Evaluating the Combined Effects of Erythromycin and Levofloxacin on the Growth of Navicula sp. and Understanding the Underlying Mechanisms
Abstract
:1. Introduction
2. Results
2.1. Influence of ERY and LEV on the Growth of Navicula
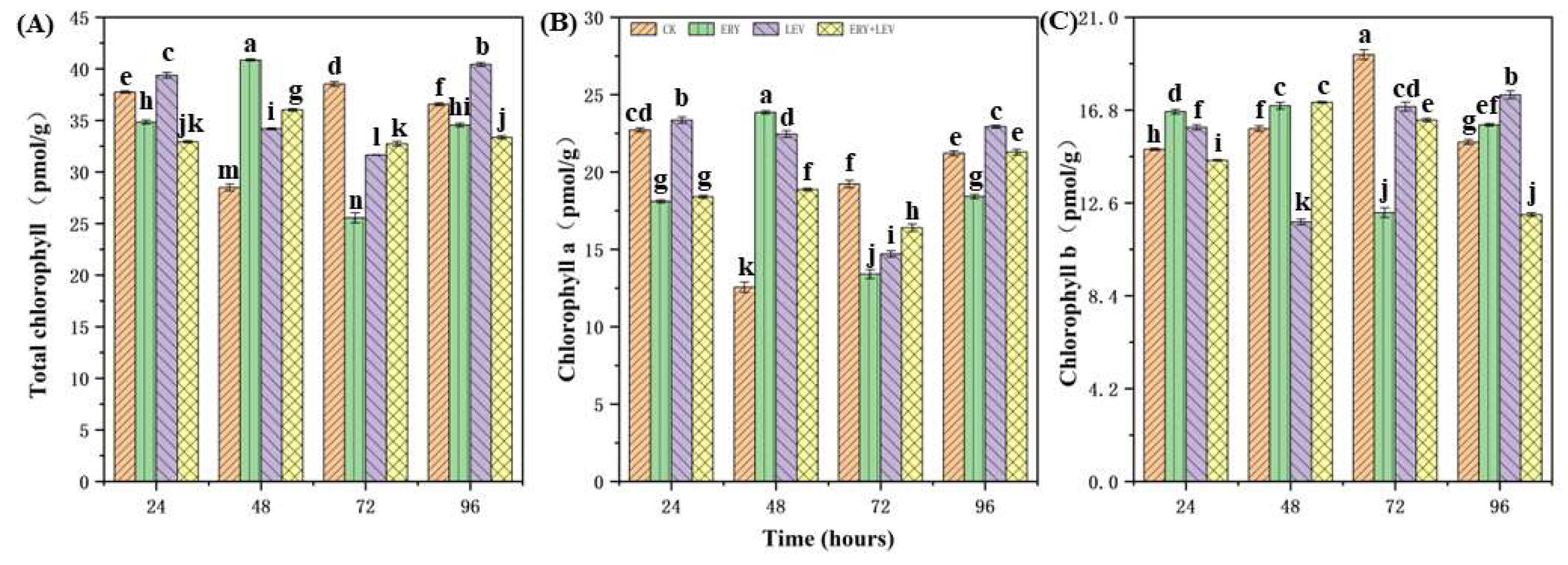
2.2. Photosynthetic Pigment Content of Navicula
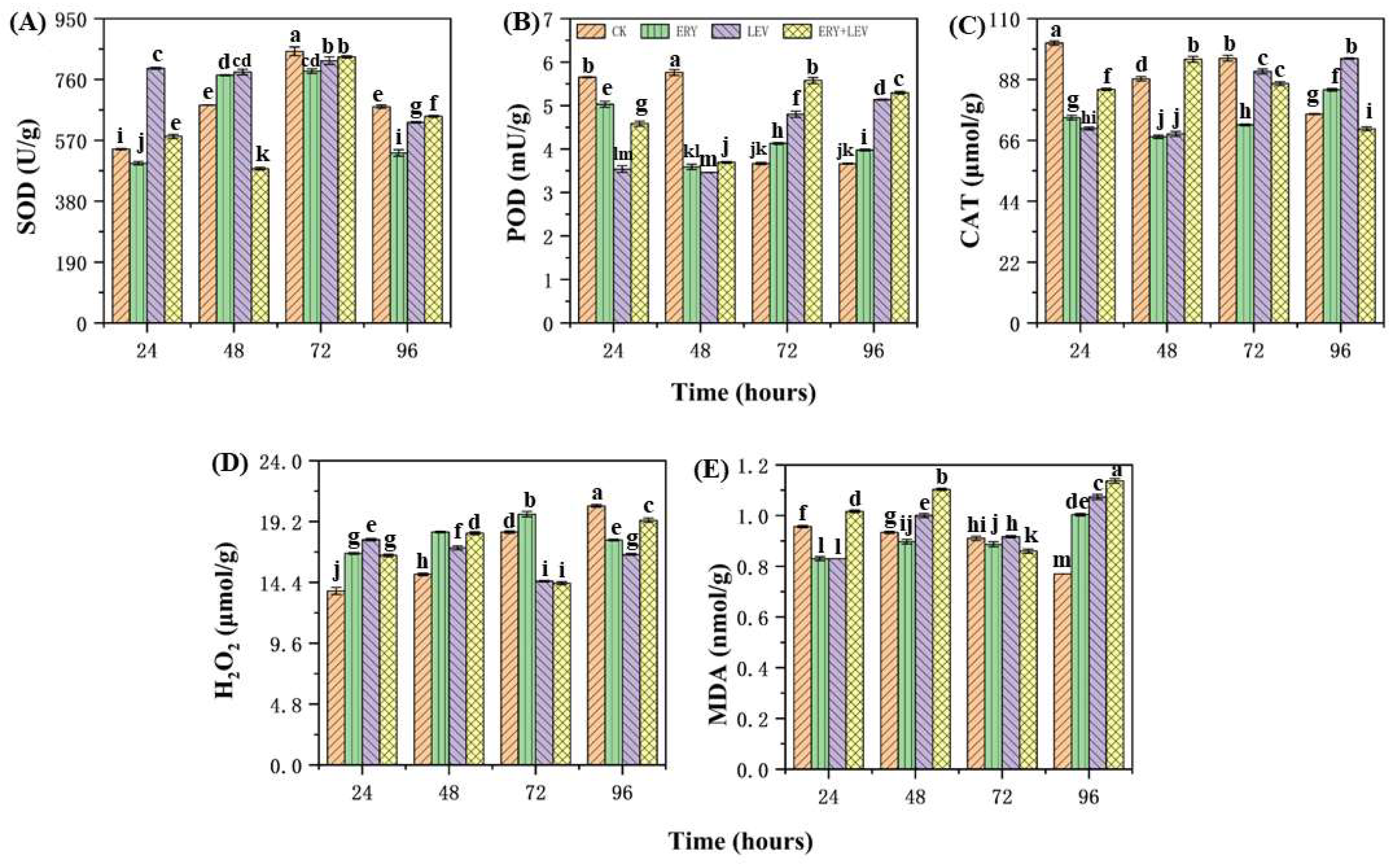
2.3. The Antioxidant System of Navicula
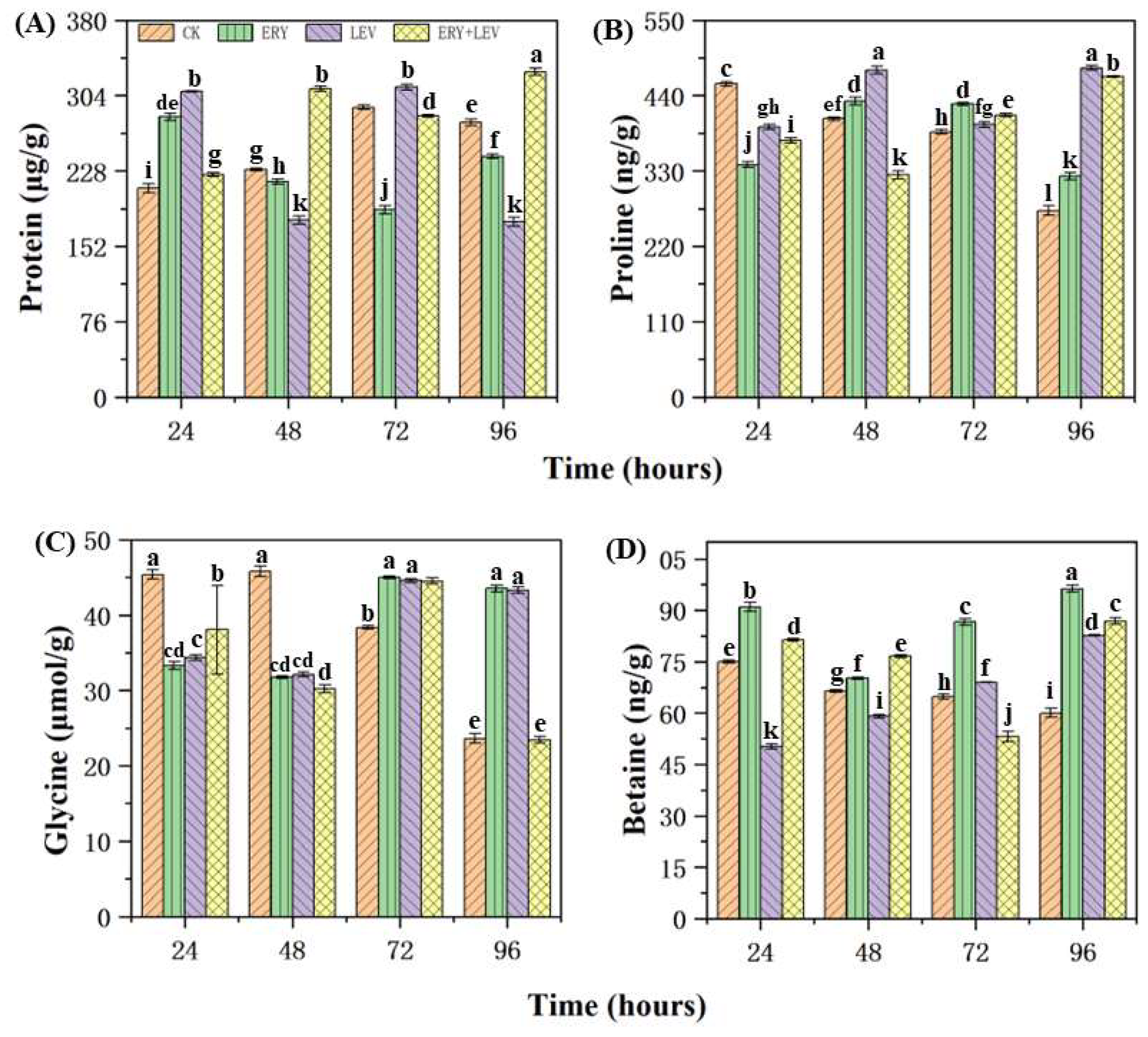
2.4. Partial Osmotic Substances of Navicula
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Microalgal Pre-Culture
3.3. Procedures for the Exposure Experiments
3.4. Measurement of Cell Density-Spectrophotometry
3.5. Chlorophyll Determination
3.6. Determination of Antioxidant Activities
3.7. Determination of Osmoregulation Substances
3.8. Statistical Analyses
4. Discussion
4.1. Effect on the Growth of Navicula
4.2. Effects on the Photosynthetic Pigment Content of Navicula
4.3. Effects on the Antioxidant Responses of Navicula
4.4. Effects of Partial Osmotic Substances of Navicula
5. Conclusions
6. Environmental Implication
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Osuoha, J.O.; Anyanwu, B.O.; Ejileugha, C. Pharmaceuticals and Personal Care Products as Emerging Contaminants: Need for Combined Treatment Strategy. J. Hazard. Mater. Adv. 2023, 9, 100206. [Google Scholar] [CrossRef]
- Liu, X.; Lu, S.; Guo, W.; Xi, B.; Wang, W. Antibiotics in the Aquatic Environments: A Review of Lakes, China. Sci. Total Environ. 2018, 627, 1195–1208. [Google Scholar] [CrossRef] [PubMed]
- Anh, H.Q.; Le, T.P.Q.; Da Le, N.; Lu, X.X.; Duong, T.T.; Garnier, J.; Rochelle-Newall, E.; Zhang, S.; Oh, N.-H.; Oeurng, C.; et al. Antibiotics in Surface Water of East and Southeast Asian Countries: A Focused Review on Contamination Status, Pollution Sources, Potential Risks, and Future Perspectives. Sci. Total Environ. 2021, 764, 142865. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, R.; Ternes, T.; Haberer, K.; Kratz, K.-L. Occurrence of Antibiotics in the Aquatic Environment. Sci. Total Environ. 1999, 225, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Langbehn, R.K.; Michels, C.; Soares, H.M. Antibiotics in Wastewater: From Its Occurrence to the Biological Removal by Environmentally Conscious Technologies. Environ. Pollut. 2021, 275, 116603. [Google Scholar] [CrossRef]
