Real-Time PCR to Phenotype Resistance to the Citrus Nematode Tylenchulus semipenetrans Cobb.
Abstract
:1. Introduction
2. Results
2.1. qPCR Assay
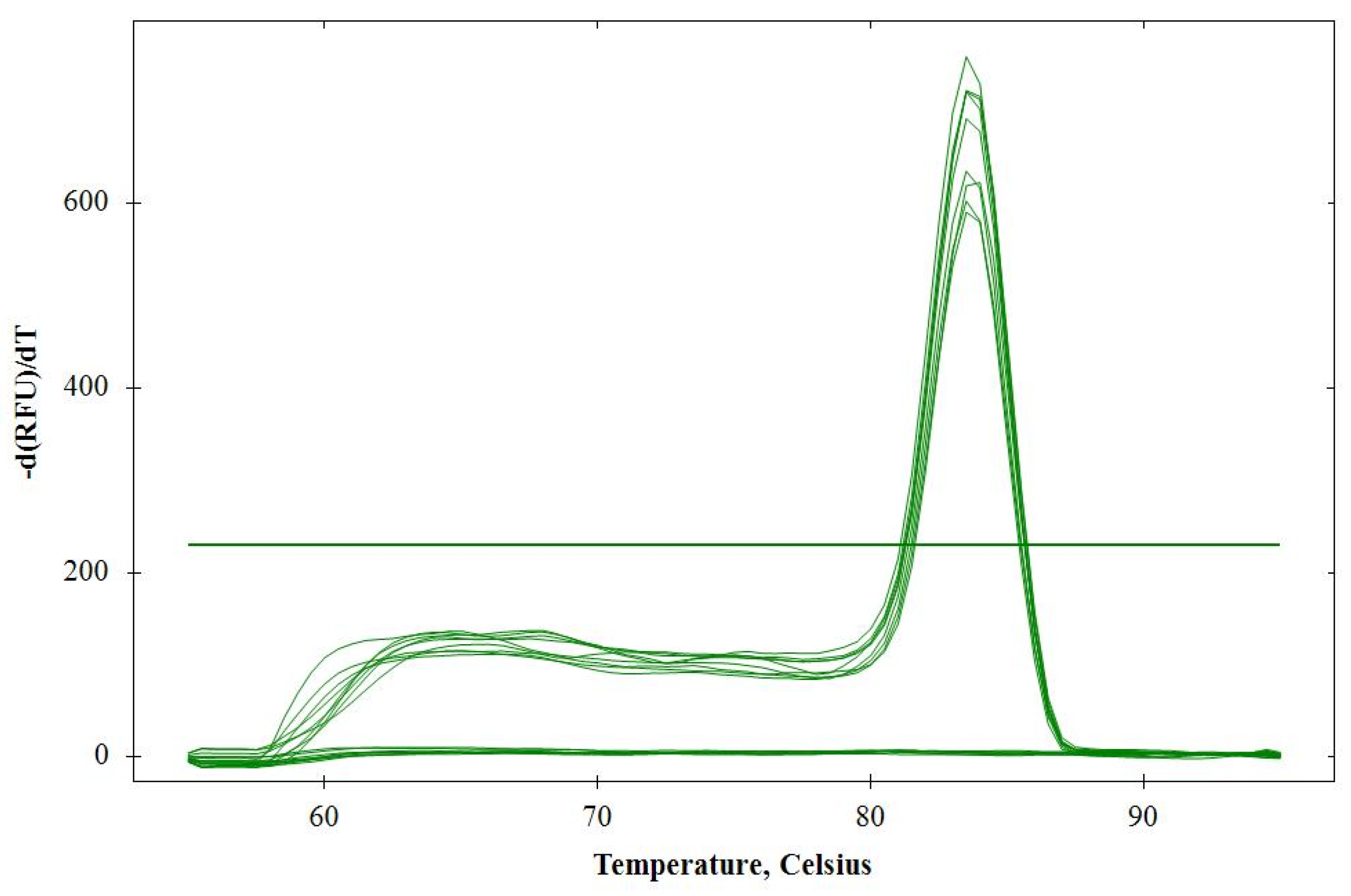
2.1.1. Specificity
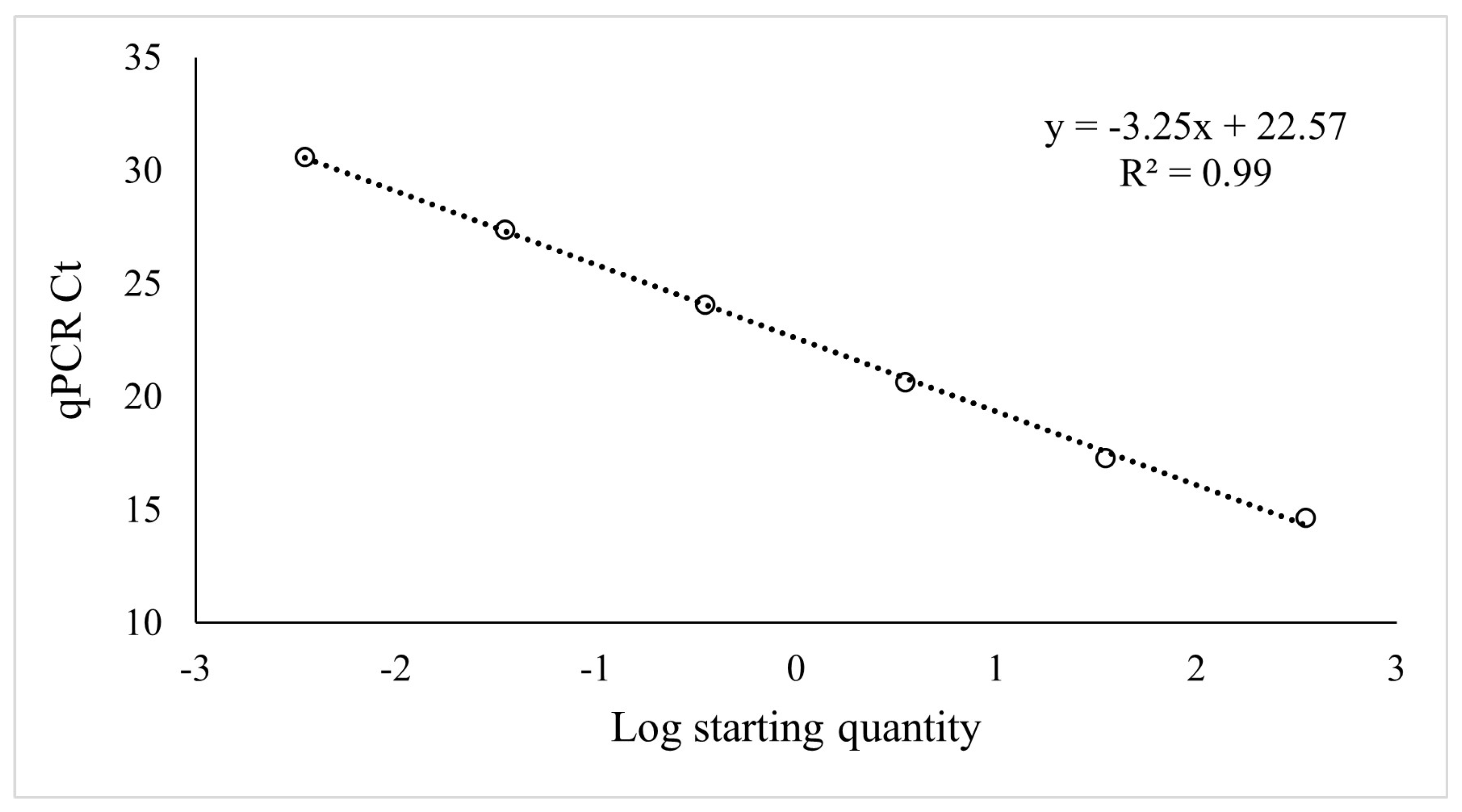
2.1.2. Level of Detection
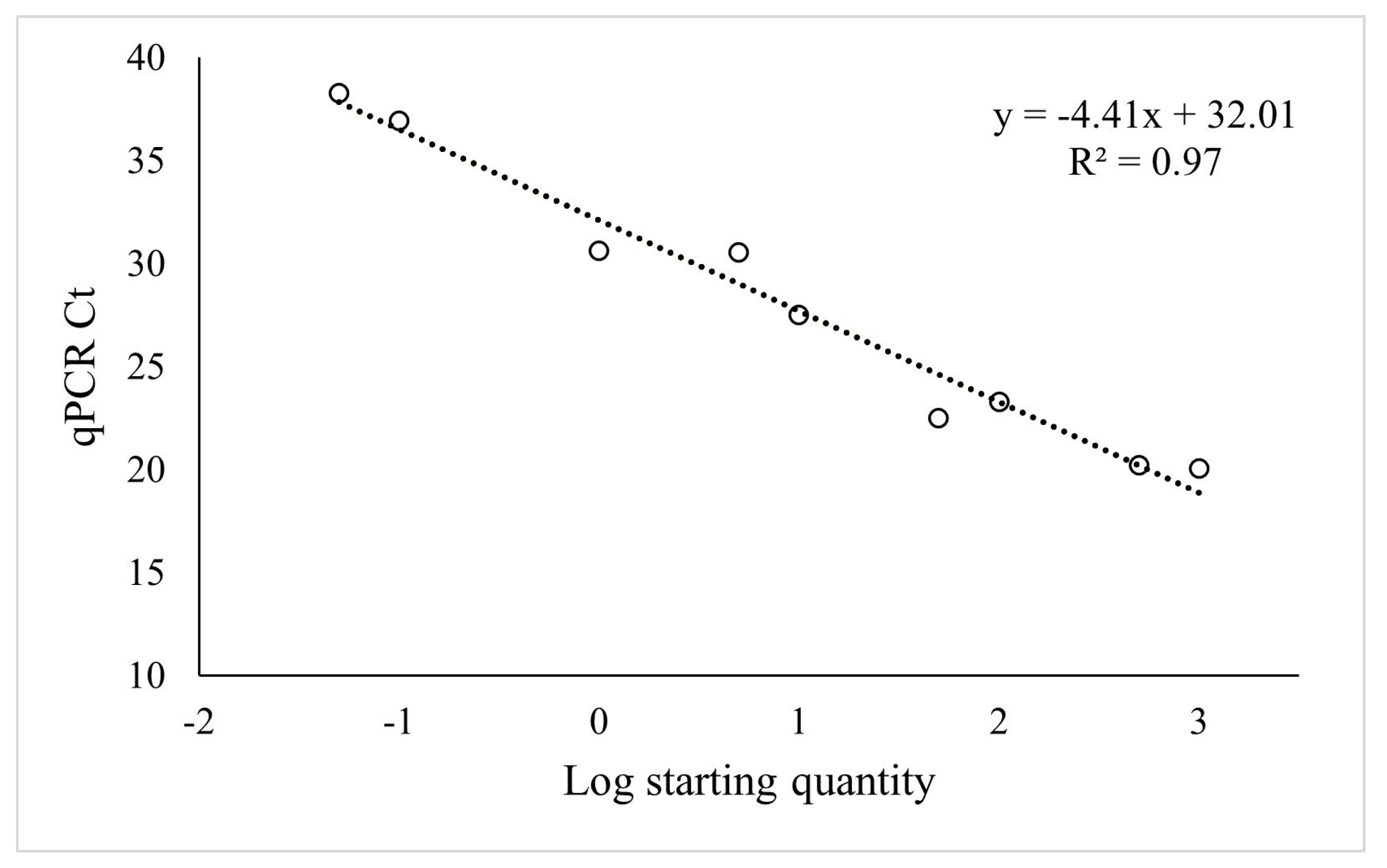
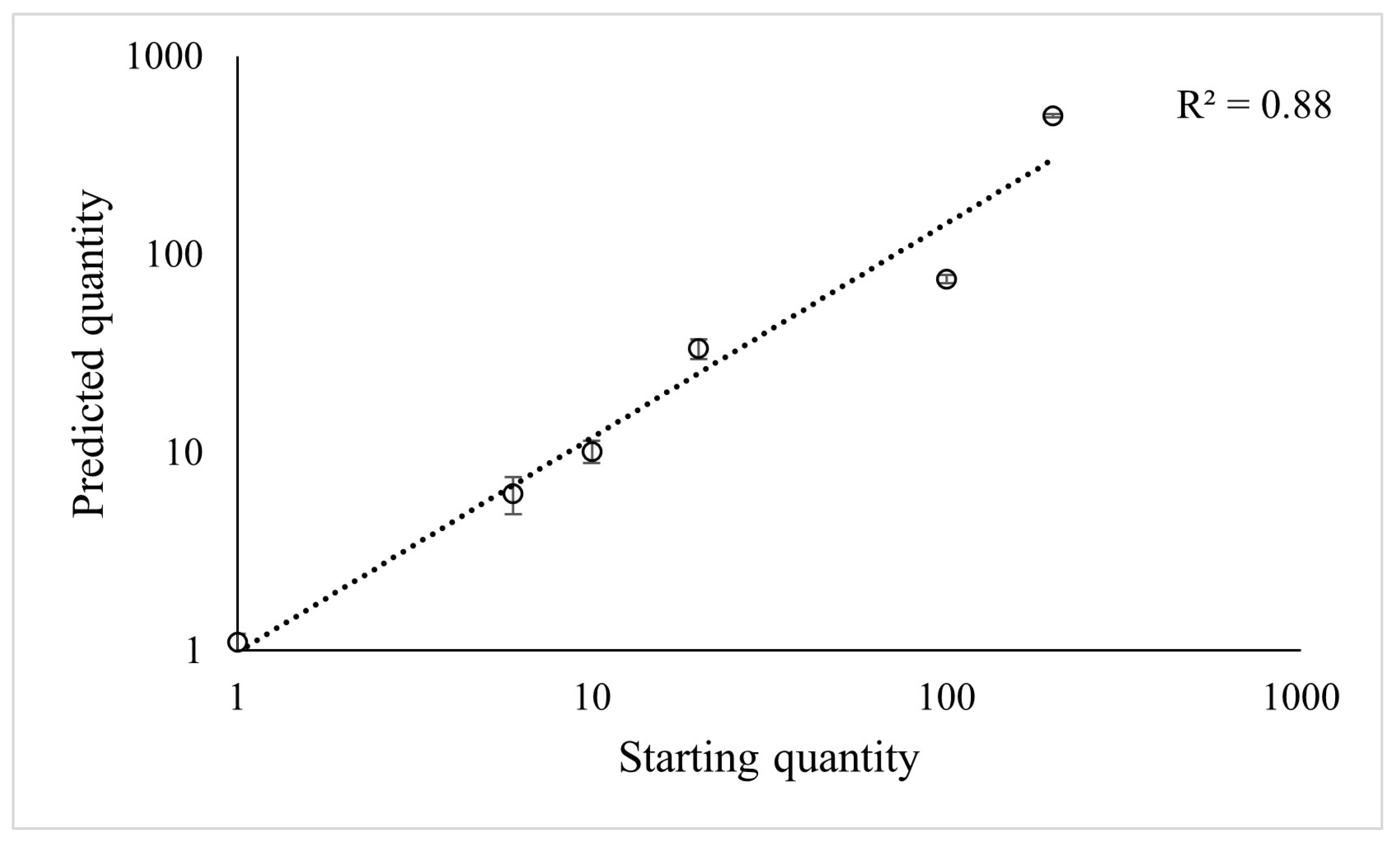
2.1.3. Predictive Power
2.2. Host Screening
2.3. Validation in Field Samples
2.4. In-Greenhouse Rearing for Inoculum Production
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Growth Conditions
4.2. Nematode Inoculum Preparation from Field Trees
4.3. Host Infestation and Incubation
4.4. In-Greenhouse Rearing for Inoculum Production
4.5. Female Nematode Extraction
4.6. Nematode Quantification
4.6.1. Based on Microscopy
4.6.2. Based on Real-Time PCR
DNA Extraction
qPCR Assay Design and Validation
4.6.3. Modeling
4.7. Statistical Analysis
4.8. Validation of Field Samples
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Duncan, L.W. Managing Nematodes In Citrus Orchards. In Integrated Management of Fruit Crops Nematodes; Ciancio, A., Mukerji, K., Eds.; Springer: Dordrecht, The Netherlands, 2009; Volume 4, pp. 135–174. [Google Scholar] [CrossRef]
- Abd-Elgawad, M.M.; Koura, F.F.; Montasser, S.A.; Hammam, M.M. Distribution and losses of Tylenchulus semipenetrans in citrus orchards on reclaimed land in Egypt. Nematology 2016, 18, 1141–1150. [Google Scholar] [CrossRef]
