Transcriptomic Analysis Reveals the Regulatory Mechanism of Color Diversity in Rhododendron pulchrum Sweet (Ericaceae)
Abstract
:1. Introduction
2. Results
2.1. The Contents of Anthocyanins Components and Total Flavonoids
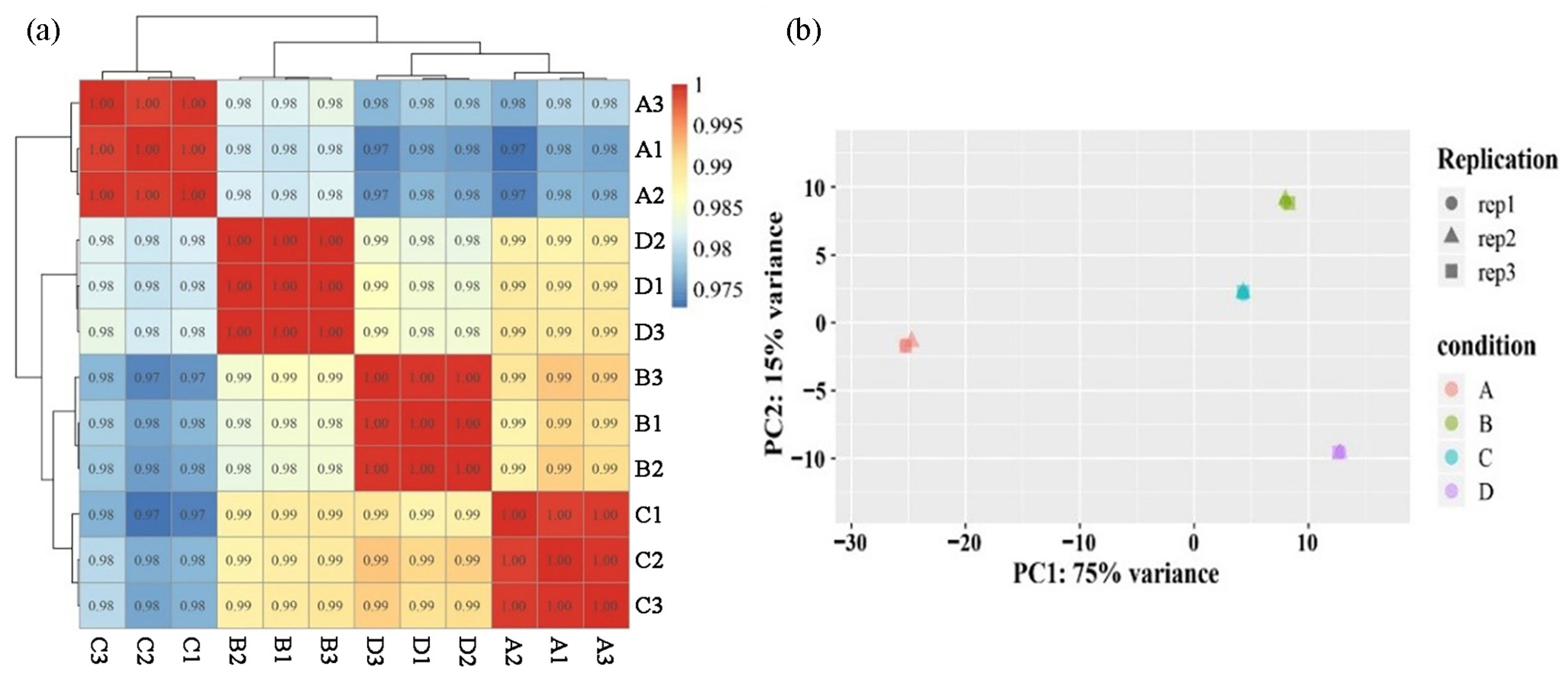
2.2. Overview of RNA-Seq Data and Sequence Assembly
2.3. Annotation of R. pulchrum Transcriptome
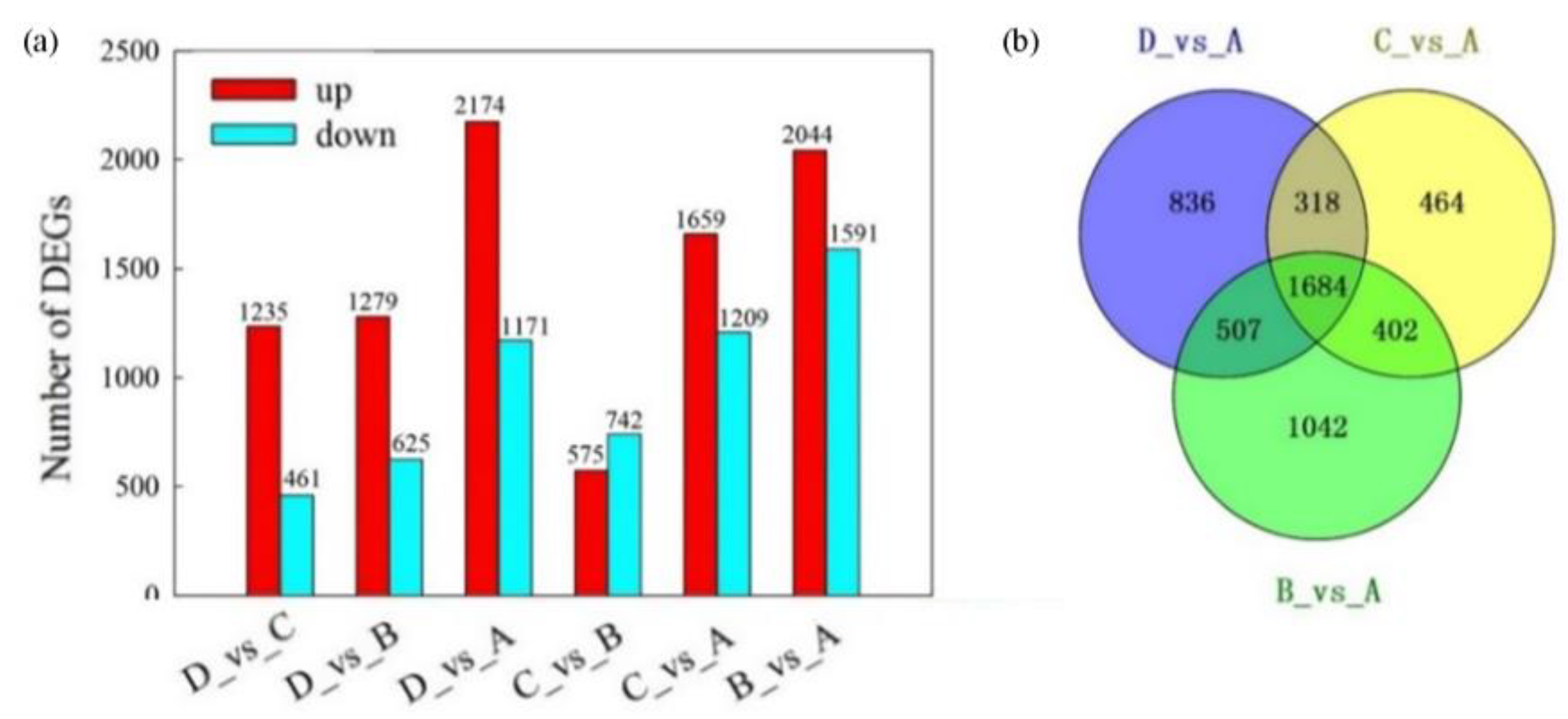
2.4. Differentially Expressed Genes Analysis
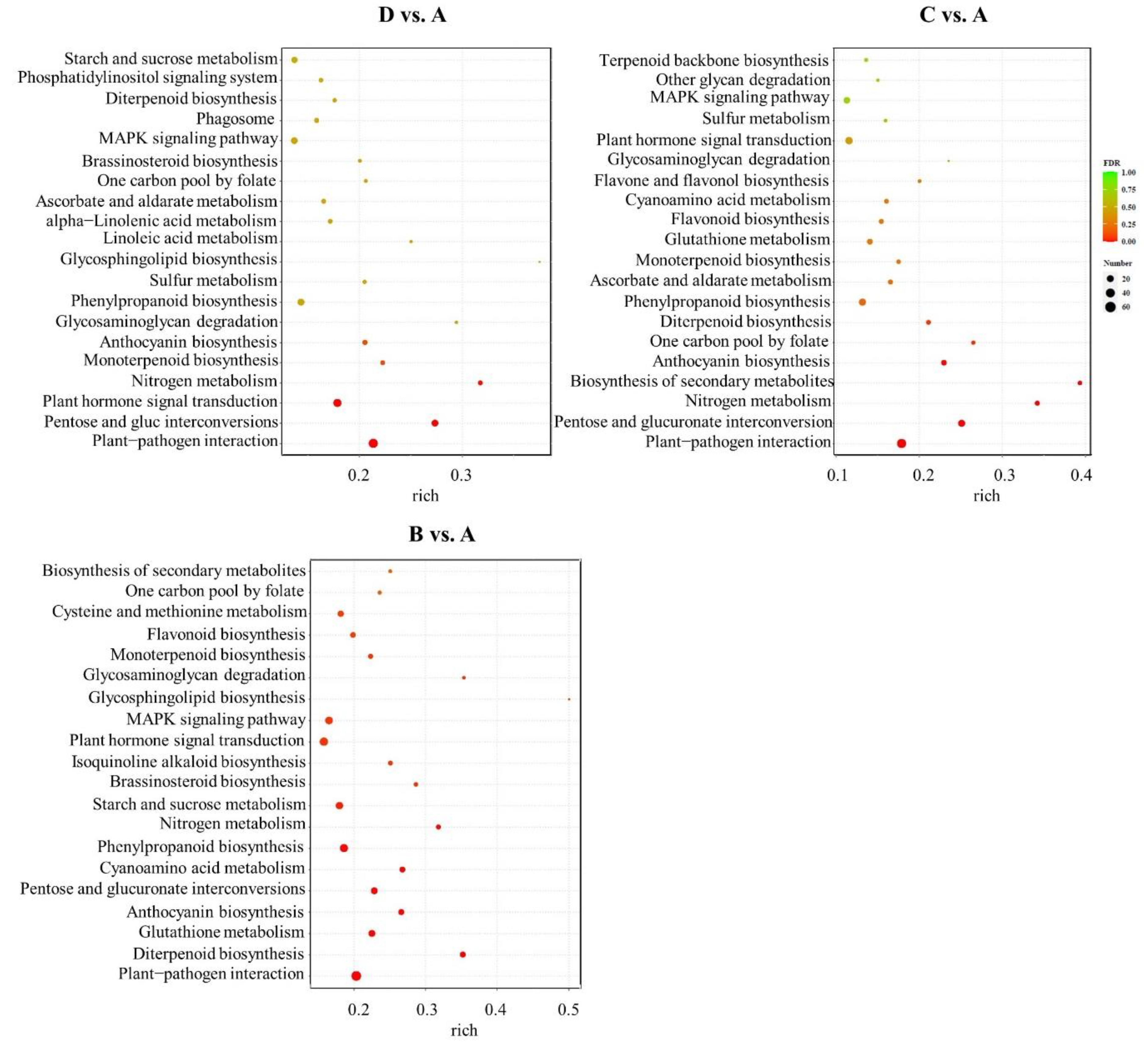
2.5. Classification of GO and KEGG Terms
2.6. The Key DEGs Involved in Anthocyanin Biosynthesis Pathway
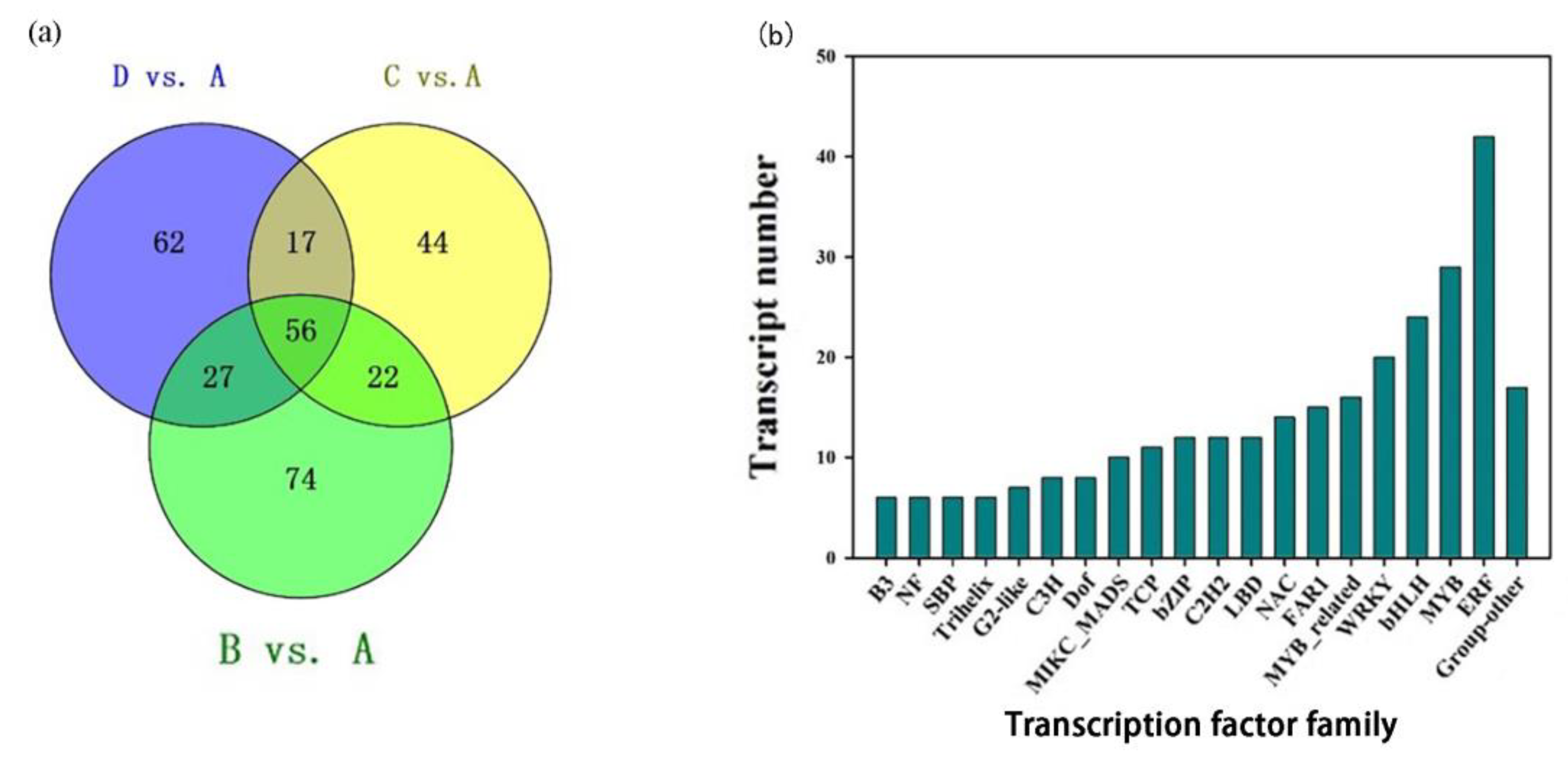
2.7. Identification of Transcription Factors Regulating Petal Color Formation
2.8. DEGs Related to Hormone Signaling
3. Discussion
4. Materials and Methods
4.1. Plant Materials
4.2. Determination of Contents of Anthocyanins Components and Total Flavonoids
4.3. RNA Extraction and Transcriptome Sequencing
4.4. RNA-Seq Data Analysis
4.5. Verification of RNA-Seq by qRT-PCR
4.6. Statistical Analyses and Bioinformatics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chang, Y.H.; Yao, G.; Neilsen, J.; Liu, D.T.; Zhang, L.; Ma, Y.P. Rhododendron kuomeianum (Ericaceae), a new species from northeastern Yunnan (China), based on morphological and genomic data. Plant Divers. 2021, 43, 292–298. [Google Scholar] [CrossRef] [PubMed]
- Shuzhen, W.; Zhiliang, L.; Weibin, J.; Yuanping, F.; Qiaofeng, Y.; Jun, X. Transcriptome analysis and identification of genes associated with flower development in Rhododendron pulchrum Sweet (Ericaceae). Gene 2018, 679, 108–118. [Google Scholar]
- Alappat, B.; Alappat, J. Anthocyanin Pigments: Beyond Aesthetics. Molecules 2020, 25, 5500. [Google Scholar] [CrossRef] [PubMed]
- Grotewold, E. The genetics and biochemistry of floral pigments. Annu. Rev. Plant Biol. 2006, 57, 761. [Google Scholar] [CrossRef]
- Noman, A.; Aqeel, M.; Deng, J.; Khalid, N.; Sanaullah, T.; Shuilin, H. Biotechnological advancements for improving floral attributes in ornamental plants. Front. Plant Sci. 2017, 8, 530. [Google Scholar] [CrossRef] [Green Version]
- Yang, F.-S.; Nie, S.; Liu, H.; Shi, T.-L.; Tian, X.-C.; Zhou, S.-S.; Bao, Y.-T.; Jia, K.-H.; Guo, J.-F.; Zhao, W.; et al. Chromosome-level genome assembly of a parent species of widely cultivated azaleas. Nat. Commun. 2020, 11, 5269. [Google Scholar] [CrossRef]
