Dittrichia viscosa Selection Strategy Based on Stress Produces Stable Clonal Lines for Phytoremediation Applications
Abstract
:1. Introduction
2. Results
2.1. Dittrichia Viscosa Clonal Populations Maintain Tolerance-Related Phenotype
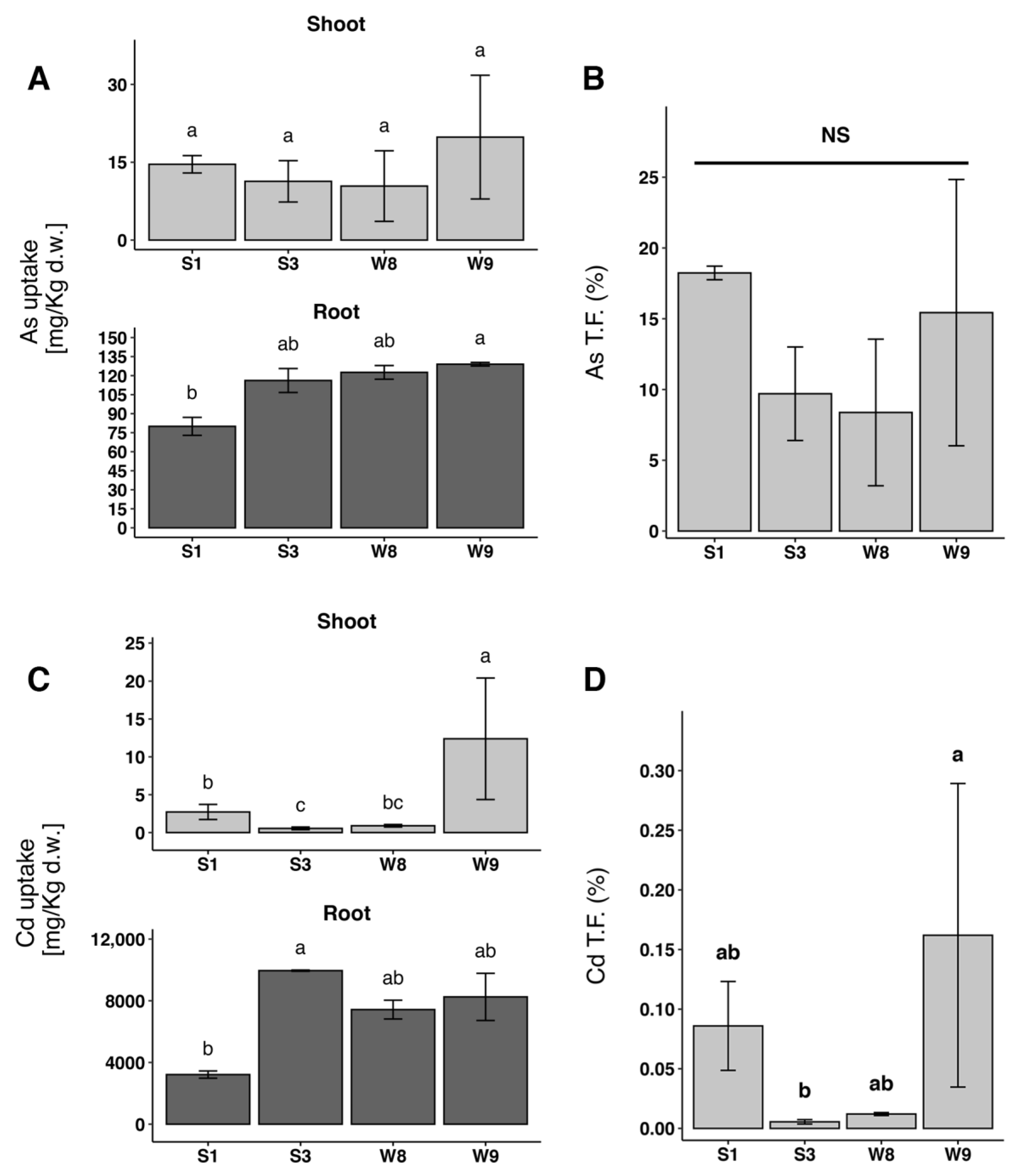
2.2. D. viscosa Clonal Individuals Accumulate HMs and Metalloids Independently from Phenotype
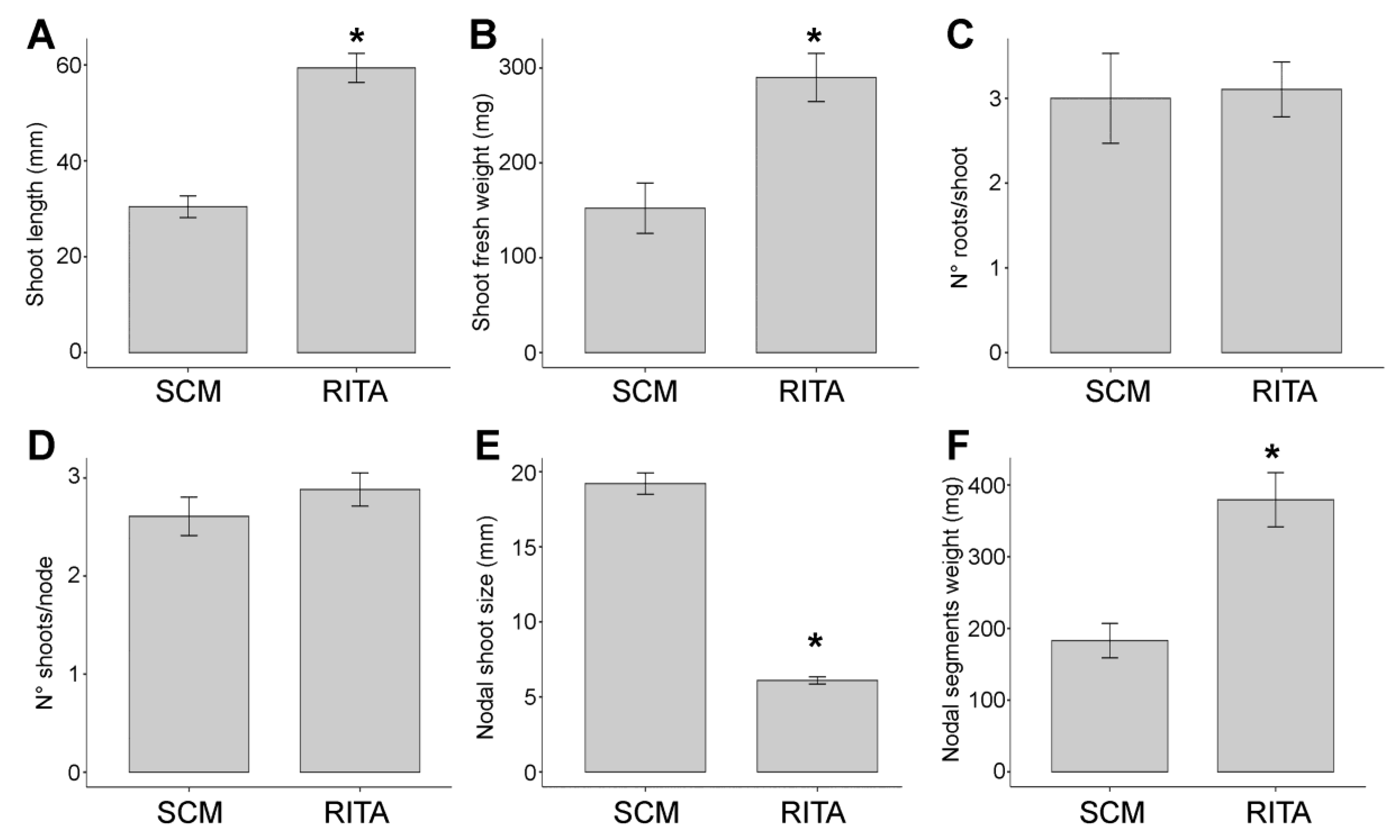
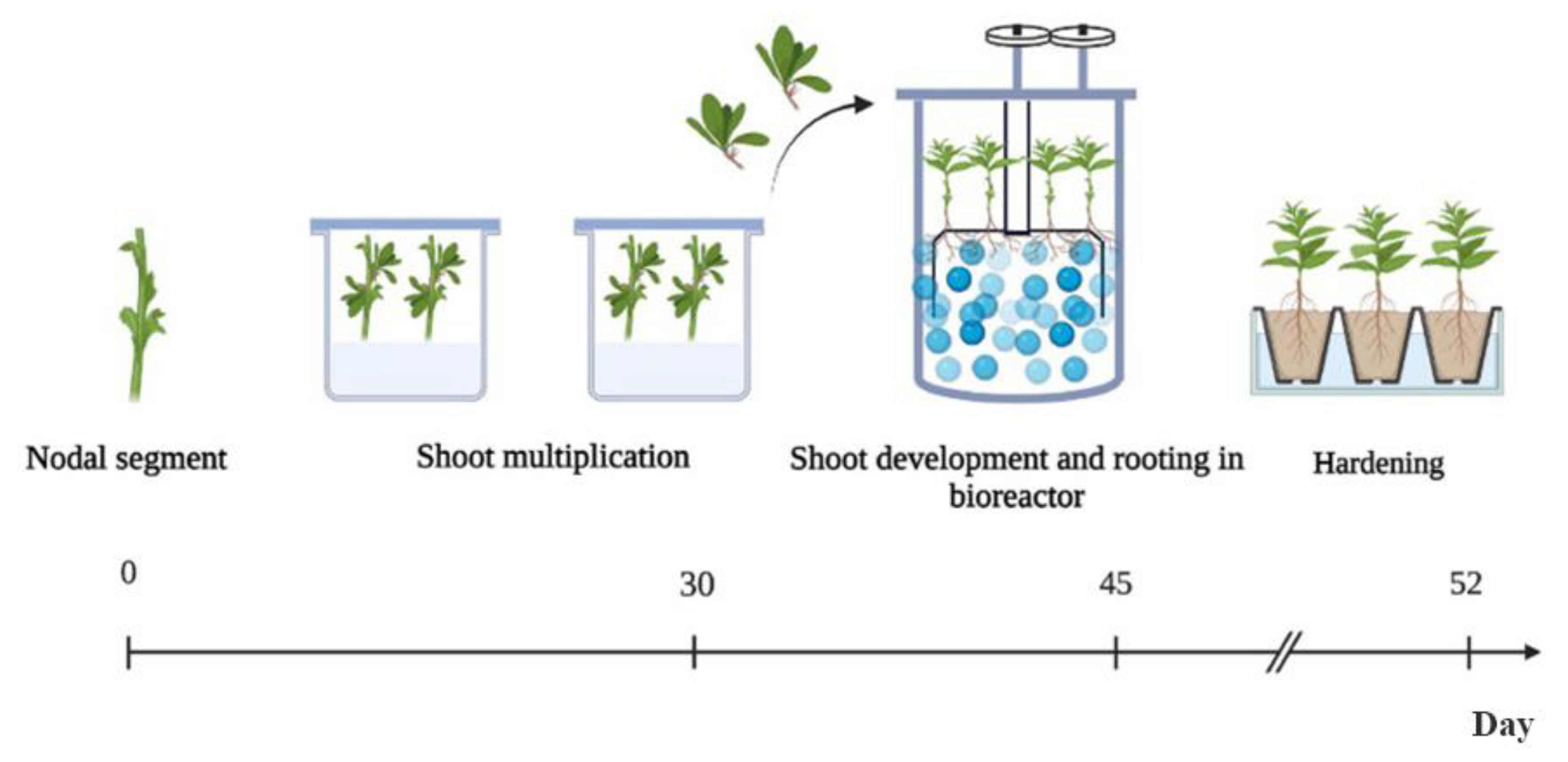
2.3. Optimization of D. viscosa Micropropagation: Effect of TIB and Solid Culture Medium on Length, Multiplication Rate, Fresh Weight, Rooting of Explants
2.4. Establishment of an Efficient Micropropagation Process Using SCM and TIB
3. Discussion
4. Materials and Methods
4.1. Plant Material for Chemicals Uptake Assay
4.2. CAT and APX Activity Measurement
4.3. Samples Mineralization and ICP Analysis
4.4. Micropropagation in Solid and TIB Culture Systems
4.5. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Landmeyer, J.E. Introduction to Phytoremediation of Contaminated Groundwater; Springer: Dordrecht, The Netherlands, 2012. [Google Scholar]
- Pandey, J.; Verma, R.K.; Singh, S. Suitability of Aromatic Plants for Phytoremediation of Heavy Metal Contaminated Areas: A Review. Int. J. Phytoremediation 2019, 21, 405–418. [Google Scholar] [CrossRef]
- Parolin, P.; Scotta, M.I.; Bresch, C. Biology of Dittrichia viscosa, a Mediterranean Ruderal Plant: A Review. Phyton-Int. J. Exp. Bot. 2014, 83, 251–262. [Google Scholar] [CrossRef]
- Papadia, P.; Barozzi, F.; Angilé, F.; Migoni, D.; Piro, G.; Fanizzi, F.P.; Di Sansebastiano, G.-P. Evaluation of Dittrichia Viscosa Performance in Substrates with Moderately Low Levels of As and Cd Contamination. Plant Biosyst. Int. J. Deal. All Asp. Plant Biol. 2020, 154, 983–989. [Google Scholar] [CrossRef]
- Hu, W.; Wang, H.; Dong, L.; Huang, B.; Borggaard, O.K.; Bruun Hansen, H.C.; He, Y.; Holm, P.E. Source Identification of Heavy Metals in Peri-Urban Agricultural Soils of Southeast China: An Integrated Approach. Environ. Pollut. 2018, 237, 650–661. [Google Scholar] [CrossRef]
