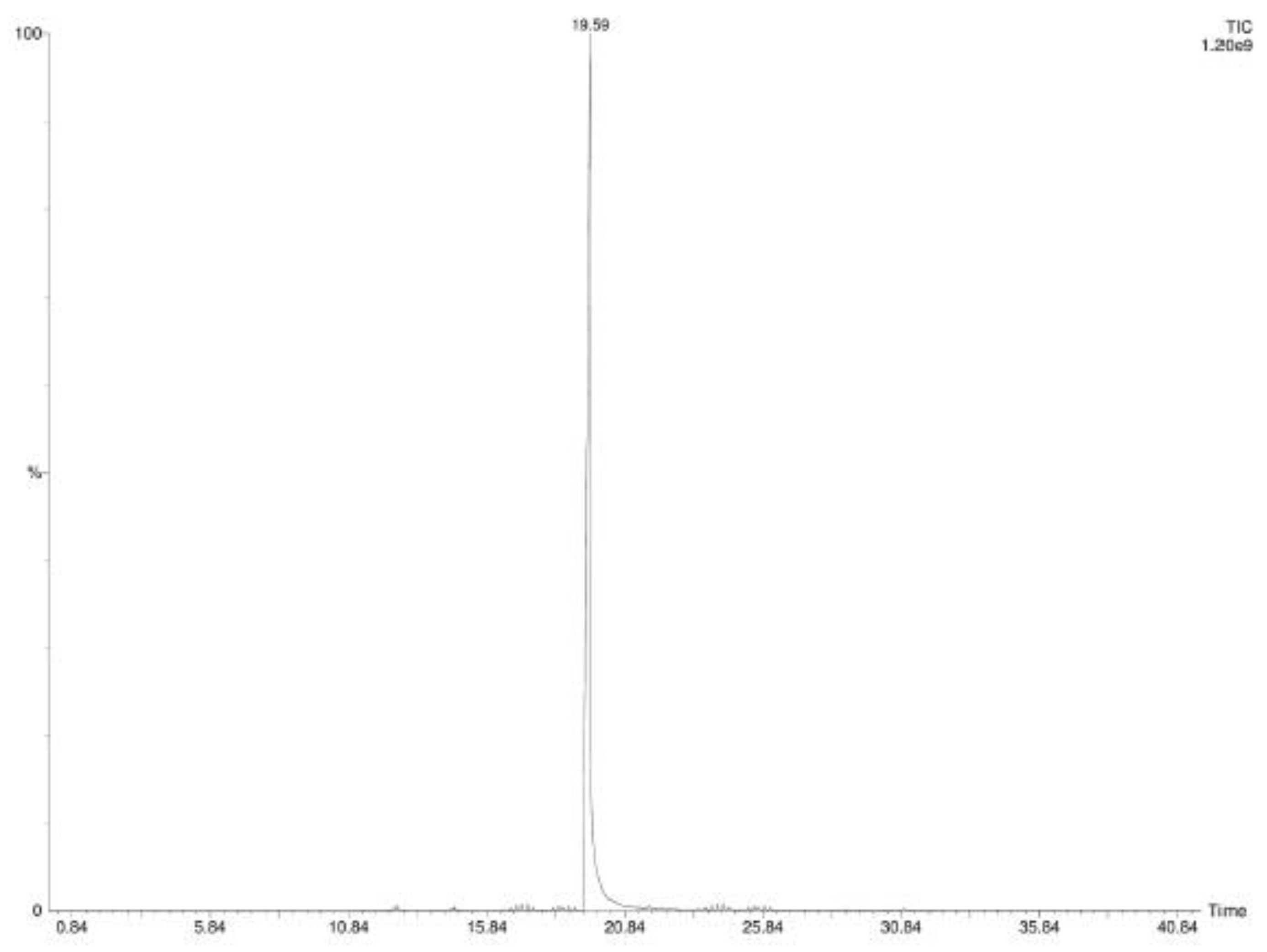

| N° | COMPONENT 1 | LRI 2 | LRI 3 | CC-EO 4 (%) | CIN-MPs 5 (%) |
|---|---|---|---|---|---|
| 1 | styrene | 894 | 898 | 1.0 ± 0.01 | - |
| 2 | α-pinene | 938 | 943 | 1.6 ± 0.02 | - |
| 3 | benzaldehyde | 940 | 945 | 2.7 ± 0.05 | - |
| 4 | camphene | 942 | 946 | 1.4 ± 0.04 | - |
| 5 | β-pinene | 978 | 986 | 0.5 ± 0.02 | - |
| 6 | p-cymene | 1018 | 1021 | 0.3 ± 0.01 | - |
| 7 | limonene | 1024 | 1026 | 0.7 ± 0.03 | - |
| 8 | salicylaldehyde | 1050 | 1057 | 0.3 ± 0.02 | - |
| 9 | phenylethyl alcohol | 1100 | 1102 | 0.7 ± 0.02 | - |
| 10 | 3-methylacetophenone | 1158 | * | 0.1 ± 0.01 | - |
| 11 | benzenepropanol | 1163 | 1162 | 0.6 ± 0.02 | - |
| 12 | borneol | 1165 | 1163 | 0.5 ± 0.02 | - |
| 13 | α-terpineol | 1190 | 1183 | 0.1 ± 0.01 | - |
| 14 | cis-cinnamaldehyde | 1210 | 1215 | 0.8 ± 0.02 | - |
| 15 | O-anisaldehyde | 1238 | 1240 | 0.8 ± 0.02 | - |
| 16 | trans-cinnamaldehyde | 1270 | 1275 | 70.4 ± 1.15 | 100.0 |
| 17 | ylangene | 1374 | 1376 | 0.4 ± 0.01 | - |
| 18 | α-copaene | 1388 | 1392 | 9.8 ± 0.05 | - |
| 19 | β-caryophyllene | 1437 | 1440 | 1.8 ± 0.03 | - |
| 20 | aromadendrene | 1455 | 1460 | 0.5 ± 0.02 | - |
| 21 | α-curcumene | 1480 | 1485 | 0.6 ± 0.02 | - |
| 22 | γ-muurolene | 1488 | 1486 | 1.1 ± 0.04 | - |
| 23 | β-bisabolene | 1498 | 1495 | 0.7 ± 0.03 | - |
| 24 | ledene | 1500 | 1496 | 0.1 ± 0.01 | - |
| 25 | α-muurolene | 1505 | * | 0.5 ± 0.03 | - |
| 26 | δ-cadinene | 1533 | 1530 | 1.7 ± 0.02 | - |
| 27 | spathulenol | 1604 | 1601 | 0.2 ± 0.02 | - |
| 28 | α-bisabolol | 1680 | 1674 | 0.1 ± 0.01 | - |
| SUM | 100.0 | 100.0 |
| Timepoints | Cumulative Percentage (% v/v) ± St. Dev. | |
|---|---|---|
| In Ethanol | In Sensititre™ YeastOne Broth | |
| 1 h | 67.13 ± 2.20 | 17.65 ± 1.75 |
| 4 h | 93.347 ± 2.16 | 21.22 ± 2.20 |
| 24 h | 99.67 ± 1.58 | 26.30 ± 2.13 |
| CIN-MPs Concentrations | Release Percentage (% v/v) ± St. Dev. | |
|---|---|---|
| 0.31 cm2 | 1.90 cm2 | |
| 600 µg/mL | 61.85 ± 1.44 | 85.66 ± 2.02 |
