From Plants to Wound Dressing and Transdermal Delivery of Bioactive Compounds
Abstract
1. Introduction
2. Natural Compound Transfer Specifics and API Delivery Methods
2.1. Skin Properties and Drug Transport Mechanism
- -
- Non-toxicity.
- -
- Low-dose administration requirements.
- -
- High lipophilicity.
- -
- Molecular weight below 500 Da.
- -
- Hydrophilic/hydrophobic balance corresponding to an n-octanol/water partition coefficient (log P) between 1 and 5 (which corresponds to moderate lipophilicity).
- -
- Acceptable water solubility (0.05 to 1 mg/mL).
- -
- Melting point below 250 °C.
2.2. Conventional and Modern Topical and TDD Methods
- -
- Toxicity and skin irritation.
- -
- Two-way skin permeabilization (increase in transepidermal water loss).
- -
- Additional pharmacological effects.
- -
- Specificity towards a certain drug.
3. Bioactive Transdermal Patches and Wound Dressing Materials
- The active component/drug, which could be a natural or a synthetic component with bioactive properties; it can be supplied from a reservoir or from another structure of the patch [27].
- Backing material, usually made of elastomers, is used to provide patches’ flexibility and protection from the outer environment and water.
- Drug-releasing membrane/matrix, made of natural/synthetic polymers/elastomers.
- Adhesive, to keep the patches’ layers together and adhered to the skin; it may also contain the permeation enhancers and/or the drug.
- The protective liner.
4. Natural Products from Plants Used in Medical Devices
4.1. Primary Metabolites as Constituents of BTP
4.1.1. Plant Mucilages
4.1.2. Plant Gums
4.1.3. Plant Cell Wall Components
- -
- Self-healing biodegradable 3D network hydrogel, based on boronic-acid-grafted CMC, used as a drug release vehicle or scaffold for tissue regeneration or bleeding control [80].
- -
- Antibacterial photo-inspired WD, based on CMC as the “network backbone” hydrogel, polyvinyl alcohol (PVA) with iodophor (KI-I2) as the antimicrobial hydrogel, and a fluorescent material (carbon quantum dots) as the doping agent. This functional material releases desired doses of an antibacterial agent (I2), and provides real-time information on the wound healing process, based on hydrogel color responsiveness to wound pH changes in UV and visible light [81].
- -
- Multilayer microneedles, based on PVA and CMC for the transdermal delivery of nicotinamide mononucleotide (antiaging agent). CMC properties, as a high water solubility and hydrophilicity, determine a higher drug release [82].
- -
- Dissolvable patch, based on sodium alginate and CMC, loaded with cephalexin monohydrate as an antimicrobial agent for skin and chronic wound infections. A sustained drug release is obtained for selected concentrations [83].
- -
- Chromogenic aerogel-like composite, based on CMC and PVA with an anthocyanin extract immobilized into the polymeric matrix, as a color indicator for wound pH and antimicrobial agent at the same time [84].
4.2. Secondary Metabolites as Constituents of BTP
4.2.1. Alkaloids
- (a)
- An adhesive layer that sticks to the skin and contains scopolamine in a polymeric gel, which provides an initial priming dose.
- (b)
- An intermediate microporous polypropylene rate-controlling membrane.
- (c)
- The scopolamine reservoir that sustains a zero-order input of a drug to the skin surface.
- (d)
- A backing of impermeable aluminized polyester film.
4.2.2. Phenolic Compounds (PC)
- -
- Phenolic acids (hydroxybenzoic and hydroxycinnamic acids).
- -
- Flavonoids (flavonols and flavan-3-ols, flavones, flavanones, isoflavones, flavanones and anthocyanidins).
- -
- Coumarins.
- -
- Lignans.
- -
- Stilbenes.
Non-Flavonoid PC
Flavonoid PC
- -
- Flavanols represented by a catechin group, containing epicatechin (EG) and specifically epigallocatechin gallate (EGCG), have attracted much attention due to their wide spectrum of biological properties, including antioxidant, photoprotective, antiviral, and antibacterial, as well as anticarcinogenic and neuroprotective, properties. EGCG is used as TP in the treatment of atopic dermatitis in the form of loaded microneedles, or with Ag nanoparticles in cotton patches for gangrene in diabetics [184,185]; EG is a TD using microneedles [114].
- -
- -
- Flavones represented by apigenin, which is a hydrophobic flavone, have been used in several formulations so far for TD, including liposomes, nanocrystal gel formulations, and self-micro-emulsifying delivery systems for anti-inflammatory and breast cancer treatment [189,190]. Luteolin is another promising flavonoid with antiarthritic activity. Topical formulations to treat psoriasis, or to keep breast cancer under control, were developed [191,192]. Due to its lipolytic character, the transport system is of a lipidic nanocarrier or niosome type [192,193,194].
- -
- Flavonols—quercetin is one of the most intensively studied and common flavonols found in nature; because this flavonoid shows a poor permeability in normal or excised human skin, it has been incorporated into various delivery systems, including nanoemulsions, nanocapsules, lipid nanoparticles, and microemulsions, to increase skin solubility and permeability [139,195]. TP with quercetin, incorporated in nanocomposite materials using microneedles, are used for the treatment of inflammation or androgenic alopecia [134,196]. Kaempferol is another well-known flavonol with antioxidant, anti-inflammatory, anticancer, and antiallergic properties, and it is used in the same TD systems compared to other flavonoids, mainly using lipid nanocarriers [197].
- -
4.2.3. Sulfur-Containing Compounds
4.2.4. Terpenoids
- -
- Hemiterpenes are the simplest terpenes, and the number of known compounds is lower than 100. Most hemiterpenes are in the form of oils and insoluble in water, with some glycosylated exceptions that are water soluble. The best-known hemiterpene is isoprene, which is the basic unit of all terpenes. Other known examples are tiglic, angelic acids, and isovaleric acid. These were used as enhancers for SC permeability in TP developed based on poly 3-hydroxybutyric acid-co-3-hydroxy valeric acid (PHBV) nanofibers, to deliver active substances (e.g., curcumin) for WD and to ensure an antimicrobial effect [223].
- -
- Monoterpenes are widely distributed in secretory tissues such as oil glands or resin chambers and ducts of higher plants. Monoterpenes are the major constituents of volatile plant oils. A number of monoterpenes are oxygenated and many exhibit biological and perfuming activities. Some are often used as medicinal agents or ingredients in cosmetic products. Monoterpenes frequently used as SC penetration agents are anethol, borneol, and campho (used in commercial TP for pain release); carvacol, carvone, and 1.8–1.4 cineol (used for TDDS in psychiatry); cymene, eugenol, fenchone, geraniol, limonene, linalool, and menthol (used as an enhancer and also for pain release); menthone, a-pinen oxide, pulegone, rose oxide, safranal, terpinene-4-ol, a-terpineol, tetra-hydrogeneraniol, and tymol (oral patches); and verbenone (perfumery and cosmetic use) [215,224,225,226,227,228,229,230,231,232,233,234,235,236,237,238,239,240,241,242].
- -
- -
- Diterpenoids belong to a versatile class of chemical constituents found in different natural sources having four isoprene units. They can be classified as linear, bicyclic, tricyclic, tetracyclic, pentacyclic, or macrocyclic diterpenes, depending on their skeletal core. In nature, they are commonly found in a polyoxygenated form, with keto and hydroxyl groups, often esterified by small-sized aliphatic or aromatic acids [244]. This class of compounds show significant biological activities including anti-inflammatory, antimicrobial, anticancer, antifungal, cardiovascular, etc. [215]. Among tricyclic diterpenoids, abietic acid was used for wound healing and tanshinone IIA—with numerous therapeutic uses—was also included in TD systems as nanoparticles or microneedles [246,247,248].
- -
- Triterpenoids are a major class of SM and contain compounds with a carbon skeleton based on six isoprene units, derived biosynthetically from the acyclic C30 hydrocarbon, squalene. They have relatively complex cyclic structures, most being either alcohols, aldehydes, or carboxylic acids [215]. Sterols are triterpenes that are based on the cyclopentane perhydrophenanthrene ring system. Plant sterols called “phytosterols”, e.g., sitosterol, stigmasterol, and campesterol, are widespread in higher plants. Triterpenes have many active sites for glycosylation, which converts them into another class of compounds, namely, saponins (triterpene glycoside). Examples of triterpenoids of medical interest are squalene used in scaffold emulgel for wound healing, ursolic acid used in niosomal gel systems for arthritis treatment and skin diseases, oleanolic acid used in nanocarriers in TD, and sitosterol used in alopecia treatment with microneedle enhancers, or in breast cancer as nanoparticles [249,250,251,252,253,254,255].
- -
- Tetraterpenoids consist of eight isoprene units and 25 carbon atoms. The most common tetraterpenoids are carotenoids, which are natural fat soluble pigments [244]. Carotenoids are extremely hydrophobic molecules, with little or no solubility in water, and a strong tendency to aggregate in aqueous solutions. Since carotenoids are unstable at high temperatures, in the presence of light and oxygen, their incorporation into micro- and nanostructures is necessary, to increase their stability and preserve their bioactivity [256,257]. Examples of used carotenoids are as follows: lycopene loaded in a lipid nanocarrier in TDD systems; β-carotene in TDS as self-nanoemulsion or as lipid base nanocarriers, or as hydrogel with plant extracts; lutein with a high antioxidant effect is delivered in TDS using nanostructured lipid carriers, solid lipid nanoparticles, a nanosphere, a liposome, nanoemulsions, and polymeric nanoparticles for eye diseases, for anti-inflammatory effects, obesity, etc.; and crocin in a nanocellulose membrane of microbial cellulose-based TP [258,259,260,261,262,263,264].
- -
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Raju, N.S.; Krishnaswami, V.; Vijayaraghavalu, S.; Kandasamy, R. Transdermal and Bioactive Nanocarriers. In Nanocosmetics: Fundamentals, Applications and Toxicity; Elsevier Inc.: Amsterdam, The Netherlands, 2020; pp. 17–33. ISBN 9780128222867. [Google Scholar]
- Cao, X.; Sun, L.; Luo, Z.; Lin, X.; Zhao, Y. Aquaculture Derived Hybrid Skin Patches for Wound Healing. Eng. Regen. 2023, 4, 28–35. [Google Scholar] [CrossRef]
- Raghav, N.; Sharma, M.R.; Kennedy, J.F. Nanocellulose: A Mini-Review on Types and Use in Drug Delivery Systems. Carbohydr. Polym. Technol. Appl. 2021, 2, 100031. [Google Scholar] [CrossRef]
- Sousa, M.P.; Neto, A.I.; Correia, T.R.; Miguel, S.P.; Matsusaki, M.; Correia, I.J.; Mano, J.F. Bioinspired Multilayer Membranes as Potential Adhesive Patches for Skin Wound Healing. Biomater. Sci. 2018, 6, 1962–1975. [Google Scholar] [CrossRef] [PubMed]
- Amr, S.S.; Tbakhi, A. Ibn Sina (Avicenna): The Prince Of Physicians. Ann. Saudi Med. 2007, 27, 134–135. [Google Scholar] [CrossRef] [PubMed]
- Szunerits, S.; Boukherroub, R. Heat: A Highly Efficient Skin Enhancer for Transdermal Drug Delivery. Front. Bioeng. Biotechnol. 2018, 6, 15. [Google Scholar] [CrossRef] [PubMed]
- Peña-Juárez, M.C.; Guadarrama-Escobar, O.R.; Escobar-Chávez, J.J. Transdermal Delivery Systems for Biomolecules. J. Pharm. Innov. 2022, 17, 319–332. [Google Scholar] [CrossRef] [PubMed]
- Kuznetsova, D.A.; Vasilieva, E.A.; Kuznetsov, D.M.; Lenina, O.A.; Filippov, S.K.; Petrov, K.A.; Zakharova, L.Y.; Sinyashin, O.G. Enhancement of the Transdermal Delivery of Nonsteroidal Anti-Inflammatory Drugs Using Liposomes Containing Cationic Surfactants. ACS Omega 2022, 7, 25741–25750. [Google Scholar] [CrossRef] [PubMed]
- Verma, P.; Maheshwari, N.; Bairagee, D. Physiochemical Characterization and in Vitro Evaluation of Formulated Herbal Bioactive Loaded Transdermal Patches. Pharmacogn. Res. 2022, 15, 64–70. [Google Scholar] [CrossRef]
- Mohammed, Y.; Holmes, A.; Kwok, P.C.L.; Kumeria, T.; Namjoshi, S.; Imran, M.; Matteucci, L.; Ali, M.; Tai, W.; Benson, H.A.E.; et al. Advances and Future Perspectives in Epithelial Drug Delivery. Adv. Drug Deliv. Rev. 2022, 186, 114293. [Google Scholar] [CrossRef]
- Chavda, V.P.; Patel, A.B.; Mistry, K.J.; Suthar, S.F.; Wu, Z.X.; Chen, Z.S.; Hou, K. Nano-Drug Delivery Systems Entrapping Natural Bioactive Compounds for Cancer: Recent Progress and Future Challenges. Front. Oncol. 2022, 12, 867655. [Google Scholar] [CrossRef]
- Richard, C.; Cassel, S.; Blanzat, M. Vesicular Systems for Dermal and Transdermal Drug Delivery. RSC Adv. 2020, 11, 442–451. [Google Scholar] [CrossRef] [PubMed]
- Ita, K. Transcutaneous Drug Administration. In Transdermal Drug Delivery; Academic Press: Cambridge, MA, USA, 2020; pp. 1–7. [Google Scholar] [CrossRef]
- Sabbagh, F.; Kim, B.S. Recent Advances in Polymeric Transdermal Drug Delivery Systems. J. Control. Release 2022, 341, 132–146. [Google Scholar] [CrossRef]
- Alkilani, A.Z.; McCrudden, M.T.C.; Donnelly, R.F. Transdermal Drug Delivery: Innovative Pharmaceutical Developments Based on Disruption of the Barrier Properties of the Stratum Corneum. Pharmaceutics 2015, 7, 438–470. [Google Scholar] [CrossRef]
- Ramadon, D.; McCrudden, M.T.C.; Courtenay, A.J.; Donnelly, R.F. Enhancement Strategies for Transdermal Drug Delivery Systems: Current Trends and Applications. Drug Deliv. Transl. Res. 2022, 12, 758–791. [Google Scholar] [CrossRef]
- Kováčik, A.; Kopečná, M.; Vávrová, K. Permeation Enhancers in Transdermal Drug Delivery: Benefits and Limitations. Expert Opin. Drug Deliv. 2020, 17, 145–155. [Google Scholar] [CrossRef] [PubMed]
- Brown, M.B.; Martin, G.P.; Jones, S.A.; Akomeah, F.K. Dermal and Transdermal Drug Delivery Systems: Current and Future Prospects. Drug Deliv. J. Deliv. Target. Ther. Agents 2006, 13, 175–187. [Google Scholar] [CrossRef]
- Prausnitz, M.R.; Langer, R. Transdermal Drug Delivery. Nat. Biotechnol. 2008, 26, 1261–1268. [Google Scholar] [CrossRef] [PubMed]
- Alkilani, A.Z.; Nasereddin, J.; Hamed, R.; Nimrawi, S.; Hussein, G.; Abo-Zour, H.; Donnelly, R.F. Beneath the Skin: A Review of Current Trends and Future Prospects of Transdermal Drug Delivery Systems. Pharmaceutics 2022, 14, 1152. [Google Scholar] [CrossRef] [PubMed]
- Tapfumaneyi, P.; Imran, M.; Mohammed, Y.; Roberts, M.S. Recent Advances and Future Prospective of Topical and Transdermal Delivery Systems. Front. Drug Deliv. 2022, 2, 957732. [Google Scholar] [CrossRef]
- Pastore, M.N.; Kalia, Y.N.; Horstmann, M.; Roberts, M.S. Transdermal Patches: History, Development and Pharmacology. Br. J. Pharmacol. 2015, 172, 2179–2209. [Google Scholar] [CrossRef]
- Jiang, X.; Xia, W.; Pan, J.; Yang, W.; Zhang, S.; Li, C.; Zan, T.; Lai, Y.; Xu, Z.; Yu, H. Engineered Microneedle Systems for Topical Cancer Therapy. Appl. Mater. Today 2023, 31, 101774. [Google Scholar] [CrossRef]
- Wurster, D.E.; Kramer, S.F. Investigation of Some Factors Influencing Percutaneous Absorption. J. Pharm. Sci. 1961, 50, 288–293. [Google Scholar] [CrossRef] [PubMed]
- Bioactive Definition & Meaning-Merriam-Webster. Available online: https://www.merriam-webster.com/dictionary/bioactive (accessed on 12 April 2023).
