Effect of Zinc Foliar Fertilization Alone and Combined with Trehalose on Maize (Zea mays L.) Growth under the Drought
Abstract
:1. Introduction
2. Results
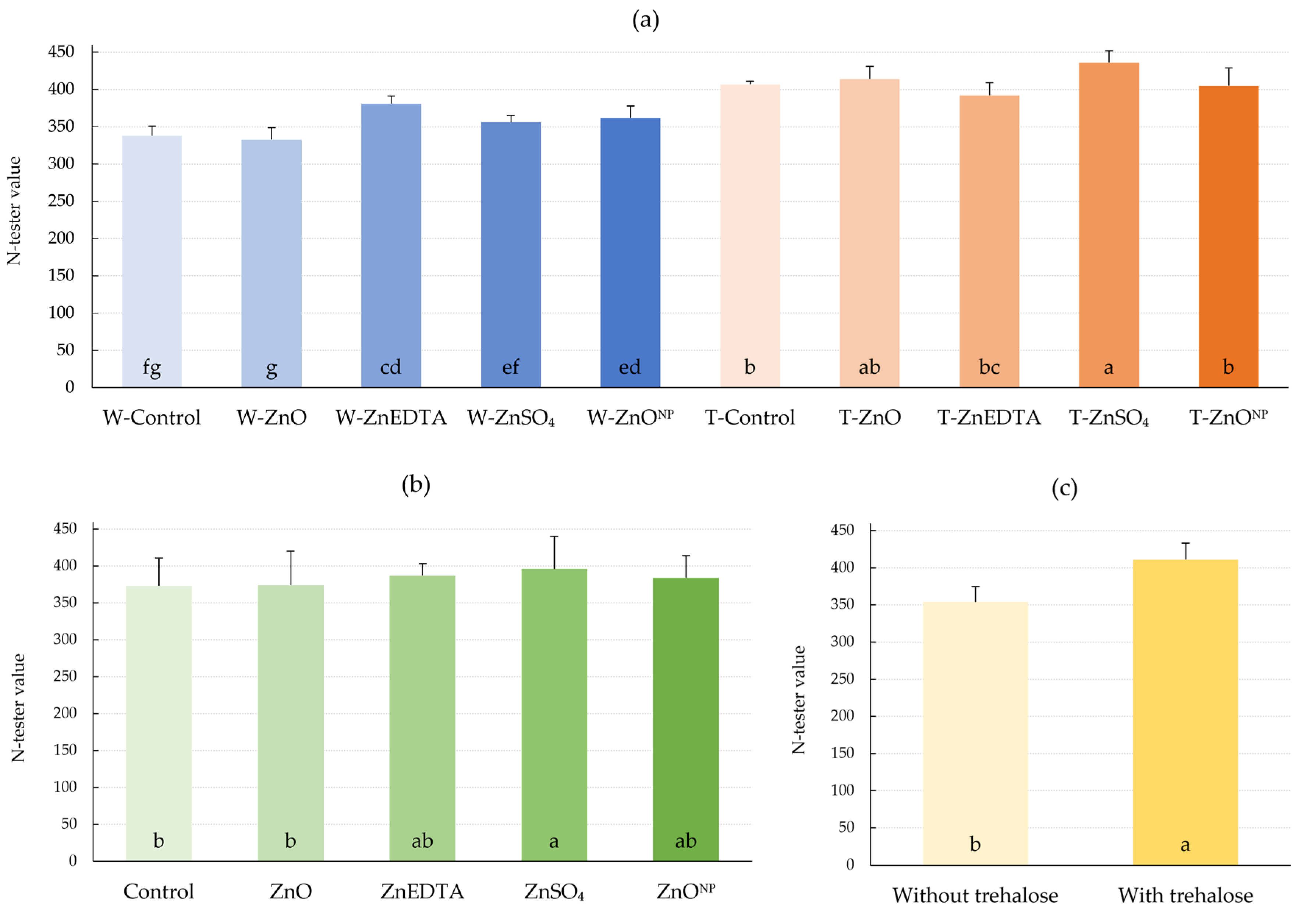
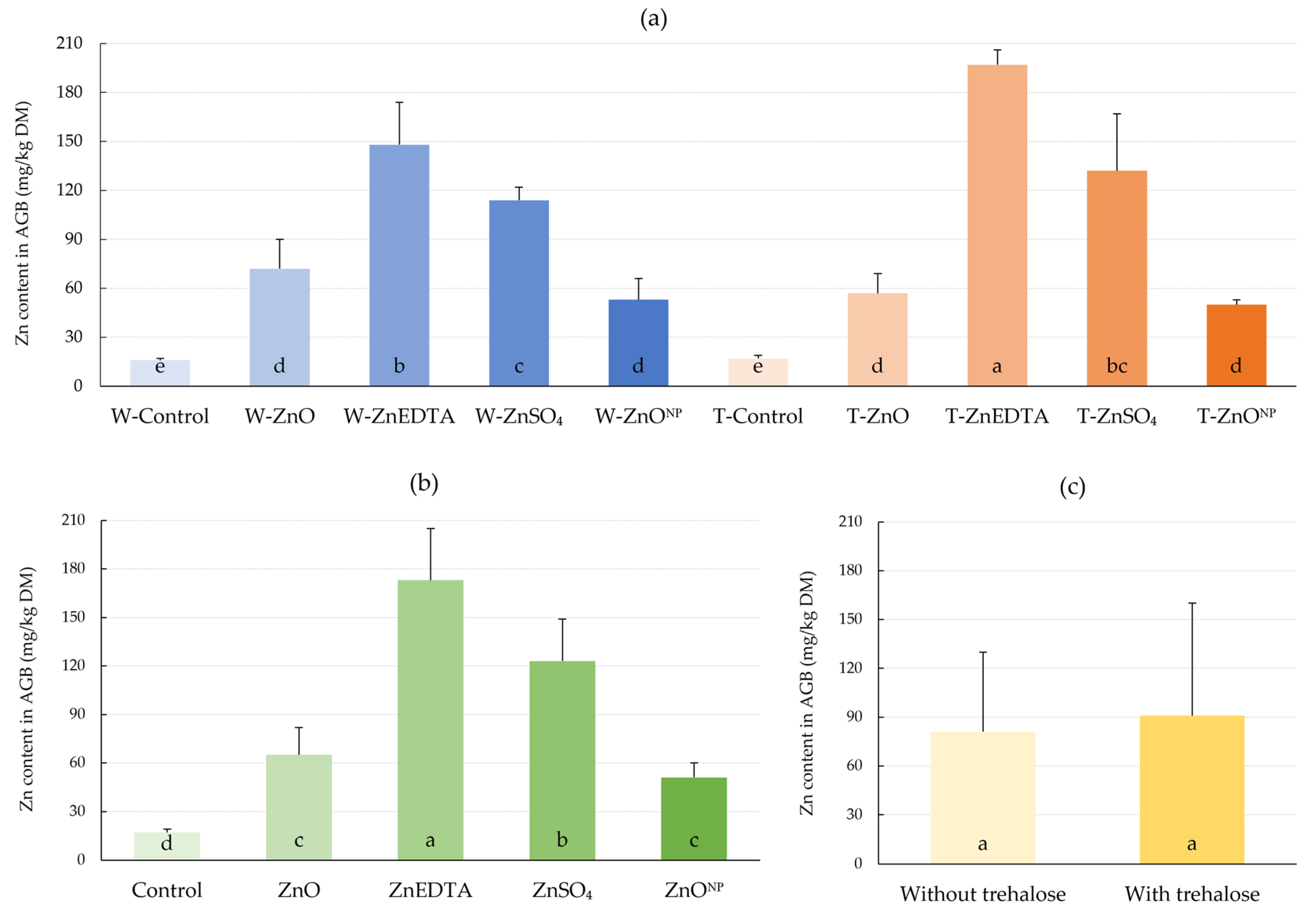
2.1. N-Tester Value and Zinc Content in Plant
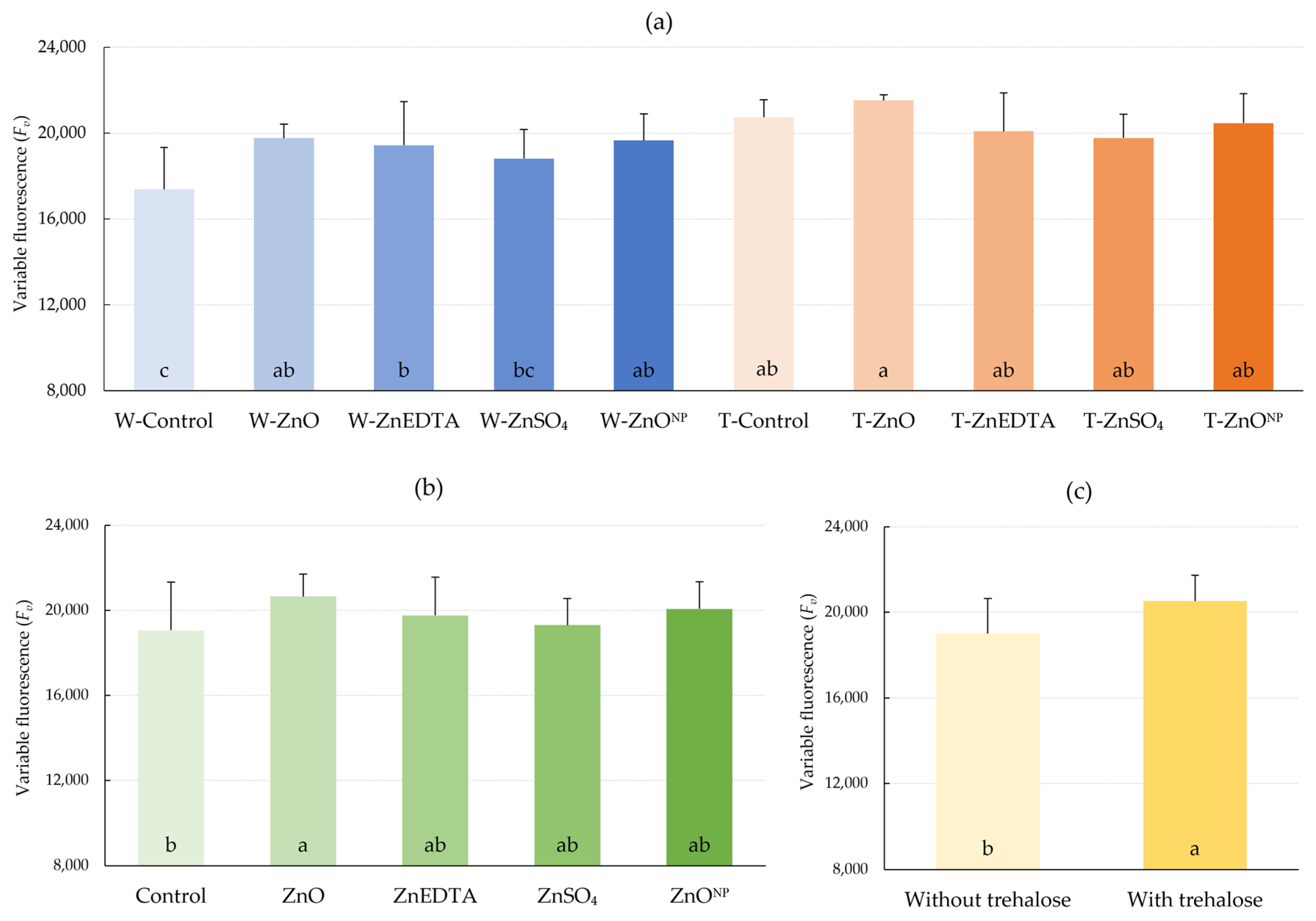
2.2. Chlorophyll Fluorescence Parameters
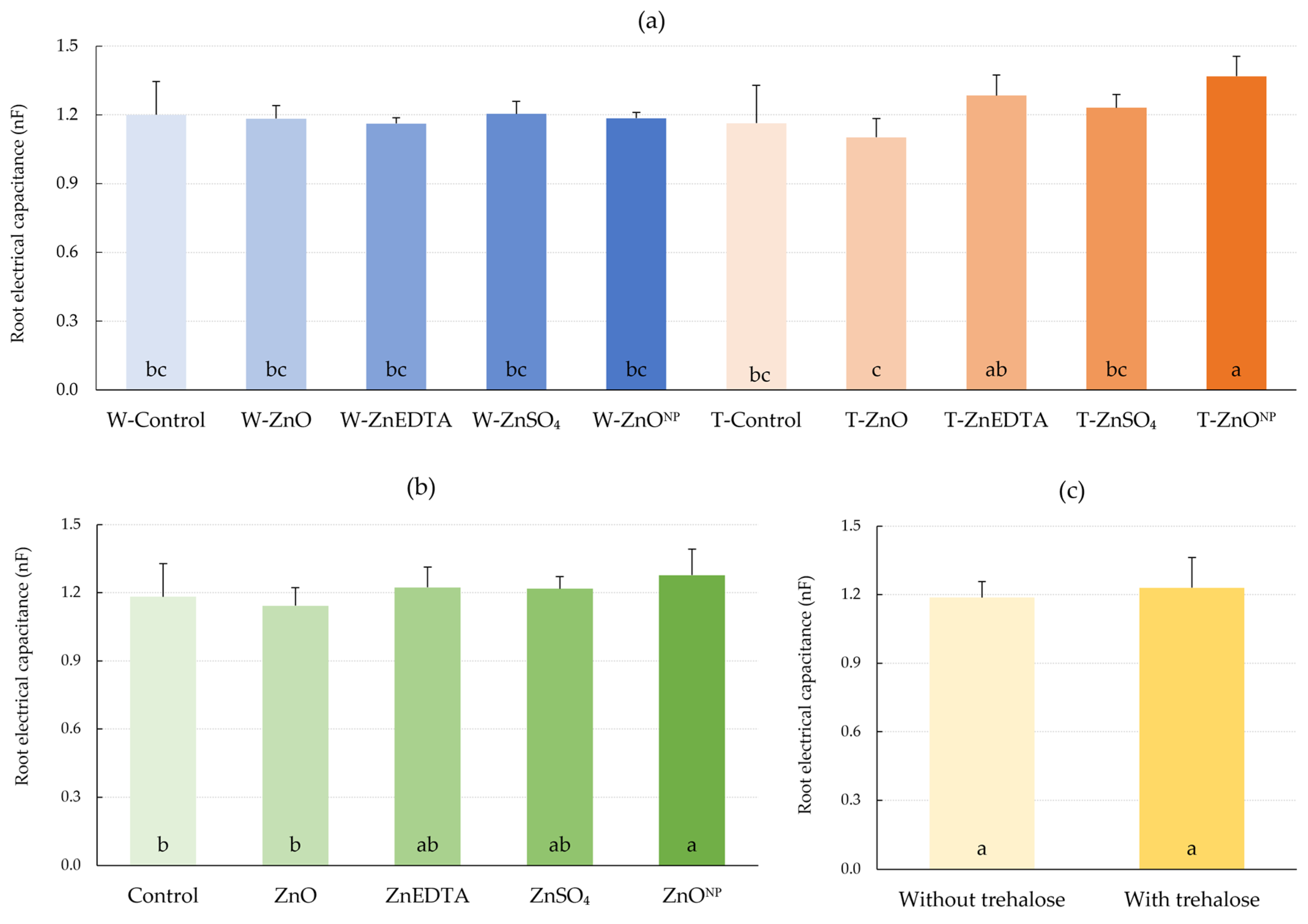
2.3. Root Electrical Capacitance (CR)
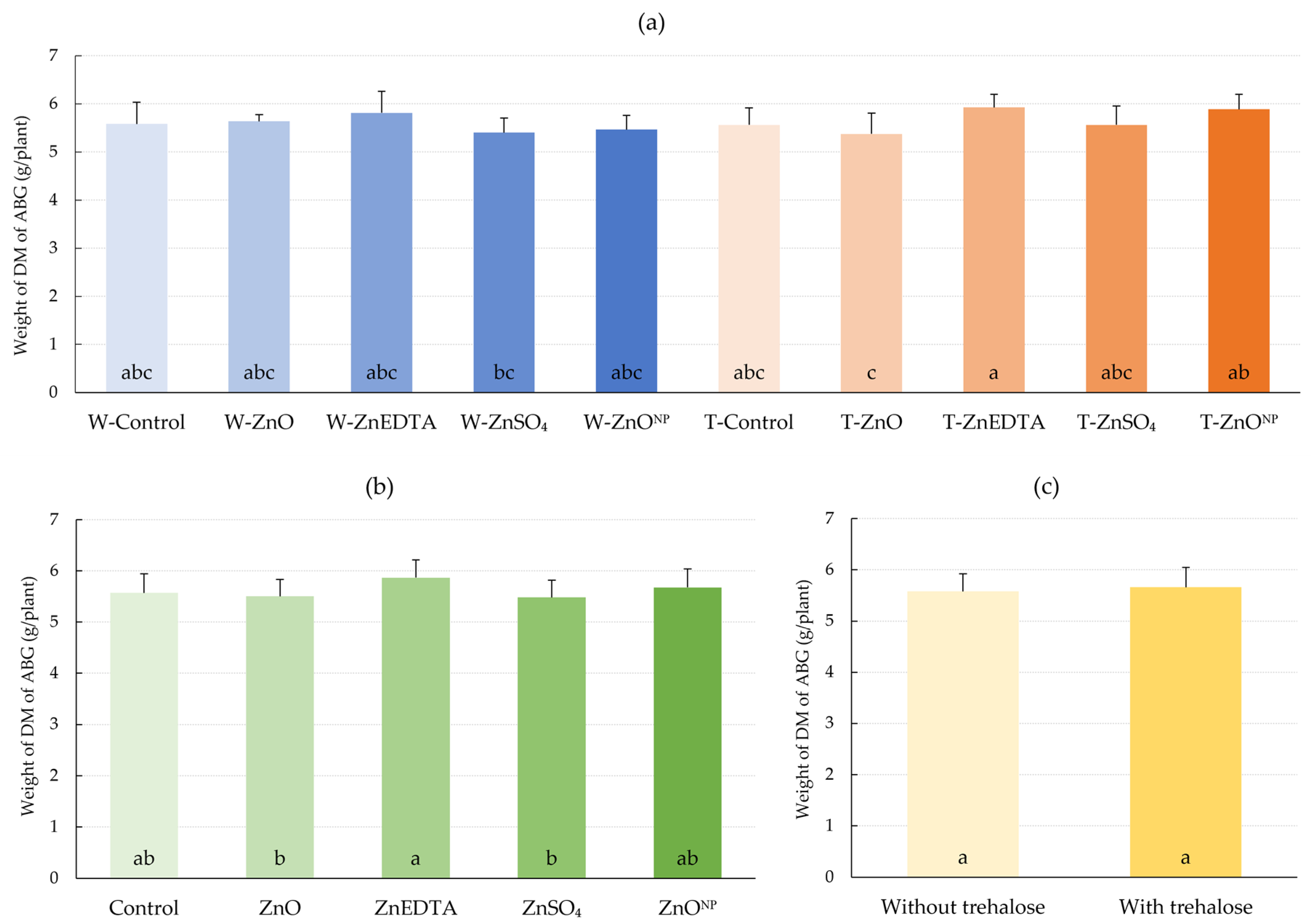
2.4. The Weight of Dry Matter of Maize Aboveground Biomass
3. Discussion
3.1. N-Tester Value and Zn Content in Plant
3.2. Chlorophyll Fluorescence Parameters
3.3. Root Capacity
3.4. Dry Matter Weight
4. Materials and Methods
4.1. Materials and Experimental Design
4.2. Measurement of Selected Parameters of Photosynthesis and Plant Growth in Maize
4.2.1. Chlorophyll Content (N-Tester Value)
4.2.2. Chlorophyll Fluorescence Parameters
4.2.3. Root Electrical Capacitance
4.2.4. Dry Weight of Aboveground Plant Biomass, Plant Zinc Content
4.3. Statistical Data Processing
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cairns, J.E.; Sanchez, C.; Vargas, M.; Ordoñez, R.; Araus, J.L. Dissecting Maize Productivity: Ideotypes Associated with Grain Yield under Drought Stress and Well-watered Conditions. J. Integr. Plant Biol. 2012, 54, 1007–1020. [Google Scholar] [CrossRef] [PubMed]
- Waraich, E.A.; Ahmad, R.; Saifullah; Ashraf, M.Y. Ehsanullah Role of mineral nutrition in alleviation of drought stress in plants. Aust. J. Crop Sci. 2011, 5, 764–777. [Google Scholar] [CrossRef]
- Bista, D.R.; Heckathorn, S.A.; Jayawardena, D.M.; Mishra, S.; Boldt, J.K. Effects of Drought on Nutrient Uptake and the Levels of Nutrient-Uptake Proteins in Roots of Drought-Sensitive and -Tolerant Grasses. Plants 2018, 7, 28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hassan, M.U.; Aamer, M.; Chattha, M.U.; Haiying, T.; Shahzad, B.; Barbanti, L.; Nawaz, M.; Rasheed, A.; Afzal, A.; Liu, Y.; et al. The Critical Role of Zinc in Plants Facing the Drought Stress. Agriculture 2020, 10, 396. [Google Scholar] [CrossRef]
