Internode Length Is Correlated with GA3 Content and Is Crucial to the Harvesting Performance of Tea-Picking Machines
Abstract
1. Introduction
2. Results
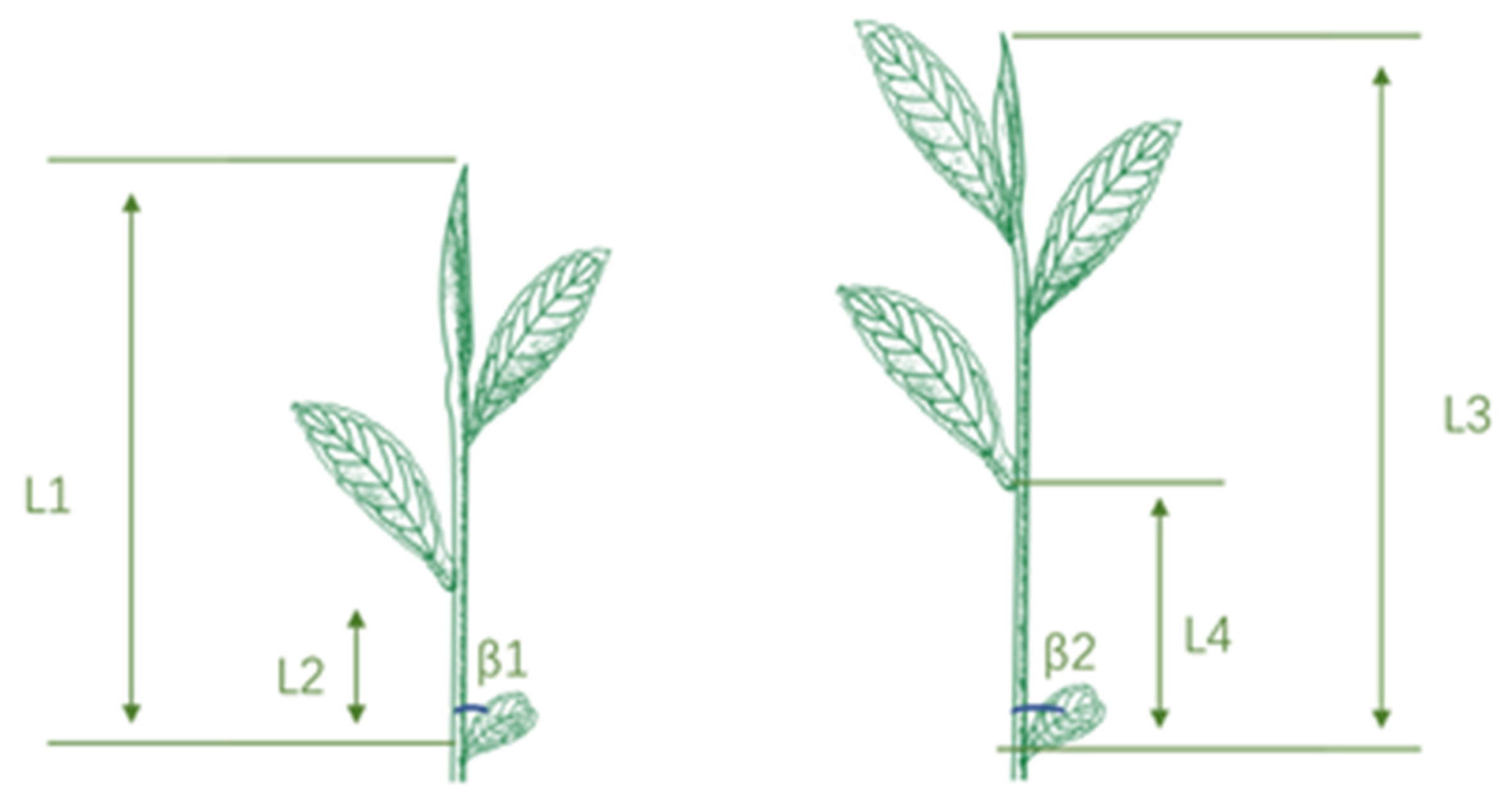
2.1. New Shoot Growth Analysis
2.2. The Effect of Internode Length and Blade Angle on Machine-Picking Efficiency
2.3. GAs and IAA Content along Internode Length
3. Discussion
3.1. A Scientific and Reasonable Comprehensive Evaluation System of Machine-Harvested Tea
3.2. The Influence of Hormones on Internode Distance
4. Materials and Methods
4.1. Design of the Experiment
4.2. Harvest Standards
- ①
- Standard leaf bud = one bud and two leaves + one bud and three leaves;
- ②
- Intact leaf buds = one bud and one leaf + one bud and two leaves + one bud and three leaves + one bud and four leaves.
