Co-Inoculation with Arbuscular Mycorrhizal Fungi and Dark Septate Endophytes under Drought Stress: Synergistic or Competitive Effects on Maize Growth, Photosynthesis, Root Hydraulic Properties and Aquaporins?
Abstract
:1. Introduction
2. Results
2.1. AMF and DSE Colonization
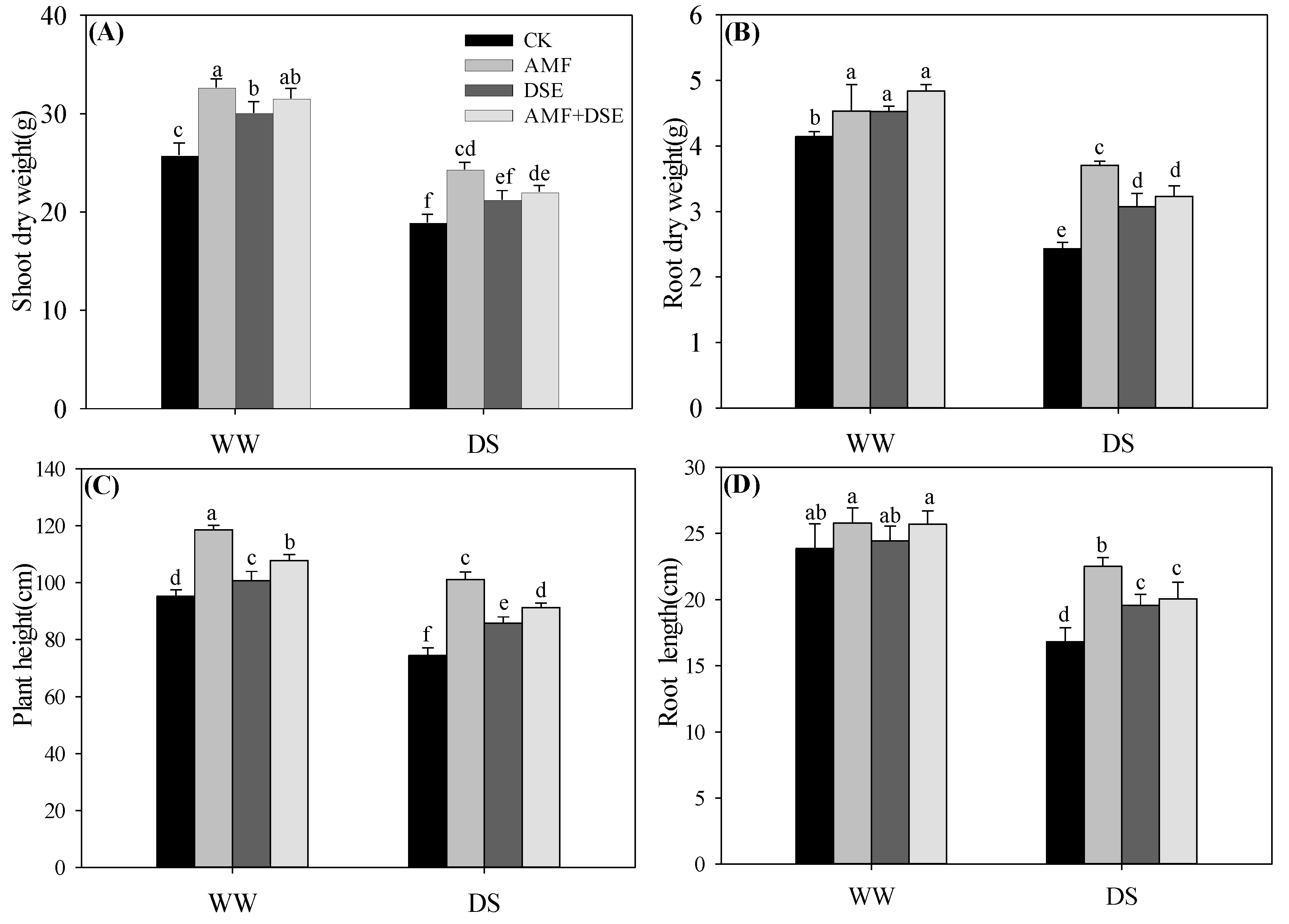
2.2. Maize Growth
2.3. Membrane Electrolyte Leakage (EL) and Oxidative Damage
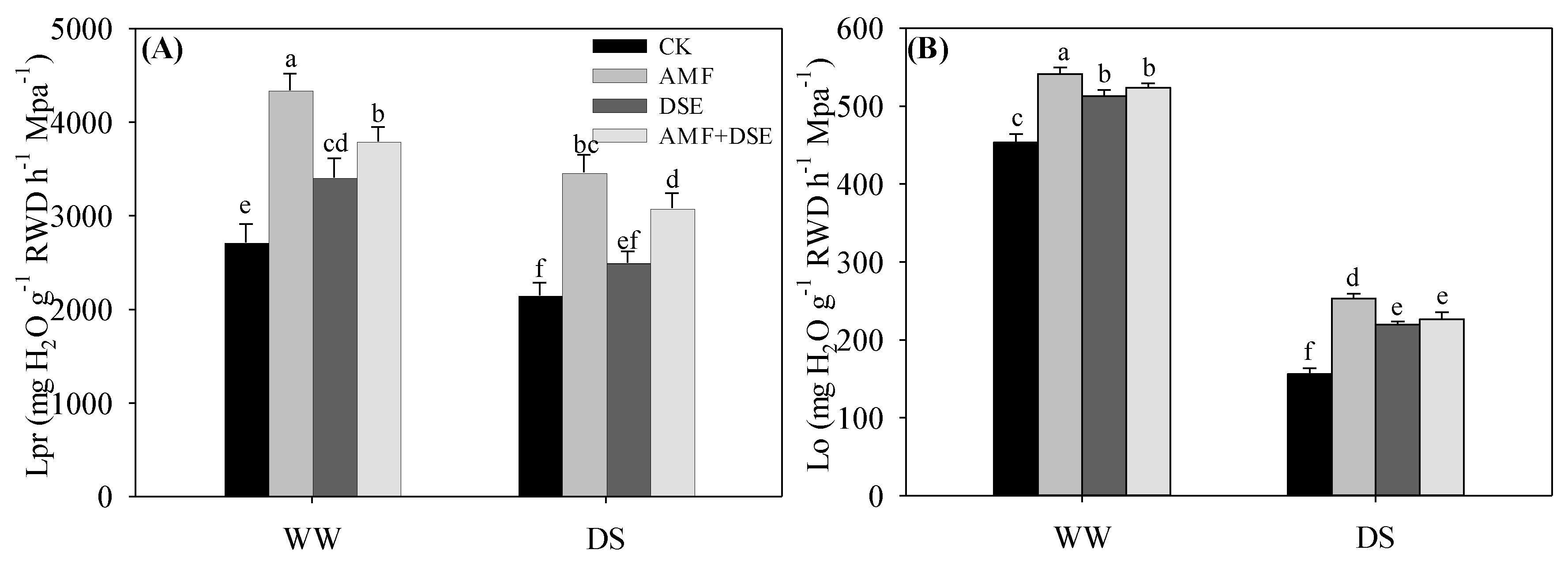
2.4. Root Hydraulic Conductivity
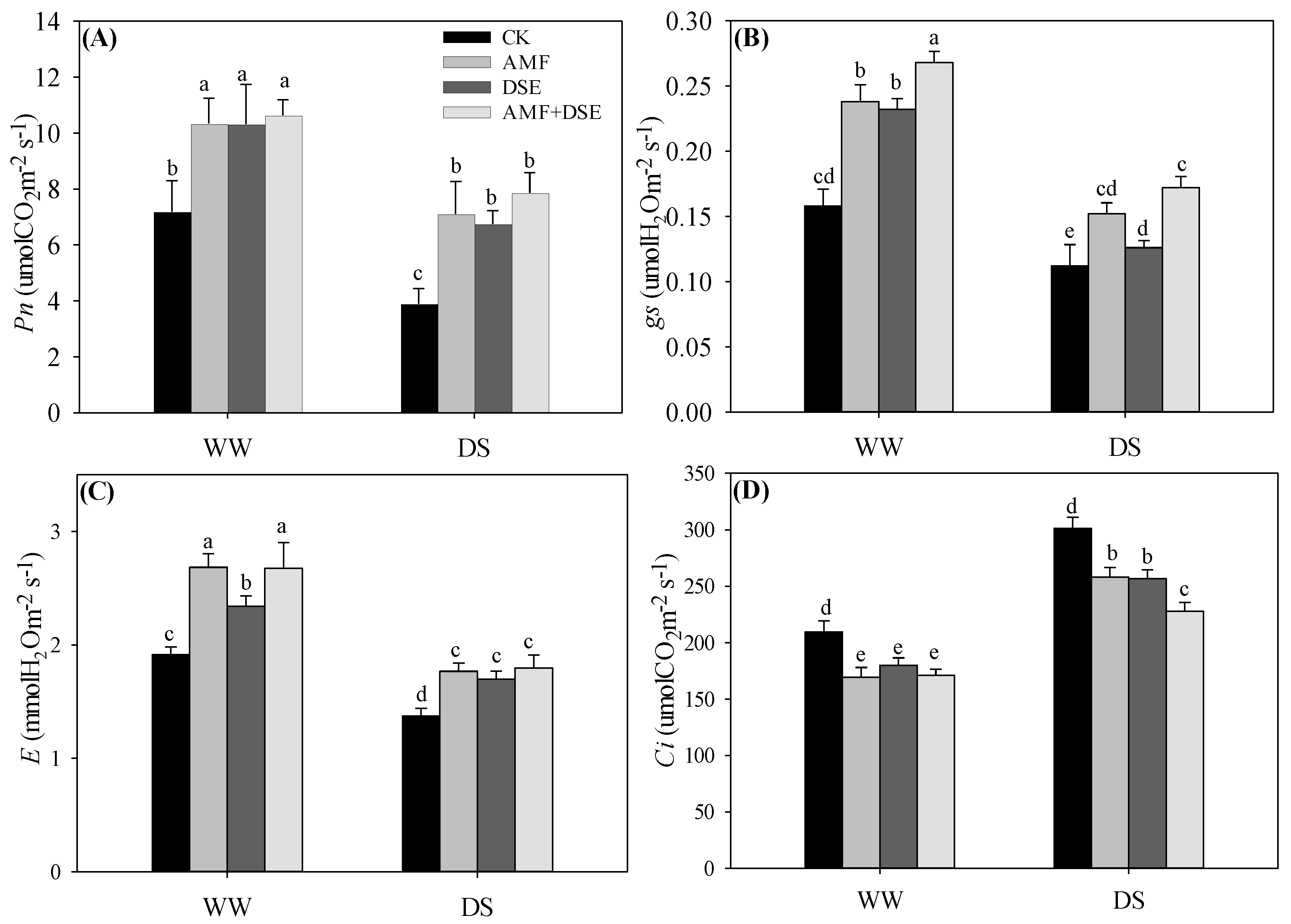
2.5. Gas Exchange
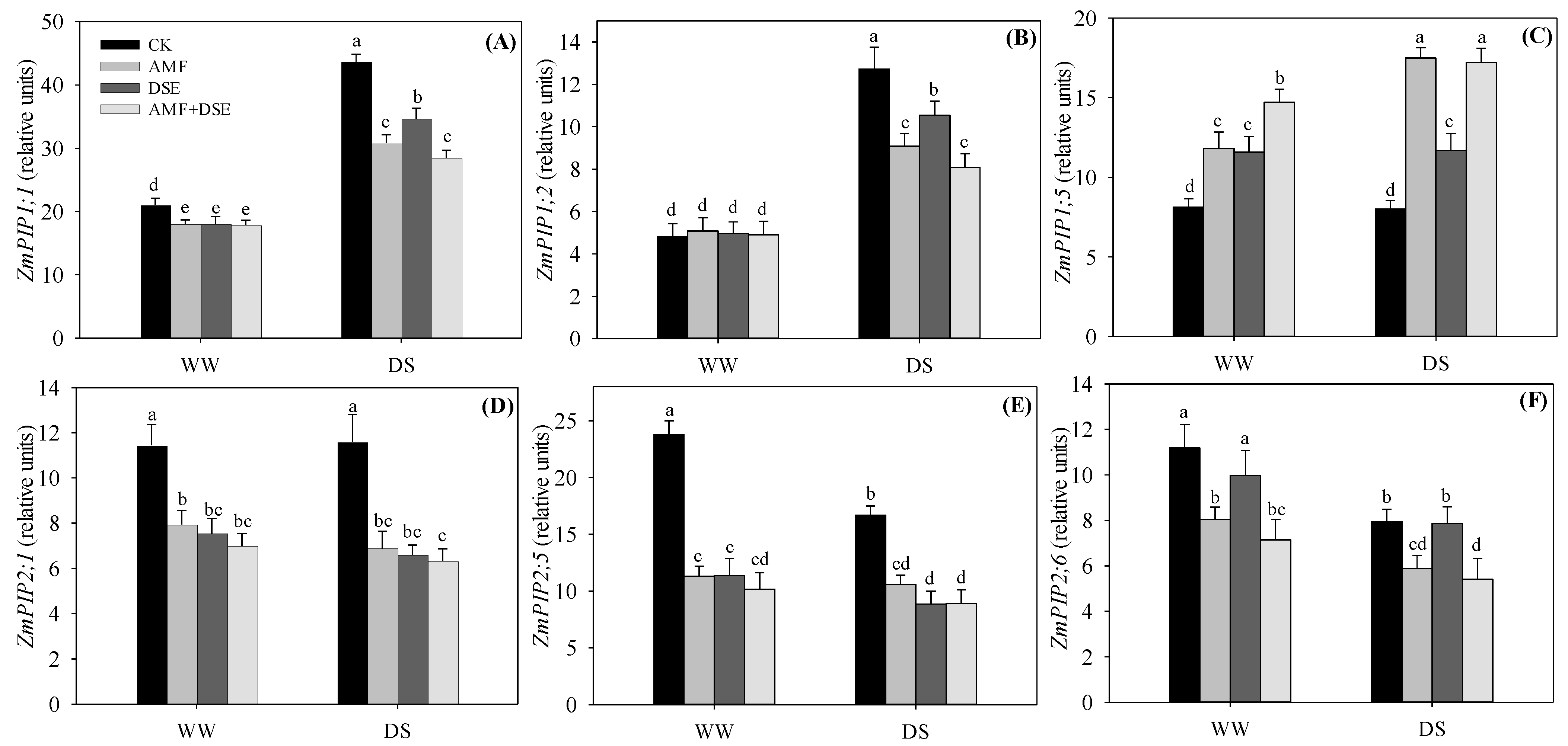
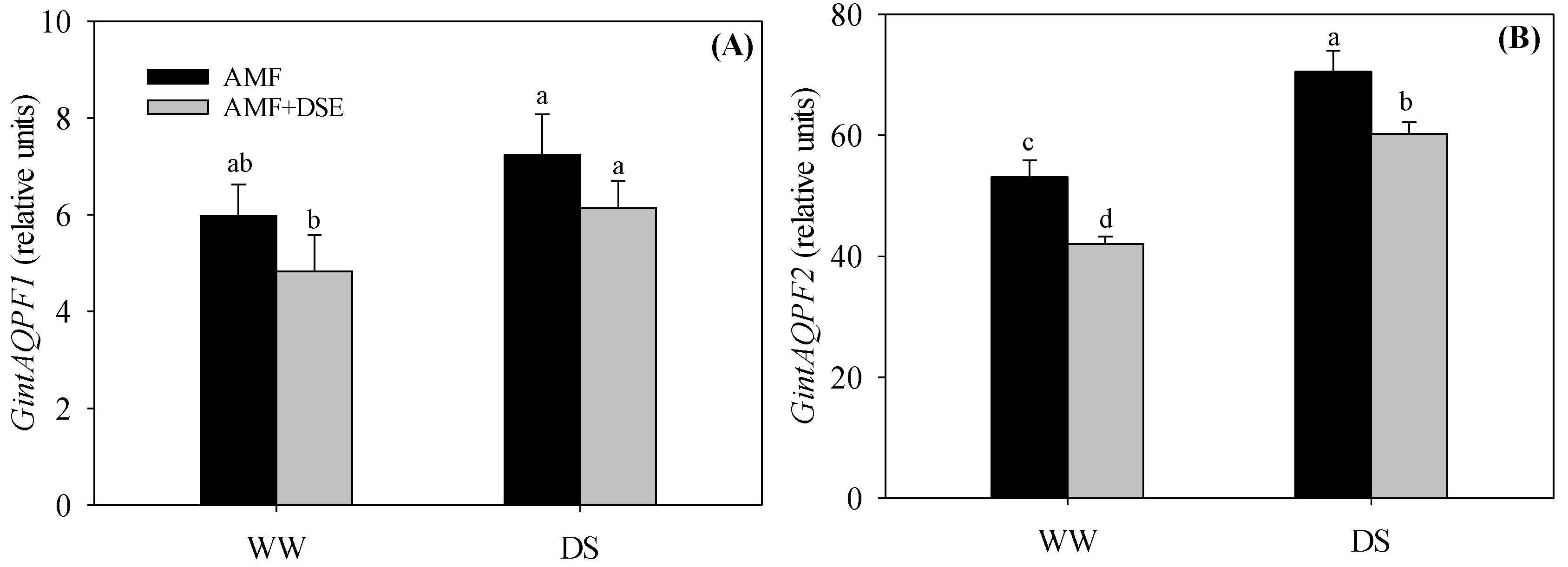
2.6. Gene Expression of Maize Aquaporins
2.7. Gene Expression of AMF Aquaporins
3. Discussion
4. Materials and Methods
4.1. Experimental Design and Statistical Analysis
4.2. Biological Material
4.3. Growth Conditions
4.4. Measurements
4.4.1. Mycorrhizal Colonization and Plant Growth
4.4.2. Membrane Electrolyte Leakage
4.4.3. Root Hydraulic Conductivity
4.4.4. Photosynthetic Efficiency
4.4.5. Measurement of Oxidative Damage
4.4.6. Gene Expression of PIP Aquaporin
4.5. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Quiroga, G.; Erice, G.; Aroca, R.; Chaumont, F.; Ruiz-Lozano, J.M. Enhanced drought stress tolerance by the arbuscular mycorrhizal symbiosis in a drought-sensitive maize cultivar is related to a broader and differential regulation of host plant aquaporins than in a drought-tolerant cultivar. Front. Plant Sci. 2017, 8, 1056. [Google Scholar] [CrossRef] [Green Version]
- Singh, N.; Mittal, S.; Thirunavukkarasu, N. Effect of drought stress and utility of transcriptomics in identification of drought tolerance mechanisms in maize. In Genetic Enhancement of Crops for Tolerance to Abiotic Stress: Mechanisms and Approaches; Springer: Berlin, Germany, 2019; pp. 73–97. [Google Scholar]
- Bárzana, G.; Aroca, R.; Ruiz-Lozano, J.M. Localized and non-localized effects of arbuscular mycorrhizal symbiosis on accumulation of osmolytes and aquaporins and on antioxidant systems in maize plants subjected to total or partial root drying. Plant Cell. Environ. 2015, 38, 1613–1627. [Google Scholar] [CrossRef]