- Alloway, B.J. Soil factors associated with zinc deficiency in crops and humans. Environ. Geochem. Health 2009, 31, 537–548. [Google Scholar] [CrossRef]
- Sadeghzadeh, B. A review of zinc nutrition and plant breeding. J. Soil Sci. Plant Nutr. 2013, 13, 907–927. [Google Scholar] [CrossRef] [Green Version]
- Noulas, C.; Tziouvalekas, M.; Karyotis, T. Zinc in soils, water and food crops. J. Trace Elem. Med. Biol. 2018, 49, 252–260. [Google Scholar] [CrossRef]
- Singh, B.; Natesan, S.K.A.; Singh, B.K.; Usha, K. Improving zinc efficiency of cereals under zinc deficiency. Curr. Sci. 2005, 88, 36–44. [Google Scholar]
- Subbaiah, L.V.; Prasad, T.N.V.K.V.; Krishna, T.G.; Sudhakar, P.; Reddy, B.R.; Pradeep, T. Novel Effects of Nanoparticulate Delivery of Zinc on Growth, Productivity, and Zinc Biofortification in Maize (Zea mays L.). J. Agric. Food Chem. 2016, 64, 3778–3788. [Google Scholar] [CrossRef]
- Ivanov, K.; Vasilev, A.; Mitkov, A.; Nguyen, N.; Tonev, T. Application of Zn-containing foliar fertilisers for recovery of the grain productivity potential of Zn-deficient maize plants. Ital. J. Agron. 2021, 16. [Google Scholar] [CrossRef]
- Anees, M.A.; Ali, A.; Shakoor, U.; Ahmed, F.; Hasnain, Z.; Hussain, A. Foliar applied potassium and zinc enhances growth and yield performance of maize under rainfed conditions. Int. J. Agric. Biol. 2016, 18, 1025–1032. [Google Scholar] [CrossRef]
- González-Caballo, P.; Barrón, V.; Torrent, J.; del Campillo, M.C.; Sánchez-Rodríguez, A.R. Wheat and Maize Grown on Two Contrasting Zinc-deficient Calcareous Soils Respond Differently to Soil and Foliar Application of Zinc. J. Soil Sci. Plant Nutr. 2022, 22, 1718–1731. [Google Scholar] [CrossRef]
- Imran, M.; Rehim, A. Zinc fertilization approaches for agronomic biofortification and estimated human bioavailability of zinc in maize grain. Arch. Agron. Soil Sci. 2017, 63, 106–116. [Google Scholar] [CrossRef]
- Umar, W.; Hameed, M.K.; Aziz, T.; Maqsood, M.A.; Bilal, H.M.; Rasheed, N. Synthesis, characterization and application of ZnO nanoparticles for improved growth and Zn biofortification in maize. Arch. Agron. Soil Sci. 2020, 67, 1164–1176. [Google Scholar] [CrossRef]
