Optimization of In Vitro Germination, Viability Tests and Storage of Paeonia ostii Pollen
Abstract
:1. Introduction
2. Results
2.1. Optimization of In Vitro Germination Medium for Pollen
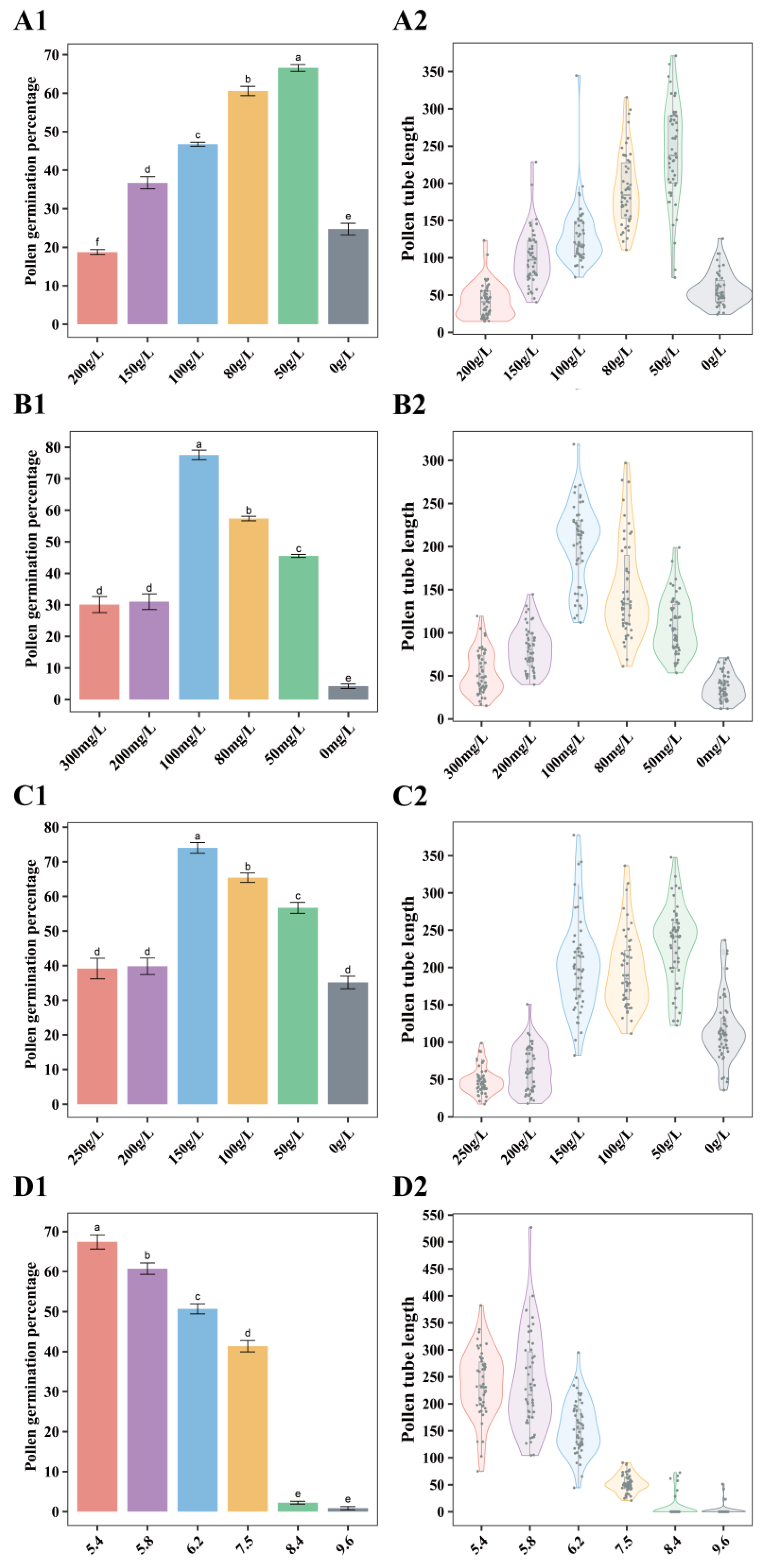
2.1.1. Effects of Individual Components of Medium on Pollen Germination
2.1.2. Optimization of In Vitro Germination Medium for Pollen
2.2. Pollen Germination and Pollen Tube Growth In Vitro at Different Temperatures
2.3. Comparison of Pollen Vitality Staining Methods
2.4. Thawing and Rehydration of Pollen Germination after Cryopreservation
2.5. Pollen Viability after Preservation at Different Temperature
3. Discussion
4. Materials and Methods
4.1. Plant Material and Pollen Grain Collection
4.2. Pollen Germination Medium
4.3. Pollen Germination Temperature
4.4. Pollen Staining
4.5. Thawing and Rehydration of Pollen after Cryopreservation and In Vitro Germination
4.6. Pollen Storage Conditions
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhang, Q.; Yu, R.; Sun, D.; Rahman, M.M.; Xie, L.; Hu, J.; He, L.; Kilaru, A.; Niu, L.; Zhang, Y. Comparative Transcriptome Analysis Reveals an Efficient Mechanism for α-Linolenic Acid Synthesis in Tree Peony Seeds. Int. J. Mol. Sci. 2019, 20, 65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Q.-Y.; Yu, R.; Xie, L.-H.; Rahman, M.M.; Kilaru, A.; Niu, L.-X.; Zhang, Y.-L. Fatty Acid and Associated Gene Expression Analyses of Three Tree Peony Species Reveal Key Genes for α-Linolenic Acid Synthesis in Seeds. Front. Plant Sci. 2018, 9, 106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, L.; Hu, J.; Zhang, Q.; Sun, Q.; Zhang, Y.; Niu, L. Influence of Pollen Sources on the Expression of FA and TAG Biosynthetic Pathway Genes in Seeds of Paeonia Rockii during the Rapid Oil Accumulation. Sci. Hortic. 2019, 243, 477–483. [Google Scholar] [CrossRef]
- Zhang, K.; He, C.; Wang, S.; Hou, X. Influence of Pollination Methods on Fruit Development, Fruit Yield and Oil Quality in Oil Tree Peony. Sci. Hortic. 2022, 295, 110877. [Google Scholar] [CrossRef]
- Kumar, A.; Chowdhury, R.K.; Dahiya, O.S. Pollen Viability and Stigma Receptivity in Relation to Meteorological Parameters in Pearl Millet. Seed Sci. Technol. 1995, 23, 147–156. [Google Scholar]
- Mazzeo, A.; Palasciano, M.; Gallotta, A.; Camposeo, S.; Pacifico, A.; Ferrara, G. Amount and Quality of Pollen Grains in Four Olive (Olea europaea L.) Cultivars as Affected by ‘on’ and ‘off’ Years. Sci. Hortic. 2014, 170, 89–93. [Google Scholar] [CrossRef]
