Intra- and Interspecies Differences of Two Cecropia Species from Tabasco, Mexico, Determined through the Metabolic Analysis and 1H-NMR-Based Fingerprinting of Hydroalcoholic Extracts
Abstract
:1. Introduction
2. Results and Discussion
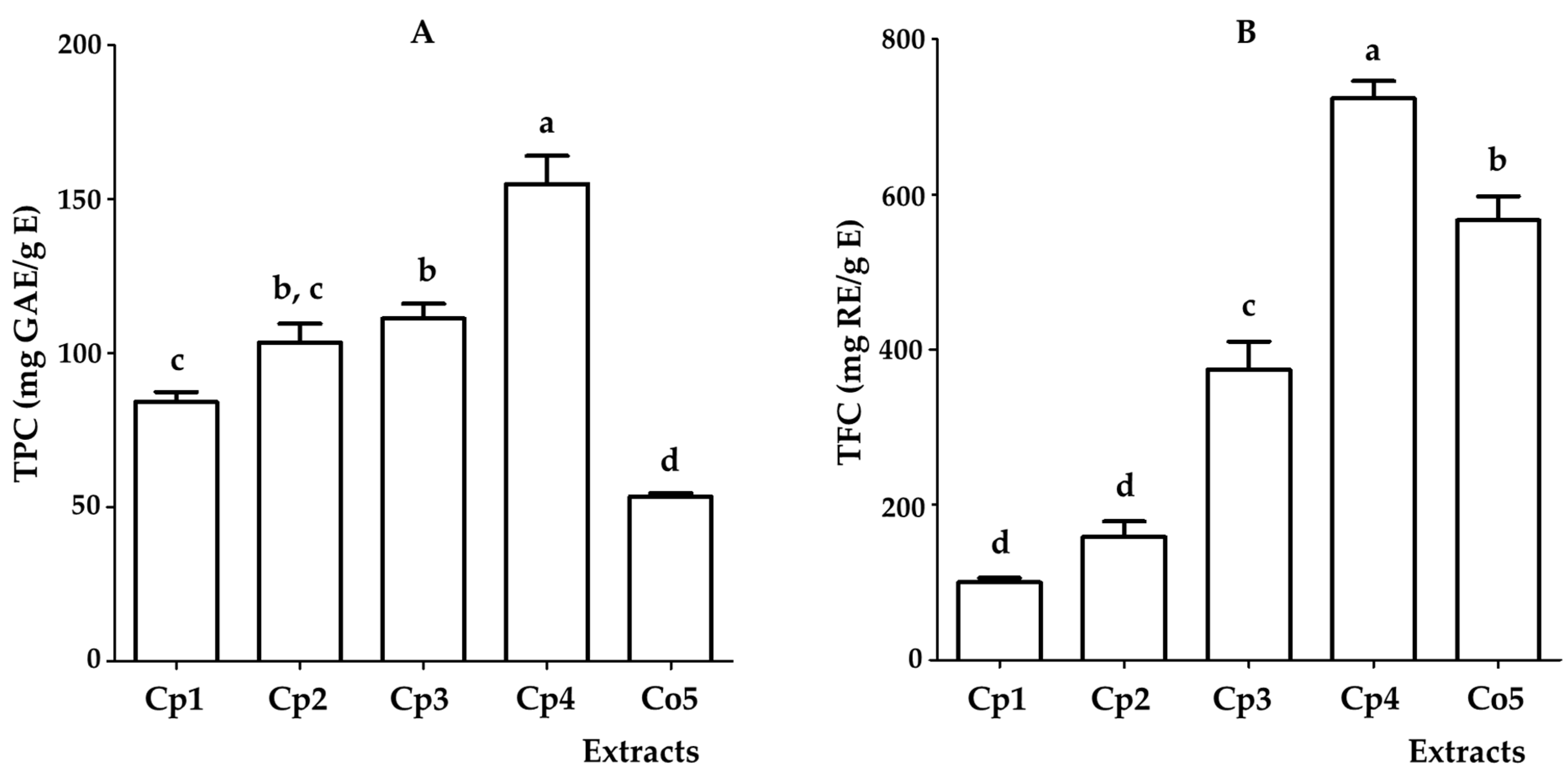
2.1. Total Phenol Content (TPC) and Total Flavonoid Content (TFC) of Hydroalcoholic Extracts from Cecropia Species
2.2. Preliminary HPLC Analysis of Hydroalcoholic Extracts from Cecropia Species
2.3. Multivariate Analysis of 1H-NMR Profiles
3. Materials and Methods
3.1. Plant Material and Extraction of Cecropia Species
3.2. Total Phenol Content of the Hydroalcoholic Extracts of C. peltata and C. obtusifolia
3.3. Total Flavonoid Content of the Hydroalcoholic Extracts of C. peltata and C. obtusifolia
3.4. Preliminary HPLC Analysis and the Quantification of Chlorogenic Acid, Isoorientin, Orientin, and the Vitexin Analog in Hydroalcoholic Extracts of C. peltata and C. obtusifolia
3.5. 1H-NMR Fingerprinting and Multivariate Analysis of the Hydroalcoholic Extracts of C. peltata and C. obtusifolia
3.6. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Razzaq, A.; Sadia, B.; Raza, A.; Khalid Hameed, M.; Saleem, F. Metabolomics: A Way Forward for Crop Improvement. Metabolites 2019, 9, 303. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Piasecka, A.; Kachlicki, P.; Stobiecki, M. Analytical Methods for Detection of Plant Metabolomes Changes in Response to Biotic and Abiotic Stresses. Int. J. Mol. Sci. 2019, 20, 379. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, S.; Micallef, S.A. Environmental Metabolomics of the Tomato Plant Surface Provides Insights on Salmonella enterica Colonization. Appl. Environ. Microbiol. 