- Bird, D.; Ravindra, N.M. Transdermal Drug Delivery and Patches—An Overview. Med. Devices Sens. 2020, 3, e10069. [Google Scholar] [CrossRef]
- Wang, C.; Bai, J.; Tian, P.; Xie, R.; Duan, Z.; Lv, Q.; Tao, Y. The Application Status of Nanoscale Cellulose-Based Hydrogels in Tissue Engineering and Regenerative Biomedicine. Front. Bioeng. Biotechnol. 2021, 9, 732513. [Google Scholar] [CrossRef] [PubMed]
- Abozenadah, H.; Bishop, A.; Bittner, S.; Lopez, O.; Wiley, C.; Flatt, P.M. CH105: Chapter 6-A Brief History of Natural Products and Organic Chemistry-Chemistry. In Consumer Chemistry: How Organic Chemistry Impacts Our Lives; Western Oregon University: Monmouth, OR, USA, 2017. [Google Scholar]
- Cheng, Y.C.; Li, T.S.; Su, H.L.; Lee, P.C.; Wang, H.M.D. Transdermal Delivery Systems of Natural Products Applied to Skin Therapy and Care. Molecules 2020, 25, 5051. [Google Scholar] [CrossRef] [PubMed]
- Atanasov, A.G.; Zotchev, S.B.; Dirsch, V.M.; Orhan, I.E.; Banach, M.; Rollinger, J.M.; Barreca, D.; Weckwerth, W.; Bauer, R.; Bayer, E.A.; et al. Natural Products in Drug Discovery: Advances and Opportunities. Nat. Rev. Drug Discov. 2021, 20, 200–216. [Google Scholar] [CrossRef]
- Adetunji, C.O.; Palai, S.; Ekwuabu, C.P.; Egbuna, C.; Adetunji, J.B.; Ehis-Eriakha, C.B.; Kesh, S.S.; Mtewa, A.G. General Principle of Primary and Secondary Plant Metabolites: Biogenesis, Metabolism, and Extraction. In Preparation of Phytopharmaceuticals for the Management of Disorders: The Development of Nutraceuticals and Traditional Medicine; Elsevier Inc.: Amsterdam, The Netherlands, 2020; pp. 3–23. ISBN 9780128202845. [Google Scholar]
- Dybka-Stępień, K.; Otlewska, A.; Góźdź, P.; Piotrowska, M. The Renaissance of Plant Mucilage in Health Promotion and Industrial Applications: A Review. Nutrients 2021, 13, 3354. [Google Scholar] [CrossRef]
- Goksen, G.; Demir, D.; Dhama, K.; Kumar, M.; Shao, P.; Xie, F.; Echegaray, N.; Lorenzo, J.M. Mucilage Polysaccharide as a Plant Secretion: Potential Trends in Food and Biomedical Applications. Int. J. Biol. Macromol. 2023, 230, 123146. [Google Scholar] [CrossRef]
- Filho, J.G.D.O.; Lira, M.M.; de Sousa, T.L.; Campos, S.B.; Lemes, A.C.; Egea, M.B. Plant-Based Mucilage with Healing and Anti-Inflammatory Actions for Topical Application: A Review. Food Hydrocoll. Health 2021, 1, 100012. [Google Scholar] [CrossRef]
- Ahuja, M.; Kumar, S.; Yadav, M. Evaluation of Mimosa Seed Mucilage as Bucoadhesive Polymer. Yakugaku Zasshi 2010, 130, 937–944. [Google Scholar] [CrossRef]
- Ahad, H.A.; Chinthaginjala, H.; Sai Priyanka, M.; Raghav, D.R.; Gowthami, M.; Naga Jyothi, V. Datura Stramonium Leaves Mucilage Aided Buccoadhesive Films of Aceclofenac Using 32 Factorial Design with Design-Expert Software. Indian J. Pharm. Educ. Res. 2021, 55, s396–s404. [Google Scholar] [CrossRef]
- Sen, S.; Bal, T.; Rajora, A.D. Green Nanofiber Mat from HLM–PVA–Pectin (Hibiscus Leaves Mucilage–Polyvinyl Alcohol–Pectin) Polymeric Blend Using Electrospinning Technique as a Novel Material in Wound-Healing Process. Appl. Nanosci. 2022, 12, 237–250. [Google Scholar] [CrossRef] [PubMed]
- Mehnath, S.; Chitra, K.; Karthikeyan, K.; Jeyaraj, M. Localized Delivery of Active Targeting Micelles from Nanofibers Patch for Effective Breast Cancer Therapy. Int. J. Pharm. 2020, 584, 119412. [Google Scholar] [CrossRef] [PubMed]
- Saju, F.; Sivaraman, C.M. Scope of Herbal Mucilage in Pharmaceutical Formulations. A Review. Herba Pol. 2021, 67, 46–57. [Google Scholar] [CrossRef]
- Ahad, H.A.; Kumar, C.S.; Ravindra, B.V.; Sasidhar, C.G.S.; Ramakrishna, G.; Venkatnath, L.; Gangadhar, P.; Navya, K. Characterization and Permeation Studies of Diltiazem Hydrochloride-Ficus Reticuleta Fruit Mucilage Transdermal Patches. Int. J. Pharm. Sci. Rev. Res. 2010, 1, 32–37. [Google Scholar]
- Rangari, N.T.; Kalyankar, T.M.; Puranik, P.K.; Chaudhari, S.R. Permeation Studies of Pioglitazone HCL from Ficus Carica Fruit Mucilage Matrix Transdermal Patches. Int. J. Pharm. Sci. Res. 2012, 3, 3927–3931. [Google Scholar] [CrossRef]
- Ahad, H.A.; Ishaq, B.M.; Shaik, M.; Bandagisa, F. Designing and Characterizing of Tramadol Hydrochloride Transdermal Patches Prepared with Ficus Carica Fruit Mucilage and Povidone. Pak. J. Pharm. Sci. 2016, 29, 945–951. [Google Scholar]
- Siddique, A.; Ratnamala, K.V. Formulation and Evaluation of Naproxen Sodium Transdermal Patch Using Natural Polymers. Hum. J. Res. Artic. Dec. 2017, 11, 160–176. [Google Scholar]
- Marthadu, S.; Medindla, J.; Sreelekha, B.; Nishkala, B. Aceclofenac Penetration Studies from Transdermal Patch Prepared with Ficus Benghalensis Fruit Mucilage as Matrix Forming Polymer. Int. J. Pharma Sci. Res. 2020, 11, 8–11. [Google Scholar]
- Baral, P.; Bhattarai, A.; Shahi, L.; Shrestha, R.; Adhikari, S.; Giri, A. Formulation and Characterization of Diclofenac Potassium Transdermal Patch-Es Prepared with Ficus Auriculata Fruit Mucilage and Hydroxypropyl Methyl Cellulose K4M. J. Pharm. Drug Dev. 2020, 7, 1–11. [Google Scholar]
- Hosseini, M.S.; Nabid, M.R. Synthesis of Chemically Cross-Linked Hydrogel Films Based on Basil Seed (Ocimum Basilicum L.) Mucilage for Wound Dressing Drug Delivery Applications. Int. J. Biol. Macromol. 2020, 163, 336–347. [Google Scholar] [CrossRef] [PubMed]
- Tantiwatcharothai, S.; Prachayawarakorn, J. Property Improvement of Antibacterial Wound Dressing from Basil Seed (O. Basilicum L.) Mucilage-ZnO Nanocomposite by Borax Crosslinking. Carbohydr. Polym. 2020, 227, 115360. [Google Scholar] [CrossRef] [PubMed]
- Sadat Hosseini, M.; Kamali, B.; Nabid, M.R. Multilayered Mucoadhesive Hydrogel Films Based on Ocimum Basilicum Seed Mucilage/Thiolated Alginate/Dopamine-Modified Hyaluronic Acid and PDA Coating for Sublingual Administration of Nystatin. Int. J. Biol. Macromol. 2022, 203, 93–104. [Google Scholar] [CrossRef]
- Izadyari Aghmiuni, A.; Heidari Keshel, S.; Sefat, F.; Akbarzadeh Khiyavi, A. Quince Seed Mucilage-Based Scaffold as a Smart Biological Substrate to Mimic Mechanobiological Behavior of Skin and Promote Fibroblasts Proliferation and h-ASCs Differentiation into Keratinocytes. Int. J. Biol. Macromol. 2020, 142, 668–679. [Google Scholar] [CrossRef] [PubMed]
- Oran, D.; Unal, S.; Gunduz, O. Evaluation of Bacterial Cellulose/Quince Seed Mucilage Composite Scaffold for Wound Dressing. Emergent Mater. 2022, 5, 315–321. [Google Scholar] [CrossRef]
- Nilani, P.; Pranavi, A.; Duraisamy, B.; Damodaran, P.; Subhashini, V.; Elango, K. Assessment of Wound Healing Potential of Dermal Patches. Int. J. Pharm. Pharmacol. 2021, 10, 001–006. [Google Scholar]
- Ramos, I.F.d.S.; Magalhães, L.M.; O Pessoa, C.D.; Ferreira, P.M.P.; Rizzo, M.D.S.; Osajima, J.A.; Silva-Filho, E.C.; Nunes, C.; Raposo, F.; Coimbra, M.A.; et al. New Properties of Chia Seed Mucilage (Salvia Hispanica L.) and Potential Application in Cosmetic and Pharmaceutical Products. Ind. Crop. Prod. 2021, 171, 113981. [Google Scholar] [CrossRef]
- Massey, S.; Iqbal, F.; Rehman, A.U.; Iqbal, M.S.; Iram, F. Preparation, Characterization and Biological Evaluation of Silver Nanoparticles and Drug Loaded Composites for Wound Dressings Formed from Lallemantia Royleana Seeds’ Mucilage. J. Biomater. Sci. Polym. Ed. 2022, 33, 481–498. [Google Scholar] [CrossRef]
- Hassan, I.; Gani, A.; Ahmad, M.; Banday, J. Extraction of Polysaccharide from Althea Rosea and Its Physicochemical, Anti-Diabetic, Anti-Hypertensive and Antioxidant Properties. Sci. Rep. 2022, 12, 17116. [Google Scholar] [CrossRef]
- Pereira, R.; Carvalho, A.; Vaz, D.C.; Gil, M.H.; Mendes, A.; Bártolo, P. Development of Novel Alginate Based Hydrogel Films for Wound Healing Applications. Int. J. Biol. Macromol. 2013, 52, 221–230. [Google Scholar] [CrossRef]
- Nair, R.S.; Ling, T.N.; Abdul Shukkoor, M.S.; Manickam, B. Matrix Type Transdermal Patches of Captopril: Ex Vivo Permeation Studies through Excised Rat Skin. J. Pharm. Res. 2013, 6, 774–779. [Google Scholar] [CrossRef]
- Chelu, M.; Musuc, A.M.; Aricov, L.; Ozon, E.A.; Iosageanu, A.; Stefan, L.M.; Prelipcean, A.M.; Popa, M.; Moreno, J.C. Antibacterial Aloe Vera Based Biocompatible Hydrogel for Use in Dermatological Applications. Int. J. Mol. Sci. 2023, 24, 3893. [Google Scholar] [CrossRef] [PubMed]
- Rana, V.; Rai, P.; Tiwary, A.K.; Singh, R.S.; Kennedy, J.F.; Knill, C.J. Modified Gums: Approaches and Applications in Drug Delivery. Carbohydr. Polym. 2011, 83, 1031–1047. [Google Scholar] [CrossRef]
- George, B.; Suchithra, T.V. Plant-Derived Bioadhesives for Wound Dressing and Drug Delivery System. Fitoterapia 2019, 137, 104241. [Google Scholar] [CrossRef]
- Ray, P.; Chatterjee, S.; Saha, P. Screening of Polysaccharides from Fruit Pulp of Ziziphus Mauritiana L. and Artocarpus Heterophyllus L. as Natural Mucoadhesives. Futur. J. Pharm. Sci. 2021, 7, 29. [Google Scholar] [CrossRef]
- Sadat Hosseini, M.; Hemmati, K.; Ghaemy, M. Synthesis of Nanohydrogels Based on Tragacanth Gum Biopolymer and Investigation of Swelling and Drug Delivery. Int. J. Biol. Macromol. 2016, 82, 806–815. [Google Scholar] [CrossRef]
- Pathania, D.; Verma, C.; Negi, P.; Tyagi, I.; Asif, M.; Kumar, N.S.; Al-Ghurabi, E.H.; Agarwal, S.; Gupta, V.K. Novel Nanohydrogel Based on Itaconic Acid Grafted Tragacanth Gum for Controlled Release of Ampicillin. Carbohydr. Polym. 2018, 196, 262–271. [Google Scholar] [CrossRef]
- Sheorain, J.; Mehra, M.; Thakur, R.; Grewal, S.; Kumari, S. In Vitro Anti-Inflammatory and Antioxidant Potential of Thymol Loaded Bipolymeric (Tragacanth Gum/Chitosan) Nanocarrier. Int. J. Biol. Macromol. 2019, 125, 1069–1074. [Google Scholar] [CrossRef]
- Gupta, V.K.; Sood, S.; Agarwal, S.; Saini, A.K.; Pathania, D. Antioxidant Activity and Controlled Drug Delivery Potential of Tragacanth Gum-Cl-Poly (Lactic Acid-Co-Itaconic Acid) Hydrogel. Int. J. Biol. Macromol. 2018, 107, 2534–2543. [Google Scholar] [CrossRef]
- Roy, D.; Bal, T.; Swain, S. Fabrication and Evaluation of PH-Sensitive Biocompatible Microwave Irradiated Moringa Barkgum-Carrageenan (MOG-CRG-IPN) Interpenetrating Isotropic Polymeric Network for Controlled Delivery of Pharmaceuticals. Sustain. Chem. Pharm. 2020, 18, 100325. [Google Scholar] [CrossRef]
- Giri, A.; Bhunia, T.; Mishra, S.R.; Goswami, L.; Panda, A.B.; Pal, S.; Bandyopadhyay, A. Acrylic Acid Grafted Guargum-Nanosilica Membranes for Transdermal Diclofenac Delivery. Carbohydr. Polym. 2013, 91, 492–501. [Google Scholar] [CrossRef]
- Dutta, K.; Das, B.; Orasugh, J.T.; Mondal, D.; Adhikari, A.; Rana, D.; Banerjee, R.; Mishra, R.; Kar, S.; Chattopadhyay, D. Bio-Derived Cellulose Nanofibril Reinforced Poly(N-Isopropylacrylamide)-g-Guar Gum Nanocomposite: An Avant-Garde Biomaterial as a Transdermal Membrane. Polymer 2018, 135, 85–102. [Google Scholar] [CrossRef]