- Quiroga, G.; Erice, G.; Aroca, R.; Delgado-Huertas, A.; Ruiz-Lozano, J.M. Elucidating the Possible Involvement of Maize Aquaporins and Arbuscular Mycorrhizal Symbiosis in the Plant Ammonium and Urea Transport under Drought Stress Conditions. Plants 2020, 9, 148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hasanuzzaman, M.; Nahar, K.; Gill, S.S.; Fujita, M. Drought stress responses inplants, oxidative stress, and antioxidant defense. In Climate Change and Plant Abiotic Stress Tolerance; John Wiley & Sons: Hoboken, NJ, USA, 2014; Volume 9, pp. 209–249. [Google Scholar]
- Ruiz-Lozano, J.M.; Alguacil, M.D.M.; Bárzana, G.; Vernieri, P.; Aroca, R. Exogenous ABA accentuates the differences in root hydraulic properties between mycorrhizal and non mycorrhizal maize plants through regulation of PIP aquaporins. Plant Mol. Biol. 2009, 70, 565–579. [Google Scholar] [CrossRef] [PubMed]
- Quiroga, G.; Erice, G.; Ding, L.; Chaumont, F.; Aroca, R.; Ruiz-Lozano, J.M. The arbuscular mycorrhizal symbiosis regulates aquaporins activity and improves root cell water permeability in maize plants subjected to water stress. Plant. Cell. Environ. 2019, 42, 2274–2290. [Google Scholar] [CrossRef] [PubMed]
- Daryanto, S.; Wang, L.; Jacinthe, P.A. Global synthesis of drought effects on maize and wheat production. PLoS ONE 2016, 11, e0156362. [Google Scholar] [CrossRef] [Green Version]
- Quiroga, G.; Erice, G.; Aroca, R.; Zamarreño, Á.M.; García-Mina, J.M.; Ruiz-Lozano, J.M. Radial water transport in arbuscular mycorrhizal maize plants under drought stress conditions is affected by indole-acetic acid (IAA) application. J. Plant Physiol. 2020, 246–247, 153115. [Google Scholar] [CrossRef]
- Anjum, S.A.; Tanveer, M.; Ashraf, U.; Hussain, S.; Shahzad, B.; Khan, I.; Wang, L. Effect of progressive drought stress on growth, leaf gas exchange, and antioxidant production in two maize cultivars. Environ. Sci. Pollut. Res. 2016, 23, 17132–17141. [Google Scholar] [CrossRef]
- Min, H.; Chen, C.; Wei, S.; Shang, X.; Sun, M.; Xia, R.; Liu, X.; Hao, D.; Chen, H.; Xie, Q. Identifification of drought tolerant mechanisms in maize seedlings based on transcriptome analysis of recombination inbred lines. Front. Plant Sci. 2016, 7, 1080. [Google Scholar] [CrossRef] [Green Version]
- Clifton, P.; Mesquita, B.D.; Cormac, M.; Río, M.D.; Katharine, N.; Suding, S.K.; Schmidt, S.K. Rapid temporal changes in root colonization by arbuscular mycorrhizal fungi and fine root endophytes, not dark septate endophytes, track plant activity and environment in an alpine ecosystem. Mycorrhiza 2018, 28, 717–726. [Google Scholar]