2016, 82, 3131–3142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Plaza, A.; Rodríguez, L.; Concha-Meyer, A.A.; Cabezas, R.; Zurob, E.; Merlet, G.; Palomo, I.; Fuentes, E. Effects of Extraction Methods on Phenolic Content, Antioxidant and Antiplatelet Activities of Tomato Pomace Extracts. Plants 2023, 12, 1188. [Google Scholar] [CrossRef] [PubMed]
- Zheleva-Dimitrova, D.; Petrova, A.; Zengin, G.; Sinan, K.I.; Balabanova, V.; Joubert, O.; Zidorn, C.; Voynikov, Y.; Simeonova, R.; Gevrenova, R. Metabolite profiling and bioactivity of Cicerbita alpina (L.) Wallr. (Asteraceae, Cichorieae). Plants 2023, 12, 1009. [Google Scholar] [CrossRef]
- Krishnan, P.; Kruger, N.J.; Ratcliffe, R.G. Metabolite fingerprinting and profiling in plants using NMR. J. Exp. Bot. 2005, 56, 255–265. [Google Scholar] [CrossRef] [Green Version]
- Kumar, R.; Bohra, A.; Pandey, A.K.; Pandey, M.K.; Kumar, A. Metabolomics for Plant Improvement: Status and Prospects. Front. Plant Sci. 2017, 8, 1302. [Google Scholar] [CrossRef] [Green Version]
- Patel, M.K.; Pandey, S.; Kumar, M.; Haque, M.I.; Pal, S.; Yadav, N.S. Plants Metabolome Study: Emerging Tools and Techniques. Plants 2021, 10, 2409. [Google Scholar] [CrossRef]
- Li, Y.; He, Q.; Geng, Z.; Du, S.; Deng, Z.; Hasi, E. NMR-based metabolomic profiling of Peganum harmala L. reveals dynamic variations between different growth stages. R. Soc. Open Sci. 2018, 5, 171722. [Google Scholar] [CrossRef] [Green Version]
- Alejandro, M.A.M.; Campillo, L.M.G.; Méndez, R.M. El Uso de las Plantas Medicinales en las Comunidades Maya-Chontales de Nacajuca, Tabasco, México. Polibotánica 2010, 29, 213–2062. [Google Scholar]
- Berg, C.C.; Franco, P. Cecropia. In Organization for Flora Neotropica Monograph 94 Bronx; The New York Botanical Garden Press: New York, NY, USA, 2005. [Google Scholar]