- Lv, J. Multivariate Receptor Models and Robust Geostatistics to Estimate Source Apportionment of Heavy Metals in Soils. Environ. Pollut. 2019, 244, 72–83. [Google Scholar] [CrossRef]
- Shi, T.; Ma, J.; Wu, F.; Ju, T.; Gong, Y.; Zhang, Y.; Wu, X.; Hou, H.; Zhao, L.; Shi, H. Mass Balance-Based Inventory of Heavy Metals Inputs to and Outputs from Agricultural Soils in Zhejiang Province, China. Sci. Total Environ. 2019, 649, 1269–1280. [Google Scholar] [CrossRef]
- Migoni, D.; Papadia, P.; Cannito, F.; Fanizzi, F.P. Sequential Extraction Analysis of Arsenic in Soil Samples Collected in an Agricultural Area of Brindisi, Apulia (Italy), in the Proximity of a Coal-Burning Power Plant. Appl. Sci. 2021, 11, 2115. [Google Scholar] [CrossRef]
- De Benedictis, M.; Gallo, A.; Migoni, D.; Papadia, P.; Roversi, P.; Santino, A. Cadmium Treatment Induces Endoplasmic Reticulum Stress and Unfolded Protein Response in Arabidopsis Thaliana. Plant Physiol. Biochem. 2023, 196, 281–290. [Google Scholar] [CrossRef]
- Ghuge, S.A.; Nikalje, G.C.; Kadam, U.S.; Suprasanna, P.; Hong, J.C. Comprehensive Mechanisms of Heavy Metal Toxicity in Plants, Detoxification, and Remediation. J. Hazard. Mater. 2023, 450, 131039. [Google Scholar] [CrossRef]
- Barbafieri, M.; Dadea, C.; Tassi, E.; Bretzel, F.; Fanfani, L. Uptake of Heavy Metals by Native Species Growing in a Mining Area in Sardinia, Italy: Discovering Native Flora for Phytoremediation. Int. J. Phytoremediation 2011, 13, 985–997. [Google Scholar] [CrossRef]
- Jiménez, M.N.; Bacchetta, G.; Casti, M.; Navarro, F.B.; Lallena, A.M.; Fernández-Ondoño, E. Potential Use in Phytoremediation of Three Plant Species Growing on Contaminated Mine-Tailing Soils in Sardinia. Ecol. Eng. 2011, 37, 392–398. [Google Scholar] [CrossRef]