- Dong, X.M.; Zhang, W.; Zhang, S.B. Selection and validation of reference genes for quantitative real-time PCR analysis of development and tissue-dependent flower color formation in Cymbidium lowianum. Int. J. Mol. Sci. 2022, 23, 738. [Google Scholar] [CrossRef]
- Liu, Q.; Liaquat, F.; He, Y.; Munis, M.F.H.; Zhang, C. Functional annotation of a full-length transcriptome and identification of genes associated with flower development in Rhododendron simsii (Ericaceae). Plants 2021, 10, 649. [Google Scholar] [CrossRef]
- Du, H.; Lai, L.; Wang, F.; Sun, W.; Zhang, L.; Li, X.; Wang, L.; Jiang, L.; Zheng, Y. Characterisation of flower colouration in 30 Rhododendron species via anthocyanin and flavonol identification and quantitative traits. Plant Biol. 2018, 20, 121–129. [Google Scholar] [CrossRef]
- Fleschhut, J.; Kratzer, F.; Rechkemmer, G.; Kulling, S.E. Stability and biotransformation of various dietary anthocyanins in vitro. Eur. J. Nutr. 2006, 45, 7–18. [Google Scholar] [CrossRef]
- Naing, A.H.; Kang, H.H.; Jeong, H.Y.; Soe, M.T.; Kim, C.K. Overexpression of the Raphanus sativus RsMYB1 using the flower-specific promoter (InMYB1) enhances anthocyanin accumulation in flowers of transgenic Petunia and their hybrids. Mol. Breed. 2020, 40, 97. [Google Scholar] [CrossRef]
- Xia, X.; Gong, R.; Zhang, C.Y. Integrative analysis of transcriptome and metabolome reveals flavonoid biosynthesis regulation in Rhododendron pulchrum petals. BMC Plant Biol. 2022, 22, 401. [Google Scholar] [CrossRef]
- Deng, X.; Bashandy, H.; Ainasoja, M.; Kontturi, J.; Pietiäinen, M.; Laitinen, R.A.E.; Albert, V.A.; Valkonen, J.P.T.; Elomaa, P.; Teeri, T.H. Functional diversification of duplicated chalcone synthase genes in anthocyanin biosynthesis of Gerbera hybrida. New Phytol. 2014, 201, 1469–1483. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Liu, T.; Nan, W.; Jeewani, D.C.; Niu, Y.; Li, C.; Wang, Y.; Shi, X.; Wang, C.; Wang, J.; et al. Two transcription factors TaPpm1 and TaPpb1 co-regulate anthocyanin biosynthesis in purple pericarps of wheat. J. Exp. Bot. 2018, 69, 2555–2567. [Google Scholar] [CrossRef] [Green Version]
- Fujino, N.; Tenma, N.; Waki, T.; Ito, K.; Komatsuzaki, Y.; Sugiyama, K.; Yamazaki, T.; Yoshida, S.; Hatayama, M.; Yamashita, S.; et al. Physical interactions among flavonoid enzymes in snapdragon and torenia reveal the diversity in the flavonoid metabolon organization of different plant species. Plant J. 2018, 94, 372–392. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Springob, K.; Nakajima, J.; Yamazaki, M.; Saito, K. Recent advances in the biosynthesis and accumulation of anthocyanins. Nat. Prod. Rep. 2003, 20, 288–303. [Google Scholar] [CrossRef]
