Higenamine Reduces Fine-Dust-Induced Matrix Metalloproteinase (MMP)-1 in Human Keratinocytes
Abstract
:1. Introduction
2. Results
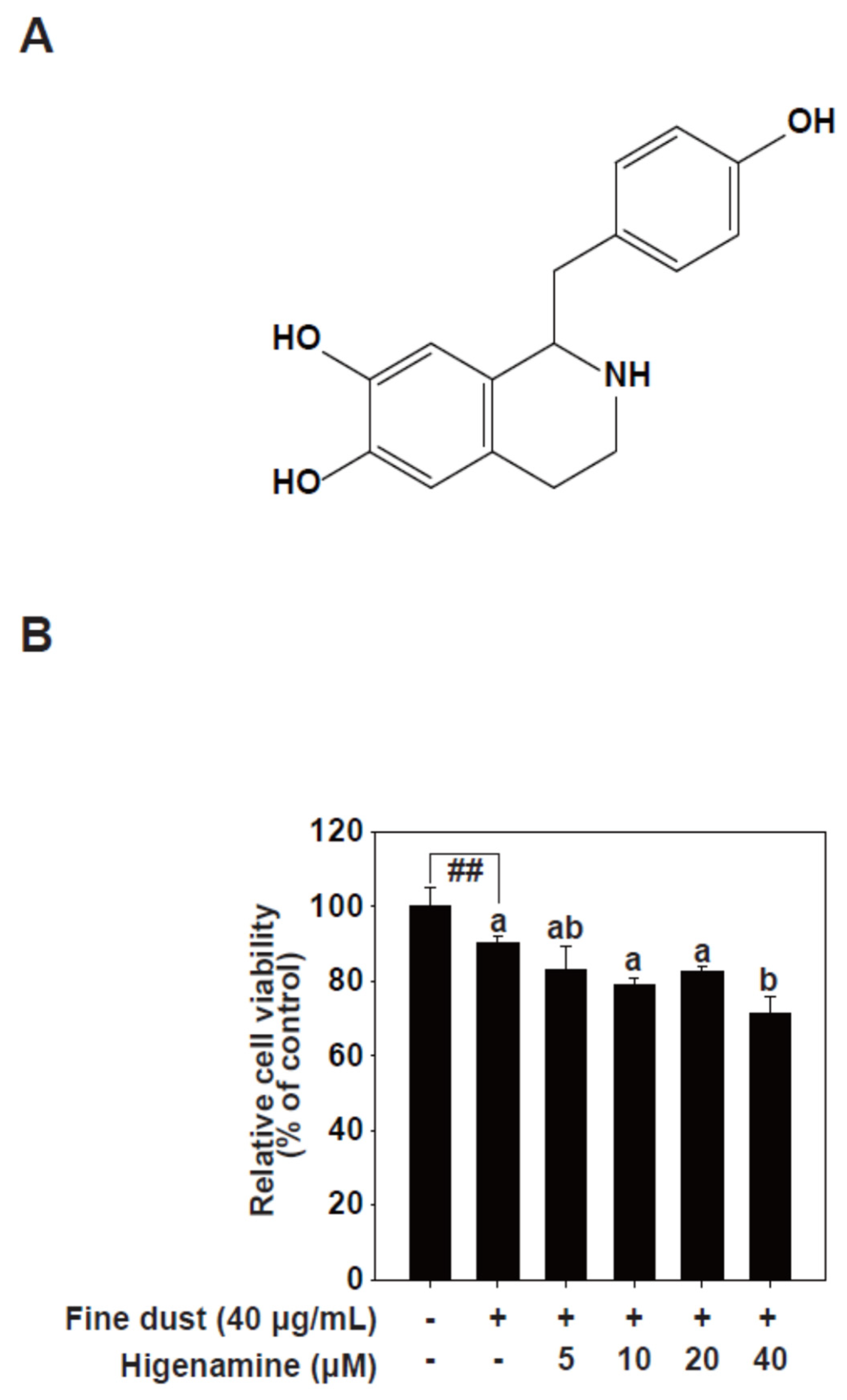
2.1. Higenamine Attenuates Fine-Dust-Induced MMP-1 Protein Expression
2.2. Higenamine Modulates Fine-Dust-Induced AP-1 and NF-κB Transactivation
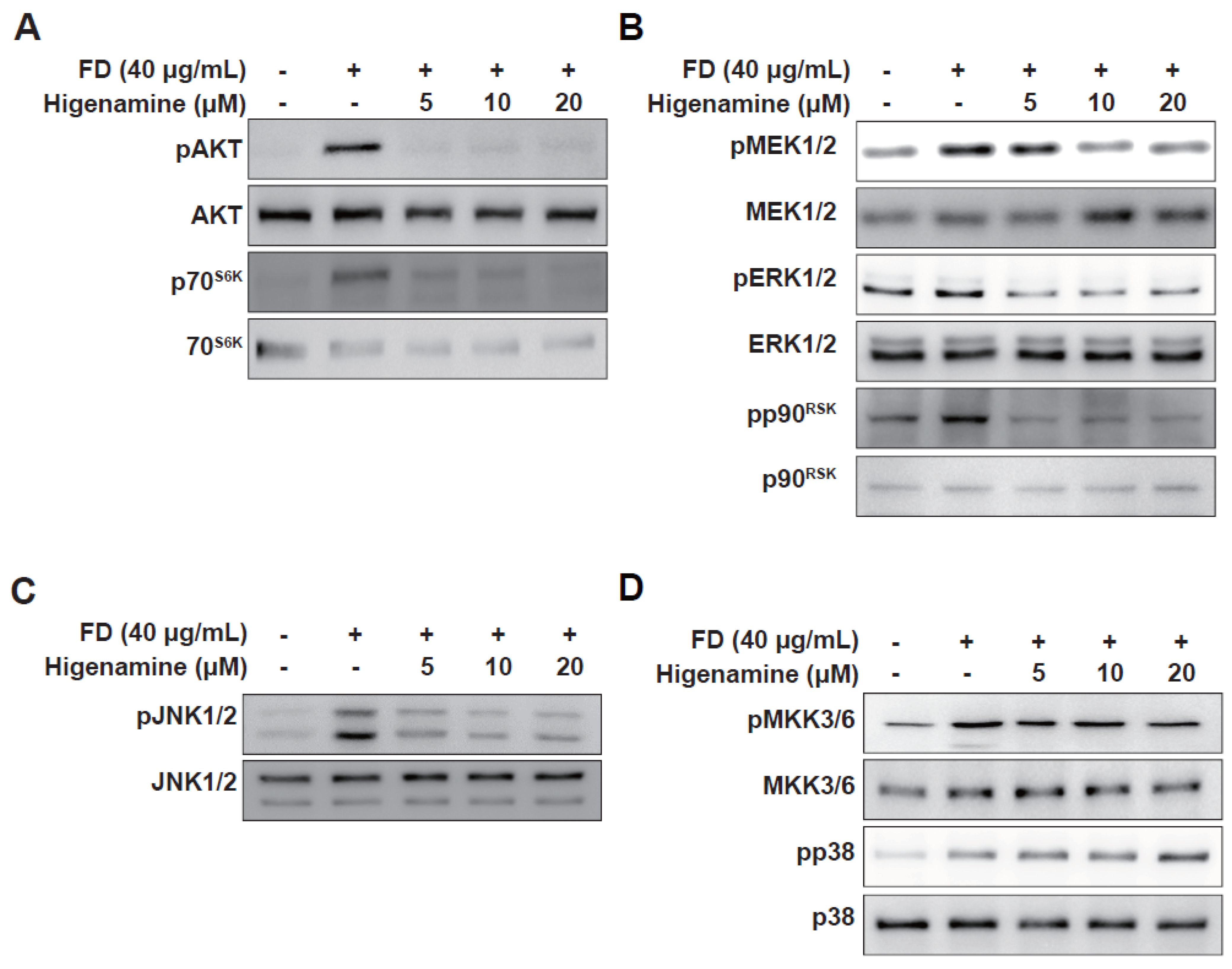
2.3. Higenamine Impedes Fine-Dust-Induced MAPK Phosphorylation in HaCaT cells and HDFs
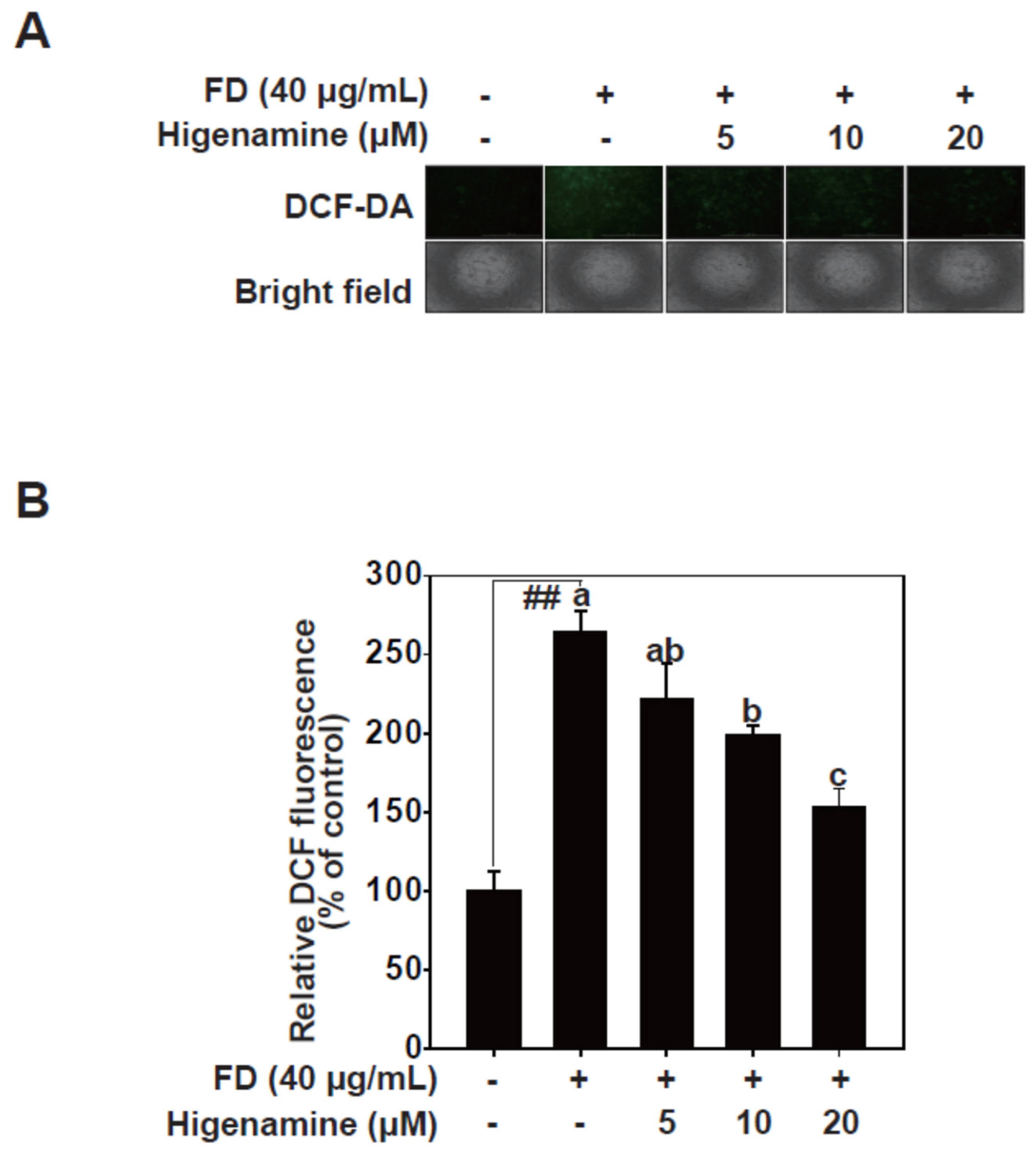
2.4. Higenamine Alleviates Fine-Dust-Induced Oxidative Damage in HaCaT Cells
3. Discussion
4. Materials and Methods
4.1. Chemicals and Materials
4.2. Cell Culture
4.3. Preparation of Fine Dust
4.4. Cell Viability
4.5. ROS Measurement
4.6. Western Blot
4.7. GFP Reporter Gene Assay
4.8. ROS Measurement
4.9. Statistical Analysis
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Vierkötter, A.; Schikowski, T.; Ranft, U.; Sugiri, D.; Matsui, M.; Krämer, U.; Krutmann, J. Airborne Particle Exposure and Extrinsic Skin Aging. J. Investig. Dermatol. 2010, 130, 2719–2726. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fang, D.; Chen, B.; Hubacek, K.; Ni, R.; Chen, L.; Feng, K.; Lin, J. Clean air for some: Unintended spillover effects of regional air pollution policies. Sci. Adv. 2019, 5, eaav4707. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, K.; Yang, W. Psychological Changes and Cancer Occurrence in Seoul Citizens Due to Changes in Fine Dust Concentration before Seoul Fine Dust Policy. Int. J. Environ. Res. Public Health 2021, 18, 1210. [Google Scholar] [CrossRef] [PubMed]
- Seok, J.K.; Lee, J.W.; Kim, Y.M.; Boo, Y.C. Punicalagin and (-)-Epigallocatechin-3-Gallate Rescue Cell Viability and Attenuate Inflammatory Responses of Human Epidermal Keratinocytes Exposed to Airborne Particulate Matter PM10. Ski. Pharm. Physiol. 2018, 31, 134–143. [Google Scholar] [CrossRef]