- Buscaroli, A.; Zannoni, D.; Menichetti, M.; Dinelli, E. Assessment of Metal Accumulation Capacity of Dittrichia viscosa (L.) Greuter in Two Different Italian Mine Areas for Contaminated Soils Remediation. J. Geochem. Explor. 2017, 182, 123–131. [Google Scholar] [CrossRef]
- Pérez-Sirvent, C.; Martínez-Sánchez, M.J.; Martínez-López, S.; Bech, J.; Bolan, N. Distribution and Bioaccumulation of Arsenic and Antimony in Dittrichia Viscosa Growing in Mining-Affected Semiarid Soils in Southeast Spain. J. Geochem. Explor. 2012, 123, 128–135. [Google Scholar] [CrossRef]
- Guarino, F.; Conte, B.; Improta, G.; Sciarrillo, R.; Castiglione, S.; Cicatelli, A.; Guarino, C. Genetic Characterization, Micropropagation, and Potential Use for Arsenic Phytoremediation of Dittrichia viscosa (L.) Greuter. Ecotoxicol. Environ. Saf. 2018, 148, 675–683. [Google Scholar] [CrossRef] [PubMed]
- De Paolis, A.; De Caroli, M.; Rojas, M.; Curci, L.M.; Piro, G.; Di Sansebastiano, G.-P. Evaluation of Dittrichia viscosa Aquaporin Nip1.1 Gene as Marker for Arsenic-Tolerant Plant Selection. Plants 2022, 11, 1968. [Google Scholar] [CrossRef]
- Etienne, H.; Berthouly, M. Temporary Immersion Systems in Plant Micropropagation. Plant Cell Tissue Organ Cult. 2002, 69, 215–231. [Google Scholar] [CrossRef]
- Georgiev, V.; Schumann, A.; Pavlov, A.; Bley, T. Temporary Immersion Systems in Plant Biotechnology. Eng. Life Sci. 2014, 14, 607–621. [Google Scholar] [CrossRef]
- Boonne, C.; Wacquant, J.P.; Jonard, R. In-Vitro Cloning of Dittrichia viscosa for Screening Nutritional Ecotypes. Plant Soil 1992, 142, 323–328. [Google Scholar] [CrossRef]
- Romano, A. Callus Induction and Micropropagation of Dittrichia viscosa (L.) W. Greuter. In II WOCMAP Congress Medicinal and Aromatic Plants, Part 3: Agricultural Production, Post Harvest Techniques, Biotechnology; Acta Horticulturae; International Society for Horticultural Science (ISHS): Leuven, Belgium, 1999; pp. 353–356. [Google Scholar]