- Kumar, M.; Jaiswal, S.; Sodhi, K.K.; Shree, P.; Singh, D.K.; Agrawal, P.K.; Shukla, P. Antibiotics Bioremediation: Perspectives on Its Ecotoxicity and Resistance. Environ. Int. 2019, 124, 448–461. [Google Scholar] [CrossRef]
- Yu, C.; Pang, H.; Wang, J.-H.; Chi, Z.-Y.; Zhang, Q.; Kong, F.-T.; Xu, Y.-P.; Li, S.-Y.; Che, J. Occurrence of Antibiotics in Waters, Removal by Microalgae-Based Systems, and Their Toxicological Effects: A Review. Sci. Total Environ. 2022, 813, 151891. [Google Scholar] [CrossRef]
- Yang, L.; Ren, L.; Tan, X.; Chu, H.; Chen, J.; Zhang, Y.; Zhou, X. Removal of Ofloxacin with Biofuel Production by Oleaginous Microalgae Scenedesmus Obliquus. Bioresour. Technol. 2020, 315, 123738. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, S.; Wang, J.; Yao, Y.; Chen, Y.; Xu, Q.; Zhao, Z.; Chen, N. Diatom Biodiversity and Speciation Revealed by Comparative Analysis of Mitochondrial Genomes. Front. Plant Sci. 2022, 13, 749982. [Google Scholar] [CrossRef]
- Benoiston, A.-S.; Ibarbalz, F.M.; Bittner, L.; Guidi, L.; Jahn, O.; Dutkiewicz, S.; Bowler, C. The Evolution of Diatoms and Their Biogeochemical Functions. Philos. Trans. R. Soc. B Biol. Sci. 2017, 372, 20160397. [Google Scholar] [CrossRef] [Green Version]
- Soininen, J.; Teittinen, A. Fifteen Important Questions in the Spatial Ecology of Diatoms. Freshw. Biol. 2019, 64, 2071–2083. [Google Scholar] [CrossRef] [Green Version]
- Hamed, S.M.; Zinta, G.; Klöck, G.; Asard, H.; Selim, S.; AbdElgawad, H. Zinc-Induced Differential Oxidative Stress and Antioxidant Responses in Chlorella Sorokiniana and Scenedesmus Acuminatus. Ecotoxicol. Environ. Saf. 2017, 140, 256–263. [Google Scholar] [CrossRef] [PubMed]
- Ding, T.; Wang, S.; Yang, B.; Li, J. Biological Removal of Pharmaceuticals by Navicula Sp. and Biotransformation of Bezafibrate. Chemosphere 2020, 240, 124949. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Li, Y.; Guan, F.; Zhang, L.; Li, J.; Tai, Y.; Ren, H.; Duan, J. Variations in Microbial Community on Different Materials in Sanya Marine Environment Experimental Station, China. Can. J. Microbiol. 2022, 68, 447–455. [Google Scholar] [CrossRef]