- Ha, J.W.; Song, H.; Hong, S.S.; Boo, Y.C. Marine Alga Ecklonia cava Extract and Dieckol Attenuate Prostaglandin E(2) Production in HaCaT Keratinocytes Exposed to Airborne Particulate Matter. Antioxidants 2019, 8, 190. [Google Scholar] [CrossRef] [Green Version]
- Fernando, I.P.S.; Dias, M.; Madusanka, D.M.D.; Kim, H.S.; Han, E.J.; Kim, M.J.; Seo, M.J.; Ahn, G. Effects of (-)-Loliolide against Fine Dust Preconditioned Keratinocyte Media-Induced Dermal Fibroblast Inflammation. Antioxidants 2021, 10, 675. [Google Scholar] [CrossRef]
- Dijkhoff, I.M.; Drasler, B.; Karakocak, B.B.; Petri-Fink, A.; Valacchi, G.; Eeman, M.; Rothen-Rutishauser, B. Impact of airborne particulate matter on skin: A systematic review from epidemiology to in vitro studies. Part. Fibre Toxicol. 2020, 17, 35. [Google Scholar] [CrossRef]
- Ryu, Y.S.; Kang, K.A.; Piao, M.J.; Ahn, M.J.; Yi, J.M.; Hyun, Y.M.; Kim, S.H.; Ko, M.K.; Park, C.O.; Hyun, J.W. Particulate matter induces inflammatory cytokine production via activation of NFkappaB by TLR5-NOX4-ROS signaling in human skin keratinocyte and mouse skin. Redox Biol. 2019, 21, 101080. [Google Scholar] [CrossRef]
- Angnunavuri, P.N.; Attiogbe, F.; Mensah, B. Particulate plastics in drinking water and potential human health effects: Current knowledge for management of freshwater plastic materials in Africa. Environ. Pollut. 2023, 316, 120714. [Google Scholar] [CrossRef]
- Kim, J.E.; Lee, K.W. Molecular Targets of Phytochemicals for Skin Inflammation. Curr. Pharm. Des. 2018, 24, 1533–1550. [Google Scholar] [CrossRef]