4.3. Gibberellin and IAA Extraction and Quantification
4.4. RNA Extraction and Quantitative Real-Time PCR
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, X.C.; Cao, X.J.; Xia, Y.H.; Ban, Q.Y.; Cao, L.; Li, S.Y.; Li, Y.Y. CsCBF5 depletion impairs cold tolerance in tea plants. Plant Sci. 2022, 325, 111463. [Google Scholar] [CrossRef]
- Xu, W.; Song, Q.; Li, D.; Wan, X. Discrimination of the production season of Chinese green tea by chemical analysis in combination with supervised pattern recognition. J. Agric. Food Chem. 2012, 60, 7064–7070. [Google Scholar] [CrossRef]
- Zhang, X.C.; Chen, K.Y.; Zhao, Z.Y.; Li, S.Y.; Li, Y.Y. A novel LED light radiation approach enhances growth in green and albino tea cultivar. Plants 2023, 12, 988. [Google Scholar] [CrossRef]
- Zhu, Y.P.; Wu, C.Y.; Tong, J.H.; Chen, J.N.; He, L.Y.; Wang, R.Y.; Jia, J.M. Deviation tolerance performance evaluation and experiment of picking end effector for famous tea. Agriculture 2021, 11, 128. [Google Scholar] [CrossRef]
- Chen, Y.T.; Chen, S.F. Localizing plucking points of tea leaves using deep convolutional neural networks. Comput. Electron. Agric. 2020, 171, 105298. [Google Scholar] [CrossRef]
- Wang, X.Y.; Tao, D.D. Research progress on mechanical tea plucking. Acta Tea Sinica 2022, 63, 275–282. (In Chinese) [Google Scholar]
- Madamombe, G.; Tesfamariam, E.; Taylor, N. Yield decline in mechanically harvested clonal tea (Camellia sinensis (L) O. Kuntze) as influenced by changes in source/sink and radiation Interception dynamics in the canopy. Sci. Hortic. 2015, 194, 286–294. [Google Scholar] [CrossRef]
- Han, Y.; Xiao, H.R.; Qin, G.M.; Song, Z.Y.; Ding, W.Q.; Mei, S. Developing situations of tea plucking machine. Engineering 2014, 6, 268–273. [Google Scholar] [CrossRef]
- Lin, Y.K.; Chen, S.F.; Kuo, Y.F.; Liu, T.L.; Lee, S.Y. Developing a guiding and growth status monitoring system for riding-type tea plucking machine using fully convolutional networks. Comput. Electron. Agric. 2021, 191, 106540. [Google Scholar] [CrossRef]
- Yan, C.Y.; Chen, Z.H.; Li, Z.L.; Liu, R.X.; Li, Y.X.; Xiao, H.; Lu, P.; Xie, B.L. Tea sprout picking point identification based on improved DeepLabV3+. Agriculture 2022, 12, 1594. [Google Scholar] [CrossRef]
- Li, Y.T.; Wu, S.K.; He, L.Y.; Tong, J.H.; Zhao, R.M.; Jia, J.M.; Chen, J.N.; Wu, C.Y. Development and field evaluation of a robotic harvesting system for plucking high-quality tea. Comput. Electron. Agric. 2023, 206, 107659. [Google Scholar] [CrossRef]
- Tian, J.; Zhu, H.L.; Liang, W.J.; Chen, J.S.; Wen, F.J.; Zhang, L. Research on the application of machine vision in tea autonomous picking. J. Phys. Conf. Ser. 2021, 1952, 022063. [Google Scholar] [CrossRef]