- Cazzonelli, C.; Pogson, B.J. Source to sink: Regulation of carotenoid biosynthesis in plants. Trends Plant Sci. 2010, 15, 266. [Google Scholar] [CrossRef] [PubMed]
- Vogt, T. Phenylpropanoid biosynthesis. Mol. Plant 2010, 3, 2–20. [Google Scholar] [CrossRef] [Green Version]
- Dao, T.T.; Linthorst, H.J.; Verpoorte, R. Chalcone synthase and its functions in plant resistance. Phytochem. Rev. 2011, 10, 397–412. [Google Scholar] [CrossRef] [Green Version]
- Jaakola, L. New insights into the regulation of anthocyanin biosynthesis in fruits. Trends Plant Sci. 2013, 18, 477–483. [Google Scholar] [CrossRef] [Green Version]
- Sun, Y.; Zhang, D.; Zheng, H.; Wu, Y.; Mei, J.; Ke, L.; Yu, D.; Sun, Y. Biochemical and expression analyses revealed the involvement of proanthocyanidins and/or their derivatives in fiber pigmentation of Gossypium stocksii. Int. J. Mol. Sci. 2022, 23, 1008. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, Y.; Sasaki, N.; Ohmiya, A. Biosynthesis of plant pigments: Anthocyanins, betalains and carotenoids. Plant J. 2008, 54, 733–749. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Dubos, C.; Lepiniec, L. Transcriptional control of flavonoid biosynthesis by MYB-bHLH-WDR complexes. Trends Plant Sci. 2015, 20, 176–185. [Google Scholar] [CrossRef] [PubMed]
- Lloyd, A.; Brockman, A.; Aguirre, L.; Campbell, A.; Bean, A.; Cantero, A.; Gonzalez, A. Advances in the MYB-bHLH-WD repeat (MBW) pigment regulatory model: Addition of a WRKY factor and co-option of an anthocyanin MYB for betalain regulation. Plant Cell Physiol. 2017, 58, 1431–1441. [Google Scholar] [CrossRef] [Green Version]
- Yuting, L.; Yuhan, T.; Xin, W.; Cong, X.; Jun, T.; Daqiu, Z. Tree peony R2R3-MYB transcription factor PsMYB30 promotes petal blotch formation by activating the transcription of the anthocyanin synthase gene. Plant Cell Physiol. 2022, 63, 1101–1116. [Google Scholar]
- Zimmermann, I.M.; Heim, M.A.; Weisshaar, B.; Uhrig, J.F. Comprehensive identification of Arabidopsis thaliana MYB transcription factors interacting with R/B-like BHLH proteins. Plant J. 2004, 40, 22–34. [Google Scholar] [CrossRef]