- Dickinson, D.B. Rapid Starch Synthesis Associated with Increased Respiration in Germinating Lily Pollen 1. Plant Physiol. 1968, 43, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, H.; Yin, H.; Chen, J.; Liu, X.; Gao, Y.; Wu, J.; Zhang, S. Gene-Expression Profile of Developing Pollen Tube of Pyrus Bretschneideri. Gene Expr. Patterns 2016, 20, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Kumari, M.; Prasad, A.; ur Rahman, L.; Mathur, A.K.; Mathur, A. In Vitro Germination, Storage and Microscopic Studies of Pollen Grains of Four Ocimum Species. Ind. Crops Prod. 2022, 177, 114445. [Google Scholar] [CrossRef]
- Zhang, D.; Wengier, D.; Shuai, B.; Gui, C.-P.; Muschietti, J.; McCormick, S.; Tang, W.-H. The Pollen Receptor Kinase LePRK2 Mediates Growth-Promoting Signals and Positively Regulates Pollen Germination and Tube Growth. Plant Physiol. 2008, 148, 1368–1379. [Google Scholar] [CrossRef] [Green Version]
- Lora, J.; de Oteyza, M.A.P.; Fuentetaja, P.; Hormaza, J.I. Low Temperature Storage and in Vitro Germination of Cherimoya (Annona Cherimola Mill.) Pollen. Sci. Hortic. 2006, 108, 91–94. [Google Scholar] [CrossRef]
- Karimi, H.R.; Zeraatkar, H. Effects of Artificial Pollination Using Pollen Suspension Spray on Nut and Kernel Quality of Pistachio Cultivar Owhadi. Int. J. Fruit Sci. 2016, 16, 171–181. [Google Scholar] [CrossRef]
- Ćalić, D.; Milojević, J.; Belić, M.; Miletić, R.; Zdravković-Korać, S. Impact of Storage Temperature on Pollen Viability and Germinability of Four Serbian Autochthon Apple Cultivars. Front. Plant Sci. 2021, 12, 709231. [Google Scholar] [CrossRef]
- Buitink, J.; Claessens, M.M.A.E.; Hemminga, M.A.; Hoekstra, F.A. Influence of Water Content and Temperature on Molecular Mobility and Intracellular Glasses in Seeds and Pollen1. Plant Physiol. 1998, 118, 531–541. [Google Scholar] [CrossRef] [Green Version]
- Machado, C.D.A.; Moura, C.R.F.; de Lemos, E.E.P.; Ramos, S.R.R.; Ribeiro, F.E.; Lédo, A.D.S. Pollen Grain Viability of Coconut Accessions at Low Temperatures. Acta Sci. Agron. 2014, 36, 227–232. [Google Scholar] [CrossRef] [Green Version]
- Zambon, C.R.; Silva, L.F.D.O.D.; Pio, R.; de Figueiredo, M.A.; Silva, K.N. Establishment of Growth Medium and Quantification of Germination of Pollen Grains of Quince Tree Cultivars. Rev. Bras. Frutic. 2014, 36, 400–407. [Google Scholar] [CrossRef] [Green Version]
- Boavida, L.C.; McCormick, S. Technical Advance: Temperature as a Determinant Factor for Increased and Reproducible in Vitro Pollen Germination in Arabidopsis Thaliana. Plant J. 2007, 52, 570–582. [Google Scholar] [CrossRef]