- Dias, S.F.L.; Nogueira, S.S.; De França Dourado, F.; Guimarães, M.A.; De Oliveira Pitombeira, N.A.; Gobbo, G.G.; Primo, F.L.; De Paula, R.C.M.; Feitosa, J.P.A.; Tedesco, A.C.; et al. Acetylated Cashew Gum-Based Nanoparticles for Transdermal Delivery of Diclofenac Diethyl Amine. Carbohydr. Polym. 2016, 143, 254–261. [Google Scholar] [CrossRef] [PubMed]
- Tosif, M.M.; Najda, A.; Bains, A.; Kaushik, R.; Dhull, S.B.; Chawla, P.; Walasek-Janusz, M. A Comprehensive Review on Plant-Derived Mucilage: Characterization, Functional Properties, Applications, and Its Utilization for Nanocarrier Fabrication. Polymers 2021, 13, 1066. [Google Scholar] [CrossRef] [PubMed]
- Houston, K.; Tucker, M.R.; Chowdhury, J.; Shirley, N.; Little, A. The Plant Cell Wall: A Complex and Dynamic Structure as Revealed by the Responses of Genes under Stress Conditions. Front. Plant Sci. 2016, 7, 984. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Gao, Y.; Zhang, L.; Zhou, Y. The Plant Cell Wall: Biosynthesis, Construction, and Functions. J. Integr. Plant Biol. 2021, 63, 251–272. [Google Scholar] [CrossRef]
- Mohd Zuki, S.A.; Abd Rahman, N.; Abu Bakar, N.F. Nanocrystal Cellulose as Drug Excipient in Transdermal Patch for Wound Healing: An Overview. IOP Conf. Ser. Mater. Sci. Eng. 2018, 334, 012046. [Google Scholar] [CrossRef]
- Latif, M.S.; Azad, A.K.; Nawaz, A.; Rashid, S.A.; Rahman, M.H.; Al Omar, S.Y.; Bungau, S.G.; Aleya, L.; Abdel-Daim, M.M. Ethyl Cellulose and Hydroxypropyl Methyl Cellulose Blended Methotrexate-Loaded Transdermal Patches: In Vitro and Ex Vivo. Polymers 2021, 13, 3455. [Google Scholar] [CrossRef]
- Manna, S.; Dhanalakshmi, D.; Bhowmik, M.; Jana, S.; Jana, S. Cellulose Derivative-Based Bioadhesive Blend Patch for Transdermal Drug Delivery. Front. Mater. 2022, 9, 835507. [Google Scholar] [CrossRef]
- Koochaki, A.; Shahgholi, M.; Sajadi, S.M.; Babadi, E.; Inc, M. Investigation of the Mechanical Stability of Polyethylene Glycol Hydrogel Reinforced with Cellulose Nanofibrils for Wound Healing: Molecular Dynamics Simulation. Eng. Anal. Bound. Elem. 2023, 151, 1–7. [Google Scholar] [CrossRef]
- Razack, S.A.; Lee, Y.; Shin, H.; Duraiarasan, S.; Chun, B.S.; Kang, H.W. Cellulose Nanofibrils Reinforced Chitosan-Gelatin Based Hydrogel Loaded with Nanoemulsion of Oregano Essential Oil for Diabetic Wound Healing Assisted by Low Level Laser Therapy. Int. J. Biol. Macromol. 2023, 226, 220–239. [Google Scholar] [CrossRef] [PubMed]
- Dong, D.; Chen, R.; Jia, J.; Zhao, C.; Chen, Z.; Lu, Q.; Sun, Y.; Huang, W.; Wang, C.; Li, Y.; et al. Tailoring and Application of a Multi-Responsive Cellulose Nanofibre-Based 3D Nanonetwork Wound Dressing. Carbohydr. Polym. 2023, 305, 120542. [Google Scholar] [CrossRef]
- Ding, M.; Wang, X.; Man, J.; Li, J.; Qiu, Y.; Zhang, Y.; Ji, M.; Li, J. Antibacterial and Hemostatic Polyvinyl Alcohol/Microcrystalline Cellulose Reinforced Sodium Alginate Breathable Dressing Containing Euphorbia Humifusa Extract Based on Microfluidic Spinning Technology. Int. J. Biol. Macromol. 2023, 239, 124167. [Google Scholar] [CrossRef]
- Pourmadadi, M.; Rahmani, E.; Shamsabadipour, A.; Samadi, A.; Esmaeili, J.; Arshad, R.; Rahdar, A.; Tavangarian, F.; Pandey, S. Novel Carboxymethyl Cellulose Based Nanocomposite: A Promising Biomaterial for Biomedical Applications. Process Biochem. 2023, 130, 211–226. [Google Scholar] [CrossRef]
- Zhang, K.; Wu, D.; Chang, L.; Duan, W.; Wang, Y.; Li, W.; Qin, J. Cellulose Based Self-Healing Hydrogel through Boronic Ester Connections for Wound Healing and Antitumor Applications. Int. J. Biol. Macromol. 2023, 230, 123294. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zhang, C.; Gong, M.; Zhan, Y.; Yu, Z.; Shen, C.; Zhang, Y.; Yu, L.; Chen, Z. Integrated Photo-Inspired Antibacterial Polyvinyl Alcohol/Carboxymethyl Cellulose Hydrogel Dressings for PH Real-Time Monitoring and Accelerated Wound Healing. Int. J. Biol. Macromol. 2023, 238, 124123. [Google Scholar] [CrossRef] [PubMed]
- Sabbagh, F.; Kim, B.-S. Ex Vivo Transdermal Delivery of Nicotinamide Mononucleotide Using Polyvinyl Alcohol Microneedles. Polymers 2023, 15, 2031. [Google Scholar] [CrossRef]
- Feketshane, Z.; Adeyemi, S.A.; Ubanako, P.; Ndinteh, D.T.; Ray, S.S.; Choonara, Y.E.; Aderibigbe, B.A. Dissolvable Sodium Alginate-Based Antibacterial Wound Dressing Patches: Design, Characterization, and in Vitro Biological Studies. Int. J. Biol. Macromol. 2023, 232, 123460. [Google Scholar] [CrossRef]
- Alsahag, M.; Alisaac, A.; Al-Hazmi, G.A.A.; Pashameah, R.A.; Attar, R.M.S.; Saad, F.A.; El-Metwaly, N.M. Preparation of Carboxymethyl Cellulose/Polyvinyl Alcohol Wound Dressing Composite Immobilized with Anthocyanin Extract for Colorimetric Monitoring of Wound Healing and Prevention of Wound Infection. Int. J. Biol. Macromol. 2023, 224, 233–242. [Google Scholar] [CrossRef]
- Aldossary, H.A.; Khalaf, M.M.; Gouda, M.; Elmushyakhi, A.; Abou Taleb, M.F.; Abd El-Lateef, H.M. Wound Dressing Candidate Materials Based on Casted Films of Cellulose Acetate Modified with Zirconium Oxide (ZrO2), and Gallium Oxide (Ga2O3). Mater. Today Commun. 2023, 34, 105299. [Google Scholar] [CrossRef]
- Sathe, T.; Bodas, D. Development and Characterization of a Polydimethylsiloxane-Cellulose Acetate Hybrid Membrane for Application in Organ-on-a-Chip. Mater. Sci. Eng. B Solid-State Mater. Adv. Technol. 2023, 291, 116366. [Google Scholar] [CrossRef]
- Kumar, G.; Khan, F.G.; Abro, M.I.; Aftab, U.; Jatoi, A.W. Development of Cellulose Acetate/CuO/AgNP Nanofibers Based Effective Antimicrobial Wound Dressing. Compos. Commun. 2023, 39, 101550. [Google Scholar] [CrossRef]
- Graça, M.F.P.; Melo, B.L.; Lima-Sousa, R.; Ferreira, P.; Moreira, A.F.; Correia, I.J. Reduced Graphene Oxide-Enriched Chitosan Hydrogel/Cellulose Acetate-Based Nanofibers Application in Mild Hyperthermia and Skin Regeneration. Int. J. Biol. Macromol. 2023, 229, 224–235. [Google Scholar] [CrossRef] [PubMed]
- Le, K.T.; Nguyen, C.T.; Lac, T.D.; Nguyen, L.G.T.; Tran, T.L.; Tran-Van, H. Facilely Preparing Carboxymethyl Chitosan/Hydroxyethyl Cellulose Hydrogel Films for Protective and Sustained Release of Fibroblast Growth Factor 2 to Accelerate Dermal Tissue Repair. J. Drug Deliv. Sci. Technol. 2023, 82, 104318. [Google Scholar] [CrossRef]
- Nawaz, A.; Farid, A.; Safdar, M.; Latif, M.S.; Ghazanfar, S.; Akhtar, N.; Al Jaouni, S.K.; Selim, S.; Khan, M.W. Formulation Development and Ex-Vivo Permeability of Curcumin Hydrogels under the Influence of Natural Chemical Enhancers. Gels 2022, 8, 384. [Google Scholar] [CrossRef]
- Natori, N.; Shibano, Y.; Hiroki, A.; Taguchi, M.; Miyajima, A.; Yoshizawa, K.; Kawano, Y.; Hanawa, T. Preparation and Evaluation of Hydrogel Film Containing Tramadol for Reduction of Peripheral Neuropathic Pain. J. Pharm. Sci. 2023, 112, 132–137. [Google Scholar] [CrossRef]
- Elsherbiny, D.A.; Abdelgawad, A.M.; Shaheen, T.I.; Abdelwahed, N.A.M.; Jockenhoevel, S.; Ghazanfari, S. Thermoresponsive Nanofibers Loaded with Antimicrobial α-Aminophosphonate-o/w Emulsion Supported by Cellulose Nanocrystals for Smart Wound Care Patches. Int. J. Biol. Macromol. 2023, 233, 123655. [Google Scholar] [CrossRef]
- Buonvino, S.; Ciocci, M.; Nanni, F.; Cacciotti, I.; Melino, S. New Vegetable-Waste Biomaterials by Lupin Albus L. as Cellular Scaffolds for Applications in Biomedicine and Food. Biomaterials 2023, 293, 121984. [Google Scholar] [CrossRef]
- Ahmad, N.; Tayyeb, D.; Ali, I.; Alruwaili, N.K.; Ahmad, W.; ur Rehman, A.; Khan, A.H.; Iqbal, M.S. Development and Characterization of Hemicellulose-Based Films for Antibacterial Wound-Dressing Application. Polymers 2020, 12, 548. [Google Scholar] [CrossRef]
- Li, Y.; Yao, M.; Luo, Y.; Li, J.; Wang, Z.; Liang, C.; Qin, C.; Huang, C.; Yao, S. Polydopamine-Reinforced Hemicellulose-Based Multifunctional Flexible Hydrogels for Human Movement Sensing and Self-Powered Transdermal Drug Delivery. ACS Appl. Mater. Interfaces 2023, 15, 5883–5896. [Google Scholar] [CrossRef]
- Anjum, S.; Hasan, N.; Ilmi, R. Pectin-Based Nanoformulations for Therapeutic Applications. Polym. Nanosyst. 2023, 1, 405–439. [Google Scholar] [CrossRef]
- Phonrachom, O.; Charoensuk, P.; Kiti, K.; Saichana, N. International Journal of Biological Macromolecules Potential Use of Propolis-Loaded Quaternized Chitosan/Pectin Hydrogel Films as Wound Dressings: Preparation, Characterization, Antibacterial Evaluation, and in Vitro Healing Assay. Int. J. Biol. Macromol. 2023, 241, 124633. [Google Scholar] [CrossRef] [PubMed]
- Raghavendra Naveen, N.; Anitha, P.; Gowthami, B.; Goudanavar, P.; Fattepur, S. QbD Assisted Formulation Design and Optimization of Thiol Pectin Based Polyethyleneglycol and Montmorillonite (PEG/MMT) Nanocomposite Films of Neomycin Sulphate for Wound Healing. J. Drug Deliv. Sci. Technol. 2023, 82, 104348. [Google Scholar] [CrossRef]
- Otles, S.; Özyurt, V.H. Biotransformation in the Production of Secondary Metabolites. Stud. Nat. Prod. Chem. 2021, 68, 435–457. [Google Scholar] [CrossRef]
- Kuete, V. Health Effects of Alkaloids from African Medicinal Plants. In Toxicological Survey of African Medicinal Plants; Elsevier: Amsterdam, The Netherlands, 2014; pp. 611–633. ISBN 9780128004753. [Google Scholar]
- Mastrangelo, S.; Capozza, M.A.; Triarico, S.; Attinà, G.; Maurizi, P.; Romano, A.; Ruggiero, A. Opioid Transdermal Delivery System: A Useful Method for Pain Management in Children. Ann. Transl. Med. 2021, 9, 185. [Google Scholar] [CrossRef]
- Swaminathan, S.K.; Strasinger, C.; Kelchen, M.; Carr, J.; Ye, W.; Wokovich, A.; Ghosh, P.; Rajagopal, S.; Ueda, K.; Fisher, J.; et al. Determination of Rate and Extent of Scopolamine Release from Transderm Scōp® Transdermal Drug Delivery Systems in Healthy Human Adults. AAPS PharmSciTech 2020, 21, 117. [Google Scholar] [CrossRef]
- Shim, K.H.; Kang, M.J.; Sharma, N.; An, S.S.A. Beauty of the Beast: Anticholinergic Tropane Alkaloids in Therapeutics. Nat. Prod. Bioprospect. 2022, 12, 33. [Google Scholar] [CrossRef]
- Pensado, A.; Hattam, L.; White, K.A.J.; McGrogan, A.; Bunge, A.L.; Guy, R.H.; Delgado-Charro, M.B. Skin Pharmacokinetics of Transdermal Scopolamine: Measurements and Modeling. Mol. Pharm. 2021, 18, 2714–2723. [Google Scholar] [CrossRef]
- Westerling, D.; Hoglund, P.; Lundin, S.; Svedman, P. Transdermal Administration of Morphine to Healthy Subjects. Br. J. Clin. Pharmacol. 1994, 37, 571–576. [Google Scholar] [CrossRef]
- Ahn, J.S.; Lin, J.; Ogawa, S.; Yuan, C.; O’Brien, T.; Le, B.H.C.; Bothwell, A.M.; Moon, H.; Hadjiat, Y.; Ganapathi, A. Transdermal Buprenorphine and Fentanyl Patches in Cancer Pain: A Network Systematic Review. J. Pain Res. 2017, 10, 1963–1972. [Google Scholar] [CrossRef]
- Inui, N.; Kato, T.; Uchida, S.; Chida, K.; Takeuchi, K.; Kimura, T.; Watanabe, H. Novel Patch for Transdermal Administration of Morphine. J. Pain Symptom Manag. 2012, 44, 479–485. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.; Lane, M.E. Topical and Transdermal Delivery of Caffeine. Int. J. Pharm. 2015, 490, 155–164. [Google Scholar] [CrossRef]