- Shokoohi, E.; Duncan, L.W. Nematodes parasites of citrus. In Plant Parasitic Nematodes in Subtropical and Tropical Agriculture, 3rd ed.; Sikora, R., Coyne, D., Hallmann, J., Timper, P., Eds.; CABI: Boston, MA, USA, 2018; pp. 446–476. [Google Scholar]
- Duncan, L.W. Nematode diseases of citrus. In Citrus Health Management; Timmer, L.W., Duncan, L.W., Eds.; American Phytopathological Society Press: St. Paul, MN, USA, 1999; pp. 136–148. [Google Scholar]
- Sorribas, F.J.; Verdejo-Lucas, S.; Pastor, J.; Ornat, C.; Pons, J.; Valero, J. Population densities of Tylenchulus semipenetrans related to physicochemical properties of soil and yield of clementine mandarin in Spain. Plant Dis. 2008, 92, 445–450. [Google Scholar] [CrossRef] [Green Version]
- Liu, G.; Chen, J.; Xiao, S.; Zhang, S.S.; Pan, D.M. Development of Species-Specific PCR Primers and Sensitive Detection of the Tylenchulus semipenetrans in China. Agric. Sci. China 2011, 10, 252–258. [Google Scholar] [CrossRef]
- Song, Z.Q.; Cheng, J.E.; Cheng, F.X.; Zhang, D.Y.; Liu, Y. Development and Evaluation of Loop-Mediated Isothermal Amplification Assay for Rapid Detection of Tylenchulus semipenetrans Using DNA Extracted from Soil. Plant Pathol. J. 2017, 33, 184–192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Gundy, S.D. The life history of the citrus nematode, Tylenchulus semipenetrans. Nematologica 1958, 3, 283–294. [Google Scholar] [CrossRef] [Green Version]