- Herrera-Arellano, A.; Aguilar-Santamaría, L.; García-Hernández, B.; Nicasio-Torres, P.; Tortoriello, J. Clinical trial of Cecropia obtusifolia and Marrubium vulgare leaf extracts on blood glucose and serum lipids in type 2 diabetics. Phytomedicine 2004, 11, 561–566. [Google Scholar] [CrossRef] [PubMed]
- Andrade-Cetto, A.; Cárdenas, R.; Ramírez-Reyes, B. Hypoglycemic efect of Cecropia peltata L. on N5-STZ type 2 diabetic rats. Pharmacologyonline 2007, 3, 203–210. [Google Scholar]
- Vidrio, H.; García-Márquez, F.; Reyes, J.; Soto, R.M. Hypotensive activity of Cecropia obtusifolia. J. Pharm. Sci. 1982, 71, 475–476. [Google Scholar] [CrossRef]
- Salas, I.; Brenes, J.R.; Morales, O.M. Antihypertensive effect of Cecropia obtusifolia (Moraceae) leaf extract on rats. Rev. Biol. Trop. 1987, 35, 127–130. [Google Scholar]
- Pérez-Guerrero, C.; Herrera, M.D.; Ortiz, R.; de Sotomayor, M.A.; Fernández, M.A. A pharmacological study of Cecropia obtusifolia Bertol aqueous extract. J. Ethnopharmacol. 2001, 76, 279–284. [Google Scholar] [CrossRef] [PubMed]
- Rojas, J.J.; Ochoa, V.J.; Ocampo, S.A.; Muñoz, J.F. Screening for antimicrobial activity of ten medicinal plants used in Colombian folkloric medicine: A possible alternative in the treatment of non-nosocomial infections. BMC Complement. Altern. Med. 2006, 6, 2. [Google Scholar] [CrossRef] [Green Version]
- Rivera-Mondragón, A.; Tuenter, E.; Bijttebier, S.; Cos, P.; Apers, S.; Caballero-George, C.; Foubert, K.; Pieters, L. Two new antiplasmodial flavonolignans from the leaves of Cecropia obtusifolia. Phytochem. Lett. 2019, 31, 118–120. [Google Scholar] [CrossRef]
- GBIG. Global Biodiversity Information Facility. Available online: https://www.gbif.org/species/2984473 (accessed on 8 March 2023).
