DREB1 and DREB2 Genes in Garlic (Allium sativum L.): Genome-Wide Identification, Characterization, and Stress Response
Abstract
:1. Introduction
2. Results
2.1. Identification and Characterization of DREB1 and DREB2 Genes in the A. sativum cv. Ershuizao Genome
2.2. Characterization of Putative AsaDREB1 and AsaDREB2 Proteins
2.3. Analysis of AsaDREB1 and AsaDREB2 Promoter Regions
2.4. AsaDREB Expression in the Organs of Garlic Cultivars Ershuizao and Sarmat
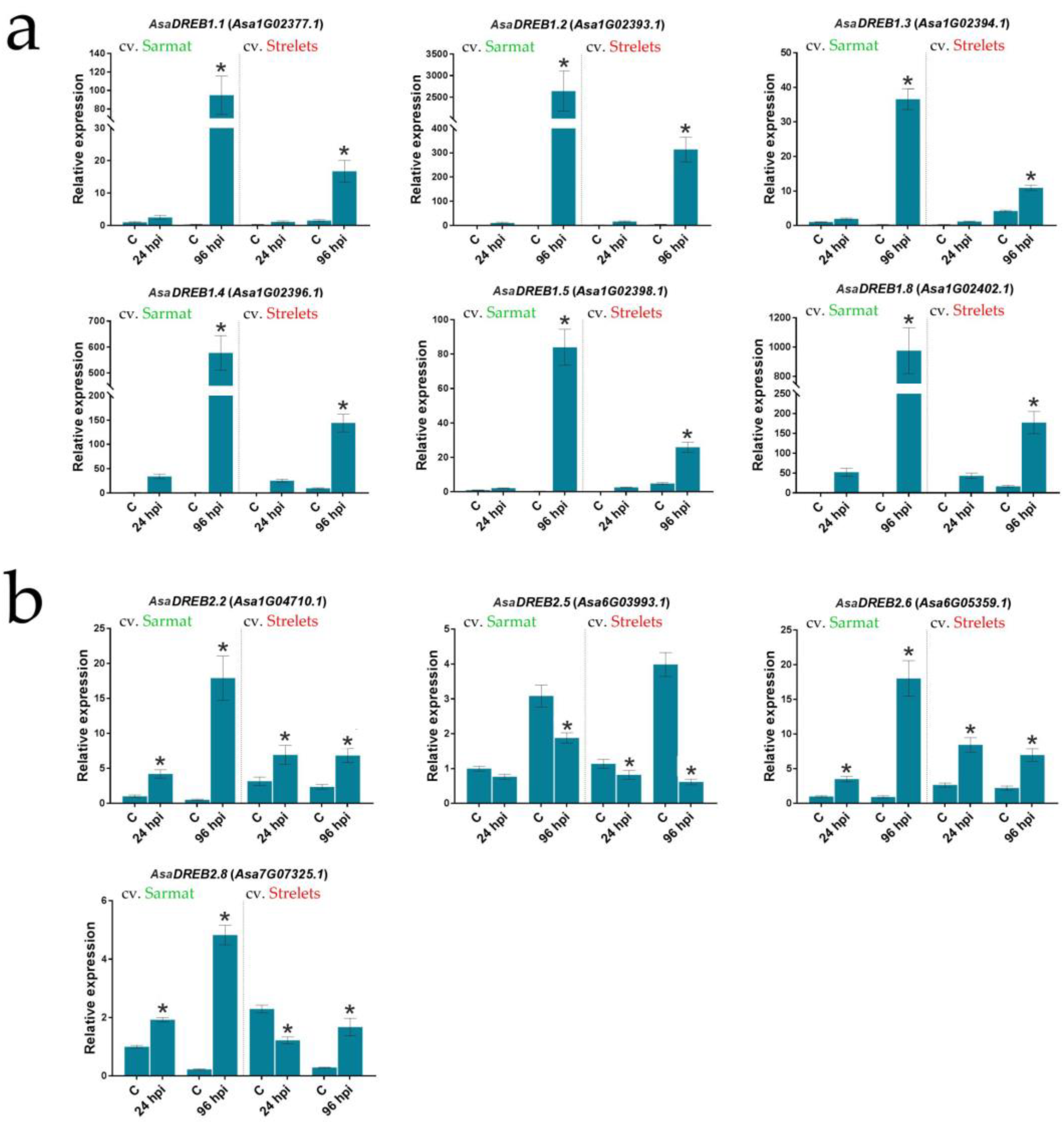
2.5. AsaDREB1 and AsaDREB2 Expression in Garlic Seedlings in Response to Fusarium Infection
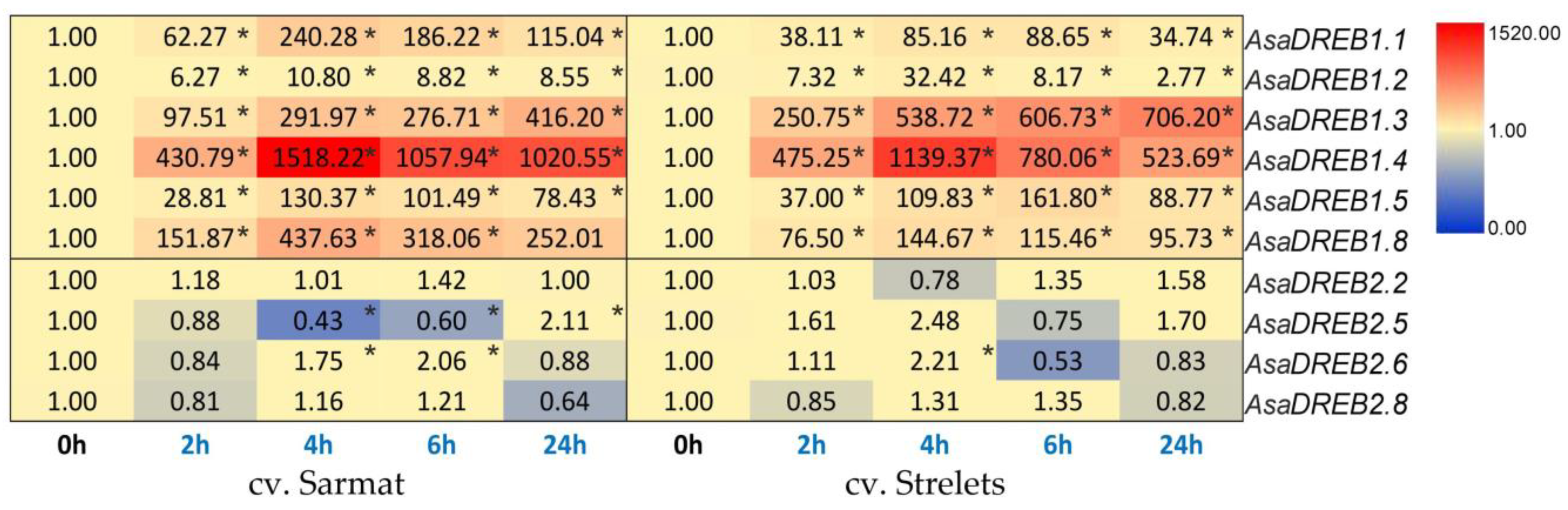
2.6. AsaDREB1 and AsaDREB2 Expression in Garlic Seedlings in Response to Cold Stress
3. Discussion
4. Materials and Methods
4.1. AsaDREB1 and AsaDREB2 Gene Subfamilies: Identification and Analysis
4.2. AsaDREB1 and AsaDREB2 Gene Expression: In Silico Analysis
4.3. cis-Regulatory Elements in the Promoters of the AsaDREB1 and AsaDREB2 Genes: In Silico Search
4.4. Garlic Cultivars and F. proliferatum Strain
4.5. Stress Assays
4.6. AsaDREB Gene Expression Pattern in Garlic Cultivars: Quantitative (q) Real-Time (RT)-PCR Analysis
4.7. Statistical Analysis of the AsaDREB Gene Expression Data
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sun, X.; Zhu, S.; Li, N.; Cheng, Y.; Zhao, J.; Qiao, X.; Lu, L.; Liu, S.; Wang, Y.; Liu, C.; et al. A Chromosome-level genome assembly of garlic (Allium sativum) provides insights into genome evolution and allicin biosynthesis. Mol. Plant 2020, 13, 1328–1339. [Google Scholar] [CrossRef]
- Kıraç, H.; Dalda Şekerci, A.; Coşkun, Ö.F.; Gülşen, O. Morphological and molecular characterization of garlic (Allium sativum L.) genotypes sampled from Turkey. Genet. Resour. Crop Evol. 2022, 69, 1833–1841. [Google Scholar] [CrossRef] [PubMed]
- Shemesh, E.; Scholten, O.; Rabinowitch, H.D.; Kamenetsky, R. Unlocking variability: Inherent variation and developmental traits of garlic plants originated from sexual reproduction. Planta 2008, 227, 1013–1024. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Q.; Guo, C.; Yang, S.; Zhong, Q.; Tian, J. Screening and verification of reference genes for analysis of gene expression in garlic (Allium sativum L.) under cold and drought stress. Plants 2023, 12, 763. [Google Scholar] [CrossRef] [PubMed]