- Grafton-Cardwell, E.E.; Baldwin, R.A.; Becker, J.O.; Eskalen, A.; Lovatt, C.J.; Rios, S.; Adaskaveg, J.E.; Faber, B.A.; Haviland, D.R.; Hembree, K.J.; et al. UC IPM Pest Management Guidelines: Citrus; UC ANR Publication: Davis, CA, USA, 2021; p. 3441. [Google Scholar]
- Kaplan, D.T. Characterization of citrus rootstock responses to Tylenchulus semipenetrans (Cobb). J. Nematol. 1981, 13, 492–498. [Google Scholar]
- Van Gundy, S.D.; Kirkpatrick, J.D. Nature of resistance in certain citrus root-stocks to citrus nematode. Phytopathology 1964, 54, 419–427. [Google Scholar]
- Sato, K.; Kadota, Y.; Shirasu, K. Plant Immune Responses to Parasitic Nematodes. Front. Plant Sci. 2019, 10, 1165. [Google Scholar] [CrossRef] [Green Version]
- Verdejo-Lucas, S.; Sorribas, F.J.; Pons, J.; Forner, J.B.; Alcaide, A. The Mediterranean biotypes of Tylenchulus semipenetrans in Spanish citrus orchards. Fundam. Appl. Nematol. 1997, 20, 399–404. [Google Scholar]
- Baines, R.C.; Cameron, W.; Soost, R.K. Four biotypes of Tylenchulus semipenetrans in California identified, and their importance in the development of resistant citrus rootstocks. J. Nematol. 1974, 6, 63–66. [Google Scholar]
- Gottlieb, Y.; Cohn, E.; Spiegel-Roy, P. Biotypes of the citrus nematode (Tylenchulus semipenetrans Cobb) in Israel. Phytoparasitica 1986, 14, 193–198. [Google Scholar] [CrossRef]
- Murguía, C.; Abad, P.; Jordá, C.; Bello, A. Identification of the Poncirus biotype of Tylenchulus semipenetrans in Valencia, Spain. Span. J. Agric. Res. 2005, 3, 130–133. [Google Scholar] [CrossRef] [Green Version]