- Roh, E.; Kim, J.E.; Kwon, J.Y.; Park, J.S.; Bode, A.M.; Dong, Z.; Lee, K.W. Molecular mechanisms of green tea polyphenols with protective effects against skin photoaging. Crit. Rev. Food Sci. Nutr. 2017, 57, 1631–1637. [Google Scholar] [CrossRef] [PubMed]
- Fisher, G.J.; Wang, B.; Cui, Y.; Shi, M.; Zhao, Y.; Quan, T.; Voorhees, J.J. Skin aging from the perspective of dermal fibroblasts: The interplay between the adaptation to the extracellular matrix microenvironment and cell autonomous processes. J. Cell. Commun. Signal. 2023. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, A.; Rodrigues, P.M.; Pintado, M.; Tavaria, F.K. A systematic review of natural products for skin applications: Targeting inflammation, wound healing, and photo-aging. Phytomedicine 2023, 115, 154824. [Google Scholar] [CrossRef] [PubMed]
- Dańczak-Pazdrowska, A.; Gornowicz-Porowska, J.; Polańska, A.; Krajka-Kuźniak, V.; Stawny, M.; Gostyńska, A.; Rubiś, B.; Nourredine, S.; Ashiqueali, S.; Schneider, A.; et al. Cellular senescence in skin-related research: Targeted signaling pathways and naturally occurring therapeutic agents. Aging Cell. 2023, 22, e13845. [Google Scholar] [CrossRef]
- Parisi, M.; Verrillo, M.; Luciano, M.A.; Caiazzo, G.; Quaranta, M.; Scognamiglio, F.; Di Meo, V.; Villani, A.; Cantelli, M.; Gallo, L.; et al. Use of Natural Agents and Agrifood Wastes for the Treatment of Skin Photoaging. Plants 2023, 12, 840. [Google Scholar] [CrossRef]
- Dai, X.Y.; Zhu, S.Y.; Chen, J.; Li, M.Z.; Zhao, Y.; Talukder, M.; Li, J.L. Lycopene alleviates di(2-ethylhexyl) phthalate-induced splenic injury by activating P62-Keap1-NRF2 signaling. Food Chem. Toxicol. 2022, 168, 113324. [Google Scholar] [CrossRef]
- Lee, J.; Kim, H.-W.; Kim, H.; Son, D.C.; Kim, D.-K. A new record of Aconitum carmichaelii var. truppelianum (Ulbr.) W.T.Wang & P.K.Hsiao (Ranunculaceae) from the Korean Peninsula. J. Asia-Pac. Biodivers. 2020, 13, 679–686. [Google Scholar] [CrossRef]