- Hamilton, E.S.; Haswell, E.S. The Tension-Sensitive Ion Transport Activity of MSL8 Is Critical for Its Function in Pollen Hydration and Germination. Plant Cell Physiol. 2017, 58, 1222–1237. [Google Scholar] [CrossRef] [Green Version]
- Jayaprakash, P.; Sarla, N. Development of an Improved Medium for Germination of Cajanus cajan (L.) Millsp. Pollen in Vitro. J. Exp. Bot. 2001, 52, 851–855. [Google Scholar] [CrossRef] [PubMed]
- Bou Daher, F.; Chebli, Y.; Geitmann, A. Optimization of Conditions for Germination of Cold-Stored Arabidopsis Thaliana Pollen. Plant Cell Rep. 2009, 28, 347–357. [Google Scholar] [CrossRef]
- Podolyan, A.; Maksimov, N.; Breygina, M. Redox-Regulation of Ion Homeostasis in Growing Lily Pollen Tubes. J. Plant Physiol. 2019, 243, 153050. [Google Scholar] [CrossRef]
- Mesnoua, M.; Roumani, M.; Salem, A. The Effect of Pollen Storage Temperatures on Pollen Viability, Fruit Set and Fruit Quality of Six Date Palm Cultivars. Sci. Hortic. 2018, 236, 279–283. [Google Scholar] [CrossRef]
- Takahashi, T.; Mori, T.; Ueda, K.; Yamada, L.; Nagahara, S.; Higashiyama, T.; Sawada, H.; Igawa, T. The Male Gamete Membrane Protein DMP9/DAU2 Is Required for Double Fertilization in Flowering Plants. Development 2018, 145, dev170076. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liang, M.; Cao, Z.; Zhu, A.; Liu, Y.; Tao, M.; Yang, H.; Xu, Q.; Wang, S.; Liu, J.; Li, Y.; et al. Evolution of Self-Compatibility by a Mutant Sm-RNase in Citrus. Nat. Plants 2020, 6, 131–142. [Google Scholar] [CrossRef] [PubMed]
- Fayos, O.; Echávarri, B.; Vallés, M.P.; Mallor, C.; Garcés-Claver, A.; Castillo, A.M. A Simple and Efficient Method for Onion Pollen Preservation: Germination, Dehydration, Storage Conditions, and Seed Production. Sci. Hortic. 2022, 305, 111358. [Google Scholar] [CrossRef]
- Daniel, I.O. Exploring Storage Protocols for Yam (Dioscorea spp.) Pollen Genebanking. Afr. J. Biotechnol. 2011, 10, 8306–8311. [Google Scholar]
- Sousa, A.S.; Santos, M.G.M.; Pelacani, C.R.; Santos, F.d.A.R. Testing Culture Media for Pollen Germination of Elaeis Guineensis Jacq. (Oil Palm, Arecaceae). Bot. J. Linn. Soc. 2016, 182, 536–542. [Google Scholar] [CrossRef] [Green Version]
- dos Santos Sousa, A.; Rego, E.J.L.; Santos, F.d.A.R.d. Viability and Action of CPL Lectin on in Vitro Germinability of Pollen Grains of Malpighia Emarginata DC.—(Malpighiaceae). Am. J. Plant Sci. 2013, 4, 53–58. [Google Scholar] [CrossRef] [Green Version]
- Fang, K.; Xie, P.; Zhang, Q.; Xing, Y.; Cao, Q.; Qin, L. Aluminum Toxicity-Induced Pollen Tube Growth Inhibition in Apple (Malus Domestica) Is Mediated by Interrupting Calcium Dynamics and Modification of Cell Wall Components. Environ. Exp. Bot. 2020, 171, 103928. [Google Scholar] [CrossRef]