- Li, J.; Li, W.; Liu, K.; Guo, Y.; Ding, C.; Han, J.; Li, P. Global Review of Macrolide Antibiotics in the Aquatic Environment: Sources, Occurrence, Fate, Ecotoxicity, and Risk Assessment. J. Hazard. Mater. 2022, 439, 129628. [Google Scholar] [CrossRef]
- Liu, K.; Li, J.; Zhou, Y.; Li, W.; Cheng, H.; Han, J. Combined Toxicity of Erythromycin and Roxithromycin and Their Removal by Chlorella Pyrenoidosa. Ecotoxicol. Environ. Saf. 2023, 257, 114929. [Google Scholar] [CrossRef]
- Wang, G.; Zhang, Q.; Li, J.; Chen, X.; Lang, Q.; Kuang, S. Combined Effects of Erythromycin and Enrofloxacin on Antioxidant Enzymes and Photosynthesis-Related Gene Transcription in Chlorella Vulgaris. Aquat. Toxicol. 2019, 212, 138–145. [Google Scholar] [CrossRef]
- Xiong, J.-Q.; Kurade, M.B.; Jeon, B.-H. Biodegradation of Levofloxacin by an Acclimated Freshwater Microalga, Chlorella Vulgaris. Chem. Eng. J. 2017, 313, 1251–1257. [Google Scholar] [CrossRef]
- Guillard, R.R.L. Culture of Phytoplankton for Feeding Marine Invertebrates. In Culture of Marine Invertebrate Animals: Proceedings—1st Conference on Culture of Marine Invertebrate Animals Greenport; Smith, W.L., Chanley, M.H., Eds.; Springer: Boston, MA, USA, 1975; pp. 29–60. ISBN 978-1-4615-8714-9. [Google Scholar]
- Guillard, R.R.; Ryther, J.H. Studies of Marine Planktonic Diatoms. I. Cyclotella Nana Hustedt, and Detonula Confervacea (Cleve) Gran. Can. J. Microbiol. 1962, 8, 229–239. [Google Scholar] [CrossRef]
- Zhang, S.; Wu, X.; Cui, J.; Zhang, F.; Wan, X.; Liu, Q.; Zhong, Y.; Lin, T. Physiological and Transcriptomic Analysis of Yellow Leaf Coloration in Populus Deltoides Marsh. PLoS ONE 2019, 14, e0216879. [Google Scholar] [CrossRef] [Green Version]
- Jiao, Q.; Mu, Y.; Deng, J.; Yao, X.; Zhao, X.; Liu, X.; Li, X.; Jiang, X.; Zhang, F. Direct Toxicity of the Herbicide Florasulam against Chlorella Vulgaris: An Integrated Physiological and Metabolomic Analysis. Ecotoxicol. Environ. Saf. 2022, 246, 114135. [Google Scholar] [CrossRef]