- Koes, R.; Verweij, W.; Quattrocchio, F. Flavonoids: A colorful model for the regulation and evolution of biochemical pathways. Trends Plant Sci. 2005, 10, 236–242. [Google Scholar] [CrossRef]
- He, C.; Liu, X.; Da Silva, J.A.T.; Liu, N.; Zhang, M.; Duan, J. Transcriptome sequencing and metabolite profiling analyses provide comprehensive insight into molecular mechanisms of flower development in Dendrobium officinale (Orchidaceae). Plant Mol. Biol. 2020, 104, 529–548. [Google Scholar] [CrossRef]
- Mei, J.; Wu, Y.; Niu, Q.; Miao, M.; Zhang, D.; Zhao, Y.; Cai, F.; Yu, D.; Ke, L.; Feng, H.; et al. Integrative analysis of expression profiles of mRNA and microRNA provides insights of cotton response to Verticillium dahliae. Int. J. Mol. Sci. 2022, 23, 4702. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C (T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Hichri, I.; Barrieu, F.; Bogs, J.; Kappel, C.; Delrot, S.; Lauvergeat, V. Recent advances in the transcriptional regulation of the flavonoid biosynthetic pathway. J. Exp. Bot. 2011, 62, 2465–2483. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holton, T.A.; Cornish, E.C. Genetics and biochemistry of anthocyanin biosynthesis. Plant Cell 1995, 7, 1071–1083. [Google Scholar] [CrossRef] [PubMed]
- Katja, K.; Declan, J.L.; Nick, W.A.; Nelli, M.; Tony, M.; Andrew, C.A.; Bilal, M.A.; Hely, H.; Richard, V.E.; Laura, J. MYBA and MYBPA transcription factors co-regulate anthocyanin biosynthesis in blue-coloured berries. New Phytol. 2021, 232, 1350–1367. [Google Scholar]
- Peng, J.Q.; Dong, X.J.; Xue, C.; Liu, Z.M.; Cao, F.X. Exploring the molecular mechanism of blue flower color formation in Hydrangea macrophylla cv. “Forever Summer”. Front. Plant Sci. 2021, 12, 585665. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Zhang, L.Y.; Wang, S.L.; Niu, X.Y. Analysis of anthocyanins and flavonols in petals of 10 Rhododendron species from the Sygera Mountains in Southeast Tibet. Plant Physiol. Bioch. 2016, 104, 250–256. [Google Scholar] [CrossRef]
- Gichuki, D.K.; Li, Q.; Hou, Y.; Liu, Y.; Ma, M.; Zhou, H.; Xu, C.; Zhu, Z.; Wang, L.; Musila, F.M.; et al. Characterization of flavonoids and transcripts involved in their biosynthesis in different organs of Cissus rotundifolia Lam. Metabolites 2021, 11, 741. [Google Scholar] [CrossRef]