- You, X.M.; Chen, Z.H.; Zhong, Q.S.; Lin, Z.H.; Shan, R.Y.; Chen, C.S. Internode-distance and leaf-angles of eight oolong tea cultivars with potential for mechanical picking. Acta Tea Sin. 2017, 58, 21–25. (In Chinese) [Google Scholar]
- Li, J.; Li, C.Y. Seventy-year major research progress in plant hormones by Chinese scholars. Sci. Sin. 2019, 49, 1227–1281. (In Chinese) [Google Scholar]
- Chen, R.F.; Fan, Y.G.; Yan, H.F.; Zhou, H.W.; Zhou, Z.F.; Weng, M.L.; Huang, X.; Lakshmanan, P.; Li, Y.R.; Qiu, L.H.; et al. Enhanced activity of genes associated with photosynthesis, phytohormone metabolism and cell wall synthesis is involved in gibberellin-mediated sugarcane internode growth. Front. Genet. 2020, 11, 570094. [Google Scholar] [CrossRef] [PubMed]
- Yan, Q.; Li, J.; Lu, L.Y.; Gao, L.J.; Lai, D.W.; Yao, N.; Yi, X.F.; Wu, Z.Y.; Lai, Z.Q.; Zhang, J.Y. Integrated analyses of phenotype, phytohormone, and transcriptome to elucidate the mechanism governing internode elongation in two contrasting elephant grass (Cenchrus purpureus) cultivars. Ind. Crop. Prod. 2021, 170, 113693. [Google Scholar] [CrossRef]
- Zhang, T.H.; Wang, J.F.; Luo, R.; Man, J.M.; Long, Q.; Xu, N. OsHLS1 regulates plant height and development by controlling active gibberellin accumulation in rice (Oryza sativa L.). Plant Sci. 2023, 326, 111508. [Google Scholar] [CrossRef]
- Guo, L.; Wang, C.Y.; Chen, J.; Ju, Y.; Yu, F.; Jiao, C.; Fei, Z.; Ding, Y.L.; Wei, Q. Cellular differentiation, hormonal gradient, and molecular alternation between the division zone and the elongation zone of bamboo internodes. Physiol. Plant. 2022, 174, e13774. [Google Scholar] [CrossRef]
- Zheng, X.X.; Ao, C.; Mao, X.X.; Cui, H.C.; Yu, J.Z. Preliminary study on selection on machine-picked varieties of Hangzhou high-quality tea. Zhejiang Agri. Sci. 2016, 57, 661–663. (In Chinese) [Google Scholar]
- Luo, Y.P.; Song, T.T.; Wen, D.H.; Tang, M.; Cai, W.Z. The internode length and blade angle of newly-formed tea shoots on machine picking. J. Zhejiang Univ. 2009, 35, 420–424. (In Chinese) [Google Scholar]
- Zhang, L.; Zou, L.; Wu, C.; Jia, J.; Chen, J. Method of famous tea sprout identification and segmentation based on improved watershed algorithm. Comput. Electron. Agric. 2021, 184, 106108. [Google Scholar] [CrossRef]
- Wang, Z.H.; Peng, H.; Yue, C.N.; Li, W.J.; Tong, Z.F.; Yang, P.X. Selection of core evaluation indices and construction of a comprehensive evaluation method for machine-harvested tea plant cultivars. Euphytica 2022, 218, 162. [Google Scholar] [CrossRef]
- Long, L.Z.; Shi, Y.Z.; Ma, L.F.; Ruan, J.Y. Characterization of young shoot population, yield, and nitrogen demands of tea (Camellia sinensis L.) harvested under different standards. Horticulturae 2022, 8, 275. [Google Scholar] [CrossRef]