- Shao, J.; Wu, W.; Rasul, F.; Munir, H.; Huang, K.; Awan, M.I.; Albishi, T.S.; Arshad, M.; Hu, Q.; Huang, G.; et al. Trehalose induced drought tolerance in plants: Physiological and molecular responses. Not. Bot. Horti Agrobot. Cluj-Napoca 2022, 50, 12584. [Google Scholar] [CrossRef]
- Jain, N.K.; Roy, I. Effect of trehalose on protein structure. Protein Sci. 2009, 18, 24–36. [Google Scholar] [CrossRef]
- Bianchi, G.; Gamba, A.; Limiroli, R.; Pozzi, N.; Elster, R.; Salamini, F.; Bartels, D. The unusual sugar composition in leaves of the resurrection plant Myrothamnus flabellifolia. Physiol. Plant. 1993, 87, 223–226. [Google Scholar] [CrossRef]
- Lunn, J.E.; Delorge, I.; Figueroa, C.M.; Van Dijck, P.; Stitt, M. Trehalose metabolism in plants. Plant J. 2014, 79, 544–567. [Google Scholar] [CrossRef]
- Richards, A.B.; Krakowka, S.; Dexter, L.B.; Schmid, H.; Wolterbeek, A.P.M.; Waalkens-Berendsen, D.H.; Shigoyuki, A.; Kurimoto, M. Trehalose: A review of properties, history of use and human tolerance, and results of multiple safety studies. Food Chem. Toxicol. 2002, 40, 871–898. [Google Scholar] [CrossRef]
- Crowe, J.H. Trehalose as a “chemical chaperone”: Fact and fantasy. Mol. Asp. Stress Response Chaperones Membr. Netw. 2007, 594, 143–158. [Google Scholar] [CrossRef]
- Pereira, C.S.; Lins, R.D.; Chandrasekhar, I.; Freitas, L.C.G.; Hünenberger, P.H. Interaction of the Disaccharide Trehalose with a Phospholipid Bilayer: A Molecular Dynamics Study. Biophys. J. 2004, 86, 2273–2285. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crowe, J.H.; Carpenter, J.F.; Crowe, L.M. The role of vitrification in anhydrobiosis. Annu. Rev. Physiol. 1998, 60, 73–103. [Google Scholar] [CrossRef] [PubMed]
- Akram, N.A.; Noreen, S.; Noreen, T.; Ashraf, M. Exogenous application of trehalose alters growth, physiology and nutrient composition in radish (Raphanus sativus L.) plants under water-deficit conditions. Braz. J. Bot. 2015, 38, 431–439. [Google Scholar] [CrossRef]
- Alam, M.M.; Nahar, K.; Hasanuzzaman, M.; Fujita, M. Trehalose-induced drought stress tolerance: A comparative study among different Brassica species. Plant Omics 2014, 7, 271–283. [Google Scholar]