- Kuroki, K.; Takemura, Y.; Mingfeng, J.; Marumori, H.; Teratani, N.; Matsumoto, K.; Matsumoto, T.; Tamura, F. Pear Pollen Selection Using Higher Germination Properties at Low Temperatures and the Effect on the Fruit Set and Quality of Japanese Pear Cultivars. Sci. Hortic. 2017, 216, 200–204. [Google Scholar] [CrossRef]
- Wood, B.W. Flavonoids, Alkali Earth, and Rare Earth Elements Affect Pecan Pollen Germination. HortScience 2017, 52, 85–88. [Google Scholar] [CrossRef]
- Okusaka, K.; Hiratsuka, S. Fructose Inhibits Pear Pollen Germination on Agar Medium without Loss of Viability. Sci. Hortic. 2009, 122, 51–55. [Google Scholar] [CrossRef]
- Fang, K.F.; Du, B.S.; Zhang, Q.; Xing, Y.; Cao, Q.Q.; Qin, L. Boron Deficiency Alters Cytosolic Ca2+ Concentration and Affects the Cell Wall Components of Pollen Tubes in Malus Domestica. Plant Biol. 2019, 21, 343–351. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Lu, L.; Wu, X.; Li, Y.; Lin, J. Boron Influences Pollen Germination and Pollen Tube Growth in Picea Meyeri. Tree Physiol. 2003, 23, 345–351. [Google Scholar] [CrossRef] [Green Version]
- Shivanna, K.R.; Sawhney, V.K. Polyethylene Glycol Improves the in Vitro Growth of Brassica Pollen Tubes without Loss in Germination. J. Exp. Bot. 1995, 46, 1771–1774. [Google Scholar] [CrossRef]
- Sakhanokho, H.F.; Rajasekaran, K. Pollen Biology of Ornamental Ginger (Hedychium spp. J. Koenig). Sci. Hortic. 2010, 125, 129–135. [Google Scholar] [CrossRef]
- Cameron, C.; Geitmann, A. Cell Mechanics of Pollen Tube Growth. Curr. Opin. Genet. Dev. 2018, 51, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Alexander, L.W. Optimizing Pollen Germination and Pollen Viability Estimates for Hydrangea Macrophylla, Dichroa Febrifuga, and Their Hybrids. Sci. Hortic. 2019, 246, 244–250. [Google Scholar] [CrossRef]
- Hebbar, K.B.; Rose, H.M.; Nair, A.R.; Kannan, S.; Niral, V.; Arivalagan, M.; Gupta, A.; Samsudeen, K.; Chandran, K.P.; Chowdappa, P.; et al. Differences in in Vitro Pollen Germination and Pollen Tube Growth of Coconut (Cocos nucifera L.) Cultivars in Response to High Temperature Stress. Environ. Exp. Bot. 2018, 153, 35–44. [Google Scholar] [CrossRef]
- Çetinbaş-Genç, A.; Cai, G.; Vardar, F.; Ünal, M. Differential Effects of Low and High Temperature Stress on Pollen Germination and Tube Length of Hazelnut (Corylus avellana L.) Genotypes. Sci. Hortic. 2019, 255, 61–69. [Google Scholar] [CrossRef]