- El-Saber Batiha, G.; Magdy Beshbishy, A.; Wasef, L.G.; Elewa, Y.H.A.; Al-Sagan, A.A.; Abd El-Hack, M.E.; Taha, A.E.; Abd-Elhakim, Y.M.; Prasad Devkota, H. Chemical constituents and pharmacological activities of garlic (Allium sativum L.): A Review. Nutrients 2020, 12, 872. [Google Scholar] [CrossRef] [Green Version]
- Luo, M.; Wang, Z.; Yang, B.; Zheng, L.; Yao, Z.; Ahmet Seyrek, U.; Chung, H.; Wei, H. Effects of winter cover crops on rice pests, natural enemies, and grain yield in a rice rotation system. J. Insect. Sci. 2019, 19, 25. [Google Scholar] [CrossRef]
- Nishioka, T.; Marian, M.; Kobayashi, I.; Kobayashi, Y.; Yamamoto, K.; Tamaki, H.; Suga, H.; Shimizu, M. Microbial basis of Fusarium wilt suppression by Allium cultivation. Sci. Rep. 2019, 9, 1715. [Google Scholar] [CrossRef]
- Bian, X.; Yang, X.; Li, Q.; Sun, X. Effects of planting of two common crops, Allium fistulosum and Brassica napus, on soil properties and microbial communities of ginseng cultivation in northeast China. BMC Microbiol. 2022, 22, 182. [Google Scholar] [CrossRef]
- Pavlou, G.C.; Vakalounakis, D.J.; Ligoxigakis, E.K. Control of root and stem rot of cucumber, caused by Fusarium oxysporum f. sp. radicis-cucumerinum, by grafting onto resistant rootstocks. Plant Dis. 2002, 86, 379–382. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Wang, T.; He, C.; Cheng, K.; Zeng, R.; Song, Y. Control of Panama disease of banana by intercropping with Chinese chive (Allium tuberosum Rottler): Cultivar differences. BMC Plant Biol. 2020, 20, 432. [Google Scholar] [CrossRef]
- Egea, L.A.; Mérida-García, R.; Kilian, A.; Hernandez, P.; Dorado, G. Assessment of genetic diversity and structure of large garlic (Allium sativum L.) germplasm bank, by diversity arrays technology “Genotyping-by-Sequencing” platform (DArTseq). Front. Genet. 2017, 8, 98. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Casals, J.; Rivera, A.; Campo, S.; Aymerich, E.; Isern, H.; Fenero, D.; Garriga, A.; Palou, A.; Monfort, A.; Howad, W.; et al. Phenotypic diversity and distinctiveness of the Belltall garlic landrace. Front. Plant Sci. 2023, 13, 1004069. [Google Scholar] [CrossRef] [PubMed]
- Son, J.H.; Park, K.C.; Lee, S.I.; Kim, H.H.; Kim, J.H.; Kim, S.H.; Kim, N.S. Isolation of cold-responsive genes from garlic, Allium sativum. Genes Genom. 2012, 34, 93–101. [Google Scholar] [CrossRef]
- Figueroa, T.; López-Hernández, L.; Lopez, M.G.; Hurtado, M.D.; Vázquez-Barrios, M.E.; Guevara-Olvera, L.; Guevara González, R.G.; Rivera-Pastrana, D.M.; Torres-Robles, H.; Mercado-Silva, E.M. Conditioning garlic “seed” cloves at low temperature modifies plant growth, sugar, fructan content, and sucrose sucrose fructosyl transferase (1-SST) expression. Sci. Hortic. 2015, 189, 150–158. [Google Scholar] [CrossRef]