- Zou, Y.; Wu, Q.; Kuča, K. Unraveling the role of arbuscular mycorrhizal fungi in mitigating the oxidative burst of plants under drought stress. Plant Biol. 2020, 23, 50–57. [Google Scholar] [CrossRef]
- Ruiz-Lozano, J.M.; Aroca, R. Plant aquaporins and mycorrhizae: Their regulation and involvement in plant physiology and performance. In Plant Aquaporins, Signaling and Communication in Plants; Chaumont, F., Tyerman, S.D., Eds.; Springer International Publishing AG: Cham, Switzerland, 2017; pp. 333–353. [Google Scholar]
- Hachez, C.; Heinen, R.B.; Draye, X.; Chaumont, F. The expression pattern of plasma membrane aquaporins in maize leaf highlights their role in hydraulic regulation. Plant. Mol. Biol. 2008, 68, 337. [Google Scholar] [CrossRef]
- Bárzana, G.; Aroca, R.; Bienert, G.P.; Chaumont, F.; Ruiz-Lozano, J.M. New insights into the regulation of aquaporins by the arbuscular mycorrhizal symbiosis in maize plants under drought stress and possible implications for plant performance. Mol. Plant Microbe Interact. 2014, 27, 349–363. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aroca, R.; Ruiz-Lozano, J.M. Root water transport under abiotic stress conditions. In New Plant Physiology Research; Devane, R.T., Ed.; Nova Science Publisher: New York, NY, USA, 2009; pp. 97–110. [Google Scholar]
- Jumpponen, A. Dark septate endophytes-Are they mycorrhizal? Mycorrhiza 2001, 11, 207–211. [Google Scholar] [CrossRef]
- Claassens, A.; Nock, C.J.; Zwieten, L.V.; Rose, T.J. Mycorrhizal fungi interactions with nutrients, pests and pathogens in sugarcane: A review. In Proceedings of the Australian Society of Sugar Cane Technologists, Cairns, QLD, Australia, 3–5 May 2017; Volume 39, pp. 326–332. [Google Scholar]
- Ban, Y.; Xu, Z.; Yang, Y.; Zhang, H.; Chen, H.; Tang, M. Effect of dark septate endophytic fungus Gaeumannomyces cylindrosporus on plant growth, photosynthesis and Pb tolerance of maize (Zea mays L.). Pedosphere 2017, 27, 283–292. [Google Scholar] [CrossRef]
- He, Y.M.; Fan, X.M.; Zhang, G.Q.; Li, B.; Li, T.G.; Zu, Y.Q.; Zhan, F.D. Effects of arbuscular mycorrhizal fungi and dark septate endophytes on maize performance and root traits under a high cadmium stress. S. Afr. J. Bot. 2019, 134, 415–423. [Google Scholar] [CrossRef]