- Impe, D.; Reitz, J.; Köpnick, C.; Rolletschek, H.; Börner, A.; Senula, A.; Nagel, M. Assessment of Pollen Viability for Wheat. Front. Plant Sci. 2020, 10, 1588. [Google Scholar] [CrossRef] [PubMed]
- Kosel, J.; Vižintin, L.; Majer, A.; Bohanec, B. Staining for Viability Testing, Germination and Maturation of Sambucus nigra L. Pollen in Vitro. Biotech. Histochem. 2018, 93, 258–266. [Google Scholar] [CrossRef] [PubMed]
- dos Santos, K.S.; Passos, A.R.; Serejo, J.A.d.S.; Lino, L.S.M.; Figueiredo, M.C.C.; Santos, R.M.F. Microsporogenesis and Pollen Viability in Physalis Ixocarpa. Cytologia 2017, 82, 363–367. [Google Scholar] [CrossRef] [Green Version]
- Grela, E.; Kozłowska, J.; Grabowiecka, A. Current Methodology of MTT Assay in Bacteria—A Review. Acta Histochem. 2018, 120, 303–311. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.-Z.; Jin, Q.-Y.; Ye, H.-L.; Zhu, T.-J. A Novel in Vitro Germination Method Revealed the Influence of Environmental Variance on the Pecan Pollen Viability. Sci. Hortic. 2015, 181, 43–51. [Google Scholar] [CrossRef]
- Polesi, L.G.; Goeten, D.; Fraga, H.P.d.F.; Steiner, N.; Guerra, M.P. Enzymatic Antioxidant System Activation Assures the Viability of Guadua Chacoensis (Bambusoideae, Poaceae) Embryogenic Cultures during Cryopreservation. Plants 2023, 12, 673. [Google Scholar] [CrossRef]
- Ferrara, G.; Camposeo, S.; Palasciano, M.; Godini, A. Production of Total and Stainable Pollen Grains in Olea europaea L. Grana 2007, 46, 85–90. [Google Scholar] [CrossRef]
- Koga, Y.; Akihama, T.; Fujimaki, H.; Yokoo, M. Studies on the Longevity of Pollen Grains of Rice, Oriza sativa L. Cytologia 1971, 36, 104–110. [Google Scholar] [CrossRef] [Green Version]
- Du, G.; Xu, J.; Gao, C.; Lu, J.; Li, Q.; Du, J.; Lv, M.; Sun, X. Effect of Low Storage Temperature on Pollen Viability of Fifteen Herbaceous Peonies. Biotechnol. Rep. 2019, 21, e00309. [Google Scholar] [CrossRef]
- van Gelderen, E.; Fossey, A.; Robbertse, P.J. The Criteria of Measurement of the Inorganic Acid Test of Pollen Viability. S. Afr. J. Bot. 1995, 61, 253–259. [Google Scholar] [CrossRef] [Green Version]
- Mukherjee, S.; Datta, A.K. Induced Genetic Male Sterility in Nigella sativa L. (Black Cumin). Cytologia 2013, 78, 105–111. [Google Scholar] [CrossRef] [Green Version]
- Luo, S.; Zhang, K.; Zhong, W.-P.; Chen, P.; Fan, X.-M.; Yuan, D.-Y. Optimization of in Vitro Pollen Germination and Pollen Viability Tests for Castanea Mollissima and Castanea Henryi. Sci. Hortic. 2020, 271, 109481. [Google Scholar] [CrossRef]
- Cran—Package Survival. Available online: https://cran.r-project.org/web/packages/survival/index.html (accessed on 15 March 2023).