- Chen, H.X.; Tang, X.; Zhou, Y.; Wen, W.Q.; Wang, Z.Y.; Hu, C.; Yang, C.Q. Selection of suitable machine-harvest tea cultivar in Sichuan. Chin. Tea 2019, 2, 23–31. (In Chinese) [Google Scholar]
- Castro-Camba, R.; Sánchez, C.; Vidal, N.; Vielba, J.M. Plant development and crop yield: The role of gibberellins. Plants 2022, 11, 2650. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Soto, D.; Allona, I.; Perales, M. Flowing LOCUS T2 promotes shoot apex development and restricts internode elongation via the 13-Hydroxylation gibberellin biosynthesis pathway in Poplar. Front. Plant Sci. 2022, 12, 814195. [Google Scholar] [CrossRef]
- Sun, W.; Ma, Z.; Chen, H.; Liu, M. MYB gene family in potato (Solanum tuberosum L.): Genome-wide identification of hormone-responsive reveals their potential functions in growth and development. Int. J. Mol. Sci. 2019, 20, 4847. [Google Scholar] [CrossRef] [PubMed]
- Gong, M.; Luo, H.; Wang, A.; Zhou, Y.; Huang, W.; Zhu, P.; He, L. Phytohormone profiling during tuber development of Chinese Yam by ultra-high performance liquid chromatography-triple quadrupole tandem mass spectrometry. J. Plant Growth Regul. 2016, 36, 362–373. [Google Scholar] [CrossRef]
- Binenbaum, J.; Weinstain, R.; Shani, E. Gibberellin Localization and Transport in Plants. Trends Plant Sci. 2018, 23, 410–421. [Google Scholar] [CrossRef] [PubMed]
- Shan, F.X.; Zhang, R.; Zhang, J.; Wang, C.; Lyu, X.C.; Xin, T.Y.; Yan, C.; Dong, S.K.; Ma, C.M.; Gong, Z.P. Study on the regulatory effects of GA3 on soybean internode elongation. Plants 2021, 10, 1737. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.Y.; Wen, J.; Ke, X.C.; Zhang, J.; Sun, X.D.; Wang, C.T.; Yang, Y.P. Gibberellin inhibition of taproot formation by modulation of DELLA-NAC complex activity in turnip (Brassica rapa var. rapa). Protoplasma 2021, 258, 925–934. [Google Scholar] [CrossRef]
- Pan, C.; Tian, K.H.; Ban, Q.Y.; Wang, L.G.; Sun, Q.L.; He, Y.; Yang, Y.F.; Pan, Y.T.; Li, Y.Y.; Jiang, J.Y.; et al. Genome-wide analysis of the biosynthesis and deactivation of gibberellin-dioxygenases gene family in Camellia sinensis (L.) O. Kuntze. Genes 2017, 8, 235. [Google Scholar] [CrossRef] [PubMed]
- Chu, Y.; Xu, N.; Wu, Q.; Yu, B.; Li, X.; Chen, R.; Huang, J. Rice transcription factor OsMADS57 regulates plant height by modulating gibberellin catabolism. Rice 2019, 12, 38. [Google Scholar] [CrossRef] [PubMed]
- Paciorek, T.; Chiapelli, B.J.; Wang, J.Y.; Paciorek, M.; Yang, H.; Sant, A.; Val, D.L.; Boddu, J.; Liu, K.; Gu, C.; et al. Targeted suppression of gibberellin biosynthetic genes ZmGA20ox3 and ZmGA20ox5 produces a short stature maize ideotype. Plant Biotechnol. J. 2022, 20, 1140–1153. [Google Scholar] [CrossRef]