- Kaplan, D.T. Screening for resistance to Tylenchulus semipenetrans and Radopholus species. In Methods for Evaluating Plant Species for Resistance to Plant Parasitic Nematodes; Starr, J.L., Ed.; Society of Nematology: Hyattsville, MD, USA, 1990; pp. 51–57. [Google Scholar]
- Ling, P.; Duncan, L.W.; Deng, Z.; Dunn, D.; Hu, X.; Huang, S.; Gmitter, F.G., Jr. Inheritance of citrus nematode resistance and its linkage with molecular markers. Theor. Appl. Genet. 2000, 100, 1010–1017. [Google Scholar] [CrossRef]
- Xiang, X.; Deng, Z.; Chen, C.; Gmitter, F.; Bowman, K. Marker Assisted Selection in Citrus Rootstock Breeding Based on a Major Gene Locus ‘Tyr1’ Controlling Citrus Nematode Resistance. Agric. Sci. China 2010, 9, 557–567. [Google Scholar] [CrossRef]
- Xu, X.; Zhan’ao, D.; Qifa, Z.; Cunxian, C.; Gmitter, F.G. Developing specific markers and improving genetic mapping for a major locus Tyr1 of Citrus nematode resistance. Mol. Plant Breed. 2009, 7, 497–504. [Google Scholar]
- Niles, R.K.; Freckman, D.W.; Roose, M.L. Use of trifoliate orange as a comparative standard for assessing the resistance of citrus rootstocks to citrus nematode. Plant Dis. 1995, 79, 813–818. [Google Scholar] [CrossRef]
- Ye, J.; Coulouris, G.; Zaretskaya, I.; Cutcutache, I.; Rozen, S.; Madden, T. Primer-BLAST: A tool to design target-specific primers for polymerase chain reaction. BMC Bioinform. 2012, 13, 134. [Google Scholar] [CrossRef] [Green Version]