- Sun, T.; Zhang, J.; Zhang, Q.; Li, X.; Li, M.; Yang, Y.; Zhou, J.; Wei, Q.; Zhou, B. Transcriptome and Metabolome Analyses Revealed the Response Mechanism of Apple to Different Phosphorus Stresses. Plant Physiol. Biochem. 2021, 167, 639–650. [Google Scholar] [CrossRef] [PubMed]
- León-Vaz, A.; Romero, L.C.; Gotor, C.; León, R.; Vigara, J. Effect of Cadmium in the Microalga Chlorella Sorokiniana: A Proteomic Study. Ecotoxicol. Environ. Saf. 2021, 207, 111301. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, S.; Hu, X.; Zhou, Q. Envelopment–Internalization Synergistic Effects and Metabolic Mechanisms of Graphene Oxide on Single-Cell Chlorella Vulgaris Are Dependent on the Nanomaterial Particle Size. ACS Appl. Mater. Interfaces 2015, 7, 18104–18112. [Google Scholar] [CrossRef] [PubMed]
- Qian, L.; Qi, S.; Cao, F.; Zhang, J.; Zhao, F.; Li, C.; Wang, C. Toxic Effects of Boscalid on the Growth, Photosynthesis, Antioxidant System and Metabolism of Chlorella Vulgaris. Environ. Pollut. 2018, 242, 171–181. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Liu, K.; Li, W.; Zhang, M.; Li, P.; Han, J. Removal Mechanisms of Erythromycin by Microalgae Chlorella Pyrenoidosa and Toxicity Assessment during the Treatment Process. Sci. Total Environ. 2022, 848, 157777. [Google Scholar] [CrossRef]
- Lin, S.; Yu, X.; Fang, J.; Fan, J. Influences of the Micropollutant Erythromycin on Cyanobacteria Treatment with Potassium Permanganate. Water Res. 2020, 177, 115786. [Google Scholar] [CrossRef]
- Xiong, J.-Q.; Kim, S.-J.; Kurade, M.B.; Govindwar, S.; Abou-Shanab, R.A.I.; Kim, J.-R.; Roh, H.-S.; Khan, M.A.; Jeon, B.-H. Combined Effects of Sulfamethazine and Sulfamethoxazole on a Freshwater Microalga, Scenedesmus Obliquus: Toxicity, Biodegradation, and Metabolic Fate. J. Hazard. Mater. 2019, 370, 138–146. [Google Scholar] [CrossRef]
- Li, J.; Min, Z.; Li, W.; Xu, L.; Han, J.; Li, P. Interactive Effects of Roxithromycin and Freshwater Microalgae, Chlorella Pyrenoidosa: Toxicity and Removal Mechanism. Ecotoxicol. Environ. Saf. 2020, 191, 110156. [Google Scholar] [CrossRef]