- Arencibia, A.; Rodriguez, C.; Roco, L.; Vergara, C.; Gonzalez-Soto, N.; Garcia-Gonzalez, R. Tolerance to Heavy Metal Stress in Seedlings of Three Pine Species from Contrasting Environmental Conditions in Chile. iForest Biogeosci. For. 2016, 9, 937–945. [Google Scholar] [CrossRef]
- Hauser, M.-T.; Aufsatz, W.; Jonak, C.; Luschnig, C. Transgenerational Epigenetic Inheritance in Plants. Biochim. et Biophys. Acta Gene Regul. Mech. 2011, 1809, 459–468. [Google Scholar] [CrossRef]
- Shim, I.-S.; Momose, Y.; Yamamoto, A.; Kim, D.-W.; Usui, K. Inhibition of Catalase Activity by Oxidative Stress and Its Relationship to Salicylic Acid Accumulation in Plants. Plant Growth Regul. 2003, 39, 285–292. [Google Scholar] [CrossRef]
- Mishra, N.P.; Mishra, R.K.; Singhal, G.S. Changes in the Activities of Anti-Oxidant Enzymes during Exposure of Intact Wheat Leaves to Strong Visible Light at Different Temperatures in the Presence of Protein Synthesis Inhibitors. Plant Physiol. 1993, 102, 903–910. [Google Scholar] [CrossRef]
- Caverzan, A.; Passaia, G.; Rosa, S.B.; Ribeiro, C.W.; Lazzarotto, F.; Margis-Pinheiro, M. Plant Responses to Stresses: Role of Ascorbate Peroxidase in the Antioxidant Protection. Genet. Mol. Biol. 2012, 35, 1011–1019. [Google Scholar] [CrossRef] [PubMed]
- Shigeoka, S.; Ishikawa, T.; Tamoi, M.; Miyagawa, Y.; Takeda, T.; Yabuta, Y.; Yoshimura, K. Regulation and Function of Ascorbate Peroxidase Isoenzymes. J. Exp. Bot. 2002, 53, 1305–1319. [Google Scholar] [CrossRef]
- Espinosa, F.; Ortega, A.; Espinosa-Vellarino, F.L.; Garrido, I. Effect of Thallium(I) on Growth, Nutrient Absorption, Photosynthetic Pigments, and Antioxidant Response of Dittrichia Plants. Antioxidants 2023, 12, 678. [Google Scholar] [CrossRef] [PubMed]
- Barozzi, F.; Papadia, P.; Stefano, G.; Renna, L.; Brandizzi, F.; Migoni, D.; Fanizzi, F.P.; Piro, G.; Di Sansebastiano, G.-P. Variation in Membrane Trafficking Linked to SNARE AtSYP51 Interaction with Aquaporin NIP1;1. Front. Plant Sci. 2019, 9, 1949. [Google Scholar] [CrossRef]