- Londzin, P.; Zamora, M.; Kąkol, B.; Taborek, A.; Folwarczna, J. Potential of Caffeine in Alzheimer’s Disease—A Review of Experimental Studies. Nutrients 2021, 13, 537. [Google Scholar] [CrossRef]
- Abebe, M.W.; Appiah-Ntiamoah, R.; Kim, H. Gallic Acid Modified Alginate Self-Adhesive Hydrogel for Strain Responsive Transdermal Delivery. Int. J. Biol. Macromol. 2020, 163, 147–155. [Google Scholar] [CrossRef]
- Aboumanei, M.H.; Mahmoud, A.F. Design and Development of a Proniosomal Transdermal Drug Delivery System of Caffeine for Management of Migraine: In Vitro Characterization, 131I-Radiolabeling and in Vivo Biodistribution Studies. Process Biochem. 2020, 97, 201–212. [Google Scholar] [CrossRef]
- Setyawan, E.I.; Rohman, A.; Setyowati, E.P.; Nugroho, A.K. The Combination of Simplex Lattice Design and Chemometrics in the Formulation of Green Tea Leaves as Transdermal Matrix Patch. Pharmacia 2021, 68, 275–282. [Google Scholar] [CrossRef]
- Vanderpluym, J.H.; Halker Singh, R.B.; Urtecho, M.; Morrow, A.S.; Nayfeh, T.; Torres Roldan, V.D.; Farah, M.H.; Hasan, B.; Saadi, S.; Shah, S.; et al. Acute Treatments for Episodic Migraine in Adults: A Systematic Review and Meta-Analysis. JAMA-J. Am. Med. Assoc. 2021, 325, 2357–2369. [Google Scholar] [CrossRef] [PubMed]
- Violante, C.; Lagoa, R.; Marques-da-Silva, D. Promotion of Dermal Permeation of Bioactive Compounds Using a Microneedle Device. In Proceedings of the Biosystems in Toxicology and Pharmacology—Current Challenges, Online, 8–9 September 2022; MDPI: Basel, Switzerland, 2022; p. 4. [Google Scholar]
- Chandran, R.; Mohd Tohit, E.R.; Stanslas, J.; Salim, N.; Tuan Mahmood, T.M. Investigation and Optimization of Hydrogel Microneedles for Transdermal Delivery of Caffeine. Tissue Eng. Part C Methods 2022, 28, 545–556. [Google Scholar] [CrossRef]
- Chai, S.H.; Leventhal, A.M.; Kirkpatrick, M.G.; Eisenlohr-Moul, T.A.; Rapkin, A.J.; D’Orazio, L.; Pang, R.D. Effectiveness of Transdermal Nicotine Patch in Premenopausal Female Smokers Is Moderated by Within-Subject Severity of Negative Affect and Physical Symptoms. Psychopharmacology 2020, 237, 1737–1744. [Google Scholar] [CrossRef]
- Niu, J.; Yuan, M.; Chen, C.; Wang, L.; Tang, Z.; Fan, Y.; Liu, X.; Ma, Y.J.; Gan, Y. Berberine-Loaded Thiolated Pluronic F127 Polymeric Micelles for Improving Skin Permeation and Retention. Int. J. Nanomed. 2020, 15, 9987–10005. [Google Scholar] [CrossRef]
- Cometa, S.; Bonifacio, M.A.; Licini, C.; Bellissimo, A.; Pinto, L.; Baruzzi, F.; Mattioli-Belmonte, M.; De Giglio, E. Innovative Eco-Friendly Hydrogel Film for Berberine Delivery in Skin Applications. Molecules 2021, 26, 4901. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Qin, Y.; Dai, D.; Wang, P.; Shi, M.; Gao, J.; Yang, J.; Xiao, W.; Song, P.; Xu, R. Transdermal Delivery of Therapeutic Compounds with Nanotechnological Approaches in Psoriasis. Front. Bioeng. Biotechnol. 2022, 9, 804415. [Google Scholar] [CrossRef] [PubMed]
- Parhi, R.; Sahoo, S.K.; Das, A. Applications of Polysaccharides in Topical and Transdermal Drug Delivery: A Recent Update of Literature. Braz. J. Pharm. Sci. 2022, 58, 1–38. [Google Scholar] [CrossRef]
- Ameen, D.; Michniak-Kohn, B. Development and in Vitro Evaluation of Pressure Sensitive Adhesive Patch for the Transdermal Delivery of Galantamine: Effect of Penetration Enhancers and Crystallization Inhibition. Eur. J. Pharm. Biopharm. 2019, 139, 262–271. [Google Scholar] [CrossRef]
- Kandil, L.S.; Hanafy, A.S.; Abdelhady, S.A. Galantamine Transdermal Patch Shows Higher Tolerability over Oral Galantamine in Rheumatoid Arthritis Rat Model. Drug Dev. Ind. Pharm. 2020, 46, 996–1004. [Google Scholar] [CrossRef]
- Sharma, S.; Sharma, S.; Chourasia, R.; Pandey, A.; Rai, A.K.; Sahoo, D. Alzheimer’s Disease. In Naturally Occurring Chemicals Against Alzheimer’s Disease; Elsevier: Amsterdam, The Netherlands, 2021; pp. 11–28. ISBN 9780128192122. [Google Scholar]
- Rorabaugh, B. Ephedrine. In xPharm: The Comprehensive Pharmacology Reference; Elsevier: Amsterdam, The Netherlands, 2007; pp. 1–6. ISBN 9780080552323. [Google Scholar]
- Jain, S.K.; Vyas, S.P.; Dixit, V.K. Effective and Controlled Transdermal Delivery of Ephedrine. J. Control. Release 1990, 12, 257–263. [Google Scholar] [CrossRef]
- Samiullah; Jan, S.U.; Gul, R.; Jalaludin, S.; Asmathullah. Formulation and Evaluation of Transdermal Patches of Pseudoephedrine HCL. Int. J. Appl. Pharm. 2020, 12, 121–127. [Google Scholar] [CrossRef]
- Cristofoli, M.; Kung, C.P.; Hadgraft, J.; Lane, M.E.; Sil, B.C. Ion Pairs for Transdermal and Dermal Drug Delivery: A Review. Pharmaceutics 2021, 13, 909. [Google Scholar] [CrossRef]
- de la Rosa, L.A.; Moreno-Escamilla, J.O.; Rodrigo-García, J.; Alvarez-Parrilla, E. Phenolic Compounds. In Postharvest Physiology and Biochemistry of Fruits and Vegetables; Elsevier: Amsterdam, The Netherlands, 2019; pp. 253–271. ISBN 9780128132784. [Google Scholar]
- Pinto, T.; Aires, A.; Cosme, F.; Bacelar, E.; Morais, M.C.; Oliveira, I.; Ferreira-Cardoso, J.; Anjos, R.; Vilela, A.; Gonçalves, B. Bioactive (Poly)Phenols, Volatile Compounds from Vegetables, Medicinal and Aromatic Plants. Foods 2021, 10, 106. [Google Scholar] [CrossRef]
- Bieżanowska-Kopeć, R.; Piatkowska, E. Total Polyphenols and Antioxidant Properties of Selected Fresh and Dried Herbs and Spices. Appl. Sci. 2022, 12, 4876. [Google Scholar] [CrossRef]
- Ali, A.; Wu, H.; Ponnampalam, E.N.; Cottrell, J.J.; Dunshea, F.R.; Suleria, H.A.R. Comprehensive Profiling of Most Widely Used Spices for Their Phenolic Compounds through Lc-Esi-Qtof-Ms2 and Their Antioxidant Potential. Antioxidants 2021, 10, 721. [Google Scholar] [CrossRef]
- Zeng, Y.; Zhou, W.; Yu, J.; Zhao, L.; Wang, K.; Hu, Z.; Liu, X. By-Products of Fruit and Vegetables: Antioxidant Properties of Extractable and Non-Extractable Phenolic Compounds. Antioxidants 2023, 12, 418. [Google Scholar] [CrossRef]
- Gan, R.Y.; Chan, C.L.; Yang, Q.Q.; Li, H.B.; Zhang, D.; Ge, Y.Y.; Gunaratne, A.; Ge, J.; Corke, H. Bioactive Compounds and Beneficial Functions of Sprouted Grains; Elsevier Inc.: Amsterdam, The Netherlands, 2018; ISBN 9780128115251. [Google Scholar]
- Zhang, Z.; Li, W.; Chang, D.; Wei, Z.; Wang, E.; Yu, J.; Xu, Y.; Que, Y.; Chen, Y.; Fan, C.; et al. A Combination Therapy for Androgenic Alopecia Based on Quercetin and Zinc/Copper Dual-Doped Mesoporous Silica Nanocomposite Microneedle Patch. Bioact. Mater. 2023, 24, 81–95. [Google Scholar] [CrossRef] [PubMed]
- Nisar, A. Medicinal Plants and Phenolic Compounds; IntechOpen: London, UK, 2022; Volume 11, p. 13. ISBN 0000957720. [Google Scholar]
- Gugleva, V.; Ivanova, N.; Sotirova, Y.; Andonova, V. Dermal Drug Delivery of Phytochemicals with Phenolic Structure via Lipid-based Nanotechnologies. Pharmaceuticals 2021, 14, 837. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Yadav, S.S. A Review on Health Benefits of Phenolics Derived from Dietary Spices. Curr. Res. Food Sci. 2022, 5, 1508–1523. [Google Scholar] [CrossRef]
- Rahman, M.; Rahaman, S.; Islam, R.; Rahman, F.; Mithi, F.M.; Alqahtani, T.; Almikhlafi, M.A.; Alghamdi, S.Q.; Alruwaili, A.S.; Hossain, S.; et al. Role of Phenolic Compounds in Human Disease: Current. Molecules 2022, 27, 233. [Google Scholar] [CrossRef] [PubMed]
- Rathod, N.B.; Elabed, N.; Punia, S.; Ozogul, F.; Kim, S.-K.; Rocha, J.M. Recent Developments in Polyphenol Applications on Human Health: A Review with Current Knowledge. Plants 2023, 12, 1217. [Google Scholar] [CrossRef] [PubMed]
- Bié, J.; Sepodes, B.; Fernandes, P.C.B.; Ribeiro, M.H.L. Polyphenols in Health and Disease: Gut Microbiota, Bioaccessibility, and Bioavailability. Compounds 2023, 3, 40–72. [Google Scholar] [CrossRef]
- Cosme, P.; Rodríguez, A.B.; Espino, J.; Garrido, M. Plant Phenolics: Bioavailability as a Key Determinant of Their Potential Health-Promoting Applications. Antioxidants 2020, 9, 1263. [Google Scholar] [CrossRef]
- Nurzyńska-Wierdak, R. Phenolic Compounds from New Natural Sources—Plant Genotype and Ontogenetic Variation. Molecules 2023, 28, 1731. [Google Scholar] [CrossRef]
- Butkeviciute, A.; Ramanauskiene, K.; Kurapkiene, V.; Janulis, V. Dermal Penetration Studies of Potential Phenolic Compounds Ex Vivo and Their Antioxidant Activity In Vitro. Plants 2022, 11, 1901. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.; Li, Y.; Li, Y.; Zhang, Z.; Liang, Y. Antioxidant and Prooxidant Activities of Phenolic Acids Commonly Existed in Vegetablesand Their Relationship with Structures. Food Sci. Technol. 2022, 42, e07622. [Google Scholar] [CrossRef]
- Zhang, Y.; Cai, P.; Cheng, G.; Zhang, Y. A Brief Review of Phenolic Compounds Identified from Plants: Their Extraction, Analysis, and Biological Activity. Nat. Prod. Commun. 2022, 17, 1934578X211069721. [Google Scholar] [CrossRef]
- Luque, G.C.; Moya, M.; Picchio, M.L.; Bagnarello, V.; Valerio, I.; Bolaños, J.; Vethencourt, M.; Gamboa, S.; Tomé, L.C.; Minari, R.J.; et al. Polyphenol Iongel Patches with Antimicrobial, Antioxidant and Anti-Inflammatory Properties. Polymers 2023, 15, 1076. [Google Scholar] [CrossRef]
- Santi, T.D.; Siregar, T.N.; Sutriana, A.; Andini, R.; Candra, A. Phytochemical Test and Optimization of Transdermal Patches of Carica Papaya Extract: Formulation Design of Candidate Drug for Wound Healing. Biodiversitas 2022, 23, 2904–2913. [Google Scholar] [CrossRef]
- Hallan, S.S.; Sguizzato, M.; Mariani, P.; Cortesi, R.; Huang, N.; Simelière, F.; Marchetti, N.; Drechsler, M.; Ruzgas, T.; Esposito, E. Design and Characterization of Ethosomes for Transdermal Delivery of Caffeic Acid. Pharmaceutics 2020, 12, 740. [Google Scholar] [CrossRef]
- Viqhi, A.V.; Manggau, M.A.; Sartini, S.; Wahyudin, E.; Rahman, L.; Yulianti, R.; Permana, A.D.; Awal, S.A. Development of Propolis (Apis Trigona)-Loaded Nanoemulgel for Improved Skin Penetration of Caffeic Acid: The Effect of Variation of Oleic Acid Concentration. Open Access Maced. J. Med. Sci. 2021, 9, 1264–1278. [Google Scholar] [CrossRef]
- Contardi, M.; Lenzuni, M.; Fiorentini, F.; Summa, M.; Bertorelli, R.; Suarato, G.; Athanassiou, A. Hydroxycinnamic Acids and Derivatives Formulations for Skin Damages and Disorders: A Review. Pharmaceutics 2021, 13, 999. [Google Scholar] [CrossRef]
- Hendriati, L.; Fredericktho, F.F.; Hamid, I.S.; Widodo, T.; Kuncorojakti, S. The Antigangrene Activity of Transdermal Patch of Insulin Leaves (Smallanthus Sonchifolius) to Diabetic Gangrene on White Rats. J. Farm. Galen. (Galenika J. Pharmacy) 2022, 9, 30–40. [Google Scholar] [CrossRef]