- Guo, J.; Ma, Z.; Peng, J.; Mo, J.; Li, Q.; Guo, J.; Yang, F. Transcriptomic Analysis of Raphidocelis Subcapitata Exposed to Erythromycin: The Role of DNA Replication in Hormesis and Growth Inhibition. J. Hazard. Mater. 2021, 402, 123512. [Google Scholar] [CrossRef]
- Li, J.; Li, W.; Min, Z.; Zheng, Q.; Han, J.; Li, P. Physiological, Biochemical and Transcription Effects of Roxithromycin before and after Phototransformation in Chlorella Pyrenoidosa. Aquat. Toxicol. 2021, 238, 105911. [Google Scholar] [CrossRef] [PubMed]
- Jin, Z.P.; Luo, K.; Zhang, S.; Zheng, Q.; Yang, H. Bioaccumulation and Catabolism of Prometryne in Green Algae. Chemosphere 2012, 87, 278–284. [Google Scholar] [CrossRef]
- Xiang, R.; Shi, J.; Yu, Y.; Zhang, H.; Dong, C.; Yang, Y.; Wu, Z. The Effect of Bisphenol A on Growth, Morphology, Lipid Peroxidation, Antioxidant Enzyme Activity, and PS II in Cylindrospermopsis Raciborskii and Scenedesmus Quadricauda. Arch. Environ. Contam. Toxicol. 2018, 74, 515–526. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Liu, W.; Nie, X.; Guan, C.; Yang, Y.; Wang, Z.; Liao, W. Growth Response and Toxic Effects of Three Antibiotics on Selenastrum Capricornutum Evaluated by Photosynthetic Rate and Chlorophyll Biosynthesis. J. Environ. Sci. 2011, 23, 1558–1563. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Tang, Z.; Zhou, F.; Zhang, W.; Song, L. Toxicity Studies of Tetracycline on Microcystis Aeruginosa and Selenastrum Capricornutum. Environ. Toxicol. Pharmacol. 2013, 35, 320–324. [Google Scholar] [CrossRef]
- Liu, B.; Nie, X.; Liu, W.; Snoeijs, P.; Guan, C.; Tsui, M.T.K. Toxic Effects of Erythromycin, Ciprofloxacin and Sulfamethoxazole on Photosynthetic Apparatus in Selenastrum Capricornutum. Ecotoxicol. Environ. Saf. 2011, 74, 1027–1035. [Google Scholar] [CrossRef]
- González-Pleiter, M.; Gonzalo, S.; Rodea-Palomares, I.; Leganés, F.; Rosal, R.; Boltes, K.; Marco, E.; Fernández-Piñas, F. Toxicity of Five Antibiotics and Their Mixtures towards Photosynthetic Aquatic Organisms: Implications for Environmental Risk Assessment. Water Res. 2013, 47, 2050–2064. [Google Scholar] [CrossRef]