- Peng, Q.Z.; Zhu, Y.; Liu, Z.; Du, C.; Li, K.G.; Xie, D.Y. An integrated approach to demonstrating the ANR pathway of proanthocyanidin biosynthesis in plants. Planta 2012, 236, 901–918. [Google Scholar] [CrossRef]
- Petroni, K.; Tonelli, C. Recent advances on the regulation of anthocyanin synthesis in reproductive organs. Plant Sci. 2011, 181, 219–229. [Google Scholar] [CrossRef]
- Davies, K.M.; Schwinn, K.E.; Deroles, S.C.; Manson, D.G.; Lewis, D.H.; Bloor, S.J. Enhancing anthocyanin production by altering competition for substrate between flavonol synthase and dihydroflavonol 4-reductase. Euphytica 2003, 131, 259–268. [Google Scholar] [CrossRef]
- Jaakola, L.; Määttä, K.; Pirttilä, A.M.; Törrönen, R.; Kärenlampi, S.; Hohtola, A. Expression of genes involved in anthocyanin biosynthesis in relation to anthocyanin, proanthocyanidin, and flavonol levels during bilberry fruit development. Plant Physiol. 2002, 130, 729–739. [Google Scholar] [CrossRef] [Green Version]
- Xia, Y.; Chen, W.; Xiang, W.; Wang, D.; Xue, B.; Liu, X.; Xing, L.; Wu, D.; Wang, S.; Guo, Q.; et al. Integrated metabolic profiling and transcriptome analysis of pigment accumulation in Lonicera japonica flower petals during colour-transition. BMC Plant Biol. 2021, 21, 98. [Google Scholar] [CrossRef]
- Lu, J.; Zhang, Q.; Lang, L.; Jiang, C.; Wang, X.; Sun, H. Integrated metabolome and transcriptome analyses reveal the molecular mechanism of a color mutation in Miniature Roses. BMC Plant Biol. 2019, 20, 611. [Google Scholar]
- Pires, N.; Dolan, L. Early evolution of bHLH proteins in plants. Plant Signal Behav. 2010, 5, 911–912. [Google Scholar] [CrossRef]
- Bogs, J.; Jaffe, F.W.; Takos, A.M.; Walker, A.R.; Robinson, S.P. The grapevine transcription factor VvMYBPA1 regulates proanthocyanidin synthesis during fruit development. Plant Physiol. 2007, 143, 1347–1361. [Google Scholar] [CrossRef] [Green Version]
- Yoshida, K.; Ma, D.; Constabel, C.P. The MYB182 protein down-regulates proanthocyanidin and anthocyanin biosynthesis in poplar by repressing both structural and regulatory flavonoid genes. Plant Physiol. 2015, 3, 693–710. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.K.; Han, P.L.; Zhao, Y.W.; Ji, X.L.; Yu, J.Q.; You, C.X.; Hu, D.G.; Hao, Y.J. Auxin regulates anthocyanin biosynthesis through the auxin repressor protein MdIAA26. Biochem. Biophys. Res. Commun. 2020, 533, 717–722. [Google Scholar] [CrossRef]