- Zvezdin, V.; Peno-Mazzarino, L.; Radionov, N.; Kasatkina, T.; Kasatkin, I. Microneedle Patch Based on Dissolving, Detachable Microneedle Technology for Improved Skin Quality–Part 1: Ex Vivo Safety Evaluation. Int. J. Cosmet. Sci. 2020, 42, 369–376. [Google Scholar] [CrossRef]
- Algahtani, M.S.; Ahmad, M.Z.; Nourein, I.H.; Albarqi, H.A.; Alyami, H.S.; Alyami, M.H.; Alqahtani, A.A.; Alasiri, A.; Algahtani, T.S.; Mohammed, A.A.; et al. Preparation and Characterization of Curcumin Nanoemulgel Utilizing Ultrasonication Technique for Wound Healing: In Vitro, Ex Vivo, and In Vivo Evaluation. Gels 2021, 7, 213. [Google Scholar] [CrossRef] [PubMed]
- Kriplani, P.; Guarve, K.; Singh Baghel, U. Formulation Optimization and Characterization of Transdermal Film of Curcumin by Response Surface Methodology. Chin. Herb. Med. 2021, 13, 274–285. [Google Scholar] [CrossRef] [PubMed]
- Eckert, R.W.; Wiemann, S.; Keck, C.M. Improved Dermal and Transdermal Delivery of Curcumin with Smartfilms and Nanocrystals. Molecules 2021, 26, 1633. [Google Scholar] [CrossRef]
- Alven, S.; Nqoro, X.; Aderibigbe, B.A. Polymer-Based Materials Loaded with Curcumin for Wound Healing Applications. Polymers 2020, 12, 2286. [Google Scholar] [CrossRef] [PubMed]
- Prabhu, A.; Jose, J.; Kumar, L.; Salwa, S.; Vijay Kumar, M.; Nabavi, S.M. Transdermal Delivery of Curcumin-Loaded Solid Lipid Nanoparticles as Microneedle Patch: An In Vitro and In Vivo Study. AAPS PharmSciTech 2022, 23, 49. [Google Scholar] [CrossRef] [PubMed]
- Madamsetty, V.S.; Vazifehdoost, M.; Alhashemi, S.H.; Davoudi, H.; Zarrabi, A.; Dehshahri, A.; Fekri, H.S.; Mohammadinejad, R.; Thakur, V.K. Next-Generation Hydrogels as Biomaterials for Biomedical Applications: Exploring the Role of Curcumin. ACS Omega 2023, 8, 8960–8976. [Google Scholar] [CrossRef] [PubMed]
- Akanda, M.; Getti, G.; Douroumis, D. In Vivo Evaluation of Nanostructured Lipid Carrier Systems (NLCs) in Mice Bearing Prostate Cancer Tumours. Drug Deliv. Transl. Res. 2021, 13, 2083–2095. [Google Scholar] [CrossRef]
- Altamimi, M.A.; Hussain, A.; Mahdi, W.A.; Imam, S.S.; Alshammari, M.A.; Alshehri, S.; Khan, M.R. Mechanistic Insights into Luteolin-Loaded Elastic Liposomes for Transdermal Delivery: HSPiP Predictive Parameters and Instrument-Based Evidence. ACS Omega 2022, 7, 48202–48214. [Google Scholar] [CrossRef]
- Sabir, F.; Qindeel, M.; Rehman, A.U.; Ahmad, N.M.; Khan, G.M.; Csoka, I.; Ahmed, N. An Efficient Approach for Development and Optimisation of Curcumin-Loaded Solid Lipid Nanoparticles’ Patch for Transdermal Delivery. J. Microencapsul. 2021, 38, 233–248. [Google Scholar] [CrossRef]
- Zálešák, F.; Bon, D.J.Y.D.; Pospíšil, J. Lignans and Neolignans: Plant Secondary Metabolites as a Reservoir of Biologically Active Substances. Pharmacol. Res. 2019, 146, 104284. [Google Scholar] [CrossRef]
- Jang, W.Y.; Kim, M.Y.; Cho, J.Y. Antioxidant, Anti-Inflammatory, Anti-Menopausal, and Anti-Cancer Effects of Lignans and Their Metabolites. Int. J. Mol. Sci. 2022, 23, 15482. [Google Scholar] [CrossRef] [PubMed]
- Rybczyńska-Tkaczyk, K.; Grenda, A.; Jakubczyk, A.; Kiersnowska, K.; Bik-Małodzińska, M. Natural Compounds with Antimicrobial Properties in Cosmetics. Pathogens 2023, 12, 320. [Google Scholar] [CrossRef] [PubMed]
- Runeberg, P.; Ryabukhin, D.; Lagerquist, L.; Rahkila, J.; Eklund, P. Transformations and Antioxidative Activities of Lignans and Stilbenes at High Temperatures. Food Chem. 2023, 404, 134641. [Google Scholar] [CrossRef] [PubMed]
- Deresa, E.M.; Diriba, T.F. Phytochemicals as Alternative Fungicides for Controlling Plant Diseases: A Comprehensive Review of Their Efficacy, Commercial Representatives, Advantages, Challenges for Adoption, and Possible Solutions. Heliyon 2023, 9, e13810. [Google Scholar] [CrossRef]
- Park, R.; Park, E.J.; Cho, Y.Y.; Lee, J.Y.; Kang, H.C.; Song, I.S.; Lee, H.S. Tetrahydrofurofuranoid Lignans, Eudesmin, Fargesin, Epimagnolin a, Magnolin, and Yangambin Inhibit UDP-Glucuronosyltransferase 1A1 and 1A3 Activities in Human Liver Microsomes. Pharmaceutics 2021, 13, 3–15. [Google Scholar] [CrossRef]
- Sangiorgio, P.; Errico, S.; Verardi, A.; Moliterni, S.; Tamasi, G.; Rossi, C.; Balducchi, R. Bioactive Lignans from Flaxseed: Biological Properties and Patented Recovery Technologies. Nutraceuticals 2023, 3, 58–74. [Google Scholar] [CrossRef]
- Yeung, A.W.K.; Tzvetkov, N.T.; Balacheva, A.A.; Georgieva, M.G.; Gan, R.Y.; Jozwik, A.; Pyzel, B.; Horbańczuk, J.O.; Novellino, E.; Durazzo, A.; et al. Lignans: Quantitative Analysis of the Research Literature. Front. Pharmacol. 2020, 11, 37. [Google Scholar] [CrossRef]
- Torrisi, C.; Cardullo, N.; Russo, S.; La Mantia, A.; Acquaviva, R.; Muccilli, V.; Castelli, F.; Sarpietro, M.G. Benzo[k,l]Xanthene Lignan-Loaded Solid Lipid Nanoparticles for Topical Application: A Preliminary Study. Molecules 2022, 27, 5887. [Google Scholar] [CrossRef]
- Naik, N.J.; Abhyankar, I.; Darne, P.; Prabhune, A.; Madhusudhan, B. Sustained Transdermal Release of Lignans Facilitated by Sophorolipid Based Transferosomal Hydrogel for Cosmetic Application. Int. J. Curr. Microbiol. Appl. Sci. 2019, 8, 1783–1791. [Google Scholar] [CrossRef]
- Tasneem, R.; Khan, H.M.S.; Rasool, F.; Khan, K.U.R.; Umair, M.; Esatbeyoglu, T.; Korma, S.A. Development of Phytocosmeceutical Microemulgel Containing Flaxseed Extract and Its In Vitro and In Vivo Characterization. Pharmaceutics 2022, 14, 1656. [Google Scholar] [CrossRef]
- González-Sarrías, A.; Espín-Aguilar, J.C.; Romero-Reyes, S.; Puigcerver, J.; Alajarín, M.; Berná, J.; Selma, M.V.; Espín, J.C. Main Determinants Affecting the Antiproliferative Activity of Stilbenes and Their Gut Microbiota Metabolites in Colon Cancer Cells: A Structure–Activity Relationship Study. Int. J. Mol. Sci. 2022, 23, 15102. [Google Scholar] [CrossRef]
- Choiri, S.; Fitriastuti, R.; Faradiva, F.Z.; Rahayu, W.V. Antioxidant Activity and Nano Delivery of the Most Frequently Applied Stilbene Derivates: A Brief and Recent Review. Pharm. Sci. 2022, 28, 365–375. [Google Scholar] [CrossRef]
- Cebrián, R.; Li, Q.; Peñalver, P.; Belmonte-Reche, E.; Andrés-Bilbao, M.; Lucas, R.; De Paz, M.V.; Kuipers, O.P.; Morales, J.C. Chemically Tuning Resveratrol for the Effective Killing of Gram-Positive Pathogens. J. Nat. Prod. 2022, 85, 1459–1473. [Google Scholar] [CrossRef]
- Mattio, L.M.; Catinella, G.; Pinto, A.; Dallavalle, S. Natural and Nature-Inspired Stilbenoids as Antiviral Agents. Eur. J. Med. Chem. 2020, 202, 112541. [Google Scholar] [CrossRef]
- Aung, N.N.; Ngawhirunpat, T.; Rojanarata, T.; Patrojanasophon, P.; Opanasopit, P.; Pamornpathomkul, B. Enhancement of Transdermal Delivery of Resveratrol Using Eudragit and Polyvinyl Pyrrolidone-Based Dissolving Microneedle Patches. J. Drug Deliv. Sci. Technol. 2021, 61, 102284. [Google Scholar] [CrossRef]
- Uchida, N.; Yanagi, M.; Shimoda, K.; Hamada, H. Transdermal Delivery of Small-Sized Resveratrol Nanoparticles to Epidermis Using Anionic Phospholipids. Nat. Prod. Commun. 2020, 15, 1934578X20951443. [Google Scholar] [CrossRef]
- Zoabi, A.; Touitou, E.; Margulis, K. Recent Advances in Nanomaterials for Dermal and Transdermal Applications. Colloids Interfaces 2021, 5, 18. [Google Scholar] [CrossRef]
- Lafarge, E.; Villette, S.; Cario-André, M.; Lecomte, S.; Faure, C. Transdermal Diffusion of Resveratrol by Multilamellar Liposomes: Effect of Encapsulation on Its Stability. J. Drug Deliv. Sci. Technol. 2022, 76, 103742. [Google Scholar] [CrossRef]
- Kozłowska, A.; Szostak-Węgierek, D. Flavonoids–Food Sources, Health Benefits, and Mechanisms Involved. In Bioactive Molecules in Food; Springer: Cham, Switzerland, 2018; pp. 1–27. ISBN 9783319780306. [Google Scholar]
- Ramesh, P.; Jagadeesan, R.; Sekaran, S.; Dhanasekaran, A.; Vimalraj, S. Flavonoids: Classification, Function, and Molecular Mechanisms Involved in Bone Remodelling. Front. Endocrinol. 2021, 12, 779638. [Google Scholar] [CrossRef] [PubMed]
- Pecorini, G.; Ferraro, E.; Puppi, D. Polymeric Systems for the Controlled Release of Flavonoids. Pharmaceutics 2023, 15, 628. [Google Scholar] [CrossRef] [PubMed]
- Chiu, Y.H.; Wu, Y.W.; Hung, J.I.; Chen, M.C. Epigallocatechin Gallate/L-Ascorbic Acid–Loaded Poly-γ-Glutamate Microneedles with Antioxidant, Anti-Inflammatory, and Immunomodulatory Effects for the Treatment of Atopic Dermatitis. Acta Biomater. 2021, 130, 223–233. [Google Scholar] [CrossRef]
- Reddy, V.N.; Nyamathulla, S.; Pahirulzaman, K.A.K.; Mokhtar, S.I.; Giribabu, N.; Pasupuleti, V.R. Gallocatechin-Silver Nanoparticles Embedded in Cotton Gauze Patches Accelerated Wound Healing in Diabetic Rats by Promoting Proliferation and Inhibiting Apoptosis through the Wnt/β-Catenin Signaling Pathway. PLoS ONE 2022, 17, e0268505. [Google Scholar] [CrossRef]
- Nagula, R.L.; Wairkar, S. Cellulose Microsponges Based Gel of Naringenin for Atopic Dermatitis: Design, Optimization, in Vitro and in Vivo Investigation. Int. J. Biol. Macromol. 2020, 164, 717–725. [Google Scholar] [CrossRef] [PubMed]
- Ravetti, S.; Garro, A.G.; Gaitán, A.; Murature, M.; Galiano, M.; Brignone, S.G.; Palma, S.D. Naringin: Nanotechnological Strategies for Potential Pharmaceutical Applications. Pharmaceutics 2023, 15, 863. [Google Scholar] [CrossRef] [PubMed]
- Hering, A.; Ochocka, J.R.; Baranska, H.; Cal, K.; Stefanowicz-Hajduk, J. Mangiferin and Hesperidin Transdermal Distribution and Permeability through the Skin from Solutions and Honeybush Extracts (Cyclopia Sp.)—A Comparison Ex Vivo Study. Molecules 2021, 26, 6547. [Google Scholar] [CrossRef] [PubMed]
- Kazi, M.; Alhajri, A.; Alshehri, S.M.; Elzayat, E.M.; Al Meanazel, O.T.; Shakeel, F.; Noman, O.; Altamimi, M.A.; Alanazi, F.K. Enhancing Oral Bioavailability of Apigenin Using a Bioactive Self-Nanoemulsifying Drug Delivery System (Bio-SNEDDS): In Vitro, in Vivo and Stability Evaluations. Pharmaceutics 2020, 12, 749. [Google Scholar] [CrossRef]
- Yoon, J.H.; Kim, M.Y.; Cho, J.Y. Apigenin: A Therapeutic Agent for Treatment of Skin Inflammatory Diseases and Cancer. Int. J. Mol. Sci. 2023, 24, 1498. [Google Scholar] [CrossRef]
- Xie, J.; Huang, S.; Huang, H.; Deng, X.; Yue, P.; Lin, J.; Yang, M.; Han, L.; Zhang, D.K. Advances in the Application of Natural Products and the Novel Drug Delivery Systems for Psoriasis. Front. Pharmacol. 2021, 12, 644952. [Google Scholar] [CrossRef]
- Altamimi, M.A.; Hussain, A.; Alrajhi, M.; Alshehri, S.; Imam, S.S.; Qamar, W. Luteolin-Loaded Elastic Liposomes for Transdermal Delivery to Control Breast Cancer: In Vitro and Ex Vivo Evaluations. Pharmaceuticals 2021, 14, 1143. [Google Scholar] [CrossRef]
- Costa, R.; Costa Lima, S.A.; Gameiro, P.; Reis, S. On the Development of a Cutaneous Flavonoid Delivery System: Advances and Limitations. Antioxidants 2021, 10, 1376. [Google Scholar] [CrossRef]