- Voorend, W.; Nelissen, H.; Vanholme, R.; Vliegher, A.D.; Breusegem, F.V.; Boerjan, W.; Isabel, R.R.; Hilde, M.; Inze, D. Overexpression of GA20-OXIDASE1 impacts plant height, biomass allocation and saccharification efficiency in maize. Plant Biotechnol. J. 2016, 14, 997–1007. [Google Scholar] [CrossRef] [PubMed]
- Reinecke, D.M.; Wickramarathna, A.D.; Ozga, J.A.; Kurepin, L.V.; Jin, A.L.; Good, A.G.; Pharis, R.P. Gibberellin 3-oxidase gene expression patterns influence gibberellin biosynthesis, growth, and development in pea. Gibberellin 3-oxidase gene expression patterns influence gibberellin biosynthesis, growth, and development in pea. Plant Physiol. 2013, 163, 929–945. [Google Scholar] [CrossRef]
- Durbak, A.; Yao, H.; McSteen, P. Hormone signaling in plant development. Curr. Opin. Plant Biol. 2012, 15, 92–96. [Google Scholar] [CrossRef]
- Kou, E.; Huang, X.M.; Zhu, Y.N.; Su, W.; Liu, H.C.; Sun, G.W.; Chen, R.Y.; Hao, Y.W.; Song, S.W. Crosstalk between auxin and gibberellin during stalk elongation in flowering Chinese cabbage. Sci. Rep. 2021, 11, 3976. [Google Scholar] [CrossRef]
- Jing, S.L.; Sun, X.M.; Yu, L.; Wang, E.S.; Cheng, Z.N.; Liu, H.M.; Jiang, P.; Qin, J.; Begum, S.; Song, B.T. Transcription factor StABI5-like 1 binding to the FLOWERING LOCUS T homologs promotes early maturity in potato. Plant Physiol. 2022, 189, 1677–1693. [Google Scholar] [CrossRef]
- Wang, H.X.; Wang, M.L.; Wang, X.Z.; Ding, Y.L. Detection of seven phytohormones in peanut tissues by ultra-highperformance liquid chromatography-triple quadrupole tandem mass spectrometry. J. Integr. Agric. 2020, 19, 700–708. [Google Scholar] [CrossRef]





| Cultivar | One Bud and Two Leaves | One Bud and Three Leaves | ||||
|---|---|---|---|---|---|---|
| L1 (cm) | L2 (cm) | β1 | L3 (cm) | L4 (cm) | β2 | |
| Zhongcha111 | 9.22 ± 0.03 a | 4.16 ± 0.04 a | 22.86 ± 0.29 e | 12.2 ± 0.06 a | 4.37 ± 0.08 a | 24.61 ± 0.23 e |
| ECha1 | 7.9 ± 0.01 b | 3.6 ± 0.08 b | 33.39 ± 0.3 a | 10.46 ± 0.45 c | 3.92 ± 0.05 b | 39.43 ± 0.34 a |
| Fuzao2 | 8.02 ± 0.07 b | 3.33 ± 0.04 c | 33.27 ± 0.45 a | 10.89 ± 0.09 b | 3.99 ± 0.05 b | 33.28 ± 0.33 b |
| Yingshuang | 6.43 ± 0.09 c | 2.95 ± 0.12 d | 23.05 ± 0.12 e | 8.01 ± 0.09 d | 3.26 ± 0.01 c | 25.81 ± 0.17 d |
| Longjingchangye | 5.94 ± 0.05 d | 2.67 ± 0.09 e | 25.14 ± 0.35 d | 7.93 ± 0.04 d | 2.61 ± 0.04 d | 24.84 ± 0.41 e |
| Fuyun6 | 5.66 ± 0.21 e | 2.05 ± 0.09 f | 29.8 ± 0.48 c | 7.86 ± 0.1 d | 2.51 ± 0.04 e | 30.38 ± 1.13 c |
| Zhenong113 | 4.89 ± 0.02 f | 1.67 ± 0.03 g | 32.55 ± 0.65 b | 6.83 ± 0.05 e | 2.02 ± 0.09 f | 29.54 ± 0.33 c |
| Cultivar | One Bud and Two Leaves | One Bud and Three Leaves | ||||
|---|---|---|---|---|---|---|