- Badran, E.G.; Abogadallah, G.M.; Nada, R.M.; Nemat Alla, M.M. Role of Glycine in Improving the Ionic and ROS Homeostasis during NaCl Stress in Wheat. Protoplasma 2015, 252, 835–844. [Google Scholar] [CrossRef]
- Rajput, V.D.; Harish; Singh, R.K.; Verma, K.K.; Sharma, L.; Quiroz-Figueroa, F.R.; Meena, M.; Gour, V.S.; Minkina, T.; Sushkova, S.; et al. Recent Developments in Enzymatic Antioxidant Defence Mechanism in Plants with Special Reference to Abiotic Stress. Biology 2021, 10, 267. [Google Scholar] [CrossRef]
- Aderemi, A.O.; Novais, S.C.; Lemos, M.F.L.; Alves, L.M.; Hunter, C.; Pahl, O. Oxidative Stress Responses and Cellular Energy Allocation Changes in Microalgae Following Exposure to Widely Used Human Antibiotics. Aquat. Toxicol. 2018, 203, 130–139. [Google Scholar] [CrossRef] [Green Version]
- Xu, D.; Xiao, Y.; Pan, H.; Mei, Y. Toxic Effects of Tetracycline and Its Degradation Products on Freshwater Green Algae. Ecotoxicol. Environ. Saf. 2019, 174, 43–47. [Google Scholar] [CrossRef] [PubMed]
- Wan, J.; Guo, P.; Peng, X.; Wen, K. Effect of Erythromycin Exposure on the Growth, Antioxidant System and Photosynthesis of Microcystis Flos-Aquae. J. Hazard. Mater. 2015, 283, 778–786. [Google Scholar] [CrossRef] [PubMed]
- Xiong, J.-Q.; Kurade, M.B.; Jeon, B.-H. Ecotoxicological Effects of Enrofloxacin and Its Removal by Monoculture of Microalgal Species and Their Consortium. Environ. Pollut. 2017, 226, 486–493. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.-M.; Ren, L.-J.; Bi, Z.-Q.; Ji, X.-J.; Zhao, Q.-Y.; Huang, H. Adaptive Evolution of Microalgae Schizochytrium Sp. under High Salinity Stress to Alleviate Oxidative Damage and Improve Lipid Biosynthesis. Bioresour. Technol. 2018, 267, 438–444. [Google Scholar] [CrossRef]
- Wan, J.; Guo, P.; Zhang, S. Response of the Cyanobacterium Microcystis Flos-Aquae to Levofloxacin. Environ. Sci. Pollut. Res. 2014, 21, 3858–3865. [Google Scholar] [CrossRef]
- Wu, L.; Qiu, Z.; Zhou, Y.; Du, Y.; Liu, C.; Ye, J.; Hu, X. Physiological Effects of the Herbicide Glyphosate on the Cyanobacterium Microcystis Aeruginosa. Aquat. Toxicol. 2016, 178, 72–79. [Google Scholar] [CrossRef]