- Wang, Z.; Hu, Y.; Xue, Y.; Zhu, Z.; Wu, Y.; Zeng, Q.; Wang, Y.; Han, H.; Zhang, H.; Shen, C.; et al. Mechanism Insight on Licorice Flavonoids Release from Carbopol Hydrogels: Role of “Release Steric Hindrance” and Drug Solubility in the Release Medium. Eur. J. Pharm. Sci. 2022, 179, 106307. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, F.; Benedusi, M.; Sguizzato, M.; Cortesi, R.; Baldisserotto, A.; Buzzi, R.; Valacchi, G.; Esposito, E. Ethosomes and Transethosomes as Cutaneous Delivery Systems for Quercetin: A Preliminary Study on Melanoma Cells. Pharmaceutics 2022, 14, 1038. [Google Scholar] [CrossRef]
- Kharia, A.; Singhai, A.K.; Gilhotra, R. Formualtion and Evalaution of Transdermal Patch for the Treatment of Inflammation. J. Pharm. Sci. Res. 2020, 12, 780–788. [Google Scholar]
- Zhang, Q.; Yang, X.; Wu, Y.; Liu, C.; Xia, H.; Cheng, X.; Cheng, Y.; Xia, Y.; Wang, Y. In Vitro Evaluation of Kaempferol-Loaded Hydrogel as PH-Sensitive Drug Delivery Systems. Polymers 2022, 14, 3205. [Google Scholar] [CrossRef] [PubMed]
- Vu, Q.L.; Fang, C.W.; Suhail, M.; Wu, P.C. Enhancement of the Topical Bioavailability and Skin Whitening Effect of Genistein by Using Microemulsions as Drug Delivery Carriers. Pharmaceuticals 2021, 14, 1233. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Grewal, S.; Sharma, N.; Behl, T.; Gupta, S.; Anwer, M.K.; Vargas-De-La-Cruz, C.; Mohan, S.; Bungau, S.G.; Bumbu, A. Unveiling the Pharmacological and Nanotechnological Facets of Daidzein: Present State-of-the-Art and Future Perspectives. Molecules 2023, 28, 1765. [Google Scholar] [CrossRef]
- Dall’oglio, F.; Fabbrocini, G.; Tedeschi, A.; Donnarumma, M.; Chiodini, P.; Micali, G. Licochalcone a in Combination with Salicylic Acid as Fluid Based and Hydroxy-Complex 10% Cream for the Treatment of Mild Acne: A Multicenter Prospective Trial. Clin. Cosmet. Investig. Dermatol. 2019, 12, 961–967. [Google Scholar] [CrossRef]
- Xin, Y.; Yun, S.; Yuhe, L.; Yinxue, M.; Shurui, N.; Yue, Z.; Kunming, Q.; Weidong, L. Development of Licorice Flavonoids Loaded Microemulsion for Transdermal Delivery Using CCD-Optimal Experimental Approach: Formulation Development and Characterization. Front. Nanotechnol. 2021, 3, 103742. [Google Scholar] [CrossRef]
- Liu, Z.; Li, M.; Wang, S.; Huang, H.; Zhang, W. Sulfur-Containing Metabolites from Marine and Terrestrial Fungal Sources: Origin, Structures, and Bioactivities. Mar. Drugs 2022, 20, 765. [Google Scholar] [CrossRef]
- Ruhee, R.T.; Roberts, L.A.; Ma, S.; Suzuki, K. Organosulfur Compounds: A Review of Their Anti-Inflammatory Effects in Human Health. Front. Nutr. 2020, 7, 64. [Google Scholar] [CrossRef]
- Iciek, M.; Bilska-wilkosz, A.; Kozdrowicki, M.; Górny, M. Reactive Sulfur Compounds in the Fight against COVID-19. Antioxidants 2022, 11, 1053. [Google Scholar] [CrossRef] [PubMed]
- Osipova, V.; Polovinkina, M.; Gracheva, Y.; Shpakovsky, D.; Osipova, A.; Berberova, N. Antioxidant Activity of Some Organosulfur Compounds in Vitro. Arab. J. Chem. 2021, 14, 103068. [Google Scholar] [CrossRef]
- Klyushova, L.S.; Kandalintseva, N.V.; Grishanova, A.Y. Antioxidant Activity of New Sulphur- and Selenium-Containing Analogues of Potassium Phenosan against H2O2-Induced Cytotoxicity in Tumour Cells. Curr. Issues Mol. Biol. 2022, 44, 3131–3145. [Google Scholar] [CrossRef] [PubMed]
- Pingaew, R.; Prachayasittikul, V.; Worachartcheewan, A.; Thongnum, A.; Prachayasittikul, S.; Ruchirawat, S.; Prachayasittikul, V. Anticancer Activity and QSAR Study of Sulfur-Containing Thiourea and Sulfonamide Derivatives. Heliyon 2022, 8, e10067. [Google Scholar] [CrossRef] [PubMed]
- Haridevamuthu, B.; Manjunathan, T.; Wilson Alphonse, C.R.; Kumar, R.S.; Thanigaivel, S.; Chandra Kishore, S.; Sundaram, V.; Gopinath, P.; Arockiaraj, J.; Bellucci, S. Functionalized Sulfur-Containing Heterocyclic Analogs Induce Sub-G1 Arrest and Apoptotic Cell Death of Laryngeal Carcinoma In Vitro. Molecules 2023, 28, 1856. [Google Scholar] [CrossRef]
- Abdalla, M.A.; Mühling, K.H. Plant-Derived Sulfur Containing Natural Products Produced as a Response to Biotic and Abiotic Stresses: A Review of Their Structural Diversity and Medicinal Importance. J. Appl. Bot. Food Qual. 2019, 92, 204–215. [Google Scholar] [CrossRef]
- Miękus, N.; Marszałek, K.; Podlacha, M.; Iqbal, A.; Puchalski, C.; Świergiel, A.H. Health Benefits of Plant-Derived Sulfur Compounds, Glucosinolates, and Organosulfur Compounds. Molecules 2020, 25, 3804. [Google Scholar] [CrossRef]
- Künstler, A.; Gullner, G.; Ádám, A.L.; Nagy, J.K.; Király, L. The Versatile Roles of Sulfur-Containing Biomolecules in Plant Defense—A Road to Disease Resistance. Plants 2020, 9, 1705. [Google Scholar] [CrossRef]
- Hill, C.R.; Shafaei, A.; Balmer, L.; Lewis, J.R.; Hodgson, J.M.; Millar, A.H.; Blekkenhorst, L.C. Sulfur Compounds: From Plants to Humans and Their Role in Chronic Disease Prevention. Crit. Rev. Food Sci. Nutr. Online ahead of print. 2022. [Google Scholar] [CrossRef]
- Khabibrakhmanova, A.M.; Faizova, R.G.; Lodochnikova, O.A.; Zamalieva, R.R.; Latypova, L.Z.; Trizna, E.Y.; Porfiryev, A.G.; Tanaka, K.; Sachenkov, O.A.; Kayumov, A.R.; et al. The Novel Chiral 2(5H)-Furanone Sulfones Possessing Terpene Moiety: Synthesis and Biological Activity. Molecules 2023, 28, 2543. [Google Scholar] [CrossRef]
- Pandey, P.; Khan, F.; Alshammari, N.; Saeed, A.; Aqil, F.; Saeed, M. Updates on the Anticancer Potential of Garlic Organosulfur Compounds and Their Nanoformulations: Plant Therapeutics in Cancer Management. Front. Pharmacol. 2023, 14, 1154034. [Google Scholar] [CrossRef] [PubMed]
- Perveen, S. Introductory Chapter: Terpenes and Terpenoids. In Terpenes and Terpenoids; IntechOpen: London, UK, 2018; Volume 11, p. 13. ISBN 0000957720. [Google Scholar]
- Çalışkan, U.K.; Karakuş, M.M. Essential Oils as Skin Permeation Boosters and Their Predicted Effect Mechanisms. J. Dermatol. Ski. Sci. 2020, 2, 24–30. [Google Scholar]
- Bundrla, D.S.; Kumar, H.; Pathak, A. A Review on Natural Permeation Enhancer for Transdermal Drug Delivery System and Permeation Evaluation. Int. J. Pharm. Pharm. Res. 2020, 19, 5–9. [Google Scholar]
- Alhasso, B.; Ghori, M.U.; Conway, B.R. Systematic Review on the Effectiveness of Essential and Carrier Oils as Skin Penetration Enhancers in Pharmaceutical Formulations. Sci. Pharm. 2022, 90, 14. [Google Scholar] [CrossRef]
- Hasan, A.; Farooqui, H. A Review on Role of Essential Oil as Penetration Enhancer in Transdermal Drug Delivery System. Sys. Rev. Pharm. 2021, 12, 439–444. [Google Scholar]
- Karaca, N.; Demirci, B.; Gavahian, M.; Demirci, F. Enhanced Bioactivity of Rosemary, Sage, Lavender, and Chamomile Essential Oils by Fractionation, Combination, and Emulsification. ACS Omega 2023, 8, 10941–10953. [Google Scholar] [CrossRef]
- Demurtas, O.C.; Nicolia, A.; Diretto, G. Terpenoid Transport in Plants: How Far from the Final Picture? Plants 2023, 12, 634. [Google Scholar] [CrossRef]
- Hosseini, M.; Pereira, D.M. The Chemical Space of Terpenes: Insights from Data Science and AI. Pharmaceuticals 2023, 16, 202. [Google Scholar] [CrossRef]
- Khoshnoudi-Nia, S.; Sharif, N.; Jafari, S.M. Loading of Phenolic Compounds into Electrospun Nanofibers and Electrosprayed Nanoparticles. Trends Food Sci. Technol. 2020, 95, 59–74. [Google Scholar] [CrossRef]
- Mamatha, J.; Gadili, S.; Pallavi, K. Formulation and Evaluation of Zidovudine Transdermal Patch Using Permeation Enhancers. J. Young Pharm. 2020, 12, s45–s50. [Google Scholar] [CrossRef]
- Li, J.; Xie, Q.; Ma, R.; Li, Y.; Yuan, J.; Ren, M.; Li, H.; Wang, J.; Lu, D.; Xu, Z.; et al. Recent Progress on the Synergistic Antitumor Effect of a Borneol-Modified Nanocarrier Drug Delivery System. Front. Med. 2021, 8, 750170. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Gan, Y.; Kang, T.; Zhao, Y.; Huang, T.; Chen, Y.; Liu, J.; Ke, B. Camphor Attenuates Hyperalgesia in Neuropathic Pain Models in Mice. J. Pain Res. 2023, 16, 785–795. [Google Scholar] [CrossRef] [PubMed]
- Carreño, H.; Stashenko, E.E.; Escobar, P. Essential Oils Distilled from Colombian Aromatic Plants and Their Constituents as Penetration Enhancers for Transdermal Drug Delivery. Molecules 2023, 28, 2872. [Google Scholar] [CrossRef] [PubMed]
- Krishnaiah, Y.S.R.; Nada, A. Enantioselective Penetration Enhancing Effect of Carvone on the in Vitro Transdermal Permeation of Nicorandil. Pharm. Dev. Technol. 2012, 17, 574–582. [Google Scholar] [CrossRef]
- Isaac, M.; Holvey, C. Transdermal Patches: The Emerging Mode of Drug Delivery System in Psychiatry. Ther. Adv. Psychopharmacol. 2012, 2, 255–263. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.; Verma, P.; Gupta, S.; Pandey, S.; Ojha, S. Nanocarrier and Herbal Based Transdermal Patch: An Advantage Over Other Drug Delivery Systems. Ann. Ayurvedic Med. 2022, 11, 145–156. [Google Scholar] [CrossRef]
- Sheth, N.S.; Mistry, R.B. Formulation and Evaluation of Transdermal Patches and to Study Permeation Enhancement Effect of Eugenol. J. Appl. Pharm. Sci. 2011, 1, 96–101. [Google Scholar]
- Sugumar, V.; Hayyan, M.; Madhavan, P.; Wong, W.F.; Looi, C.Y. Current Development of Chemical Penetration Enhancers for Transdermal Insulin Delivery. Biomedicines 2023, 11, 664. [Google Scholar] [CrossRef]
- Boix-Montañés, A.; Celma-Lezcano, C.; Obach-Vidal, R.; Peraire-Guitart, C. Collaborative Permeation of Drug and Excipients in Transdermal Formulations. In Vitro Scrutiny for Ethanol:Limonene Combinations. Eur. J. Pharm. Biopharm. 2022, 181, 239–248. [Google Scholar] [CrossRef]
- Ogueta, I.A.; Brared Christensson, J.; Giménez-Arnau, E.; Brans, R.; Wilkinson, M.; Stingeni, L.; Foti, C.; Aerts, O.; Svedman, C.; Gonçalo, M.; et al. Limonene and Linalool Hydroperoxides Review: Pros and Cons for Routine Patch Testing. Contact Dermat. 2022, 87, 1–12. [Google Scholar] [CrossRef]
- Castro, E.; Dent, D. A Comparison of Transdermal Over-the-Counter Lidocaine 3.6% Menthol 1.25%, Rx Lidocaine 5% and Placebo for Back Pain and Arthritis. Pain Manag. 2017, 7, 489–498. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Ren, S.; Yang, H.; Tang, S.; Guo, C.; Liu, M.; Tao, Q.; Ming, T.; Xu, H. Peppermint Essential Oil: Its Phytochemistry, Biological Activity, Pharmacological Effect and Application. Biomed. Pharmacother. 2022, 154, 113559. [Google Scholar] [CrossRef] [PubMed]
- Salehi, B.; Upadhyay, S.; Orhan, I.E.; Jugran, A.K.; Jayaweera, S.L.D.; Dias, D.A.; Sharopov, F.; Taheri, Y.; Martins, N.; Baghalpour, N.; et al. Therapeutic Potential of α-and β-Pinene: A Miracle Gift of Nature. Biomolecules 2019, 9, 738. [Google Scholar] [CrossRef] [PubMed]
- Masood, T.; Lakatos, S.; Rosta, J. Modification of the TRP Channel TRPA1 as a Relevant Factor in Migraine-Related Intracranial Hypersensitivity. Int. J. Mol. Sci. 2023, 24, 5375. [Google Scholar] [CrossRef]