- Ali, Q.; Ashraf, M. Induction of Drought Tolerance in Maize (Zea mays L.) due to Exogenous Application of Trehalose: Growth, Photosynthesis, Water Relations and Oxidative Defence Mechanism. J. Agron. Crop Sci. 2011, 197, 258–271. [Google Scholar] [CrossRef]
- Ibrahim, H.A.; Abdellatif, Y.M.R. Effect of maltose and trehalose on growth, yield and some biochemical components of wheat plant under water stress. Ann. Agric. Sci. 2016, 61, 267–274. [Google Scholar] [CrossRef] [Green Version]
- Shafiq, S.; Akram, N.A.; Ashraf, M. Does exogenously-applied trehalose alter oxidative defense system in the edible part of radish (Raphanus sativus L.) under water-deficit conditions? Sci. Hortic. 2015, 185, 68–75. [Google Scholar] [CrossRef]
- Zulfiqar, F.; Chen, J.; Finnegan, P.M.; Younis, A.; Nafees, M.; Zorrig, W.; Hamed, K.B. Application of Trehalose and Salicylic Acid Mitigates Drought Stress in Sweet Basil and Improves Plant Growth. Plants 2021, 10, 1078. [Google Scholar] [CrossRef]
- Goltsev, V.N.; Kalaji, H.M.; Paunov, M.; Bąba, W.; Horaczek, T.; Mojski, J.; Kociel, H.; Allakhverdiev, S.I. Variable chlorophyll fluorescence and its use for assessing physiological condition of plant photosynthetic apparatus. Russ. J. Plant Physiol. 2016, 63, 869–893. [Google Scholar] [CrossRef]
- Chloupek, O.; Dostál, V.; Středa, T.; Psota, V.; Dvořáčková, O. Drought tolerance of barley varieties in relation to their root system size. Plant Breed. 2010, 129, 630–636. [Google Scholar] [CrossRef]
- Cseresnyés, I.; Vozáry, E.; Rajkai, K. Does electrical capacitance represent roots in the soil? Acta Physiol. Plant. 2020, 42, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Cseresnyés, I.; Takács, T.; Végh, K.R.; Anton, A.; Rajkai, K. Electrical impedance and capacitance method: A new approach for detection of functional aspects of arbuscular mycorrhizal colonization in maize. Eur. J. Soil Biol. 2013, 54, 25–31. [Google Scholar] [CrossRef]
- Heitholt, J.J.; Sloan, J.J.; MacKown, C.T. Copper, manganese, and zinc fertilization effects on growth of soybean on a calcareous soil. J. Plant Nutr. 2002, 25, 1727–1740. [Google Scholar] [CrossRef]