- Peng, Z.; Han, C.; Yuan, L.; Zhang, K.; Huang, H.; Ren, C. Brassinosteroid enhances jasmonate-induced anthocyanin accumulation in Arabidopsis seedlings. J. Integr. Plant Biol. 2011, 53, 632–640. [Google Scholar] [CrossRef]
- Gu, Z.Y.; Zhu, J.; Hao, Q.; Yuan, Y.W.; Duan, Y.W.; Men, S.Q.; Wang, Q.Y.; Hou, Q.Z.; Liu, Z.A.; Shu, Q.Y.; et al. A novel R2R3-MYB transcription factor contributes to petal blotch formation by regulating organ-specific expression of PsCHS in tree peony (Paeonia suffruticosa). Plant Cell Physiol. 2019, 60, 599–611. [Google Scholar] [CrossRef] [PubMed]
- Daria, N.; Robert, B.; Thomas, D.; Rares, C.L.; Heidi, H. First genome edited poinsettias: Targeted mutagenesis of flavonoid 3′-hydroxylase using CRISPR/Cas9 results in a colour shift. Plant Cell 2021, 147, 49–60. [Google Scholar]
- Tang, Y.C.; Liu, Y.J.; He, G.R.; Cao, Y.W.; Bi, M.M.; Song, M.; Yang, P.P.; Xu, L.F.; Ming, J. Comprehensive analysis of secondary metabolites in the extracts from different lily bulbs and their antioxidant ability. Antioxidants 2021, 10, 1634. [Google Scholar] [CrossRef] [PubMed]
- Wan, H.; He, Y.; He, Z.; He, F. Determination of quercetin, plumbagin and total flavonoids in Drosera peltata Smith var. glabrata Y.Z.Ruan. Phcog Mag. 2012, 8, 263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qi, S.; Yang, L.; Wen, X.; Hong, Y.; Song, X.; Zhang, M.; Dai, S. Reference gene selection for RT-qPCR analysis of flower development in Chrysanthemum morifolium and Chrysanthemum lavandulifolium. Front. Plant Sci. 2016, 7, 287. [Google Scholar] [CrossRef] [Green Version]
- Wang, M.; Ren, T.; Marowa, P.; Du, H.; Xu, Z. Identification and selection of reference genes for gene expression analysis by quantitative real-time PCR in Suaeda glauca’s response to salinity. Sci. Rep. 2021, 11, 8569. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Langmead, B.; Salzberg, S.L. HISAT: A fast spliced aligner with low memory requirements. Nat. Methods 2015, 12, 357–360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, K.; Zhang, T. Construction of customized sub-databases from NCBI-nr database for rapid annotation of huge metagenomic datasets using a combined BLAST and MEGAN approach. PLoS ONE 2013, 8, e59831. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gasteiger, E.; Jung, E.; Bairoch, A. SWISS-PROT: Connecting biomolecular knowledge via a protein database. Curr. Issues Mol. Biol. 2001, 3, 47–55. [Google Scholar] [PubMed]