| 300 µg/mL | 74.43 ± 1.57 | 92.85 ± 1.69 |
| 150 µg/mL | 89.00 ± 1.75 | 93.00 ± 1.32 |
| MFC90 μg/mL | ||||
|---|---|---|---|---|
| C. albicans R | C. albicans S | C. glabrata R | C. glabrata S | |
| CIN vehiculated | 200 ± 77 | 400 ± 155 | 400 ± 155 | 450 ± 164 |
| CIN | 350 ± 123 | 500 ± 155 | 500 ± 155 | 500 ± 155 |
| CC-EO | 400 ± 173 | 500 ± 173 | 500 ± 173 | 500 ± 173 |
| Empty MPs | >40,000 | >40,000 | >40,000 | >40,000 |
| Nucleic Acid Absorbance ± St. Dev. | ||||
|---|---|---|---|---|
| C. albicans R | C. albicans S | C. glabrata R | C. glabrata S | |
| CIN-MPs | 0.54 ± 0.07 | 0.56 ± 0.05 | 0.69 ± 0.08 | 0.82 ± 0.03 |
| CIN | 0.75 ± 0.04 | 0.94 ± 0.05 | 0.65 ± 0.03 | 0.68 ± 0.07 |
| Untreated (Control) | 0.20 ± 0.01 | 0.25 ± 0.05 | 0.26 ± 0.02 | 0.34 ± 0.04 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rizzo, S.; Di Vito, M.; Mazzinelli, E.; Favuzzi, I.; Torelli, R.; Cacaci, M.; Arcovito, A.; Sanguinetti, M.; Garzoli, S.; Nocca, G.; et al. Cinnamaldehyde Loaded Poly(lactide-co-glycolide) (PLGA) Microparticles for Antifungal Delivery Application against Resistant Candida albicans and Candida glabrata. Plants 2023, 12, 2437. https://doi.org/10.3390/plants12132437
Rizzo S, Di Vito M, Mazzinelli E, Favuzzi I, Torelli R, Cacaci M, Arcovito A, Sanguinetti M, Garzoli S, Nocca G, et al. Cinnamaldehyde Loaded Poly(lactide-co-glycolide) (PLGA) Microparticles for Antifungal Delivery Application against Resistant Candida albicans and Candida glabrata. Plants. 2023; 12(13):2437. https://doi.org/10.3390/plants12132437
Chicago/Turabian StyleRizzo, Silvia, Maura Di Vito, Elena Mazzinelli, Ilaria Favuzzi, Riccardo Torelli, Margherita Cacaci, Alessandro Arcovito, Maurizio Sanguinetti, Stefania Garzoli, Giuseppina Nocca, and et al. 2023. "Cinnamaldehyde Loaded Poly(lactide-co-glycolide) (PLGA) Microparticles for Antifungal Delivery Application against Resistant Candida albicans and Candida glabrata" Plants 12, no. 13: 2437. https://doi.org/10.3390/plants12132437