- Mosaad, I. Influence of integrated in-soil zinc application and organic fertilization on yield, nitrogen uptake and nitrogen use efficiency of rice. Egypt. J. Soil Sci. 2019, 59, 241–250. [Google Scholar] [CrossRef] [Green Version]
- Hesse, H.; Trachsel, N.; Suter, M.; Kopriva, S.; Von Ballmoos, P.; Rennenberg, H.; Brunold, C. Effect of glucose on assimilatory sulphate reduction in Arabidopsis thaliana roots. J. Exp. Bot. 2003, 54, 1701–1709. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smoleń, S.; Sady, W. Effect of foliar application of urea, molybdenum, benzyladenine, sucrose and salicylic acid on yield, nitrogen metabolism of radish plants and quality of edible roots. J. Plant Nutr. 2012, 35, 1113–1129. [Google Scholar] [CrossRef]
- Kumar, A.M.; Schaub, U.; Söll, D.; Ujwal, M.L. Glutamyl-transfer RNA: At the crossroad between chlorophyll and protein biosynthesis. Trends Plant Sci. 1996, 1, 371–376. [Google Scholar] [CrossRef]
- Sadak, M.S. Mitigation of drought stress on Fenugreek plant by foliar application of trehalose. Int. J. ChemTech Res. 2016, 9, 147–155. [Google Scholar]
- Zhou, W.; Liang, X.; Zhang, Y.; Dai, P.; Liang, B.; Li, J.; Sun, C.; Lin, X. Role of sucrose in modulating the low-nitrogen-induced accumulation of phenolic compounds in lettuce (Lactuca sativa L.). J. Sci. Food Agric. 2020, 100, 5412–5421. [Google Scholar] [CrossRef]
- Alexander, A.; Hunsche, M. Influence of formulation on the cuticular penetration and on spray deposit properties of manganese and zinc foliar fertilizers. Agronomy 2016, 6, 39. [Google Scholar] [CrossRef] [Green Version]
- Fernandez, V.; Eichert, T. Uptake of hydrophilic solutes through plant leaves: Current state of knowledge and perspectives of foliar fertilization. Crit. Rev. Plant Sci. 2009, 28, 36–68. [Google Scholar] [CrossRef] [Green Version]
- Alloway, B.J. Zinc in Soils and Crop Nutrition, 2nd ed.; Springer: Brussels, Belgium, 2008. [Google Scholar]