- Zhang, H.; Forman, H.J. Glutathione Synthesis and Its Role in Redox Signaling. Semin. Cell Dev. Biol. 2012, 23, 722–728. [Google Scholar] [CrossRef] [Green Version]
- Sies, H. Glutathione and Its Role in Cellular Functions. Free Radic. Biol. Med. 1999, 27, 916–921. [Google Scholar] [CrossRef]
- Mofeed, J.; Mosleh, Y.Y. Toxic Responses and Antioxidative Enzymes Activity of Scenedesmus Obliquus Exposed to Fenhexamid and Atrazine, Alone and in Mixture. Ecotoxicol. Environ. Saf. 2013, 95, 234–240. [Google Scholar] [CrossRef]
- Alla, N. The Influence of Naphthalic Anhydride and 1-Aminobenzotriazole on Maize Tolerance to Herbicides: A Possible Role for Glutathione S-Transferase in Herbicide Persistence and Detoxification. Agric. Mediterr. 2000, 130, 18–26. [Google Scholar]
- Guzzetti, E.; Salabery, E.; Ferriol, P.; Díaz, J.A.; Tejada, S.; Faggio, C.; Sureda, A. Oxidative Stress Induction by the Invasive Sponge Paraleucilla Magna Growing on Peyssonnelia Squamaria Algae. Mar. Environ. Res. 2019, 150, 104763. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; He, F.; Wu, C.; Li, P.; Li, N.; Deng, J.; Zhu, G.; Ren, W.; Peng, Y. Betaine in Inflammation: Mechanistic Aspects and Applications. Front. Immunol. 2018, 9, 1070. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smirnoff, N.; Cumbes, Q.J. Hydroxyl Radical Scavenging Activity of Compatible Solutes. Phytochemistry 1989, 28, 1057–1060. [Google Scholar] [CrossRef]
- Figueroa-Soto, C.G.; Valenzuela-Soto, E.M. Glycine Betaine Rather than Acting Only as an Osmolyte Also Plays a Role as Regulator in Cellular Metabolism. Biochimie 2018, 147, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Lou, Y.; Yang, Y.; Hu, L.; Liu, H.; Xu, Q. Exogenous Glycinebetaine Alleviates the Detrimental Effect of Cd Stress on Perennial Ryegrass. Ecotoxicology 2015, 24, 1330–1340. [Google Scholar] [CrossRef] [PubMed]
- Abdelaal, K.A.A.; Attia, K.A.; Alamery, S.F.; El-Afry, M.M.; Ghazy, A.I.; Tantawy, D.S.; Al-Doss, A.A.; El-Shawy, E.-S.E.; Abu-Elsaoud, A.M.; Hafez, Y.M. Exogenous Application of Proline and Salicylic Acid Can Mitigate the Injurious Impacts of Drought Stress on Barley Plants Associated with Physiological and Histological Characters. Sustainability 2020, 12, 1736. [Google Scholar] [CrossRef] [Green Version]