- Mistry, J.; Chuguransky, S.; Williams, L.; Qureshi, M.; Salazar, G.A. Pfam: The protein families database in 2021. Nucleic Acids Res. 2021, 49, 412–419. [Google Scholar] [CrossRef] [PubMed]
- The Gene Ontology Consortium. Expansion of the gene ontology knowledgebase and resources. Nucleic Acids Res. 2017, 45, 331–338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kanehisa, M.; Sato, Y.; Kawashima, M.; Furumichi, M.; Tanabe, M. KEGG as a reference resource for gene and protein annotation. Genet. Epidemiol. 2016, 44, 457–462. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Lv, J.; Liu, Z.; Wang, J.; Yang, B.; Chen, W.; Ou, L.; Dai, X.; Zhang, Z.; Zou, X. Integrative analysis of metabolome and transcriptome reveals the mechanism of color formation in pepper fruit (Capsicum Annuum L.). Food Chem. 2020, 306, 125629. [Google Scholar] [CrossRef]
- Wu, B.S.; Mansoori, M.; Trumpler, K.; Addo, P.W.; MacPherson, S.; Lefsrud, M. Effect of amber (595 nm) light supplemented with narrow blue (430 nm) light on tomato biomass. Plants 2023, 12, 2457. [Google Scholar] [CrossRef] [PubMed]
- Villagra, J.; Sancho, L.G.; Alors, D. Macrolichen communities depend on phorophyte in Conguillío national park, Chile. Plants 2023, 12, 2452. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulos, E.I.; Petraki, C.; Gregorakis, A.; Fragoulis, E.G.; Scorilas, A. Clinical evaluation of microRNA-145 expression in renal cell carcinoma: A promising molecular marker for discriminating and staging the clear cell histological subtype. Biol. Chem. 2016, 397, 529–539. [Google Scholar] [CrossRef] [PubMed]




| Sample | Total Flavonoids (QE mg/g DW) | |
|---|---|---|
| Petal | Spot | |
| A | 1.14 ± 0.12 d | 1.03 ± 0.07 b |
| B | 1.71 ± 0.06 c | 1.03 ± 0.03 b |
| C | 1.97 ± 0.06 b | 1.44 ± 0.10 a |
| D | 2.24 ± 0.06 a | 1.57 ± 0.08 a |
| Sample | Raw Reads | Clean Reads | Q30 (%) | Total Mapped | Unique Mapped |
|---|---|---|---|---|---|
| A1 | 42,949,574 | 38,765,084 (90.25%) | 91.04 | 31,266,555 (80.66%) | 29,622,723 (94.74%) |
| A2 | 39,308,136 | 36,123,834 (91.89%) | 90.85 | 29,098,513 (80.55%) | 27,596,151 (94.84%) |
| A3 | 38,671,858 | 35,545,248 (91.91%) | 90.84 | 28,562,447 (80.36%) | 27,047,544 (94.70%) |
| B1 | 40,011,542 | 36,950,600 (92.34%) | 91.08 | 29,530,300 (79.92%) | 28,227,592 (95.59%) |
| B2 | 42,082,072 | 38,694,198 (91.94%) | 89.80 | 30,691,892 (79.32%) | 29,280,727 (95.40%) |