- Xia, H.; Xue, Y.; Liu, D.; Kong, W.; Xue, Y.; Tang, Y.; Li, J.; Li, D.; Mei, P. Rational Application of Fertilizer Nitrogen to Soil in Combination with Foliar Zn Spraying Improved Zn Nutritional Quality of Wheat Grains. Front. Plant Sci. 2018, 9, 677. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, A.Q.; Tian, X.H.; Cao, Y.X.; Lu, X.C.; Liu, T. Comparison of soil and foliar zinc application for enhancing grain zinc content of wheat when grown on potentially zinc-deficient calcareous soils. J. Sci. Food Agric. 2014, 94, 2016–2022. [Google Scholar] [CrossRef] [PubMed]
- Fernández, V.; Bahamonde, H.A.; Peguero-Pina, J.J.; Gil-Pelegrín, E.; Sancho-Knapik, D.; Gil, L.; Goldbach, H.E.; Eichert, T. Physico-chemical properties of plant cuticles and their functional and ecological significance. J. Exp. Bot. 2017, 68, 5293–5306. [Google Scholar] [CrossRef] [PubMed]
- Roosta, H.R.; Estaji, A.; Niknam, F. Effect of iron, zinc and manganese shortage-induced change on photosynthetic pigments, some osmoregulators and chlorophyll fluorescence parameters in lettuce. Photosynthetica 2018, 56, 606–615. [Google Scholar] [CrossRef]
- Pilon-Smits, E.A.H.; Terry, N.; Sears, T.; Kim, H.; Zayed, A.; Hwang, S.; Van Dun, K.; Voogd, E.; Verwoerd, T.C.; Krutwagen, R.W.H.H.; et al. Trehalose-producing transgenic tobacco plants show improved growth performance under drought stress. J. Plant Physiol. 1998, 152, 525–532. [Google Scholar] [CrossRef]
- Roger, M.J.R.; Weiss, O. Fluorescence techniques. In Handbook of Plant Ecophisiolgy Techniques; Roger, M.J.R., Ed.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2001; pp. 155–171. ISBN 0306480573. [Google Scholar]
- Cherif, J.; Derbel, N.; Nakkach, M.; von Bergmann, H.; Jemal, F.; Lakhdar, Z.B. Analysis of in vivo chlorophyll fluorescence spectra to monitor physiological state of tomato plants growing under zinc stress. J. Photochem. Photobiol. B Biol. 2010, 101, 332–339. [Google Scholar] [CrossRef]
- Yusefi-Tanha, E.; Fallah, S.; Rostamnejadi, A.; Pokhrel, L.R. Responses of soybean (Glycine max [L.] Merr.) to zinc oxide nanoparticles: Understanding changes in root system architecture, zinc tissue partitioning and soil characteristics. Sci. Total Environ. 2022, 835, 155348. [Google Scholar] [CrossRef]
- Moghaddasi, S.; Fotovat, A.; Khoshgoftarmanesh, A.H.; Karimzadeh, F.; Khazaei, H.R.; Khorassani, R. Bioavailability of coated and uncoated ZnO nanoparticles to cucumber in soil with or without organic matter. Ecotoxicol. Environ. Saf. 2017, 144, 543–551. [Google Scholar] [CrossRef]