- Altschul, S.F.; Gish, W. Local alignment statistics. Methods Enzymol. 1996, 266, 460–480. [Google Scholar]
- Maafi, T.Z.; Amani, M.; Stanley, J.D.; Inserra, R.N.; Van den Berg, E.; Subbotin, S.A. Description of Tylenchulus musicola sp. n. (Nematoda: Tylenchulidae) from banana in Iran with molecular phylogeny and characterisation of species of Tylenchulus Cobb, 1913. Nematology 2012, 14, 353–369. [Google Scholar] [CrossRef] [Green Version]
- Byrd, D.W.; Kirkpatrick, T., Jr.; Barker, K.R. An improved technique for clearing and staining plant tissue for detection of nematodes. J. Nematol. 1983, 14, 142–143. [Google Scholar]
- EPPO. Nematode extraction PM 7/119 (1). EPPO Bull. 2013, 43, 471–495. [Google Scholar] [CrossRef]
- Berensmeier, S. Magnetic particles for the separation and purification of nucleic acids. Appl. Microbiol. Biotechnol. 2006, 73, 495–504. [Google Scholar] [CrossRef] [PubMed]
- Woo, P.C.; Leung, S.Y.; To, K.K.; Chan, J.F.; Ngan, A.H.; Cheng, V.C.; Lau, S.K.; Yuen, K.Y. Internal transcribed spacer region sequence heterogeneity in Rhizopus microsporus: Implications for molecular diagnosis in clinical microbiology laboratories. J. Clin. Microbiol. 2010, 48, 208–214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hodson, A.K.; Cicchetto, A.; Fierro, F.A. Real time PCR assays to detect and quantify the nematodes Pratylenchus vulnus and Mesocriconema xenoplax. Crop Prot. 2021, 145, 105617. [Google Scholar] [CrossRef]