- Diaz, R.; Chavez, E.C.; Delgado Ortiz, J.C.; Velazquez, J.J.; Roque, A.; Ochoa, Y.M. First report of Clonostachys rosea causing root rot on garlic in Mexico. Plant Dis. 2022, 106, 3000. [Google Scholar] [CrossRef]
- Han, J.; Thamilarasan, S.K.; Natarajan, S.; Park, J.I.; Chung, M.Y.; Nou, I.S. De Novo assembly and transcriptome analysis of bulb onion (Allium cepa L.) during cold acclimation using contrasting genotypes. PLoS ONE 2016, 11, e0161987. [Google Scholar] [CrossRef] [PubMed]
- Anisimova, O.K.; Kochieva, E.Z.; Shchennikova, A.V.; Filyushin, M.A. Thaumatin-like protein (TLP) genes in garlic (Allium sativum L.): Genome-wide identification, characterization, and expression in response to Fusarium proliferatum infection. Plants 2022, 11, 748. [Google Scholar] [CrossRef]
- Filyushin, M.A.; Anisimova, O.K.; Shchennikova, A.V.; Kochieva, E.Z. Genome-wide identification, expression, and response to Fusarium infection of the SWEET gene family in garlic (Allium sativum L.). Int. J. Mol. Sci. 2023, 24, 7533. [Google Scholar] [CrossRef]
- Anisimova, O.K.; Shchennikova, A.V.; Kochieva, E.Z.; Filyushin, M.A. Pathogenesis-related genes of PR1, PR2, PR4, and PR5 families are involved in the response to Fusarium infection in garlic (Allium sativum L.). Int. J. Mol. Sci. 2021, 22, 6688. [Google Scholar] [CrossRef]
- Filyushin, M.A.; Anisimova, O.K.; Kochieva, E.Z.; Shchennikova, A.V. Genome-wide identification and expression of chitinase class i genes in garlic (Allium sativum L.) cultivars resistant and susceptible to Fusarium proliferatum. Plants 2021, 10, 720. [Google Scholar] [CrossRef]
- De Vleesschauwer, D.; Xu, J.; Höfte, M. Making sense of hormone-mediated defense networking: From rice to Arabidopsis. Front. Plant Sci. 2014, 5, 611. [Google Scholar] [CrossRef]
- Waadt, R.; Seller, C.A.; Hsu, P.K.; Takahashi, Y.; Munemasa, S.; Schroeder, J.I. Plant hormone regulation of abiotic stress responses. Nat. Rev. Mol. Cell Biol. 2022, 23, 680–694. [Google Scholar] [CrossRef] [PubMed]
- Baillo, E.H.; Kimotho, R.N.; Zhang, Z.; Xu, P. transcription factors associated with abiotic and biotic stress tolerance and their potential for crops improvement. Genes 2019, 10, 771. [Google Scholar] [CrossRef] [Green Version]