- Doolette, C.L.; Read, T.L.; Li, C.; Scheckel, K.G.; Donner, E.; Kopittke, P.M.; Schjoerring, J.K.; Lombi, E. Foliar application of zinc sulphate and zinc EDTA to wheat leaves: Differences in mobility, distribution, and speciation. J. Exp. Bot. 2018, 69, 4469–4481. [Google Scholar] [CrossRef]
- Cakmak, I.; Kalayci, M.; Kaya, Y.; Torun, A.A.; Aydin, N.; Wang, Y.; Arisoy, Z.; Erdem, H.; Yazici, A.; Gokmen, O.; et al. Biofortification and Localization of Zinc in Wheat Grain. J. Agric. Food Chem. 2010, 58, 9092–9102. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.Q.; Sun, Y.X.; Ye, Y.L.; Karim, M.R.; Xue, Y.F.; Yan, P.; Meng, Q.F.; Cui, Z.L.; Cakmak, I.; Zhang, F.S.; et al. Zinc biofortification of wheat through fertilizer applications in different locations of China. Field Crops Res. 2012, 125, 1–7. [Google Scholar] [CrossRef]
- Wang, J.; Mao, H.; Zhao, H.; Huang, D.; Wang, Z. Different increases in maize and wheat grain zinc concentrations caused by soil and foliar applications of zinc in Loess Plateau, China. Field Crops Res. 2012, 135, 89–96. [Google Scholar] [CrossRef]
- Xia, H.; Kong, W.; Wang, L.; Xue, Y.; Liu, W.; Zhang, C.; Yang, S.; Li, C. Foliar Zn Spraying Simultaneously Improved Concentrations and Bioavailability of Zn and Fe in Maize Grains Irrespective of Foliar Sucrose Supply. Agronomy 2019, 9, 386. [Google Scholar] [CrossRef] [Green Version]
- Zbíral, J. Determination of plant-available micronutrients by the Mehlich 3 soil extractant—A proposal of critical values. Plant Soil Environ. 2016, 62, 527–531. [Google Scholar] [CrossRef] [Green Version]
- Zbíral, J.; Malý, S.; Váňa, M. (Eds.) Soil Analysis III, 3rd ed.; Central Institute for Supervising and Testing in Agriculture: Brno, Czech Republic, 2011; pp. 18–52. (In Czech) [Google Scholar]
- Schumacher, B.A. Methods for the Determination of Total Organic Carbon (TOC) in Soils and Sediments; United States Environmental Protection Agency, Environmental Sciences Division National, Exposure Research Laboratory: Las Vegas, NV, USA, 2002.