- Berthelot, C.; Blaudez, D.; Leyval, C. Differential growth promotion of poplar and birch inoculated with three dark septate endophytes in two trace element-contaminated soils. Int. J. Phytoremediat. 2017, 19, 1118–1125. [Google Scholar] [CrossRef] [PubMed]
- Scervino, J.M.; Gottlieb, A.; Silvani, V.A.; Pérgola, M.; Fernández, L.; Godeas, A.M. Exudates of dark septate endophyte (DSE) modulate the development of the arbuscular mycorrhizal fungus (AMF) Gigaspora rosea. Soil. Biol. Biochem. 2009, 41, 1753–1756. [Google Scholar] [CrossRef]
- Claassens, A.; Nock, C.J.; Rose, M.T.; Zwieten, L.V.; Rose, T.J. Colonisation dynamics of arbuscular mycorrhizal fungi and dark septate endophytes in the sugarcane crop cycle. Rhizosphere 2018, 7, 18–26. [Google Scholar] [CrossRef]
- Della Monica, I.F.; Saparrat, M.C.; Godeas, A.M.; Scervino, J.M. The co-existence between DSE and AMF symbionts affects plant P pools through P mineralization and solubilization processes. Fungal. Ecol. 2015, 17, 10–17. [Google Scholar] [CrossRef]
- He, Y.M.; Fan, X.M.; Zhang, G.Q.; Li, B.; Li, M.R.; Zu, Y.Q.; Zhan, F.D. Influences of arbuscular mycorrhizal fungi and dark septate endophytes on the growth, nutrition, photosynthesis and antioxidant physiology of maize. Intl. J. Agric. Biol. 2019, 22, 1071–1078. [Google Scholar]
- Marulanda, A.; Azcon, R.; Chaumont, F.; Ruiz-Lozano, J.M.; Aroca, R. Regulation of plasma membrane aquaporins by inoculation with a Bacillus megaterium strain in maize (Zea mays L.) plants under unstressed and salt-stressed conditions. Planta 2010, 232, 533–543. [Google Scholar] [CrossRef] [PubMed]
- Soteras, F.; Renison, D.; Becerra, A. Growth response, phosphorus content and root colonization of Polylepis australis bitt. seedlings inoculated with different soil types. Forest 2013, 44, 577–589. [Google Scholar] [CrossRef]
- Berthelot, C.; Blaudez, D.; Beguiristain, T.; Chalot, M.; Leyval, C. Co-inoculation of Lolium perenne with Funneliformis mosseae and the dark septate endophyte Cadophora sp. in a trace element-polluted soil. Mycorrhiza 2018, 28, 301–314. [Google Scholar] [CrossRef] [Green Version]
- Xie, L.L.; Bi, Y.L.; Ma, S.P.; Shang, J.X.; Hu, Q.C.; Peter, C. Combined inoculation with dark septate endophytes and arbuscular mycorrhizal fungi: Synergistic or competitive growth efects on maize? BMC Plant Biol. 2021, 21, 498. [Google Scholar] [CrossRef]