| L1 (cm) | L2 (cm) | β1 | L3 (cm) | L4 (cm) | β2 | |
| Zhongcha111 | 6.87 ± 0.39 a | 1.81 ± 0.09 b | 39.29 ± 3.58 ab | 9.1 ± 0.34 ab | 1.93 ± 0.21 b | 41.04 ± 1.66 ab |
| ECha1 | 6.89 ± 0.61 a | 1.9 ± 0.08 ab | 42.64 ± 0.68 a | 10.05 ± 0.86 a | 1.99 ± 0.09 b | 41.1 ± 0.34 ab |
| Fuzao2 | 6.68 ± 0.3 a | 2.1 ± 0.22 a | 34.01 ± 2.4 b | 7.84 ± 0.6 bcd | 2.01 ± 0.33 b | 37.05 ± 2.13 b |
| Yingshuang | 5.46 ± 0.22 bc | 1.68 ± 0.04 bc | 41.7 ± 5.44 a | 7.97 ± 1.04 bcd | 3.32 ± 0.44 a | 25.62 ± 7.76 c |
| Longjingchangye | 4.95 ± 0.25 c | 1.45 ± 0.1 cd | 35.47 ± 4.32 ab | 6.59 ± 0.86 d | 1.3 ± 0.11 c | 39.78 ± 3.72 ab |
| Fuyun6 | 5.71 ± 0.35 b | 1.22 ± 0.19 d | 42.68 ± 4.94 a | 8.5 ± 0.94 bc | 2.12 ± 0.42 b | 45.58 ± 2.27 a |
| Zhenong113 | 5.78 ± 0.49 b | 1.49 ± 0.2 c | 38.34 ± 2.88 ab | 7.46 ± 0.21 cd | 1.26 ± 0.22 c | 42.93 ± 1.44 ab |
| Cultivar | One Bud and Two Leaves | One Bud and Three Leaves | ||||
|---|---|---|---|---|---|---|
| L1 (cm) | L2 (cm) | β1 | L3 (cm) | L4 (cm) | β2 | |
| Zhongcha111 | 5.83 ± 0.19 a | 2.5 ± 0.16 a | 30.85 ± 3.23 a | 7.76 ± 0.84 a | 2.67 ± 0.58 a | 35.29 ± 0.68 bc |
| ECha1 | 5.5 ± 0.13 ab | 2.18 ± 0.09 a | 33.22 ± 2.15 a | 8.04 ± 0.6 a | 2.51 ± 0.21 a | 39.05 ± 3.89 ab |
| Fuzao2 | 5.49 ± 0.06 ab | 1.32 ± 0.21 bc | 30.77 ± 1.05 a | 6.85 ± 0.5 abc | 1.41 ± 0.23 b | 31.31 ± 3.71 cd |
| Yingshuang | 4.69 ± 0.56 cd | 1.65 ± 0.28 b | 34.87 ± 0.93 a | 7.29 ± 1.27 ab | 1.86 ± 0.31 b | 35.1 ± 2.22 bc |
| Longjingchangye | 4.32 ± 0.24 d | 1.58 ± 0.17 b | 26.13 ± 3.23 b | 5.27 ± 0.64 d | 1.3 ± 0.12 b | 27.26 ± 1.48 d |
| Fuyun6 | 5.06 ± 0.31 bc | 1.64 ± 0.21 b | 35.04 ± 2.53 a | 6.04 ± 0.64 bcd | 1.32 ± 0.35 b | 40.37 ± 2.83 a |
| Zhenong113 | 3.48 ± 0.31 e | 1.18 ± 0.08 c | 32.42 ± 3.14 a | 5.82 ± 0.39 cd | 1.49 ± 0.09 b | 35.63 ± 0.58 bc |
| Cultivar | Bud and Leaves | Machine-Harvest Waste | Standard Bud Leaf (%) | Intact Bud and Leaves (%) | |||||
|---|---|---|---|---|---|---|---|---|---|
| One Bud and One Leaf (%) | One Bud and Two Leaves (%) | One Bud and Three Leaves (%) | One Bud and Four Leaves (%) | Other (%) | Broken Leaves (%) | Branch (%) | |||
| Zhongcha111 | 0.44 ± 0.17 de | 43.45 ± 5.9 a | 34.46 ± 11.81 a | 2.5 ± 2.54 c | 4.68 ± 4.94 c | 14.48 ± 4.17 b | 0 ± 0 | 77.9 ± 6.16 a | 80.84 ± 8.42 a |
| ECha1 | 2.53 ± 0 b | 33.01 ± 1.29 b | 29.39 ± 6.33 a | 5.59 ± 1.59 bc | 15.84 ± 5.32 b | 13.63 ± 2.89 b | 0 ± 0 | 62.4 ± 6.22 b | 70.53 ± 7.79 ab |
| Fuzao2 | 0.06 ± 0.05 e | 20.71 ± 2.01 d | 34.18 ± 5.86 a | 12.38 ± 1.09 a | 15.67 ± 1.82 b | 14.75 ± 2.05 b | 2.24 ± 3.87 | 54.9 ± 7.86 b | 67.34 ± 6.77 b |
| Yingshuang | 1.35 ± 0.11 c | 26.1 ± 2.14 cd | 35.76 ± 5.26 a | 6.66 ± 2.61 b | 8.68 ± 5.13 bc | 21.45 ± 4.37 a | 0 ± 0 | 61.86 ± 7.08 b | 69.87 ± 9.1 ab |