- Sakuma, Y.; Liu, Q.; Dubouzet, J.G.; Abe, H.; Yamaguchi-Shinozaki, K.; Shinozaki, K. DNA-binding specificity of the ERF/AP2 domain of Arabidopsis DREBs, transcription factors involved in dehydration- and cold-inducible gene expression. Biochem. Biophys. Res. Commun. 2002, 290, 998–1009. [Google Scholar] [CrossRef]
- Shi, Y.; Ding, Y.; Yang, S. Molecular Regulation of CBF Signaling in Cold Acclimation. Trends Plant Sci. 2018, 23, 623–637. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Lee, C.M.; Doherty, C.J.; Gilmour, S.J.; Kim, Y.; Thomashow, M.F. Regulation of the Arabidopsis CBF regulon by a complex low-temperature regulatory network. Plant J. 2015, 82, 193–207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.; Fowler, S.G.; Cheng, H.; Lou, Y.; Rhee, S.Y.; Stockinger, E.J.; Thomashow, M.F. Freezing-sensitive tomato has a functional CBF cold response pathway, but a CBF regulon that differs from that of freezing-tolerant Arabidopsis. Plant J. 2004, 39, 905–919. [Google Scholar] [CrossRef]
- Shen, Y.G.; Zhang, W.K.; He, S.J.; Zhang, J.S.; Liu, Q.; Chen, S.Y. An EREBP/AP2-type protein in Triticum aestivum was a DRE-binding transcription factor induced by cold, dehydration and ABA stress. Theor. Appl. Genet. 2003, 106, 923–930. [Google Scholar] [CrossRef]
- Agarwal, P.K.; Agarwal, P.; Reddy, M.K.; Sopory, S.K. Role of DREB transcription factors in abiotic and biotic stress tolerance in plants. Plant Cell Rep. 2006, 25, 1263–1274. [Google Scholar] [CrossRef]
- Akbudak, M.A.; Filiz, E.; Kontbay, K. DREB2 (dehydration-responsive element-binding protein 2) type transcription factor in sorghum (Sorghum bicolor): Genome-wide identification, characterization and expression profiles under cadmium and salt stresses. BioTech 2018, 8, 426. [Google Scholar] [CrossRef]
- Bihani, P.; Char, B.; Bhargava, S. Transgenic expression of sorghum DREB2 in rice improves tolerance and yield under water limitation. J. Agric. Sci. 2011, 149, 95–101. [Google Scholar] [CrossRef]
- Tsutsui, T.; Kato, W.; Asada, Y.; Sako, K.; Sato, T.; Sonoda, Y.; Kidokoro, S.; Yamaguchi-Shinozaki, K.; Tamaoki, M.; Arakawa, K.; et al. DEAR1, a transcriptional repressor of DREB protein that mediates plant defense and freezing stress responses in Arabidopsis. J. Plant Res. 2009, 122, 633–643. [Google Scholar] [CrossRef]
- Maruyama, Y.; Yamoto, N.; Suzuki, Y.; Chiba, Y.; Yamazaki, K.; Sato, T.; Yamaguchi, J. The Arabidopsis transcriptional repressor ERF9 participates in resistance against necrotrophic fungi. Plant Sci. 2013, 213, 79–87. [Google Scholar] [CrossRef]