- Hamayun, M.; Khan, S.A.; Sohn, E.Y.; Lee, I.-J. Folk medicinal knowledge and conservation status of some economically valued medicinal plants of District Swat, Pakistan. Lyonia 2006, 11, 101–113. [Google Scholar]
- Hamayun, M. Ethnobotanical studies of some useful shrubs and trees of District Buner, NWFP, Pakistan. Ethnobot. Leafl. 2003, 2003, 12. [Google Scholar]
- Cohen, P.A.; Travis, J.C.; Keizers, P.H.J.; Boyer, F.E.; Venhuis, B.J. The stimulant higenamine in weight loss and sports supplements. Clin. Toxicol. 2019, 57, 125–130. [Google Scholar] [CrossRef]
- Zhang, N.N.; Li, Z.J.; Zhu, H.B. Higenamine as a Potential Pharmacologic Stress Agent in the Detection of Coronary Artery Disease. Chin. Med. Sci. J. 2022, 37, 275–281. [Google Scholar] [CrossRef] [PubMed]
- Rangelov Kozhuharov, V.; Ivanov, K.; Ivanova, S. Higenamine in Plants as a Source of Unintentional Doping. Plants 2022, 11, 354. [Google Scholar] [CrossRef] [PubMed]
- Yarmohammadi, F.; Hayes, A.W.; Karimi, G. Natural compounds against cytotoxic drug-induced cardiotoxicity: A review on the involvement of PI3K/Akt signaling pathway. J. Biochem. Mol. Toxicol. 2021, 35, e22683. [Google Scholar] [CrossRef]
- Chen, D.T.; Rao, W.; Shen, X.; Chen, L.; Wan, Z.J.; Sheng, X.P.; Fan, T.Y. Pharmacological effects of higenamine based on signalling pathways and mechanism of action. Front. Pharm. 2022, 13, 981048. [Google Scholar] [CrossRef] [PubMed]
- Wen, J.; Li, M.; Zhang, W.; Wang, H.; Bai, Y.; Hao, J.; Liu, C.; Deng, K.; Zhao, Y. Role of Higenamine in Heart Diseases: A Mini-Review. Front. Pharm. 2021, 12, 798495. [Google Scholar] [CrossRef]
- Hudzik, T.J.; Patel, M.; Brown, A. β(2) -Adrenoceptor agonist activity of higenamine. Drug Test. Anal. 2021, 13, 261–267. [Google Scholar] [CrossRef]