- Gee, G.W.; Bauder, J.W. Particle-size analysis. In Methods of Soil Analysis Part 1—Physical and Mineralogical Methods; Klute, A., Ed.; ASA; SSSA: Madison, WI, USA, 1986; pp. 383–411. [Google Scholar]
- Nachabe, B.M.H. Refining the definition of field capacity in the literature. J. Irrig. Drain. Eng. 1998, 124, 230–232. [Google Scholar] [CrossRef]
- Netto, A.T.; Campostrini, E.; De Oliveira, J.G.; Bressan-Smith, R.E. Photosynthetic pigments, nitrogen, chlorophyll a fluorescence and SPAD-502 readings in coffee leaves. Sci. Hortic. 2005, 104, 199–209. [Google Scholar] [CrossRef]
- Ortuzar-Iragorri, M.A.; Alonso, A.; Castellón, A.; Besga, G.; Estavillo, J.M.; Aizpurua, A. N-Tester use in soft winter wheat: Evaluation of nitrogen status and grain yield prediction. Agron. J. 2005, 97, 1380–1389. [Google Scholar] [CrossRef]
- Strasser, R.J.; Srivastava, A.; Tsimilli-Michael, M. The fluorescence transient as a tool to characterize and screen photosynthetic samples. In Probing Photosynthesis: Mechanism, Regulation & Adaptation; Yunus, M., Pathre, U., Monathy, P., Eds.; Taylor and Francis: London, UK, 2000; pp. 443–480. ISBN 0748408215. [Google Scholar]
- Stirbet, A.; Govindjee, G. On the relation between the Kautsky effect (chlorophyll a fluorescence induction) and Photosystem II: Basics and applications of the OJIP fluorescence transient. J. Photochem. Photobiol. B Biol. 2011, 104, 236–257. [Google Scholar] [CrossRef]
- Kalaji, H.M.; Schansker, G.; Ladle, R.J.; Goltsev, V.; Bosa, K.; Allakhverdiev, S.I.; Brestic, M.; Bussotti, F.; Calatayud, A.; Dąbrowski, P.; et al. Frequently asked questions about in vivo chlorophyll fluorescence: Practical issues. Photosynth. Res. 2014, 122, 121–158. [Google Scholar] [CrossRef]
- Genty, B.; Briantais, J.M.; Baker, N.R. The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochim. Biophys. Acta—Gen. Subj. 1989, 990, 87–92. [Google Scholar] [CrossRef]
- StatSoft, Inc. STATISTICA (Data Analysis Software System), Version 14. 2021. Available online: www.statsoft.com (accessed on 10 July 2022).


| Soil Parameter | Value | Ref. |
|---|---|---|
| pH (CaCl2) | 6.09 | [58] |
| Cox | 0.80% | [59] |
| Clay | 20% | [60] |
| Slit | 27% | |
| Sand | 53% | |
| Cation Exchange Capacity | 164 mmol/kg | [58] |
| N total | 0.19% | |
| N-NH4⁺ (K2SO4) | 1.48 mg/kg | |
| N-NO3− (K2SO4) | 17.2 mg/kg | |
| P (Mehlich 3) | 36.4 mg/kg | |
| K (Mehlich 3) | 400 mg/kg | |
| Ca (Mehlich 3) | 2720 mg/kg | |
| Mg (Mehlich 3) | 214 mg/kg | |
| Zn (Mehlich 3) | 1.8 mg/kg |
| Treatment | Form of Zinc | Foliar Spray | Zn Concentration (w/v) | Trehalose Concentration (w/v) |
|---|---|---|---|---|
| Control | - | Distilled water (W) | 0 | 0 |
| W-ZnO | ZnO | 0.1% | 0 | |
| W-ZnEDTA | Zn-EDTA | 0.1% | 0 | |
| W-ZnSO4 | ZnSO4 | 0.1% | 0 | |
| W-ZnONP | nano ZnO | 0.1% | 0 | |
| T-Control | - | Distilled water + trehalose (T) | - | 1% |
| T-ZnO | ZnO | 0.1% | 1% | |
| T-ZnEDTA | Zn-EDTA | 0.1% | 1% | |
| T-ZnSO4 | ZnSO4 | 0.1% | 1% | |
| T-ZnONP | nano ZnO | 0.1% | 1% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Klofac, D.; Antosovsky, J.; Skarpa, P. Effect of Zinc Foliar Fertilization Alone and Combined with Trehalose on Maize (Zea mays L.) Growth under the Drought. Plants 2023, 12, 2539. https://doi.org/10.3390/plants12132539
Klofac D, Antosovsky J, Skarpa P. Effect of Zinc Foliar Fertilization Alone and Combined with Trehalose on Maize (Zea mays L.) Growth under the Drought. Plants. 2023; 12(13):2539. https://doi.org/10.3390/plants12132539
Chicago/Turabian StyleKlofac, Daniel, Jiri Antosovsky, and Petr Skarpa. 2023. "Effect of Zinc Foliar Fertilization Alone and Combined with Trehalose on Maize (Zea mays L.) Growth under the Drought" Plants 12, no. 13: 2539. https://doi.org/10.3390/plants12132539