- Bose, S.K.; Nirbhavane, P.; Batra, M.; Chhibber, S.; Harjai, K. Nanolipoidal α-Terpineol Modulates Quorum Sensing Regulated Virulence and Biofilm Formation in Pseudomonas Aeruginosa. Nanomedicine 2020, 15, 1743–1761. [Google Scholar] [CrossRef]
- Su, C.W.; Tighe, S.; Sheha, H.; Cheng, A.M.S.; Tseng, S.C.G. Safety and Efficacy of 4-Terpineol against Microorganisms Associated with Blepharitis and Common Ocular Diseases. BMJ Open Ophthalmol. 2018, 3, e000094. [Google Scholar] [CrossRef]
- Khan, A.; Raja, M.H.; Khan, A.; Khan, M.W.; Khan, G.M. Synthesis and Assessment of a New Tetrahydrogeraniol Derivative as Penetration Enhancer for Transdermal Drug Delivery. J. Glycom. Metab. 2016, 1, 45–53. [Google Scholar] [CrossRef]
- Silveira, Z.d.S.; Macêdo, N.S.; dos Santos, J.F.S.; de Freitas, T.S.; Barbosa, C.R.d.S.; Júnior, D.L.d.S.; Muniz, D.F.; de Oliveira, L.C.C.; Júnior, J.P.S.; da Cunha, F.A.B.; et al. Evaluation of the Antibacterial Activity and Efflux Pump Reversal of Thymol and Carvacrol against Staphylococcus Aureus and Their Toxicity in Drosophila Melanogaster. Molecules 2020, 25, 2103. [Google Scholar] [CrossRef]
- Chadwick, M.; Trewin, H.; Gawthrop, F.; Wagstaff, C. Sesquiterpenoids Lactones: Benefits to Plants and People. Int. J. Mol. Sci. 2013, 14, 12780–12805. [Google Scholar] [CrossRef]
- Ludwiczuk, A.; Skalicka-Woźniak, K.; Georgiev, M.I. Terpenoids. In Pharmacognosy Fundamentals, Applications and Strategies; Academic Press: Cambridge, MA, USA, 2017; ISBN 9780128020999. [Google Scholar]
- Arizmendi, N.; Alam, S.B.; Azyat, K.; Makeiff, D.; Befus, A.D.; Kulka, M. The Complexity of Sesquiterpene Chemistry Dictates Its Pleiotropic Biologic Effects on Inflammation. Molecules 2022, 27, 2450. [Google Scholar] [CrossRef]
- Albahri, G.; Badran, A.; Hijazi, A.; Daou, A.; Baydoun, E.; Nasser, M.; Merah, O. The Therapeutic Wound Healing Bioactivities of Various Medicinal Plants. Life 2023, 13, 317. [Google Scholar] [CrossRef] [PubMed]
- Jeon, J.H.; Kaiser, E.E.; Waters, E.S.; Yang, X.; Lourenco, J.M.; Fagan, M.M.; Scheulin, K.M.; Sneed, S.E.; Shin, S.K.; Kinder, H.A.; et al. Tanshinone IIA-Loaded Nanoparticles and Neural Stem Cell Combination Therapy Improves Gut Homeostasis and Recovery in a Pig Ischemic Stroke Model. Sci. Rep. 2023, 13, 2520. [Google Scholar] [CrossRef] [PubMed]
- Zhan, Y.; Xu, X.; Luo, X.; Liu, R.; Lin, Y.; Zhao, P.; Shi, J. Preparation of Tanshinone IIA Self-Soluble Microneedles and Its Inhibition on Proliferation of Human Skin Fibroblasts. Chin. Herb. Med. 2023, 15, 251–262. [Google Scholar] [CrossRef] [PubMed]
- Shanmugarajan, T.S.; Selvan, N.K.; Uppuluri, V.N.V.A. Development and Characterization of Squalene-Loaded Topical Agar-Based Emulgel Scaffold: Wound Healing Potential in Full-Thickness Burn Model. Int. J. Low. Extrem. Wounds 2021, 20, 364–373. [Google Scholar] [CrossRef] [PubMed]
- Jamal, M.; Imam, S.S.; Aqil, M.; Amir, M.; Mir, S.R.; Mujeeb, M. Transdermal Potential and Anti-Arthritic Efficacy of Ursolic Acid from Niosomal Gel Systems. Int. Immunopharmacol. 2015, 29, 361–369. [Google Scholar] [CrossRef] [PubMed]
- Fonseca-Santos, B.; Araujo, G.A.; Ferreira, P.S.; Victorelli, F.D.; Pironi, A.M.; Araújo, V.H.S.; Carvalho, S.G.; Chorilli, M. Design and Characterization of Lipid-Surfactant-Based Systems for Enhancing Topical Anti-Inflammatory Activity of Ursolic Acid. Pharmaceutics 2023, 15, 366. [Google Scholar] [CrossRef]
- Krajewska, M.; Dopierała, K.; Prochaska, K. The Biomimetic System of Oleanolic Acid and Oleic Acid at the Air-Water Interface–Interactions in Terms of Nanotechnology-Based Drug Delivery Systems. Membranes 2022, 12, 1215. [Google Scholar] [CrossRef]
- Shi, Z.; Pan, S.; Wang, L.; Li, S. Topical Gel Based Nanoparticles for the Controlled Release of Oleanolic Acid: Design and in Vivo Characterization of a Cubic Liquid Crystalline Anti-Inflammatory Drug. BMC Complement. Med. Ther. 2021, 21, 224. [Google Scholar] [CrossRef]
- Prabahar, K.; Uthumansha, U.; Elsherbiny, N.; Qushawy, M. Enhanced Skin Permeation and Controlled Release of β-Sitosterol Using Cubosomes Encrusted with Dissolving Microneedles for the Management of Alopecia. Pharmaceuticals 2023, 16, 563. [Google Scholar] [CrossRef]
- Afzal, O.; Akhter, M.H.; Ahmad, I.; Muzammil, K.; Dawria, A.; Zeyaullah, M.; Altamimi, A.S.A.; Khalilullah, H.; Mir Najib Ullah, S.N.; Rahman, M.A.; et al. A β–Sitosterol Encapsulated Biocompatible Alginate/Chitosan Polymer Nanocomposite for the Treatment of Breast Cancer. Pharmaceutics 2022, 14, 1711. [Google Scholar] [CrossRef]
- Tosato, M.G.; Orallo, D.E.; Fangio, M.F.; Diz, V.; Dicelio, L.E.; Churio, M.S. Nanomaterials and Natural Products for UV-Photoprotection. In Surface Chemistry of Nanobiomaterials-Applications of Nanobiomaterials; Elsevier: Amsterdam, The Netherlands, 2016; ISBN 9780323428613. [Google Scholar]
- de Souza Guedes, L.; Martinez, R.M.; Bou-Chacra, N.A.; Velasco, M.V.R.; Rosado, C.; Baby, A.R. An Overview on Topical Administration of Carotenoids and Coenzyme Q10 Loaded in Lipid Nanoparticles. Antioxidants 2021, 10, 1034. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Sathasivam, T.; Rawat, P.; Pushpamalar, J. Lycopene-Loaded Nanostructured Lipid Carrier from Carboxymethyl Oil Palm Empty Fruit Bunch Cellulose for Topical Administration. Carbohydr. Polym. Technol. Appl. 2021, 2, 100049. [Google Scholar] [CrossRef]
- Gomes, A.; Costa, A.L.R.; Sobral, P.J.D.A.; Cunha, R.L. Delivering β-Carotene from O/W Emulsion-Based Systems: Influence of Phase Ratio and Carrier Lipid Composition. Food Hydrocoll. Health 2023, 3, 100125. [Google Scholar] [CrossRef]
- Pezeshki, A.; Hamishehkar, H.; Ghanbarzadeh, B.; Fathollahy, I.; Keivani Nahr, F.; Khakbaz Heshmati, M.; Mohammadi, M. Nanostructured Lipid Carriers as a Favorable Delivery System for β-Carotene. Food Biosci. 2019, 27, 11–17. [Google Scholar] [CrossRef]
- Plyduang, T.; Sermkeaw, N. Development and Evaluation of a Hydrogel Containing Momordica Cochinchinensis Spreng Extract for Topical Applications. Braz. J. Pharm. Sci. 2022, 58, 1–14. [Google Scholar] [CrossRef]
- Algan, A.H.; Gungor-Ak, A.; Karatas, A. Nanoscale Delivery Systems of Lutein: An Updated Review from a Pharmaceutical Perspective. Pharmaceutics 2022, 14, 1852. [Google Scholar] [CrossRef]
- Akombaetwa, N.; Ilangala, A.B.; Thom, L.; Memvanga, P.B.; Witika, B.A.; Buya, A.B. Current Advances in Lipid Nanosystems Intended for Topical and Transdermal Drug Delivery Applications. Pharmaceutics 2023, 15, 656. [Google Scholar] [CrossRef]
- Abba, M.; Ibrahim, Z.; Chong, C.S.; Zawawi, N.A.; Kadir, M.R.A.; Yusof, A.H.M.; Razak, S.I.A. Transdermal Delivery of Crocin Using Bacterial Nanocellulose Membrane. Fibers Polym. 2019, 20, 2025–2031. [Google Scholar] [CrossRef]
- Suksaeree, J.; Pichayakorn, W.; Monton, C.; Sakunpak, A.; Chusut, T.; Saingam, W. Rubber Polymers for Transdermal Drug Delivery Systems. Ind. Eng. Chem. Res. 2014, 53, 507–513. [Google Scholar] [CrossRef]
- Syed Azhar, S.N.A.; Ashari, S.E.; Tan, J.K.; Kassim, N.K.; Hassan, M.; Zainuddin, N.; Mohamad, R.; Mat Azmi, I.D. Screening and Selection of Formulation Components of Nanostructured Lipid Carriers System for Mitragyna Speciosa (Korth). Havil Drug Delivery. Ind. Crop. Prod. 2023, 198, 116668. [Google Scholar] [CrossRef]
- Al-Ouqaili, M.T.S.; Saleh, R.O.; Amin, H.I.M.; Jawhar, Z.H.; Akbarizadeh, M.R.; Naderifar, M.; Issa, K.D.; Gavilán, J.C.O.; Nobre, M.A.L.; Jalil, A.T.; et al. Synthesize of Pluronic-Based Nanovesicular Formulation Loaded with Pistacia Atlantica Extract for Improved Antimicrobial Efficiency. Arab. J. Chem. 2023, 16, 104704. [Google Scholar] [CrossRef]
- Bustos-Salgado, P.; Andrade-Carrera, B.; Domínguez-Villegas, V.; Noé, V.; Mallandrich, M.; Colom, H.; Calpena-Campmany, A.; Garduño-Ramírez, M.L. In Vitro Approaches to Explore the Anticancer Potential of One Natural Flavanone and Four Derivatives Loaded in Biopolymeric Nanoparticles for Application in Topical Delivery Treatments. Pharmaceutics 2023, 15, 1632. [Google Scholar] [CrossRef]
- Chavda, V.P.; Nalla, L.V.; Balar, P.; Bezbaruah, R.; Apostolopoulos, V.; Singla, R.K.; Khadela, A.; Vora, L.; Uversky, V.N. Advanced Phytochemical-Based Nanocarrier Systems for the Treatment of Breast Cancer. Cancers 2023, 15, 1023. [Google Scholar] [CrossRef] [PubMed]
- Chellathurai, B.J.; Anburose, R.; Alyami, M.H.; Sellappan, M.; Bayan, M.F.; Chandrasekaran, B.; Chidambaram, K.; Rahamathulla, M. Development of a Polyherbal Topical Gel for the Treatment of Acne. Gels 2023, 9, 163. [Google Scholar] [CrossRef] [PubMed]
- Chuysinuan, P.; Pengsuk, C.; Lirdprapamongkol, K.; Thanyacharoen, T.; Techasakul, S.; Svasti, J.; Nooeaid, P. Turmeric Herb Extract-Incorporated Biopolymer Dressings with Beneficial Antibacterial, Antioxidant and Anti-Inflammatory Properties for Wound Healing. Polymers 2023, 15, 1090. [Google Scholar] [CrossRef]
- Dytrych, P.; Kejík, Z.; Hajduch, J.; Kaplánek, R.; Veselá, K.; Kučnirová, K.; Skaličková, M.; Venhauerová, A.; Hoskovec, D.; Martásek, P.; et al. Therapeutic Potential and Limitations of Curcumin as Antimetastatic Agent. Biomed. Pharmacother. 2023, 163, 114758. [Google Scholar] [CrossRef]
- Platon, I.V.; Ghiorghita, C.A.; Lazar, M.M.; Raschip, I.E.; Dinu, M.V. Chitosan Sponges with Instantaneous Shape Recovery and Multistrain Antibacterial Activity for Controlled Release of Plant-Derived Polyphenols. Int. J. Mol. Sci. 2023, 24, 4452. [Google Scholar] [CrossRef]
- Sharma, A.; Dheer, D.; Singh, I.; Puri, V.; Kumar, P. Phytoconstituent-Loaded Nanofibrous Meshes as Wound Dressings: A Concise Review. Pharmaceutics 2023, 15, 1058. [Google Scholar] [CrossRef]
- d’Angelo, I.; Provenzano, R.; Florio, E.; Lombardi, A.; Trama, U.; Ungaro, F.; Quaglia, F.; Miro, A. Transmucosal Delivery of the Medical Cannabis Oil via a Nanoemulsion Formulation. J. Drug Deliv. Sci. Technol. 2023, 79, 104004. [Google Scholar] [CrossRef]
- Diniz, L.R.L.; Calado, L.L.; Duarte, A.B.S.; de Sousa, D.P. Centella Asiatica and Its Metabolite Asiatic Acid: Wound Healing Effects and Therapeutic Potential. Metabolites 2023, 13, 276. [Google Scholar] [CrossRef]
- Feng, Y.; Li, B.; Hu, J.; Liu, W.; Tian, X.; Chen, Y.; Lu, S.; Liu, Y. Sustainable Use of Chrysanthemum Indicum Var. Aromaticum as Value-Added Green Materials in Microemulsion Hydrogels. ACS Sustain. Chem. Eng. 2023, 11, 3820–3831. [Google Scholar] [CrossRef]
- Hassan, A.S.; Hofni, A.; Abourehab, M.A.; Abdel-Rahman, I.A. Ginger Extract–Loaded Transethosomes for Effective Transdermal Permeation and Anti-Inflammation in Rat Model. Int. J. Nanomed. 2023, 18, 1259–1280. [Google Scholar] [CrossRef] [PubMed]
- Hayat, M.; Nawaz, A.; Chinnam, S.; Muzammal, M.; Latif, M.S.; Yasin, M.; Ashique, S.; Zengin, G.; Farid, A. Formulation Development and Optimization of Herbo Synthetic Gel: In Vitro Biological Evaluation and in Vivo Wound Healing Studies. Process Biochem. 2023, 130, 116–126. [Google Scholar] [CrossRef]