- Reininger, V.; Sieber, T. Mycorrhiza reduces adverse effects of dark septate endophytes (DSE) on growth of conifers. PLoS ONE 2012, 7, e42865. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ważny, R.; Rozpądek, P.; Jędrzejczyk, R.J.; Śliwa, M.; Stojakowska, A.; Anielska, T.; Turnau, K. Does co-inoculation of Lactuca serriola with endophytic and arbuscular mycorrhizal fungi improve plant growth in a polluted environment? Mycorrhiza 2018, 28, 235–246. [Google Scholar] [CrossRef] [Green Version]
- Wężowicz, K.; Rozpadek, P.; Turnau, K. Interactions of arbuscular mycorrhizal and endophytic fungi improve seedling survival and growth in post-mining waste. Mycorrhiza 2017, 27, 499–511. [Google Scholar] [CrossRef] [Green Version]
- Shahabivand, S.; Maivan, H.; Goltapeh, E.; Sharifi, M.; Aliloo, A. The effects of root endophyte and arbuscular mycorrhizal fungi on growth and cadmium accumulation in wheat under cadmium toxicity. Plant Physiol. Bioch. 2012, 60, 53–58. [Google Scholar] [CrossRef]
- Ortiz, N.; Armada, E.; Duque, E.; Roldán, A.; Azcón, R. Contribution of arbuscular mycorrhizal fungi and/or bacteria to enhancing plant drought tolerance under natural soil conditions: Effectiveness of autochthonous or allochthonous strains. J. Plant Physiol. 2015, 174, 87–96. [Google Scholar] [CrossRef]
- Abid, M.; Tian, Z.; Ata-Ul-Karim, S.T.; Liu, Y.; Cui, Y.; Zahoor, R.; Jiang, D.; Dai, T. Improved tolerance to post-anthesis drought stress by pre-drought priming at vegetative stages in drought-tolerant and -sensitive wheat cultivars. Plant Physiol. Biochem. 2016, 106, 218–227. [Google Scholar] [CrossRef] [PubMed]
- Calvo-Polanco, M.; Sánchez-Castro, I.; Cantos, M.; García, J.L.; Azcón, R.; Ruiz-Lozano, J.M.; Beuzón, C.R.; Aroca, R. Effects of different arbuscular mycorrhizal fungal backgrounds and soils on olive plants growth and water relation properties under well-watered and drought conditions. Plant Cell Environ. 2016, 39, 2498–2514. [Google Scholar] [CrossRef] [Green Version]
- Khalvati, M.A.; Hu, Y.; Mozafar, A.; Schmidhalter, U. Quantification of water uptake by arbuscular mycorrhizal hyphae and its significance for leaf growth, water relations, and gas exchange of barley subjected to drought stress. Plant Biol. 2005, 7, 706–712. [Google Scholar] [CrossRef] [Green Version]