- Zhang, J.; Wei, B.; Wen, R.; Liu, Y.; Wang, Z. Genetically modified sugarcane intercropping soybean impact on rhizosphere bacterial communities and co-occurrence patterns. Front. Microbiol. 2021, 12, 742341. [Google Scholar] [PubMed]
- Choudhary, S.; Naika, M.B.N.; Sharma, R.; Meena, R.D.; Singh, R.; Lal, G. Transcriptome profiling of coriander: A dual purpose crop unravels stem gall resistance genes. J. Genet. 2019, 98, 19. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Cai, M.; Zhang, S.; Chai, J.; Sun, M.; Wang, Y.; Xie, Q.; Chen, Y.; Wang, H.; Chen, T. Genome-wide identification of AP2/ERF transcription factor family and functional analysis of DcAP2/ERF#96 associated with abiotic stress in Dendrobium catenatum. Int. J. Mol. Sci. 2022, 23, 13603. [Google Scholar]
- Li, Z.; Wang, G.; Liu, X.; Wang, Z.; Zhang, M.; Zhang, J. Genome-wide identification and expression profiling of DREB genes in Saccharum spontaneum. BMC Genom. 2021, 22, 456. [Google Scholar] [CrossRef]
- Nakano, T.; Suzuki, K.; Fujimura, T.; Shinshi, H. Genome-wide analysis of the ERF gene family in Arabidopsis and rice. Plant Physiol. 2006, 140, 411–432. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Han, G.; Sun, C.; Sui, N. Research advances of MYB transcription factors in plant stress resistance and breeding. Plant Signal Behav. 2019, 14, 1613131. [Google Scholar] [CrossRef]
- Wang, J.; Song, L.; Gong, X.; Xu, J.; Li, M. Functions of jasmonic acid in plant regulation and response to abiotic stress. Int. J. Mol. Sci. 2020, 21, 1446. [Google Scholar] [CrossRef] [Green Version]
- Fujiwara, T.; Beachy, R.N. Tissue-specific and temporal regulation of a beta-conglycinin gene: Roles of the RY repeat and other cis-acting elements. Plant Mol. Biol. 1994, 24, 261–272. [Google Scholar] [CrossRef] [PubMed]
- Ito, M. Factors controlling cyclin B expression. Plant Mol. Biol. 2000, 43, 677–690. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Lyu, H.M.; Zhu, K.; Van de Peer, Y.; Max Cheng, Z.M. The emergence and evolution of intron-poor and intronless genes in intron-rich plant gene families. Plant J. 2021, 105, 1072–1082. [Google Scholar] [CrossRef] [PubMed]
- Je, J.; Chen, H.; Song, C.; Lim, C.O. Arabidopsis DREB2C modulates ABA biosynthesis during germination. Biochem. Biophys. Res. Commun. 2014, 452, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Je, J.; Song, C.; Hwang, J.E.; Lim, C.O. A proximal promoter region of Arabidopsis DREB2C confers tissue-specific expression under heat stress. J. Integr. Plant Biol. 2012, 54, 640–651. [Google Scholar] [CrossRef]