- Juszczak, A.M.; Jakimiuk, K.; Czarnomysy, R.; Strawa, J.W.; Zovko Končić, M.; Bielawski, K.; Tomczyk, M. Wound Healing Properties of Jasione Montana Extracts and Their Main Secondary Metabolites. Front. Pharmacol. 2022, 13, 894233. [Google Scholar] [CrossRef] [PubMed]
- Lam, P.L.; Gambari, R.; Ho, Y.W.; Wong, W.Y.; Hau, D.K.P.; Leung, T.W.T.; Leung, P.H.M.; Chui, C.H. Anti-Methicillin Resistance Staphylococcus Aureus and in Vitro Toxicology Evaluation of Corilagin-Loaded Gelatin/Agar Microspheres with Potential Biotextile Applications. Int. J. Biol. Macromol. 2023, 237, 123982. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Xiao, T.; Guo, S.; Wu, Y.; Lai, R.; Liu, Z.; Luo, W.; Xu, Y. Oxymatrine-Fatty Acid Deep Eutectic Solvents as Novel Penetration Enhancers for Transdermal Drug Delivery: Formation Mechanism and Enhancing Effect. Int. J. Pharm. 2023, 637, 122880. [Google Scholar] [CrossRef]
- Ferreira, L.M.d.M.C.; Bandeira, E.d.S.; Gomes, M.F.; Lynch, D.G.; Bastos, G.N.T.; Silva-Júnior, J.O.C.; Ribeiro-Costa, R.M. Polyacrylamide Hydrogel Containing Calendula Extract as a Wound Healing Bandage: In Vivo Test. Int. J. Mol. Sci. 2023, 24, 3806. [Google Scholar] [CrossRef]
- Neimkhum, W.; Anuchapreeda, S.; Lin, W.C.; Lue, S.C.; Lee, K.H.; Chaiyana, W. Enhancement of Stability and Dermal Delivery of Carissa Carandas Linn. Leaf Extract by Liquid Crystals. J. Drug Deliv. Sci. Technol. 2023, 82, 104258. [Google Scholar] [CrossRef]
- Perużyńska, M.; Nowak, A.; Birger, R.; Ossowicz-Rupniewska, P.; Konopacki, M.; Rakoczy, R.; Kucharski, Ł.; Wenelska, K.; Klimowicz, A.; Droździk, M.; et al. Anticancer Properties of Bacterial Cellulose Membrane Containing Ethanolic Extract of Epilobium Angustifolium L. Front. Bioeng. Biotechnol. 2023, 11, 1133345. [Google Scholar] [CrossRef]
- Pulat, S.; Subedi, L.; Pandey, P.; Bhosle, S.R.; Hur, J.S.; Shim, J.H.; Cho, S.S.; Kim, K.T.; Ha, H.H.; Kim, H.; et al. Topical Delivery of Atraric Acid Derived from Stereocaulon Japonicum with Enhanced Skin Permeation and Hair Regrowth Activity for Androgenic Alopecia. Pharmaceutics 2023, 15, 340. [Google Scholar] [CrossRef]
- Sotirova, Y.; Gugleva, V.; Stoeva, S.; Kolev, I.; Nikolova, R.; Marudova, M.; Nikolova, K.; Kiselova-Kaneva, Y.; Hristova, M.; Andonova, V. Bigel Formulations of Nanoencapsulated St. John’s Wort Extract—An Approach for Enhanced Wound Healing. Gels 2023, 9, 360. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, S.; Duraipandian, C.; Alsayari, A.; Ramachawolran, G.; Wong, L.S.; Sekar, M.; Gan, S.H.; Subramaniyan, V.; Seethalakshmi, S.; Jeyabalan, S.; et al. Wound Healing Properties of a New Formulated Flavonoid-Rich Fraction from Dodonaea Viscosa Jacq. Leaves Extract. Front. Pharmacol. 2023, 14, 1096905. [Google Scholar] [CrossRef] [PubMed]
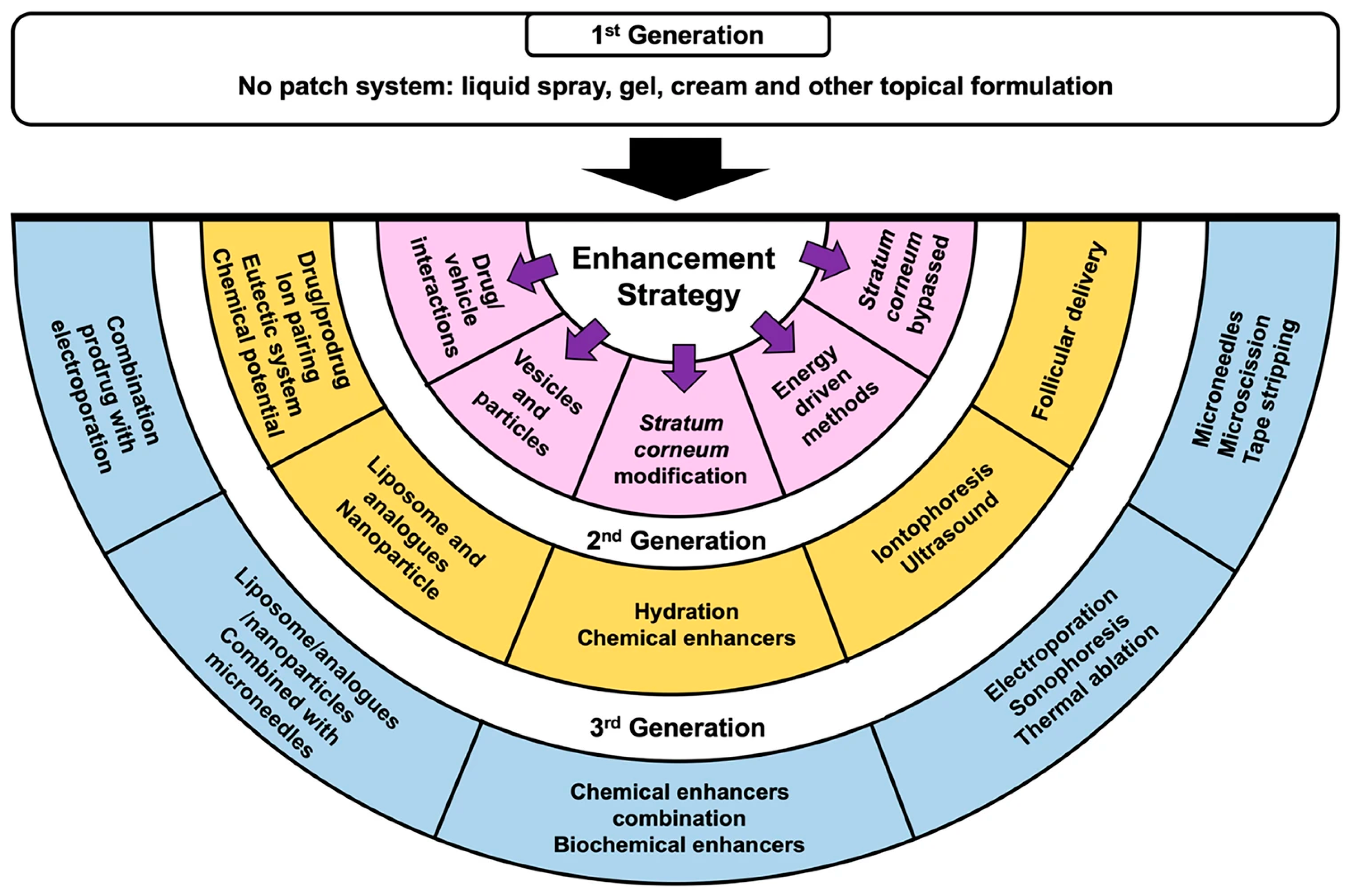
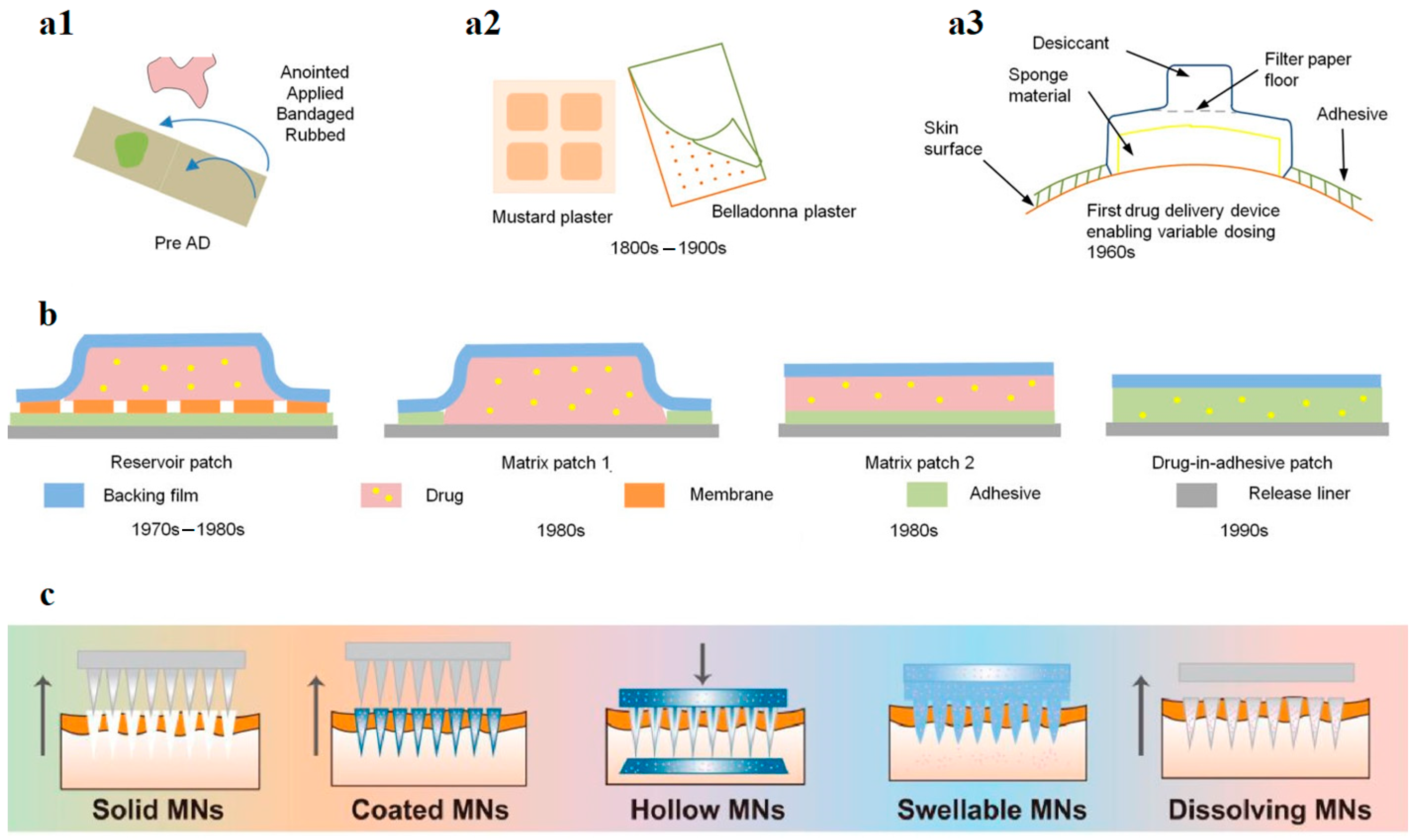
| Mucilage Plant Source | Other Constituents | Active Ingredient/Drug | Applicability and Properties | Ref. |
|---|---|---|---|---|
| Ficus reticuleta fruit | Glycerol Propylene Glycol Span-80 Propyl/Methyl paraben | Diltiazem HCl | Therapy of hypertension, angina pectoris, arrhythmia Controlled release matrix | [40] |
| Ficus carica fruit | Propylene glycol Span-80 Propyl/Methyl paraben | Pioglitazone HCl | Type II diabetes therapy Matrix-moderated transdermal patch Retardation of release | [41] |
| Povidone Propylene glycol Span-80 Propyl/Methyl paraben | Tramadol HCl | Patch for therapy of pain, inflammation, and arthritis Sustained drug release | [42] | |
| Artocarpus heterophyllus fruit | Gelatin Glycerol Dimethyl sulphoxide | Naproxen sodium | Therapy for rheumatoid arthritis, osteoarthritis, and ankylosis spondylitis Sustained drug release | [43] |
| Ficus benghalensis fruit | Eudragit L Propylene glycol Span-60 | Aceclofenac | Treatment of pain and inflammation Controlled discharge matrix transdermal patches | [44] |
| Ficus auriculata fruit | Hydroxypropyl Methyl Cellulose K4M Polyethylene glycol-400 Tween-80 | Diclofenac Potassium | Treatment of arthritis symptoms Matrix-moderated transdermal patch Controlled release diffusion | [45] |
| Ocimum basilicum seeds | Polyvinyl alcohol Glutaraldehyde Glycerol | Tetracycline Hydrochloride | Wound dressing in chronic wound management pH-sensitive cross-linked hydrogel Drug release control | [46] |
| Borax Glycerol | Zinc oxide nanoparticles | Wound dressing Crosslinked antimicrobial sponge | [47] | |
| L-cysteine-modified alginate Dopamine-modified hyaluronic acid Thiolated alginate Polydopamine | Nystatin | Oral fungal infection treatment Bi- and tri-layer mucoadhesive sublingual films Controlled release | [48] | |
| Cydonia oblonga seeds | Chitosan Poly-caprolactone Polyethylene glycol | Skin-tissue-engineered hybrid scaffolds Dressing for continuous absorption of wound exudates Smart/stimuli-responsive bio-matrix | [49] | |
| Bacterial cellulose | Composite scaffold for wound dressings | [50] | ||
| Leaves of Cocculus hirsutus | Polyvinyl alcohol | Dermal wound patches Biodegradable biopolymer | [51] | |
| Salvia hispanica seeds | Dermatological and cosmetic formulations Biopolymer with mucoadhesive properties Controlled release of bioactive compounds and drugs | [52] | ||
| Lallemantia royleana seeds | Chitosan Chitin | Ag nanoparticles Ciprofloxacin | Wound dressings Controlled drug release | [53] |
| Althea rosea plant | Functional and nutraceutical in inflammatory disorders | [54] | ||
| Aloe vera leaves (fresh or powdered) | Sodium alginate Glycerol | Improved film thermal stability and transparency Good UV light barrier Suitable for wound healing and drug delivery | [55] | |
| Hydroxypropyl methylcellulose PEG 400 | Captopril | Improved drug transdermal penetration | [56] | |
| Xanthan gum | Allantoin Natural BHA salicylic acid | Anti-inflammatory, wound epithelization, and skin regeneration hydrogel | [57] |
| SM Plant Source | Main SM | TDS | Applicability and Properties | Ref. |
|---|---|---|---|---|
| Mitragyna Speciosa leaves’ extract | Alkaloids Flavonoids Saponins | Lipidic carriers in oleic acid, Tween 80 | For topical application/wound dressing | [266] |
| Pistacia atlantica fruit extract | β–sitosterol | Lipidic carriers: span 40, pluronic F127, Cholesterol and Glycerol | Antimicrobial in topical application | [267] |
| Eysenhardtia platycarpa | Flavone | Biopolymeric nanoparticles | Topical application for cancer treatment | [268] |
| Lippia origanoides, Turnera diffusa | Eugenol Carvacrol Limonene | Carbopol hydrogel | Caffeine’s skin permeation for inflammatory diseases | [227] |
| Catharanthus roseus | Vinblastine Vincristine (alkaloids) | Lipid nanocarrier | Topical treatment of breast cancer | [269] |
| Mucuna prurita | L-Dopa | |||
| Rauvolfia serpentine | Reserpine | |||
| Taxus brevifolia | Paclitaxel | |||
| Vigna radiata | Flavonoids Phenolic acids | Aloe vera hydrogel | Topical treatment of acne vulgaris | [270] |
| Curcuma longa | Curcumin | Loaded in biopolymers (CMC-silk sericin) | Antibacterial, antioxidant, and anti-inflammatory for wound healing | [271] |
| Nanoparticles | Different types of cancer | [272] | ||
| Chitosan sponge—cryogel | Wound healing | [273] | ||
| Nanofibrous meshes | Wound healing | [274] | ||
| Cannabis sativa | Cannabinoids Terpenoids Sterols Flavonoids | Nano emulsions (Tween 80, PEG 400) | Transmucosal delivery | [275] |
| Centella asiatica | Flavonoids Plant sterols Eugenol Pentacyclic triterpenoids | Hydroalcoholic extracts | For topical application/WD | [276] |
| Nanofibrous meshes | [274] | |||
| Chrysanthemum indicum var. aromaticum | Terpenoids Flavonoids Phenols | Gel formulated with Tween-80, transcutol-P, sodium hyaluronate, and glycerol | Topical application for inflammatory skin disease, cosmetic use | [277] |
| Zingiber officinale | Sesquiterpene Monoterpene hydrocarbons | Nanocarriers | Major inflammatory disease | [278] |
| Sideroxylon mascatense | Berberine Palmatine Jatrorrhizine Saponins | Nanoemulsion gel | Wound healing | [279] |
| Jasione montana | Luteolin Luteolin 7-O-glucoside Luteolin 7-O-sambubioside | Direct application (scratch cells) | Wound healing | [280] |
| Caesalpinia coriaria | Corilagin (ellagitannin) | Gelatin/agar microsphere | Wound healing | [281] |
| Genus Sophora | Oxymatrine natural alkaloid | Deep eutectic solvent for TD Capric acid, Lauric acid, Myristic acid, Palmitic acid, and Stearic acid | Anti-pruritic and anti-inflammatory effects | [282] |
| Calendula officinalis | Carotenoids Lycopene Phenolic acids Hydroxycinnamic acids Flavonoids (rutin) Coumarins (esculetin) | Polyacrylamide hydrogel containing calendula extract | Therapeutic applications, which include tissue re-epithelialization and general wound healing action | [283] |
| Carissa carandas leaf extract | Ursolic acid Phenolic and flavonoid compounds | Liquid crystals composed of 2.5% w/w coconut oil, 30% w/w Tween® 85 | Antioxidant, anti-inflammatory, and antiaging effects, decrease inflammatory cytokine and matrix metalloproteinase (MMP) production, as well as enhance the level of ceramide and collagen in the skin | [284] |
| Epilobium angustifolium | Polyphenols (flavonoids, phenolic acids, and tannins) | Biocellulose | Anticancer properties | [285] |
| Stereocaulon japonicum | Atraric acid Phenolic compound | Volatile and nonvolatile solvents, as well as oleic acid, an effective permeation enhancer | Androgenic alopecia | [286] |
| Azadirachta indica | Alkaloids | Nanofibrous meshes | Wound healing | [274] |
| Tecomella undulata | Alkaloids | Nanofibrous meshes | Wound healing | |
| Hypericum perforatum, | Polyprenylated phloroglucinol hyperforin | Bigel nanocarriers | Wound healing | [287] |
| Dodonaea viscosa, leaf extract | Flavonoids (quercetin and kaempferol) | Direct application (scratch cells) | Wound healing | [288] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Isopencu, G.O.; Covaliu-Mierlă, C.-I.; Deleanu, I.-M. From Plants to Wound Dressing and Transdermal Delivery of Bioactive Compounds. Plants 2023, 12, 2661. https://doi.org/10.3390/plants12142661
Isopencu GO, Covaliu-Mierlă C-I, Deleanu I-M. From Plants to Wound Dressing and Transdermal Delivery of Bioactive Compounds. Plants. 2023; 12(14):2661. https://doi.org/10.3390/plants12142661
Chicago/Turabian StyleIsopencu, Gabriela Olimpia, Cristina-Ileana Covaliu-Mierlă, and Iuliana-Mihaela Deleanu. 2023. "From Plants to Wound Dressing and Transdermal Delivery of Bioactive Compounds" Plants 12, no. 14: 2661. https://doi.org/10.3390/plants12142661
APA StyleIsopencu, G. O., Covaliu-Mierlă, C.-I., & Deleanu, I.-M. (2023). From Plants to Wound Dressing and Transdermal Delivery of Bioactive Compounds. Plants, 12(14), 2661. https://doi.org/10.3390/plants12142661