- Nicasio, P.; Aguilar-Santamaría, L.; Aranda, E.; Ortiz, S.; González, M. Hypoglycemic efect and chlorogenic acid content in two Cecropia species. Phyther. Res. 2005, 19, 661–664. [Google Scholar] [CrossRef]
- Iwanycki Ahlstrand, N.; Havskov Reghev, N.; Markussen, B.; Bruun Hansen, H.C.; Eiriksson, F.F.; Thorsteinsdóttir, M.; Rønsted, N.; Barnes, C.J. Untargeted metabolic profiling reveals geography as the strongest predictor of metabolic phenotypes of a cosmopolitan weed. Ecol. Evol. 2018, 8, 6812–6826. [Google Scholar] [CrossRef] [Green Version]
- Cardoso-Taketa, A.T.; Pereda-Miranda, R.; Choi, Y.H.; Verpoorte, R.; Villarreal, M.L. Metabolic profiling of the Mexican anxiolytic and sedative plant Galphimia glauca using nuclear magnetic resonance spectroscopy and multivariate data analysis. Planta Med. 2008, 74, 1295–1301. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.K.; Khan, S.; Wilson, E.G.; Kricun, S.D.P.; Meissner, A.; Goraler, S.; Deelder, A.M.; Choi, Y.H.; Verpoorte, R. Metabolic classification of South American Ilex species by NMR-based metabolomics. Phytochemistry 2010, 71, 773–784. [Google Scholar] [CrossRef]
- Tuñón-Pablos, E. Diagnostico Estatal y Regional de los Sistemas de Ciencia, Tecnología e Innovación: Perspectivas para su Desarrollo Regional; CONACyT/ECOSUR: San Cristobal de las Casas, Chiapas, Mexico, 2012. [Google Scholar]
- Gómez-Álvarez, R. Plantas medicinales en una aldea del estado de Tabasco, México. Rev. Fitotec. Mex. 2012, 35, 43–49. [Google Scholar] [CrossRef]
- Rivera-Mondragón, A.; Ortíz, O.O.; Bijttebier, S.; Vlietinck, A.; Apers, S.; Pieters, L.; Caballero-George, C. Selection of chemical markers for the quality control of medicinal plants of the genus Cecropia. Pharm. Biol. 2017, 55, 1500–1512. [Google Scholar] [CrossRef] [Green Version]
- Rivera-Mondragón, A.; Bijttebier, S.; Tuenter, E.; Custers, D.; Ortíz, O.O.; Pieters, L.; Caballero-George, C.; Foubert, K. Phytochemical characterization and comparative studies of four Cecropia species collected in Panama using multivariate data analysis. Sci. Rep. 2019, 9, 1763. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Costa, G.M.; Ortmann, C.F.; Schenkel, E.P.; Reginatto, F.H. An HPLC-DAD method to quantification of main phenolic compounds from leaves of Cecropia species. J. Braz. Chem. Soc. 2011, 22, 1096–1102. [Google Scholar] [CrossRef]