| Longjingchangye | 1.43 ± 0.64 c | 22.15 ± 2.44 d | 32.47 ± 3.29 a | 5.45 ± 0.8 bc | 12.84 ± 4.22 bc | 25.66 ± 0.44 a | 0 ± 0 | 54.62 ± 5.61 b | 61.5 ± 4.62 b |
| Fuyun6 | 6.52 ± 0 a | 30.97 ± 3.49 bc | 27.5 ± 2.86 a | 2.64 ± 2.3 c | 6.22 ± 5.53 bc | 25.22 ± 0.98 a | 0.93 ± 1.61 | 58.46 ± 4.94 b | 67.62 ± 4.91 b |
| Zhenong113 | 0.73 ± 0.01 d | 24.45 ± 1.62 d | 14.34 ± 2.61 b | 3.03 ± 2.21 bc | 31.77 ± 7.15 a | 25.69 ± 3.67 a | 0 ± 0 | 38.79 ± 4.15 c | 42.54 ± 4.39 c |
| 9 | Bud and Leaves | Machine-Harvest Waste | Standard Bud Leaf (%) | Intact Bud and Leaves (%) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| One bud (%) | One Bud and One Leaf (%) | One Bud and Two Leaves (%) | One Bud and Three Leaves (%) | One Bud and Four Leaves (%) | Other (%) | Broken Leaves (%) | Branch (%) | |||
| Zhongcha111 | 1.21 ± 0.25 a | 9.36 ± 3.2 abc | 28.98 ± 3.19 bc | 27.97 ± 4.66 ab | 9.19 ± 2.68 a | 3.63 ± 2.32 b | 16.98 ± 2.09 d | 2.67 ± 1.52 bc | 56.96 ± 3.35 a | 76.71 ± 3.5 ab |
| ECha1 | 0.66 ± 0.18 abc | 10.33 ± 3.29 ab | 32.9 ± 1.29 ab | 25.21 ± 6.92 abc | 3.35 ± 1.49 b | 6.81 ± 2.84 ab | 16.14 ± 1.68 d | 4.61 ± 0.31 ab | 58.11 ± 5.74 a | 72.45 ± 4.02 abc |
| Fuzao2 | 0.48 ± 0.36 bc | 7.51 ± 1.77 abcd | 31.53 ± 2.6 abc | 32.26 ± 6.1 a | 7.81 ± 1.89 a | 2.3 ± 0.23 b | 16.84 ± 0.88 d | 1.26 ± 0.94 c | 63.79 ± 3.69 a | 79.6 ± 1.48 a |
| Yingshuang | 0.9 ± 0.08 abc | 10.46 ± 2.42 ab | 36.14 ± 2.16 a | 21.37 ± 6.63 bcd | 0.6 ± 1.05 b | 3.05 ± 1.1 b | 23.81 ± 3.69 bc | 3.66 ± 1.07 abc | 57.51 ± 4.96 a | 69.48 ± 3.63 bc |
| Longjingchangye | 0.32 ± 0.24 bc | 4.54 ± 1.44 d | 27.16 ± 2.78 c | 32.73 ± 4.36 a | 10.9 ± 3.42 a | 1.91 ± 0.91 b | 20.8 ± 1.73 cd | 1.65 ± 0.78 c | 59.89 ± 1.59 a | 75.64 ± 3.22 ab |
| Fuyun6 | 0.64 ± 0.18 abc | 7.23 ± 0.44 a | 32.1 ± 4 abc | 24.84 ± 4.95 abc | 3.04 ± 1.77 b | 7.88 ± 2.76 ab | 19.51 ± 4.26 cd | 4.75 ± 0.38 ab | 56.95 ± 6.2 a | 67.86 ± 6.96 c |
| Zhenong113 | 1 ± 0.78 ab | 11.04 ± 3.7 abcd | 29.54 ± 4.45 bc | 14.8 ± 3.69 d | 0.36 ± 0.63 b | 12.53 ± 6.67 a | 24.95 ± 5.63 bc | 5.78 ± 0.47 a | 44.33 ± 6.78 b | 56.74 ± 2.75 d |
| Cultivar | Bud and Leaves | Machine-Harvest Waste | Standard Bud Leaf (%) | Intact Bud and Leaves (%) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| One Bud (%) | One Bud and One Leaf (%) | One Bud and Two Leaves (%) | One Bud and Three Leaves (%) | One Bud and Four Leaves (%) | Other (%) | Broken Leaves (%) | Branch (%) | |||
| Zhongcha111 | 0 ± 0 b | 3.77 ± 1.76 c | 33.35 ± 8.44 ab | 36.97 ± 0.9 a | 6.66 ± 6.63 bc | 6.05 ± 4.55 a | 9.87 ± 1.02 e | 3.34 ± 1.71 a | 70.32 ± 8.55 a | 80.75 ± 2.6 ab |
| ECha1 | 0 ± 0 b | 3.62 ± 0.25 c | 31.71 ± 3.08 ab | 38.72 ± 3.03 a | 8.07 ± 1.26 bc | 5.91 ± 3.4 a | 11.97 ± 1.93 de | 0 ± 0 b | 70.43 ± 0.39 a | 82.12 ± 1.52 a |