- Sánchez-Romera, B.; Ruiz-Lozano, J.M.; Zamarreño, Á.M.; García-Mina, J.M.; Aroca, R. Arbuscular mycorrhizal symbiosis and methyl jasmonate avoid the inhibition of root hydraulic conductivity caused by drought. Mycorrhiza 2016, 26, 111–122. [Google Scholar] [CrossRef]
- Galmés, J.; Medrano, H.; Flexas, J. Photosynthetic limitations in response to water stress and recovery in Mediterranean plants with different growth forms. New Phytol. 2007, 175, 81–93. [Google Scholar] [CrossRef] [PubMed]
- Maurel, C.; Verdoucq, L.; Luu, D.T.; Santoni, V. Plant aquaporins: Membrane channels with multiple integrated functions. Annu. Rev. Plant Biol. 2008, 59, 595–624. [Google Scholar] [CrossRef] [Green Version]
- Maurel, C. Plant aquaporins: Novel functions and regulation properties. Fed. Eur. Biochem. Soc. Lett. 2007, 581, 2227–2236. [Google Scholar] [CrossRef]
- Venter, W.D.; Snyman, H.A.; van Rensburg, W.L.J. Photosynthetic response to water stress in Themeda triandra and Eragrostis lehmanniana. S. Afr. J. Bot. 1997, 63, 37–41. [Google Scholar] [CrossRef] [Green Version]
- Li, B.; Li, G.; Kronzucker, H.J.; Baluška, F.; Shi, W. Ammonium stress in Arabidopsis: Signaling, genetic loci, and physiological targets. Trends. Plant. Sci. 2014, 19, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Li, C.; Liang, D.; Ma, F.; Wang, S.; Wang, P.; Wang, R. Aquaporin expression in response to water-defificit stress in two Malus species: Relationship with physiological status and drought tolerance. Plant Growth Regul. 2013, 70, 187–197. [Google Scholar] [CrossRef]
- Vinnakota, R.; Ramakrishnan, A.M.; Samdani, A.; Venugopal, M.A.; Ram, B.S.; Krishnan, S.N.; Murugesan, D.; Sankaranarayanan, K. A comparison of aquaporin function in mediating stomatal aperture gating among drought-tolerant and sensitive varieties of rice (Oryza sativa L.). Protoplasma 2016, 253, 1593–1597. [Google Scholar] [CrossRef]
- Liang, W.H.; Li, L.; Zhang, F.; Liu, Y.X.; Li, M.M.; Shi, H.H.; Li, H.; Shang, F.; Lou, C.; Lin, Q.-T.; et al. Effects of abiotic stress, light, phytochromes and phytohormones on the expression of OsAQP, a rice aquaporin gene. Plant Growth Regul. 2013, 69, 21–27. [Google Scholar] [CrossRef]
- Li, T.; Hu, Y.J.; Hao, Z.P.; Li, H.; Wang, Y.S.; Chen, B.D. First cloning and characterization of two functional aquaporin genes from an arbuscular mycorrhizal fungus Glomus intraradices. New Phytol. 2013, 197, 617–630. [Google Scholar] [CrossRef]