- Hwang, J.E.; Lim, C.J.; Chen, H.; Je, J.; Song, C.; Lim, C.O. Overexpression of Arabidopsis dehydration-responsive element-binding protein 2C confers tolerance to oxidative stress. Mol. Cells. 2012, 33, 135–140. [Google Scholar] [CrossRef] [Green Version]
- Lim, C.J.; Hwang, J.E.; Chen, H.; Hong, J.K.; Yang, K.A.; Choi, M.S.; Lee, K.O.; Chung, W.S.; Lee, S.Y.; Lim, C.O. Over-expression of the Arabidopsis DRE/CRT-binding transcription factor DREB2C enhances thermotolerance. Biochem. Biophys. Res. Commun. 2007, 362, 431–436. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular evolutionary genetics analysis version 7.0. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef] [Green Version]
- Hu, B.; Jin, J.; Guo, A.Y.; Zhang, H.; Luo, J.; Gao, G. GSDS 2.0: An Upgraded Gene Feature Visualization Server. Bioinformatics 2015, 31, 1296–1297. [Google Scholar] [CrossRef] [Green Version]
- Babicki, S.; Arndt, D.; Marcu, A.; Liang, Y.; Grant, J.R.; Maciejewski, A.; Wishart, D.S. Heatmapper: Web-enabled heat mapping for all. Nucl. Acids Res. 2016, 44, W147–W153. [Google Scholar] [CrossRef]
- Schwinn, K.E.; Ngo, H.; Kenel, F.; Brummell, D.A.; Albert, N.W.; McCallum, J.A.; Pither-Joyce, M.; Crowhurst, R.N.; Eady, C.; Davies, K.M. The onion (Allium cepa L.) R2R3-MYB gene MYB1 regulates anthocyanin biosynthesis. Front. Plant Sci. 2016, 7, 1865. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, M.; Wu, Z.; Jiang, F. Selection and validation of garlic reference genes for quantitative real-time PCR normalization. Plant Cell Tissue Organ Cult. 2015, 122, 435–444. [Google Scholar] [CrossRef]
- Chen, M.; Wang, Q.; Li, Y.; Gao, L.; Lv, F.; Yang, R.; Wang, P. Candidate reference genes for quantitative gene expression analysis in Lagerstroemia indica. Mol. Biol. Rep. 2021, 48, 1677–1685. [Google Scholar] [CrossRef] [PubMed]
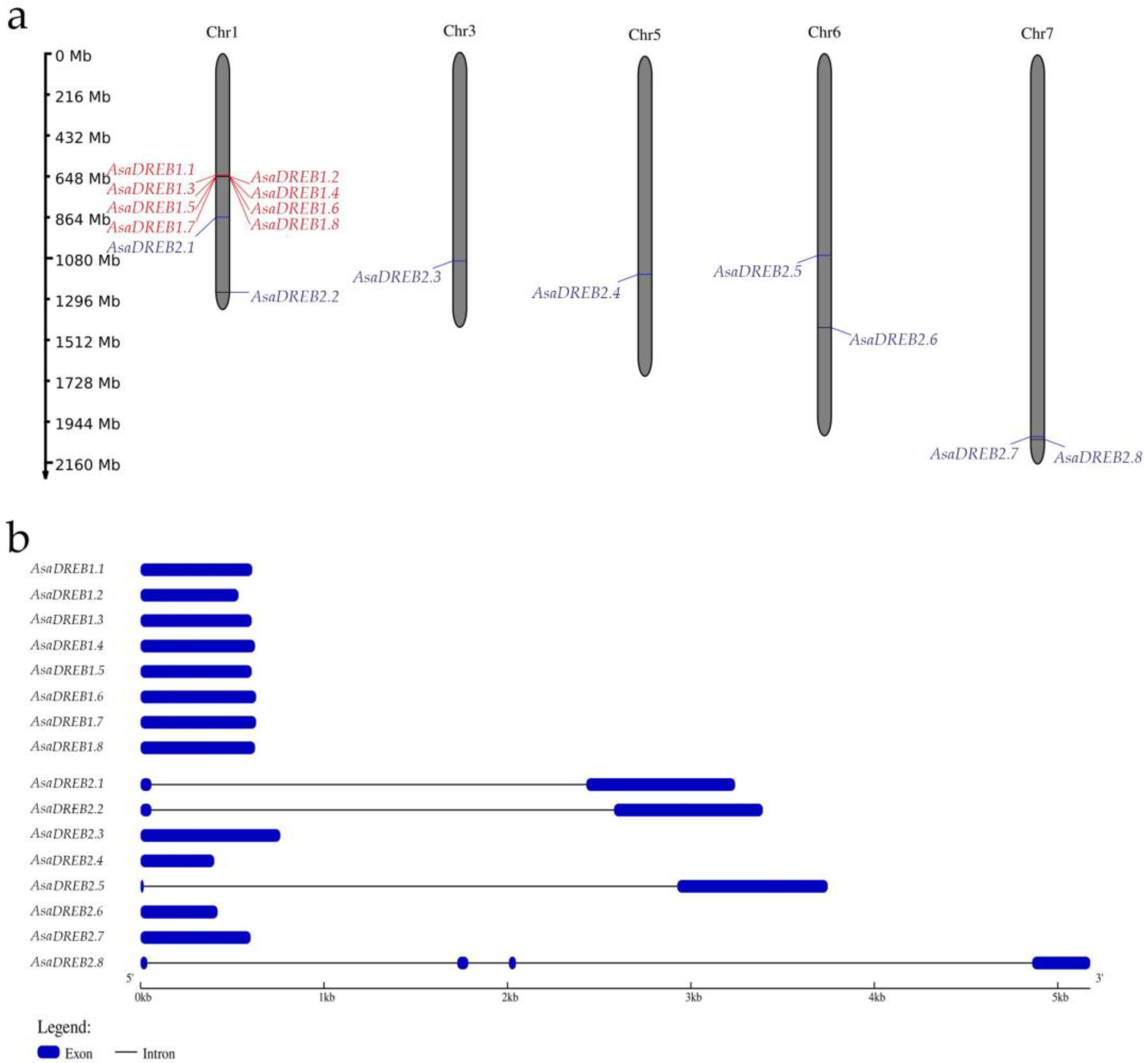
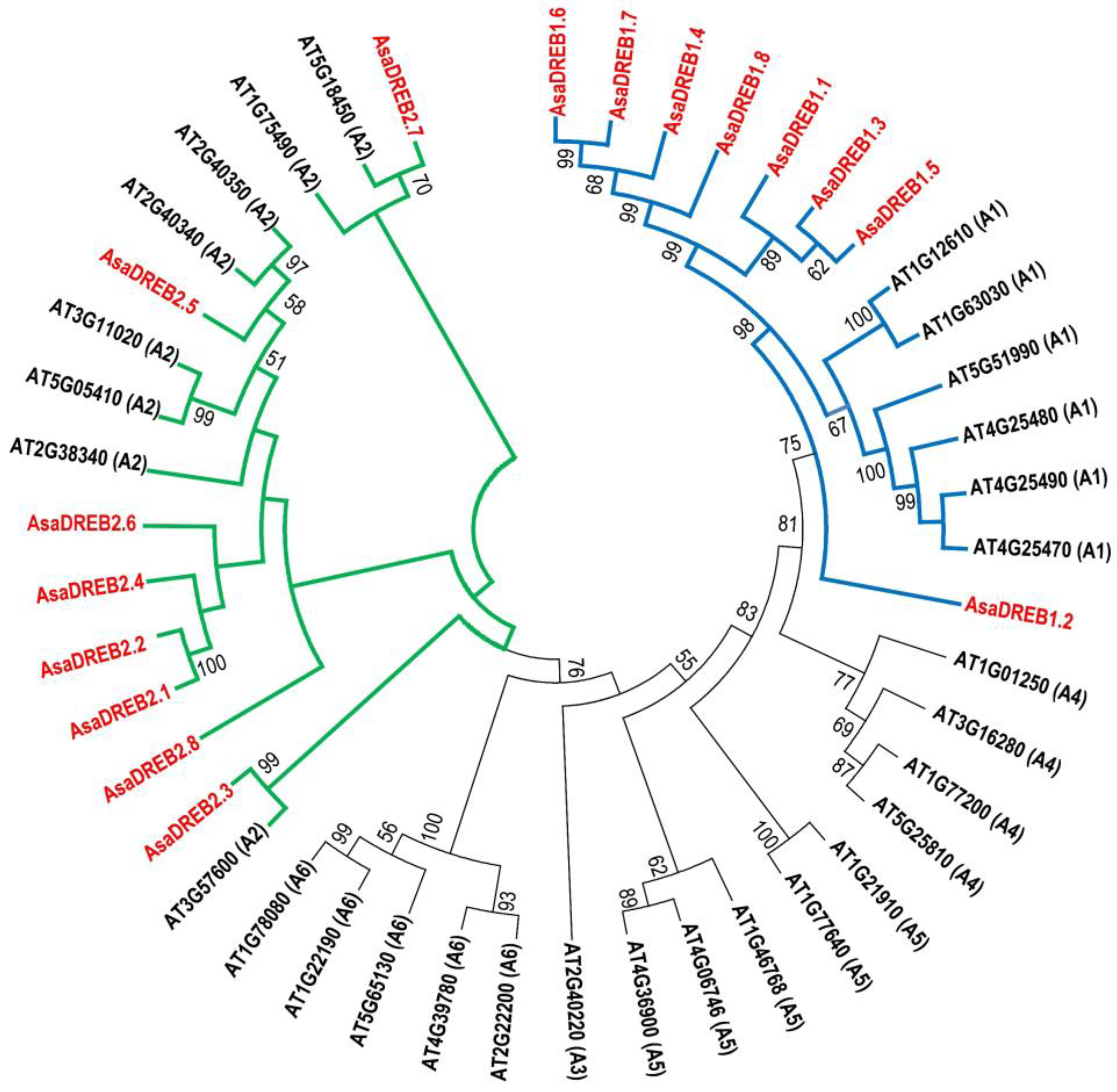
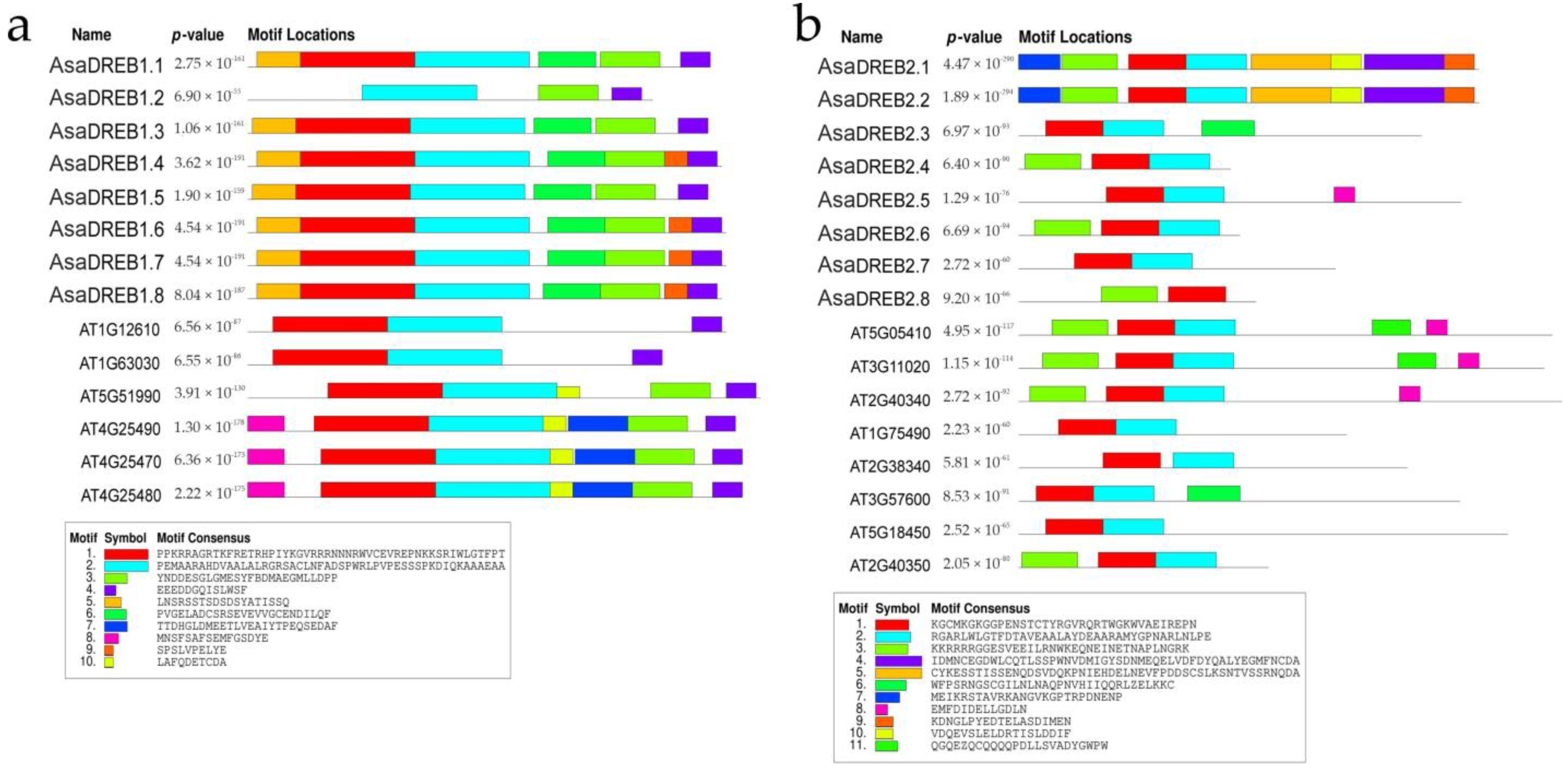

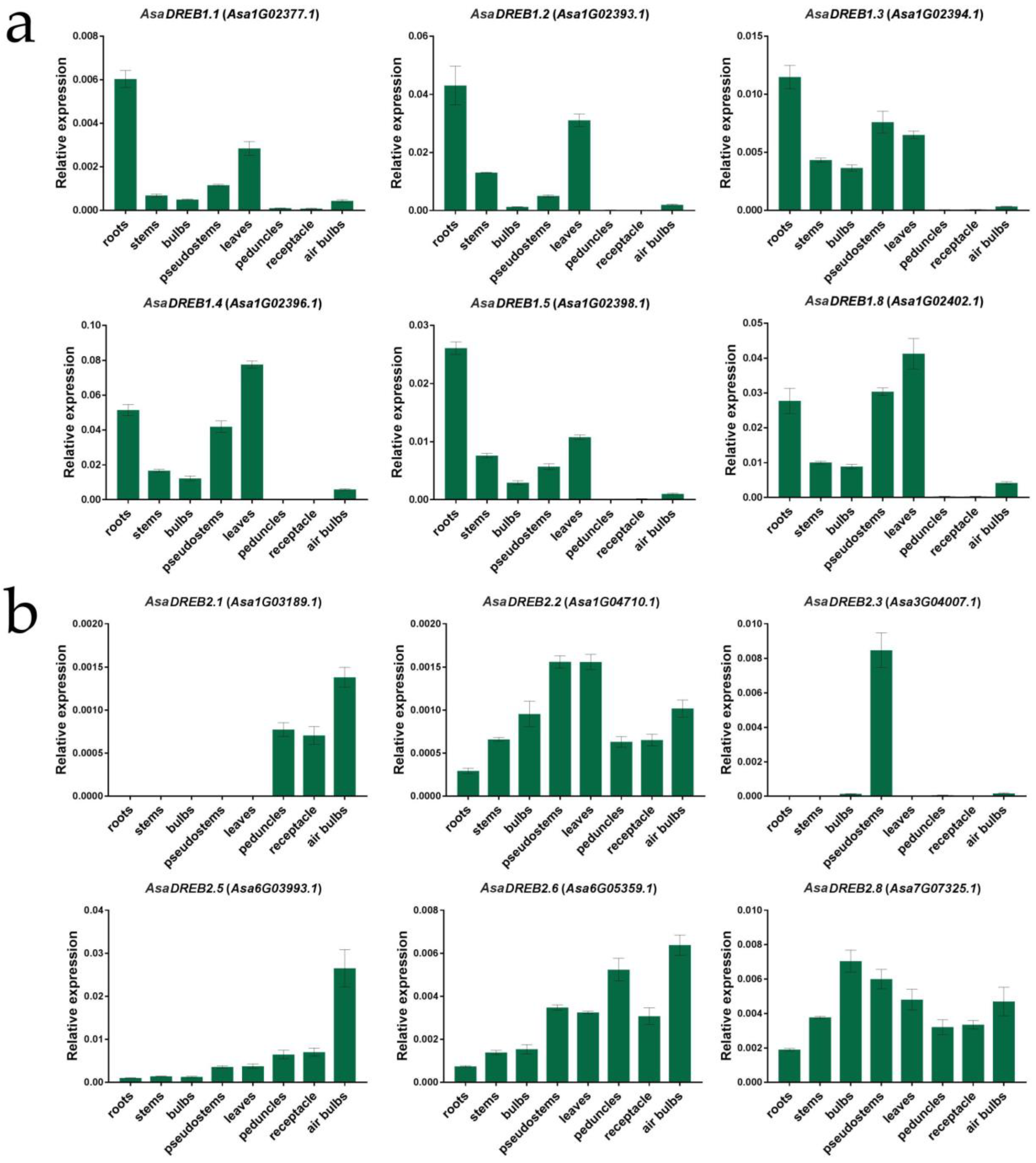
| Gene Name | Gene/Transript ID [1] | Genomic Localization | Gene, bp | CDS, bp | Protein, aa | MW, kDa | pI |
|---|---|---|---|---|---|---|---|
| DREB1 subfamily | |||||||
| AsaDREB1.1 | Asa1G02377.1/Asa2G02428.1 | chr1:643013697-643014305 | 609 | 609 | 202 | 22.68 | 5.35 |