| Levels | Sucrose (S) (g/L) | Boric Acid (B) (mg/L) | PEG6000 (P) (g/L) | pH (H) |
|---|---|---|---|---|
| 1 | 50 | 50 | 50 | 5.4 |
| 2 | 80 | 80 | 100 | 5.8 |
| 3 | 100 | 100 | 150 | 6.2 |
| Code | Factors | Germination Percentage (%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Sucrose | Boric Acid | PEG6000 | pH | Treatment | Replicate I | Replicate II | Replicate III | Replicate IV | Replicate V | Average | |
| S | B | P | H | T | |||||||
| 1 | S1 | B1 | P1 | H1 | S1B1P1H1 | 84.52 | 84.74 | 82.83 | 84.05 | 82.02 | 83.63 |
| 2 | S1 | B2 | P2 | H2 | S1B2P2H2 | 80.11 | 82.86 | 80 | 79 | 80.95 | 80.58 |
| 3 | S1 | B3 | P3 | H3 | S1B3P3H3 | 45 | 58.57 | 51.08 | 50.15 | 51.19 | 51.2 |
| 4 | S2 | B1 | P2 | H3 | S2B1P2H3 | 48.92 | 48.15 | 39.33 | 48.82 | 47.95 | 46.63 |
| 5 | S2 | B2 | P3 | H1 | S2B2P3H1 | 88.24 | 79.25 | 85.96 | 70.39 | 85.71 | 81.91 |
| 6 | S2 | B3 | P1 | H2 | S2B3P1H2 | 80.75 | 84.74 | 88.24 | 82.14 | 81.18 | 83.41 |
| 7 | S3 | B1 | P3 | H2 | S3B1P3H2 | 68.75 | 75.76 | 68.33 | 60 | 79.03 | 70.37 |
| 8 | S3 | B2 | P1 | H3 | S3B2P1H3 | 48.53 | 47.5 | 47.89 | 48.21 | 47.27 | 47.88 |
| 9 | S3 | B3 | P2 | H1 | S3B3P2H1 | 82.93 | 82.97 | 85.35 | 82.36 | 82.31 | 83.18 |
| K1 | 1077.07 | 1003.2 | 1074.61 | 1243.63 | |||||||
| K2 | 1059.77 | 1051.87 | 1052.01 | 1171.84 | |||||||
| K3 | 1007.19 | 1088.96 | 1017.41 | 728.56 | |||||||
| x1 | 71.8 | 66.88 | 71.64 | 82.91 | |||||||
| x2 | 70.65 | 70.12 | 70.13 | 78.12 | |||||||
| x3 | 67.14 | 72.6 | 67.83 | 48.57 | |||||||
| R | 4.66 | 5.72 | 1.51 | 34.34 | |||||||
| Time (min) | Culture Temperature (°C) | ||||
|---|---|---|---|---|---|
| 15 | 20 | 25 | 30 | 35 | |
| 30 | 1.7 ± 1.7 d | 11.19 ± 0.51 d | 13.67 ± 0.51 d | 6.33 ± 2.64 c | 0 ± 0 d |
| 60 | 16.33 ± 0.34 c | 39.83 ± 1.69 c | 37.69 ± 1.92 c | 26.67 ± 0.33 b | 9.09 ± 0.31 c |
| 90 | 23.18 ± 0.49 b | 58.42 ± 1.16 b | 57.82 ± 0.54 b | 50.54 ± 0.64 b | 14.08 ± 1.01 b |
| 120 | 52.22 ± 4.19 a | 73.67 ± 0.87 a | 85.05 ± 0.58 a | 66.01 ± 1.83 a | 24.23 ± 1.21 a |
| 180 | 51.04 ± 2.3 a | 74.36 ± 1.29 a | 84.34 ± 1.33 a | 65.31 ± 1.3 a | 25.2 ± 0.71 a |
| Time (min) | Culture Temperature (°C) | ||||
|---|---|---|---|---|---|
| 15 | 20 | 25 | 30 | 35 | |
| 30 | 2.54 ± 1.26 d | 46.12 ± 1.33 e | 55.69 ± 1.25 e | 67.74 ± 2.03 e | 0 ± 0 e |
| 60 | 41.73 ± 0.34 c | 88.38 ± 1.91 d | 100.57 ± 2.71 d | 107.36 ± 2.35 d | 39.11 ± 0.89 d |
| 90 | 96.82 ± 2.22 b | 123.01 ± 2.31 c | 149.16 ± 2.63 c | 145.16 ± 3.02 c | 79.79 ± 2.89 c |
| 120 | 135.73 ± 4.57 a | 156.14 ± 4.21 b | 245.06 ± 5.86 b | 251.51 ± 7.71 b | 123.67 ± 3.09 b |
| 180 | 248.24 ± 7.5 a | 284.63 ± 11.87 a | 390.76 ± 5.63 a | 331.52 ± 1.26 a | 177.47 ± 7.47 a |