- Jang, D.; Jung, Y.S.; Kim, M.S.; Oh, S.E.; Nam, T.G.; Kim, D.O. Developing and Validating a Method for Separating Flavonoid Isomers in Common Buckwheat Sprouts Using HPLC-PDA. Foods 2019, 8, 549. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- da Silva Mathias, M.; Rodrigues de Oliveira, R. Differentiation of the phenolic chemical profiles of Cecropia pachystachya and Cecropia hololeuca. Phytochem. Anal. 2019, 30, 73–82. [Google Scholar] [CrossRef] [Green Version]
- Ortiz, O.O.; Rivera-Mondragón, A.; Pieters, L.; Foubert, K.; Caballero-George, C. Cecropia telenitida Cuatrec. (Urticaceae: Cecropieae): Phytochemical diversity, chemophenetic implications and new records from Central America. Biochem. Syst. Ecol. 2019, 86, 103935. [Google Scholar] [CrossRef]
- Rivera-Mondragón, A.; Broeckx, G.; Bijttebier, S.; Naessens, T.; Fransen, E.; Kiekens, F.; Foubert, K. Ultrasound-assisted extraction optimization and validation of an HPLC-DAD method for the quantification of polyphenols in leaf extracts of Cecropia species. Sci. Rep. 2019, 9, 2028. [Google Scholar] [CrossRef] [Green Version]
- Nicasio-Torres, M.P.; Meckes-Fischer, M.; Aguilar-Santamaría, L.; Garduño-Ramírez, M.L.; Chávez-Ávila, V.M.; Cruz-Sosa, F. Production of chlorogenic acid and isoorientin hypoglycemic compounds in Cecropia obtusifolia calli and in cell suspension cultures with nitrate starvation. Acta Physiol. Plant 2012, 34, 307–316. [Google Scholar] [CrossRef]
- Andrade-Cetto, A.; Vázquez, R.C. Gluconeogenesis inhibition and phytochemical composition of two Cecropia species. J. Ethnopharmacol. 2010, 130, 93–97. [Google Scholar] [CrossRef] [PubMed]
- Costa, G.M.; Schenkel, E.P.; Reginatto, F.H. Chemical and pharmacological aspects of the genus Cecropia. Nat. Prod. Commun. 2011, 6, 913–920. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muñoz-Bernal, Ó.A.; Torres-Aguirre, G.A.; Núñez-Gastélum, J.A.; de la Rosa, L.A.; Rodrigo-García, J.; Ayala-Zavala, J.F.; Álvarez-Parrilla, E. Nuevo Acercamiento a La Interacción Del Reactivo de Folin-Ciocalteu Con Azúcares Durante La Cuantificación de Polifenoles Totales. TIP 2017, 20, 23–28. [Google Scholar] [CrossRef] [Green Version]
- Zhu, H.; Wang, Y.; Liu, Y.; Xia, Y.; Tang, T. Analysis of Flavonoids in Portulaca oleracea L. by UV–Vis Spectrophotometry with Comparative Study on Different Extraction Technologies. Food Anal. Methods 2010, 3, 90–97. [Google Scholar] [CrossRef]