| AsaDREB1.2 | Asa1G02393.1/Asa2G02432.1 | chr1:647143533-647144066 | 534 | 534 | 177 | 19.12 | 4.70 |
| AsaDREB1.3 | Asa1G02394.1/Asa2G02430.1 | chr1:647572970-647573575 | 606 | 606 | 201 | 22.44 | 5.03 |
| AsaDREB1.4 | Asa1G02396.1/Asa2G02427.1 | chr1:648016507-648017130 | 624 | 624 | 207 | 23.00 | 5.31 |
| AsaDREB1.5 | Asa1G02398.1/Asa2G02425.1 | chr1:649128626-649129231 | 606 | 606 | 201 | 22.34 | 5.18 |
| AsaDREB1.6 | Asa1G02399.1/Asa2G02422.1 | chr1:649153916-649154545 | 630 | 630 | 209 | 23.11 | 5.77 |
| AsaDREB1.7 | Asa1G02401.1/Asa2G02424.1 | chr1:649317409-649318038 | 630 | 630 | 209 | 23.11 | 5.77 |
| AsaDREB1.1 | Asa1G02377.1/Asa2G02428.1 | chr1:643013697-643014305 | 609 | 609 | 202 | 22.68 | 5.35 |
| DREB2 subfamily | |||||||
| AsaDREB2.1 | Asa1G03189.1/Asa2G01510.1 | chr1:865525192-865528432 | 3241 | 870 | 289 | 32.67 | 4.97 |
| AsaDREB2.2 | Asa1G04710.1/Asa0G03205.1 | chr1:1262498222-1262501613 | 3392 | 870 | 289 | 32.63 | 5.29 |
| AsaDREB2.3 | Asa3G04007.1/Asa1G05763.1 | chr3:1103416519-1103417280 | 762 | 762 | 253 | 28.07 | 6.11 |
| AsaDREB2.4 | Asa5G04281.1/Asa5G01946.1 | chr5:1152951019-1152951420 | 402 | 402 | 133 | 14.98 | 9.91 |
| AsaDREB2.5 | Asa6G03993.1/Asa6G04032.1 | chr6:1068494972-1068498718 | 3746 | 837 | 278 | 30.57 | 4.86 |
| AsaDREB2.6 | Asa6G05359.1/Asa6G02115.1 | chr6:1450079651-1450080070 | 420 | 420 | 139 | 15.55 | 9.69 |
| AsaDREB2.7 | Asa7G07291.1/Asa5G06740.1 | chr7_2:939903143-939903742 | 600 | 600 | 199 | 21.65 | 4.93 |
| AsaDREB2.8 | Asa7G07325.1/Asa5G06785.1 | chr7_2:949089009-949094185 | 5177 | 450 | 149 | 16.86 | 9.76 |
| Motif Name | Annotation | DREB1 | DREB2 | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AsaDREB1.1 | AsaDREB1.2 | AsaDREB1.3 | AsaDREB1.4 | AsaDREB1.5 | AsaDREB1.6 | AsaDREB1.7 | AsaDREB1.8 | AsaDREB2.1 | AsaDREB2.2 | AsaDREB2.3 | AsaDREB2.4 | AsaDREB2.5 | AsaDREB2.6 | AsaDREB2.7 | AsaDREB2.8 | ||
| Hormone response | |||||||||||||||||
| ABRE | ABA-responsive | 4 | 2 | 8 | 3 | 4 | 2 | 2 | 2 | 1 | 2 | 4 | 1 | 5 | 2 | ||
| CARE | 1 | 1 | |||||||||||||||
| TGA-element | Auxin-responsive | 1 | 1 | 1 | |||||||||||||
| CGTCA-motif | JA-responsive | 1 | 1 | 1 | 1 | 2 | 2 | 1 | 1 | 1 | 2 | ||||||
| TCA-element | 1 | 1 | 1 | ||||||||||||||
| P-box | GA-responsive | 1 | 1 | ||||||||||||||
| ERE | ET-responsive | 1 | 1 | 1 | 1 | 1 | 1 | ||||||||||
| Stress response | |||||||||||||||||
| ARE | Essential for the anaerobic induction | 4 | 1 | 1 | 1 | 5 | 5 | 1 | 1 | 1 | 1 | 2 | |||||