- Yan, G.; Smiley, R.W.; Okubara, P.A. Detection and quantification of Pratylenchus thornei in DNA extracted from soil using real-time PCR. Phytopathology 2012, 102, 14–22. [Google Scholar] [CrossRef] [Green Version]
- Min, Y.Y.; Toyota, K.; Sato, E. A novel nematode diagnostic method using the direct quantification of major plant-parasitic nematodes in soil by real-time PCR. Nematology 2012, 14, 265–276. [Google Scholar] [CrossRef]
- Sato, E.; Min, Y.Y.; Shirakashi, T.; Wada, S.; Toyota, K. Detection of the root-lesion nematode, Pratylenchus penetrans (Cobb), in a nematode community using real-time PCR. Jpn. J. Nematol. 2007, 37, 87–92. [Google Scholar] [CrossRef] [Green Version]
- Kepiro, J.L.; Roose, M.L. AFLP markers closely linked to a major gene essential for nucellar embryony (apomixis) in Citrus maxima × Poncirus trifoliata. Tree Genet. Genomes 2010, 6, 1–11. [Google Scholar] [CrossRef]
- McCarty, C.D.; Bitters, W.P.; Van Gundy, S.D. Susceptibility of 25 Citrus Rootstocks to the Citrus Nematode. HortScience 1979, 14, 54–55. [Google Scholar] [CrossRef]
- Ravichandra, N.G. Nematological techniques. In Horticultural Nematology; Springer: New Delhi, India, 2014; p. 303. [Google Scholar]
- Mai, W.F.; Mullin, P.G.; Lyon, H.H.; Loeffler, K. Plant-Parasitic Nematodes: A Pictorial Key to Genera, 4th ed.; Cornell University Press: Ithaca, NY, USA, 1996; Available online: http://www.jstor.org/stable/10.7591/j.ctv5rdz0t (accessed on 25 May 2023).