- Skoog, S.; West, D.; Holler, D.; Crouch, F. Química Analítica, 7th ed.; McGraw-Hill: Ciudad de Mexico, Mexico, 2001. [Google Scholar]
- Bubb, W.A. NMR spectroscopy in the study of carbohydrates: Characterizing the structural complexity. Concepts Magn. Reson. Part A Educ. J. 2003, 19, 1–19. [Google Scholar] [CrossRef]
- Duus, J.Ø.; Gotfredsen, C.H.; Bock, K. Carbohydrate structural determination by NMR spectroscopy: Modern methods and limitations. Chem. Rev. 2000, 100, 4589–4614. [Google Scholar] [CrossRef]
- Difonzo, G.; Crescenzi, M.A.; Piacente, S.; Altamura, G.; Caponio, F.; Montoro, P. Metabolomics Approach to Characterize Green Olive Leaf Extracts Classified Based on Variety and Season. Plants 2022, 11, 3321. [Google Scholar] [CrossRef]
- Gallegos-García, A.J.; Lobato-García, C.E.; González-Cortazar, M.; Herrera-Ruiz, M.; Zamilpa, A.; Álvarez-Fitz, P.; Pérez-García, M.D.; López-Rodríguez, R.; Ble-González, E.A.; Medrano-Sánchez, E.J.; et al. Preliminary Phytochemical Profile and Bioactivity of Inga jinicuil Schltdl & Cham. ex G. Don. Plants 2022, 11, 794. [Google Scholar]
- Qiu, X.L.; Zhang, Q.F. Identification and quantification of main flavonoids in the leaves of Bambusa multiplex cv. Fernleaf. Nat. Prod. Res. 2020, 34, 2076–2079. [Google Scholar] [CrossRef]
- Hernández-Bolio, G.I.; Fagundo-Mollineda, A.; Caamal-Fuentes, E.E.; Robledo, D.; Freile-Pelegrin, Y.; Hernández-Núñez, E. NMR metabolic profiling of sargassum species under different stabilization/extraction processes. J. Phycol. 2021, 57, 655–663. [Google Scholar] [CrossRef]




| Cp1 | Cp2 | Cp3 | Cp4 | Co5 | |
|---|---|---|---|---|---|
| TPC (mg GAE/g E) | 84.1 ± 3.3 c | 103.4 ± 6.3 b,c | 111.3 ± 4.8 b | 155 ± 9.1 a | 53.5 ± 1.1 d |
| TFC (mg RE/g E) | 100.1 ± 5.5 d | 158.8 ± 19.6 d | 374.5 ± 36.1 c | 724 ± 22.2 a | 567.1 ± 30.5 b |
| TCMC (mg/g) | 100.8 ± 4.2 b | 64.9 ± 1.0 c | 103.9 ± 4.7 b | 118.0 ± 0.7 a | 77.4 ± 1.1 c |
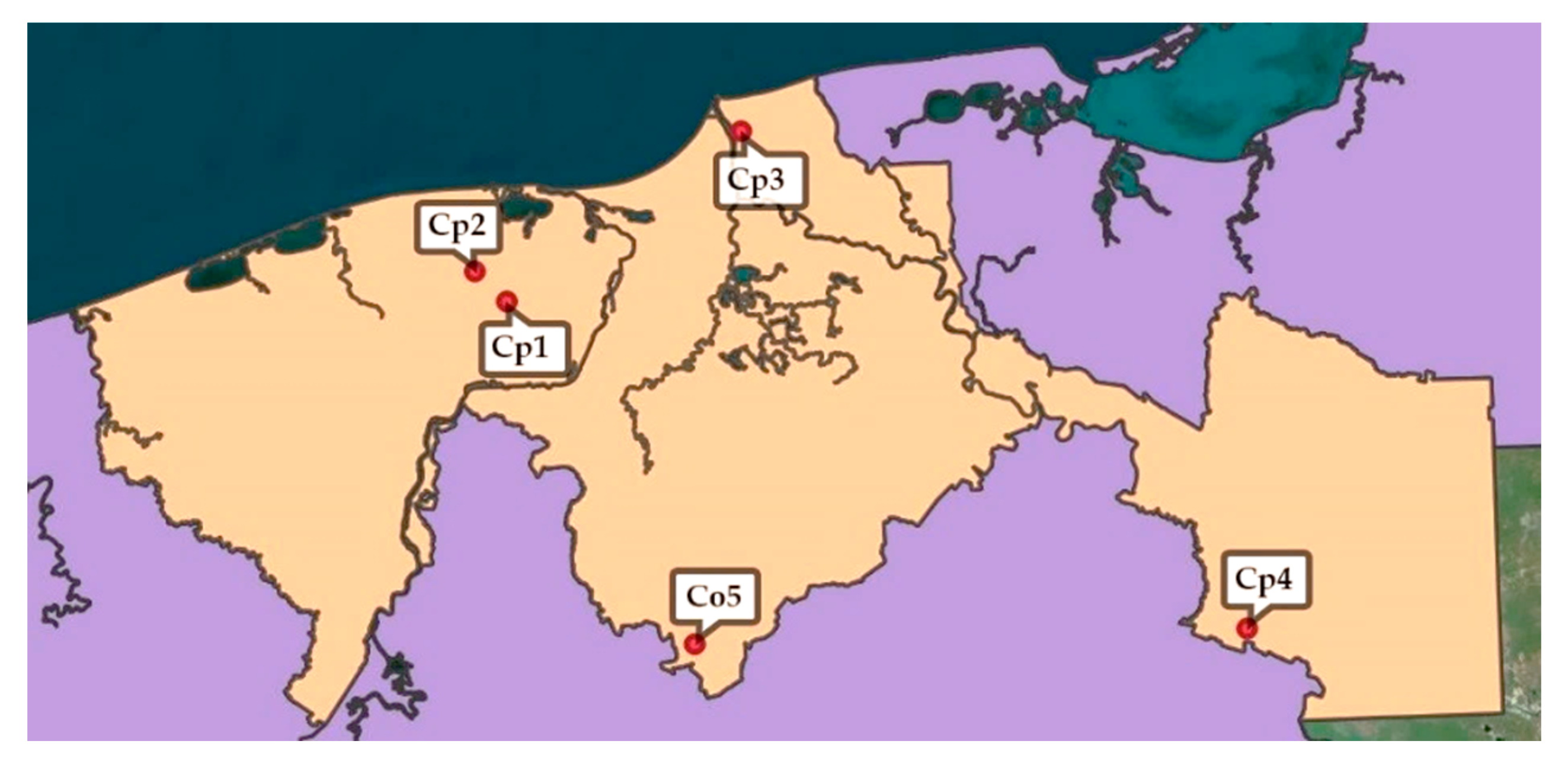
| Sub-Region | Municipality | Geographical Coordinates | Species | Voucher Number |
|---|---|---|---|---|
| “Centro” | Jalpa de Méndez | N 18°11′50.046″/W 93°10′15.2544″ | C. peltata | 36575 |
| “Chontalpa” | Comalcalco | N 18°15′47.0916″/W 93°14′22.884″ | C. peltata | 36575 |
| “Pantanos” | Centla | N 18°32′22.8084″/W 92°38′11.7852″ | C. peltata | 36666 |