| Unfreezing Time at 4 °C (min) | Rehydration Time at 25 °C (min) | Germination Percentage (%) |
|---|---|---|
| 0 | 0 | 58.15 ± 0.4 c |
| 30 | 0 | 58.94 ± 1.51 c |
| 60 | 0 | 65.68 ± 0.89 b |
| 90 | 0 | 66.94 ± 0.92 b |
| 30 | 30 | 77.18 ± 0.86 a |
| 60 | 30 | 77.25 ± 0.38 a |
| 90 | 30 | 74.34 ± 0.86 a |
| 30 | 60 | 76.68 ± 0.66 a |
| 60 | 60 | 76.97 ± 0.47 a |
| 90 | 60 | 77.76 ± 0.82 a |
| Levels | Sucrose (S) (g/L) | Boric Acid (B) (mg/L) | Calcium Nitrate (Ca) (mg/L) | Magnesium Sulfate (Mg) (mg/L) | Potassium Nitrate (K) (mg/L) | PEG6000 (P) (g/L) | pH (H) |
|---|---|---|---|---|---|---|---|
| 1 | 200 | 300 | 500 | 500 | 300 | 250 | 5.4 |
| 2 | 150 | 200 | 400 | 400 | 200 | 200 | 5.8 |
| 3 | 100 | 100 | 300 | 300 | 100 | 150 | 6.2 |
| 4 | 80 | 80 | 200 | 200 | 80 | 100 | 7.5 |
| 5 | 50 | 50 | 100 | 100 | 50 | 50 | 8.4 |
| 6 | 0 | 0 | 0 | 0 | 0 | 0 | 9.6 |
| Method | Staining Solution | Staining Conditions | |
|---|---|---|---|
| Time (min) | Temperature (°C) | ||
| Acetic carmine | 1% Acetic carmine [47] | 5 | 25 |
| Benzidine-H2O2 | 0.5% benzidine and 0.3% H2O2 [48] | 5 | 25 |
| I2-KI | 0.5% I2-KI [49] | 5 | 25 |
| Inorganic acid | 14.4% H2SO4 [50] | 5 | 25 |
| Methylene blue | 1% methylene blue [5] | 2 | 25 |
| MTT | 0.3% MTT: 0.3 g MTT dissolved in 100 mL PBS [9] | 30 | 35 |
| Peroxide | A solution: 0.2 g of benzidine was dissolved in 100 mL of 50% alcohol, 0.15 g of naphthol was dissolved in 100 mL of 50% alcohol, 0.25 g of sodium carbonate was dissolved in 100 mL of distilled water, and a mixed solution was prepared in equal amountsB solution: 0.3% H2O2 Take solution A and solution B in equal volumes during the test [51] | 30 | 35 |
| TTC | 0.5% TTC: 0.5 g TTC dissolved in 100 mL 95% alcohol [52] | 30 | 35 |
| Fluorescein diacetate (FDA) | FDA solution: 25 μg FDA dissolved in 1 mL acetone [8] | 20 | 25 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, M.; Jiang, F.; Huang, L.; Wang, H.; Song, W.; Zhang, X.; Zhang, Y.; Niu, L. Optimization of In Vitro Germination, Viability Tests and Storage of Paeonia ostii Pollen. Plants 2023, 12, 2460. https://doi.org/10.3390/plants12132460
Li M, Jiang F, Huang L, Wang H, Song W, Zhang X, Zhang Y, Niu L. Optimization of In Vitro Germination, Viability Tests and Storage of Paeonia ostii Pollen. Plants. 2023; 12(13):2460. https://doi.org/10.3390/plants12132460
Chicago/Turabian StyleLi, Mengchen, Fengfei Jiang, Linbo Huang, Hui Wang, Wenqing Song, Xiaoxiao Zhang, Yanlong Zhang, and Lixin Niu. 2023. "Optimization of In Vitro Germination, Viability Tests and Storage of Paeonia ostii Pollen" Plants 12, no. 13: 2460. https://doi.org/10.3390/plants12132460