| Fuzao2 | 0.43 ± 0.26 a | 12.24 ± 3.22 a | 36.87 ± 4.94 a | 19.32 ± 5.03 d | 4.88 ± 6.13 bc | 1.88 ± 0.66 ab | 24.38 ± 3.81 b | 0 ± 0 b | 56.19 ± 2.34 cd | 73.74 ± 3.16 c |
| Yingshuang | 0 ± 0 b | 6.03 ± 1.82 bc | 37.01 ± 1.85 a | 30 ± 1.97 bc | 8.99 ± 3.17 b | 1.96 ± 1.64 ab | 15.5 ± 2.64 cd | 0.51 ± 0.88 b | 67.01 ± 2.63 ab | 82.03 ± 1.78 a |
| Longjingchangye | 0.14 ± 0.24 b | 4.94 ± 0.89 bc | 26.19 ± 5.21 b | 31.82 ± 0.68 b | 17.62 ± 4.64 a | 0.76 ± 1.32 c | 18.45 ± 2.35 c | 0.09 ± 0.16 b | 58.01 ± 4.6 bcd | 80.7 ± 2.18 ab |
| Fuyun6 | 0 ± 0 b | 8.26 ± 0.96 b | 37.53 ± 3.06 a | 25.24 ± 2.84 c | 3.92 ± 2.25 bc | 5.35 ± 2.87 ab | 19.71 ± 2.56 c | 0 ± 0 b | 62.77 ± 5.45 abc | 74.94 ± 5.41 bc |
| Zhenong113 | 0 ± 0 b | 12.83 ± 2.89 a | 37.35 ± 3.9 a | 13.92 ± 3.41 e | 0.9 ± 0.94 c | 4.57 ± 1.4 ab | 29.05 ± 2.82 a | 1.39 ± 2.41 ab | 51.26 ± 7.3 d | 64.99 ± 5.15 d |
| Cultivar | Content (ng/g) | |||
|---|---|---|---|---|
| GA1 | GA3 | GA4 | IAA | |
| Zhongcha111 | 2.118 ± 0.14 b | 1.458 ± 0.21 b | 2.195 ± 0.18 a | 28.513 ± 2.65 d |
| Echa1 | 2.395 ± 0.59 b | 1.444 ± 0.41 b | 0.898 ± 0.08 e | 42.959 ± 4.96 b |
| Fuzao2 | 4.283 ± 0.58 a | 2.544 ± 0.5 a | 1.199 ± 0.1 c | 102.382 ± 3.52 a |
| Yingshuang | 1.577 ± 0.15 c | 1.113 ± 0.22 bc | 1.705 ± 0.11 b | 21.323 ± 1.75 e |
| Longjingchangye | 1.373 ± 0.4 c | 1.05 ± 0.25 bc | 1.037 ± 0.07 d | 25.092 ± 2.54 d |
| Fuyun6 | 1.448 ± 0.07 c | 0.52 ± 0.28 d | 0.681 ± 0.07 g | 10.014 ± 1.11 f |
| Zhenong113 | 1.442 ± 0.32 c | 0.989 ± 0.19 c | 2.18 ± 0.06 a | 39.206 ± 1.59 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, Y.; Yu, Q.; Xie, Y.; Xu, C.; Cheng, L.; Shi, Q.; Li, Y.; Zhang, X.; Shen, Z. Internode Length Is Correlated with GA3 Content and Is Crucial to the Harvesting Performance of Tea-Picking Machines. Plants 2023, 12, 2508. https://doi.org/10.3390/plants12132508
Luo Y, Yu Q, Xie Y, Xu C, Cheng L, Shi Q, Li Y, Zhang X, Shen Z. Internode Length Is Correlated with GA3 Content and Is Crucial to the Harvesting Performance of Tea-Picking Machines. Plants. 2023; 12(13):2508. https://doi.org/10.3390/plants12132508
Chicago/Turabian StyleLuo, Yao, Qianqian Yu, Yinghua Xie, Chaojie Xu, Letian Cheng, Qing Shi, Yeyun Li, Xianchen Zhang, and Zhougao Shen. 2023. "Internode Length Is Correlated with GA3 Content and Is Crucial to the Harvesting Performance of Tea-Picking Machines" Plants 12, no. 13: 2508. https://doi.org/10.3390/plants12132508
APA StyleLuo, Y., Yu, Q., Xie, Y., Xu, C., Cheng, L., Shi, Q., Li, Y., Zhang, X., & Shen, Z. (2023). Internode Length Is Correlated with GA3 Content and Is Crucial to the Harvesting Performance of Tea-Picking Machines. Plants, 12(13), 2508. https://doi.org/10.3390/plants12132508