| “Ríos” | Tenosique | N 18°11′50.046″/W 93°10′15.2544″ | C. peltata | 36575 |
| “Sierra” | Tacotalpa | N 17°27′28.2024″/W 92°46′46.2108 | C. obtusifolia | 36667 |
| Sub-Region | Label * | Average Yield % |
|---|---|---|
| “Centro” | Cp1a–c | 9.3 ± 2.5 |
| “Chontalpa” | Cp2a–c | 10.5 ± 3.2 |
| “Pantanos” | Cp3a–c | 13.2 ± 2.6 |
| “Ríos” | Cp4a–c | 8.6 ± 1.8 |
| “Sierra” | Co5a–c | 10.5 ± 2.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Medrano-Sánchez, E.J.; Hernández-Bolio, G.I.; Lobato-García, C.E.; González-Cortazar, M.; Antunez-Mojica, M.; Gallegos-García, A.J.; Barredo-Hernández, C.O.; López-Rodríguez, R.; Aguilar-Sánchez, N.C.; Gómez-Rivera, A. Intra- and Interspecies Differences of Two Cecropia Species from Tabasco, Mexico, Determined through the Metabolic Analysis and 1H-NMR-Based Fingerprinting of Hydroalcoholic Extracts. Plants 2023, 12, 2440. https://doi.org/10.3390/plants12132440
Medrano-Sánchez EJ, Hernández-Bolio GI, Lobato-García CE, González-Cortazar M, Antunez-Mojica M, Gallegos-García AJ, Barredo-Hernández CO, López-Rodríguez R, Aguilar-Sánchez NC, Gómez-Rivera A. Intra- and Interspecies Differences of Two Cecropia Species from Tabasco, Mexico, Determined through the Metabolic Analysis and 1H-NMR-Based Fingerprinting of Hydroalcoholic Extracts. Plants. 2023; 12(13):2440. https://doi.org/10.3390/plants12132440
Chicago/Turabian StyleMedrano-Sánchez, Eric Jaziel, Gloria Ivonne Hernández-Bolio, Carlos Ernesto Lobato-García, Manasés González-Cortazar, Mayra Antunez-Mojica, Ammy Joana Gallegos-García, Cristian Octavio Barredo-Hernández, Ricardo López-Rodríguez, Nelly Cristina Aguilar-Sánchez, and Abraham Gómez-Rivera. 2023. "Intra- and Interspecies Differences of Two Cecropia Species from Tabasco, Mexico, Determined through the Metabolic Analysis and 1H-NMR-Based Fingerprinting of Hydroalcoholic Extracts" Plants 12, no. 13: 2440. https://doi.org/10.3390/plants12132440
APA StyleMedrano-Sánchez, E. J., Hernández-Bolio, G. I., Lobato-García, C. E., González-Cortazar, M., Antunez-Mojica, M., Gallegos-García, A. J., Barredo-Hernández, C. O., López-Rodríguez, R., Aguilar-Sánchez, N. C., & Gómez-Rivera, A. (2023). Intra- and Interspecies Differences of Two Cecropia Species from Tabasco, Mexico, Determined through the Metabolic Analysis and 1H-NMR-Based Fingerprinting of Hydroalcoholic Extracts. Plants, 12(13), 2440. https://doi.org/10.3390/plants12132440