- Braun-Kiewnick, A.; Viaene, N.; Folcher, L.; Ollivier, F.; Anthoine, G.; Niere, B.; Sapp, M.; van de Vossenberg, B.; Toktay, H.; Kiewnick, S. Assessment of a new qPCR tool for the detection and identification of the root-knot nematode Meloidogyne enterolobii by an international test performance study. Eur. J. Plant Pathol. 2016, 144, 97–108. [Google Scholar] [CrossRef]
- Untergasser, A.; Cutcutache, I.; Koressaar, T.; Ye, J.; Faircloth, B.C.; Remm, M.; Rozen, S.G. Primer3 new capabilities and interfaces. Nucleic Acids Res. 2012, 40, e115. [Google Scholar] [CrossRef] [Green Version]


| Primer Name | Sequences (5′→3′) | Target | Reference |
|---|---|---|---|
| TS18SAJ966511.1-228F | ATCGTATGGGCTTGTCCCGA | 18s rRNA | This study |
| TS18SAJ966511.1-433R | TCGTCACTACCTCCTCGTGC | ||
| TW81 | GTTTCCGTAGGTGAACCTGC | ITS rRNA | [24] |
| Semipenetrans specific | GGACTCTGCTCAACCTGGTAGA |
| Host | 1 Females/Root | Whole-Root FW (g) | Fibrous Root FW (g) |
|---|---|---|---|
| Rich—2 R | 0.00 a | 9.38 ab | 4.07 a |
| Pom—R | 0.00 a | 12.03 a | 5.87 ab |
| Ch × tf–60—U | 0.00 a | 17.04 bc | 7.44 bc |
| Swin—R | 0.00 a | 19.46 c | 11.66 d |
| ASRT—U | 0.00 a | 14.05 abc | 7.36 bc |
| C-57—R | 0.29 a | 25.20 d | 11.74 d |
| Ch × tf–84—U | 0.46 a | 16.82 bc | 8.68 c |
| CC—S | 84.55 bc | 17.53 bc | 8.72 c |
| C-22—S | 61.93 b | 13.85 abc | 7.31 bc |
| Volk—S | 189.59 cd | 15.01 abc | 6.20 abc |
| ASwo—S | 341.33 d | 17.26 bc | 8.56 bc |
| Average | 5.35 | 16.15 | 7.96 |
| ANOVA | F = 136.26, p < 0.001 | F = 4.33, p < 0.001 | F = 7.00, p < 0.001 |
| Host | 1,2 Females Counted | 1,3 Females Predicted with Ct Values | qPCR Ct Value |
|---|---|---|---|
| Rich—4 R | 0.00 | 0.04 | 38.3 |
| Pom—R | 0.00 | 0.05 | 38.4 |
| Ch × tf–60—U | 0.00 | 0.06 | 38.1 |
| Swin—R | 0.00 | 0.51 | 35.9 |
| ASRT—U | 0.12 | 0.87 | 34.5 |
| C-57—R | 0.00 | 1.26 | 34.0 |
| Ch × tf–84—U | 0.15 | 3.57 *** | 31.2 *** |
| CC—S | 17.06 *** | 6.39 *** | 28.7 *** |
| C-22—S | 12.59 *** | 8.65 *** | 28.0 *** |
| Volk—S | 38.51 *** | 18.15 *** | 26.7 *** |
| ASwo—S | 68.89 *** | 31.86 *** | 25.5 *** |
| Average R vs. S | 0.03 vs. 27.69 | 0.33 vs. 13.56 | 37.36 vs. 27.22 |
| ANOVA | F = 343.65, p < 0.001 | F = 163.58, p < 0.001 | F = 66.80, p < 0.001 |
| Dunnett’s | 3.050 98, 0.001 |
| Rootstock | Pedigree | 1 Ct value | 2 Sampling Location |
|---|---|---|---|
| Koethen sweet orange | C. sinensis L. Osbeck | 24.0 | LR |