- Ji, R.; Zhou, L.; Liu, J.; Wang, Y.; Yang, L.; Zheng, Q.; Zhang, C.; Zhang, B.; Ge, H.; Yang, Y.; et al. Calcium-Dependent Protein Kinase CPK31 Interacts with Arsenic Transporter AtNIP1;1 and Regulates Arsenite Uptake in Arabidopsis thaliana. PLoS ONE 2017, 12, e0173681. [Google Scholar] [CrossRef]
- Kamiya, T.; Tanaka, M.; Mitani, N.; Ma, J.F.; Maeshima, M.; Fujiwara, T. NIP1;1, an Aquaporin Homolog, Determines the Arsenite Sensitivity of Arabidopsis thaliana. J. Biol. Chem. 2009, 284, 2114–2120. [Google Scholar] [CrossRef]
- Kamiya, T.; Fujiwara, T. Arabidopsis NIP1;1 Transports Antimonite and Determines Antimonite Sensitivity. Plant Cell Physiol. 2009, 50, 1977–1981. [Google Scholar] [CrossRef]
- Ariani, A.; Barozzi, F.; Sebastiani, L.; di Toppi, L.S.; di Sansebastiano, G.P.; Andreucci, A. AQUA1 Is a Mercury Sensitive Poplar Aquaporin Regulated at Transcriptional and Post-Translational Levels by Zn Stress. Plant Physiol. Biochem. 2019, 135, 588–600. [Google Scholar] [CrossRef]
- Akdemir, H.; Süzerer, V.; Onay, A.; Tilkat, E.; Ersali, Y.; Çiftçi, Y.O. Micropropagation of the Pistachio and Its Rootstocks by Temporary Immersion System. Plant Cell Tissue Organ Cult. 2014, 117, 65–76. [Google Scholar] [CrossRef]
- Mordocco, A.M.; Brumbley, J.A.; Lakshmanan, P. Development of a Temporary Immersion System (RITA®) for Mass Production of Sugarcane (Saccharum spp. Interspecific Hybrids). Vitr. Cell. Dev. Biol. Plant 2009, 45, 450–457. [Google Scholar] [CrossRef]
- Sinha, D.; Datta, S.; Mishra, R.; Agarwal, P.; Kumari, T.; Adeyemi, S.B.; Kumar Maurya, A.; Ganguly, S.; Atique, U.; Seal, S.; et al. Negative Impacts of Arsenic on Plants and Mitigation Strategies. Plants 2023, 12, 1815. [Google Scholar] [CrossRef] [PubMed]
- Aebi, H. [13] Catalase In Vitro. In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 1984; Volume 105, pp. 121–126. ISBN 0076-6879. [Google Scholar]