- Tsukiyama, M.; Ueki, T.; Yasuda, Y.; Kikuchi, H.; Akaishi, T.; Okumura, H.; Abe, K. Beta2-adrenoceptor-mediated tracheal relaxation induced by higenamine from Nandina domestica Thunberg. Planta Med. 2009, 75, 1393–1399. [Google Scholar] [CrossRef]
- Kashiwada, Y.; Aoshima, A.; Ikeshiro, Y.; Chen, Y.P.; Furukawa, H.; Itoigawa, M.; Fujioka, T.; Mihashi, K.; Cosentino, L.M.; Morris-Natschke, S.L.; et al. Anti-HIV benzylisoquinoline alkaloids and flavonoids from the leaves of Nelumbo nucifera, and structure-activity correlations with related alkaloids. Bioorg. Med. Chem. 2005, 13, 443–448. [Google Scholar] [CrossRef]
- Kam, S.C.; Do, J.M.; Choi, J.H.; Jeon, B.T.; Roh, G.S.; Chang, K.C.; Hyun, J.S. The relaxation effect and mechanism of action of higenamine in the rat corpus cavernosum. Int. J. Impot. Res. 2012, 24, 77–83. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Lee, W.; Cui, Y.R.; Ahn, G.; Jeon, Y.J. Protective effect of green tea catechin against urban fine dust particle-induced skin aging by regulation of NF-κB, AP-1, and MAPKs signaling pathways. Environ. Pollut. 2019, 252, 1318–1324. [Google Scholar] [CrossRef]
- Fang, Y.; Xing, C.; Wang, X.; Cao, H.; Zhang, C.; Guo, X.; Zhuang, Y.; Hu, R.; Hu, G.; Yang, F. Activation of the ROS/HO-1/NQO1 signaling pathway contributes to the copper-induced oxidative stress and autophagy in duck renal tubular epithelial cells. Sci. Total. Environ. 2021, 757, 143753. [Google Scholar] [CrossRef] [PubMed]
- Pittayapruek, P.; Meephansan, J.; Prapapan, O.; Komine, M.; Ohtsuki, M. Role of Matrix Metalloproteinases in Photoaging and Photocarcinogenesis. Int. J. Mol. Sci. 2016, 17, 868. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernando, I.P.S.; Sanjeewa, K.K.A.; Lee, H.G.; Kim, H.S.; Vaas, A.; De Silva, H.I.C.; Nanayakkara, C.M.; Abeytunga, D.T.U.; Lee, W.W.; Lee, D.S.; et al. Characterization and cytoprotective properties of Sargassum natans fucoidan against urban aerosol-induced keratinocyte damage. Int. J. Biol. Macromol. 2020, 159, 773–781. [Google Scholar] [CrossRef] [PubMed]
- Dias, M.; Madusanka, D.M.D.; Han, E.J.; Kim, H.S.; Jeon, Y.J.; Jee, Y.; Kim, K.N.; Lee, K.; Fernando, I.P.S.; Ahn, G. Sargassum horneri (Turner) C. Agardh ethanol extract attenuates fine dust-induced inflammatory responses and impaired skin barrier functions in HaCaT keratinocytes. J. Ethnopharmacol. 2021, 273, 114003. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Liu, H.T. MAPK signal pathways in the regulation of cell proliferation in mammalian cells. Cell. Res. 2002, 12, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Paudel, K.R.; Panth, N. Phytochemical Profile and Biological Activity of Nelumbo nucifera. Evid. Based Complement. Altern. Med. 2015, 2015, 789124. [Google Scholar] [CrossRef] [Green Version]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, D.; Yun, J.; Roh, E.; Shin, H.-S.; Kim, J.-E. Higenamine Reduces Fine-Dust-Induced Matrix Metalloproteinase (MMP)-1 in Human Keratinocytes. Plants 2023, 12, 2479. https://doi.org/10.3390/plants12132479
Kim D, Yun J, Roh E, Shin H-S, Kim J-E. Higenamine Reduces Fine-Dust-Induced Matrix Metalloproteinase (MMP)-1 in Human Keratinocytes. Plants. 2023; 12(13):2479. https://doi.org/10.3390/plants12132479
Chicago/Turabian StyleKim, DongHyeon, JeaHyeok Yun, Eunmiri Roh, Han-Seung Shin, and Jong-Eun Kim. 2023. "Higenamine Reduces Fine-Dust-Induced Matrix Metalloproteinase (MMP)-1 in Human Keratinocytes" Plants 12, no. 13: 2479. https://doi.org/10.3390/plants12132479