- Allen, M.F. Mycorrhizal fungi: Highways for water and nutrients in arid soils. Vadose Zone J. 2007, 6, 291–297. [Google Scholar] [CrossRef]
- Hoagland, D.R.; Arnon, D.I. The water-culture method for growing plants without soil. Calif. Agric. Exp. Stn. Circ. 1950, 347, 1–32. [Google Scholar]
- Phillips, J.M.; Hayman, D.S. Improved procedures for clearing roots and staining parasitic and vesicular-arbuscular mycorrhizal fungi for rapid assessment of infection. Trans. Br. Mycol. Soc. 1970, 55, 158–161. [Google Scholar] [CrossRef]
- Giovannetti, M.; Mosse, B. An evaluation of techniques for measuring vesicular arbuscular mycorrhizal infection in roots. New Phytol. 1980, 84, 489–500. [Google Scholar] [CrossRef]
- Bárzana, G.; Aroca, R.; Paz, J.A.; Chaumont, F.; Martinez-Ballesta, M.C.; Carvajal, M.; Ruiz-Lozano, J.M. Arbuscular mycorrhizal symbiosis increases relative apoplastic water flow in roots of the host plant under both well-watered and drought stress conditions. Ann. Bot. 2012, 109, 1009–1017. [Google Scholar] [CrossRef]
- Benabdellah, K.; Ruiz-Lozano, J.M.; Aroca, R. Hydrogen peroxide effects on root hydraulic properties and plasma membrane aquaporin regulation in Phaseolus vulgaris. Plant Mol. Biol. 2009, 70, 647–661. [Google Scholar] [CrossRef]
- Aroca, R.; Porcel, R.; Ruiz-Lozano, J.M. How does arbuscular mycorrhizal symbiosis regulate root hydraulic properties and plasma membrane aquaporins in Phaseolus vulgaris under drought, cold or salinity stresses? New Phytol. 2007, 173, 808–816. [Google Scholar] [CrossRef]
- Janero, D.R. Malondialdehyde and thiobarbituric acid-reactivity as diagnostic indices of lipid peroxidation and peroxidative tissue injury. Free Radic. Biol. Med. 1990, 9, 515–540. [Google Scholar] [CrossRef]
- Wang, A.G.; Luo, G.H. Quantitative relation between the reaction of hydroxylamine and superoxide anion radicals in plants. Plant Physiol Commun. 1990, 26, 55–57. [Google Scholar]
- Elavarthi, S.; Martin, B. Spectrophotometric assays for antioxidant enzymes in plants. Methods Mol. Biol. 2010, 639, 273–280. [Google Scholar] [PubMed]