| DRE1/DRE core | Drought responsive | 1 | |||||||||||||||
| LTR | Low-temperature response | 1 | 2 | 4 | 1 | 1 | 1 | 1 | |||||||||
| STRE | Response to heat, osmotic stress, low pH, and nutrient starvation | 2 | 5 | 1 | 1 | 2 | 3 | ||||||||||
| TC-rich repeats | Defense and stress response | 1 | 1 | 1 | |||||||||||||
| W-box | WRKY binding site; defense and biotic and abiotic stress response | 1 | 1 | 1 | 1 | 1 | |||||||||||
| Wun-motif | Wounding and pathogen response | 1 | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | |||||||
| WRE3 | 1 | 1 | 2 | 2 | 1 | 1 | 1 | ||||||||||
| Box S | 1 | ||||||||||||||||
| Developmental processes | |||||||||||||||||
| O2-site | Nitrogen-responsive; endosperm-specific gene expression | 3 | 1 | 1 | 1 | 1 | |||||||||||
| GCN4 motif | 1 | ||||||||||||||||
| CCGTCC motif | Meristem-specific gene expression | 1 | 1 | 1 | |||||||||||||
| RY-element | Seed-specific gene expression | 1 | 1 | ||||||||||||||
| MSA-like | Cell cycle regulation | 1 | 1 | ||||||||||||||
| Other cis-elements | |||||||||||||||||
| CCAAT-box/MYB/MRE/MBS1 | MYB-binding site | 2 | 2 | 5 | 1 | 7 | 1 | 4 | 3 | 3 | 3 | 2 | 5 | 2 | 5 | 2 | 3 |
| Re2f-1 | E2F-binding sites; seed-specific, cell-cycle regulated gene expression | 1 | 1 | ||||||||||||||
| E2Fb | 1 | 1 | 1 | 1 | |||||||||||||
| MYC | MYC-binding site | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 4 | 3 | 1 | 4 | 1 | ||||
| CTAG-motif | Unknown | 1 | 1 | 1 | |||||||||||||
| A-box (TACGTA) | bZIP-binding site | 1 | |||||||||||||||
| HD-Zip IV | HD-Zip IV-binding site | 1 | |||||||||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Filyushin, M.A.; Anisimova, O.K.; Shchennikova, A.V.; Kochieva, E.Z. DREB1 and DREB2 Genes in Garlic (Allium sativum L.): Genome-Wide Identification, Characterization, and Stress Response. Plants 2023, 12, 2538. https://doi.org/10.3390/plants12132538
Filyushin MA, Anisimova OK, Shchennikova AV, Kochieva EZ. DREB1 and DREB2 Genes in Garlic (Allium sativum L.): Genome-Wide Identification, Characterization, and Stress Response. Plants. 2023; 12(13):2538. https://doi.org/10.3390/plants12132538
Chicago/Turabian StyleFilyushin, Mikhail A., Olga K. Anisimova, Anna V. Shchennikova, and Elena Z. Kochieva. 2023. "DREB1 and DREB2 Genes in Garlic (Allium sativum L.): Genome-Wide Identification, Characterization, and Stress Response" Plants 12, no. 13: 2538. https://doi.org/10.3390/plants12132538