| Rangpur lime | C. limonia | 24.2 | SCREC |
| Alemow | C. macrophylla W | 25.2 | LR |
| Volkamer lemon | C. volkameriana | 25.7 | CVARS |
| Alemow | C. macrophylla W | 26.1 | LR |
| Yuma ponderosa | C. maxima ×? | 26.4 | SCREC |
| Troyer citrange | C. sinensis × P. trifoliata | 26.4 | LREC |
| Schaub rough lemon | C. jambhiri Lush | 27.5 | LR |
| C-32 | C. sinensis × P. trifoliata | 28.3 | LR |
| Carrizo citrange | C. sinensis × P. trifoliata | 28.4 | SCREC |
| Tosu hybrid | Citrus × aurantium L. | 28.4 | LR |
| Carrizo citrange | C. sinensis × P. trifoliata | 28.5 | CVARS |
| C-32 | C. sinensis × P. trifoliata | 28.9 | SCREC |
| C-35 | C. sinensis × P. trifoliata | 31.2 | CVARS |
| C-35 | C. sinensis × P. trifoliata | 31.7 | LREC |
| Flying dragon | P. trifoliata L. Raf | 32.0 | SCREC |
| Rootstock | Species/Pedigree | Abbreviation | 1 Host Suitability | References |
|---|---|---|---|---|
| Rich 16-6 trifoliate | Poncirus trifoliata (L.) Raf | Rich | R | [19] |
| Pomeroy trifoliate | Pom | R | [19,34] | |
| Swingle citrumelo | Citrus paradisi × P. trifoliata Macfad. | Swin | R | [19,21,34] |
| Furr C-57 citrandarin | C. sunki (Hayata) hort ex. Tanaka × P. trifoliata ‘Swingle’ | C-57 | R | [21] |
| African shaddock × Rubidoux trifoliate | C. maxima Merr. × P. trifoliata | ASRT | U | [21] |
| Carrizo citrange | C. sinensis Osb. × P. trifoliata | CC | S | [19,21,34] |
| Bitters C-22 citrandarin | C. sunki × P. trifoliata ‘Swingle’ | C-22 | S | [21] |
| Volkamer lemon | C. limonia Osb. | Volk | S | [19] |
| Argentina sweet orange | C. sinensis Osb. | ASwo | S | [19,21,34] |
| Chandler × trifoliate–84 | C. maxima Merr × P. trifoliata | Ch × tf–84 | U | [33] |
| Chandler × trifoliate–60 | Ch × tf–60 | U | [33] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruiz, M.; Vo, A.D.; Becker, J.O.; Roose, M.L. Real-Time PCR to Phenotype Resistance to the Citrus Nematode Tylenchulus semipenetrans Cobb. Plants 2023, 12, 2543. https://doi.org/10.3390/plants12132543
Ruiz M, Vo AD, Becker JO, Roose ML. Real-Time PCR to Phenotype Resistance to the Citrus Nematode Tylenchulus semipenetrans Cobb. Plants. 2023; 12(13):2543. https://doi.org/10.3390/plants12132543
Chicago/Turabian StyleRuiz, Marta, Annie Du Vo, J. Ole Becker, and Mikeal L. Roose. 2023. "Real-Time PCR to Phenotype Resistance to the Citrus Nematode Tylenchulus semipenetrans Cobb." Plants 12, no. 13: 2543. https://doi.org/10.3390/plants12132543