- Dubrovna, O.V.; Mykhalska, S.I.; Komisarenko, A.G. Using Proline Metabolism Genes in Plant Genetic Engineering. Cytol. Genet. 2022, 56, 361–378. [Google Scholar] [CrossRef]
- Hossain, M.A.; Hasanuzzaman, M.; Fujita, M. Coordinate Induction of Antioxidant Defense and Glyoxalase System by Exogenous Proline and Glycinebetaine Is Correlated with Salt Tolerance in Mung Bean. Front. Agric. China 2011, 5, 1–14. [Google Scholar] [CrossRef]
- Zhang, Y.; Skirycz, A.; Fernie, A.R. An Abundance and Interaction Encyclopedia of Plant Protein Function. Trends Plant Sci. 2020, 25, 627–630. [Google Scholar] [CrossRef]
- Xiong, Q.; Liu, Y.-S.; Hu, L.-X.; Shi, Z.-Q.; Ying, G.-G. Levofloxacin and Sulfamethoxazole Induced Alterations of Biomolecules in Pseudokirchneriella Subcapitata. Chemosphere 2020, 253, 126722. [Google Scholar] [CrossRef]


| Compound | Molecular Formula | Molecular Structure | Molecular Weight (g/mol) |
|---|---|---|---|
| Erythromycin | C37H67NO13 |  | 733.94 |
| Levofloxacin | C18H20FN3O4 | 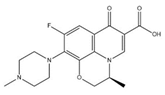 | 361.37 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, J.; Ahmed, W.; Mehmood, S.; Ou, W.; Li, J.; Xu, W.; Wang, L.; Mahmood, M.; Li, W. Evaluating the Combined Effects of Erythromycin and Levofloxacin on the Growth of Navicula sp. and Understanding the Underlying Mechanisms. Plants 2023, 12, 2547. https://doi.org/10.3390/plants12132547
Yang J, Ahmed W, Mehmood S, Ou W, Li J, Xu W, Wang L, Mahmood M, Li W. Evaluating the Combined Effects of Erythromycin and Levofloxacin on the Growth of Navicula sp. and Understanding the Underlying Mechanisms. Plants. 2023; 12(13):2547. https://doi.org/10.3390/plants12132547
Chicago/Turabian StyleYang, Jie, Waqas Ahmed, Sajid Mehmood, Wenjie Ou, Jiannan Li, Wenxin Xu, Lu Wang, Mohsin Mahmood, and Weidong Li. 2023. "Evaluating the Combined Effects of Erythromycin and Levofloxacin on the Growth of Navicula sp. and Understanding the Underlying Mechanisms" Plants 12, no. 13: 2547. https://doi.org/10.3390/plants12132547