| B3 | 43,723,232 | 40,563,542 (92.77%) | 91.24 | 32,648,377 (80.49%) | 31,192,551 (95.54%) |
| C1 | 42,767,546 | 39,166,056 (91.57%) | 91.52 | 31,415,529 (80.21%) | 29,824,376 (94.94%) |
| C2 | 43,187,530 | 39,626,174 (91.75%) | 91.14 | 31,744,384 (80.11%) | 30,137,984 (94.94%) |
| C3 | 41,563,920 | 38,298,794 (92.14%) | 90.59 | 30,513,325 (79.67%) | 28,991,127 (95.01%) |
| D1 | 42,163,278 | 38,866,774 (92.18%) | 91.29 | 31,243,679 (80.39%) | 29,832,406 (95.48%) |
| D2 | 41,325,128 | 38,027,950 (92.02%) | 91.07 | 30,559,996 (80.36%) | 29,179,418 (95.48%) |
| D3 | 40,312,100 | 37,029,356 (91.85%) | 91.05 | 29,625,922 (80.01%) | 28,282,098 (95.46%) |
| Annotated Databases | Gene Number | Matching Proportion (%) |
|---|---|---|
| Nr | 28,273 | 85.68 |
| Swiss-Prot | 18,054 | 54.71 |
| Pfam | 24,301 | 73.64 |
| GO | 19,099 | 57.88 |
| KEGG | 11,507 | 34.87 |
| Id | log2(Fold Change) | Description | ||
|---|---|---|---|---|
| D vs. A | C vs. A | B vs. A | ||
| Rhsim04G0145800 | 1.02 | 1.00 | PAL | |
| Rhsim01G0211600 | 1.44 | 1.68 | 4CL | |
| Rhsim07G0135800 | −1.08 | 4CL | ||
| Rhsim13G0208200 | −5.79 | F3′5′H | ||
| Rhsim04G0208200 | −2.05 | F3′5′H | ||
| RhsimUnG0095500 | 2.12 | 1.75 | 1.52 | FLS |
| RhsimUnG0143300 | 3.35 | 2.01 | 3.29 | FLS |
| RhsimUnG0134400 | 2.97 | 1.68 | FLS | |
| Rhsim04G0219900 | 3.64 | FLS | ||
| RhsimUnG0007900 | 1.18 | 2.15 | FLS | |
| RhsimUnG0105700 | 3.54 | FLS | ||
| Rhsim12G0144700 | 2.70 | 2.94 | 3.48 | Leucoanthocyanidin reductase |
| Rhsim06G0030600 | −4.36 | −4.22 | −6.99 | DFR |
| Rhsim06G0030500 | −1.22 | −2.45 | −2.55 | DFR |
| Rhsim06G0030400 | −1.15 | −1.30 | DFR | |
| Rhsim07G0096600 | 1.33 | ANS | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, N.; Zhou, C. Transcriptomic Analysis Reveals the Regulatory Mechanism of Color Diversity in Rhododendron pulchrum Sweet (Ericaceae). Plants 2023, 12, 2656. https://doi.org/10.3390/plants12142656
Zhu N, Zhou C. Transcriptomic Analysis Reveals the Regulatory Mechanism of Color Diversity in Rhododendron pulchrum Sweet (Ericaceae). Plants. 2023; 12(14):2656. https://doi.org/10.3390/plants12142656
Chicago/Turabian StyleZhu, Nanyan, and Chunhua Zhou. 2023. "Transcriptomic Analysis Reveals the Regulatory Mechanism of Color Diversity in Rhododendron pulchrum Sweet (Ericaceae)" Plants 12, no. 14: 2656. https://doi.org/10.3390/plants12142656