- Hachez, C.; Moshelion, M.; Zelazny, E.; Cavez, D.; Chaumont, F. Localization and quantifification of plasma membrane aquaporin expression in maize primary root: A clue to understanding their role as cellular plumbers. Plant Mol. Biol. 2006, 62, 305–323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kay, R.; Chan, A.; Daly, M.; McPherson, J. Duplication of CaMV 35S promoter sequences creates a strong enhancer for plant genes. Science 1987, 236, 1299–1302. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCt method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]

| Water Conditions | Inoculation | Number of AMF Spore (Number /gDW) | Arbuscule Colonization (%) | AMF Colonization(%) | DSE Colonization (%) |
|---|---|---|---|---|---|
| WS | CK | - | - | - | - |
| AMF | 25.67 ± 2.08 a | 23 ± 2.00 a | 42.42 ± 1.53 a | - | |
| DSE | - | - | - | 42.92 ± 2.57 a | |
| AMF + DSE | 20.67 ± 2.52 ab | 17.67 ± 1.53 b | 33.42 ± 1.29 b | 36.42 ± 1.42 b | |
| DS | CK | - | - | - | - |
| AMF | 18.33 ± 1.53 b | 16.67 ± 1.53 b | 29.58 ± 1.88 c | - | |
| DSE | - | - | - | 31.42 ± 1.13 c | |
| AMF + DSE | 12.67 ± 1.53 c | 10 ± 1.00 c | 18.5 ± 1.00 d | 24.33 ± 1.51 d |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gong, M.; Bai, N.; Wang, P.; Su, J.; Chang, Q.; Zhang, Q. Co-Inoculation with Arbuscular Mycorrhizal Fungi and Dark Septate Endophytes under Drought Stress: Synergistic or Competitive Effects on Maize Growth, Photosynthesis, Root Hydraulic Properties and Aquaporins? Plants 2023, 12, 2596. https://doi.org/10.3390/plants12142596
Gong M, Bai N, Wang P, Su J, Chang Q, Zhang Q. Co-Inoculation with Arbuscular Mycorrhizal Fungi and Dark Septate Endophytes under Drought Stress: Synergistic or Competitive Effects on Maize Growth, Photosynthesis, Root Hydraulic Properties and Aquaporins? Plants. 2023; 12(14):2596. https://doi.org/10.3390/plants12142596
Chicago/Turabian StyleGong, Minggui, Na Bai, Pengfei Wang, Jiajie Su, Qingshan Chang, and Qiaoming Zhang. 2023. "Co-Inoculation with Arbuscular Mycorrhizal Fungi and Dark Septate Endophytes under Drought Stress: Synergistic or Competitive Effects on Maize Growth, Photosynthesis, Root Hydraulic Properties and Aquaporins?" Plants 12, no. 14: 2596. https://doi.org/10.3390/plants12142596
APA StyleGong, M., Bai, N., Wang, P., Su, J., Chang, Q., & Zhang, Q. (2023). Co-Inoculation with Arbuscular Mycorrhizal Fungi and Dark Septate Endophytes under Drought Stress: Synergistic or Competitive Effects on Maize Growth, Photosynthesis, Root Hydraulic Properties and Aquaporins? Plants, 12(14), 2596. https://doi.org/10.3390/plants12142596