- Elavarthi, S.; Martin, B. Spectrophotometric Assays for Antioxidant Enzymes in Plants. In Plant Stress Tolerance: Methods and Protocols; Sunkar, R., Ed.; Humana Press: Totowa, NJ, USA, 2010; pp. 273–280. ISBN 978-1-60761-702-0. [Google Scholar]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein Measurement with the Folin Phenol Reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2023. [Google Scholar]
- RStudio Team. RStudio: Integrated Development Environment for R; RStudio, PBC: Boston, MA, USA, 2020. [Google Scholar]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 Years of Image Analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anglana, C.; Capaci, P.; Barozzi, F.; Migoni, D.; Rojas, M.; Stigliano, E.; Fanizzi, F.P.; Di Sansebastiano, G.P.; Papadia, P. Dittrichia viscosa Selection Strategy Based on Stress Produces Stable Clonal Lines for Phytoremediation Applications. Plants 2023, 12, 2499. https://doi.org/10.3390/plants12132499
Anglana C, Capaci P, Barozzi F, Migoni D, Rojas M, Stigliano E, Fanizzi FP, Di Sansebastiano GP, Papadia P. Dittrichia viscosa Selection Strategy Based on Stress Produces Stable Clonal Lines for Phytoremediation Applications. Plants. 2023; 12(13):2499. https://doi.org/10.3390/plants12132499
Chicago/Turabian StyleAnglana, Chiara, Piergiorgio Capaci, Fabrizio Barozzi, Danilo Migoni, Makarena Rojas, Egidio Stigliano, Francesco Paolo Fanizzi, Gian Pietro Di Sansebastiano, and Paride Papadia. 2023. "Dittrichia viscosa Selection Strategy Based on Stress Produces Stable Clonal Lines for Phytoremediation Applications" Plants 12, no. 13: 2499. https://doi.org/10.3390/plants12132499
APA StyleAnglana, C., Capaci, P., Barozzi, F., Migoni, D., Rojas, M., Stigliano, E., Fanizzi, F. P., Di Sansebastiano, G. P., & Papadia, P. (2023). Dittrichia viscosa Selection Strategy Based on Stress Produces Stable Clonal Lines for Phytoremediation Applications. Plants, 12(13), 2499. https://doi.org/10.3390/plants12132499








