Response of Medical Cannabis to Magnesium (Mg) Supply at the Vegetative Growth Phase
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Growing Conditions
2.2. Inorganic Mineral Analysis
2.3. Determination of the Osmotic Potential, Relative Water Content, and Membrane Leakage
2.4. Photosynthetic Pigments and Gas-Exchange Parameters
2.5. Plant Architecture and Development
2.6. Statistical Analyses
3. Results
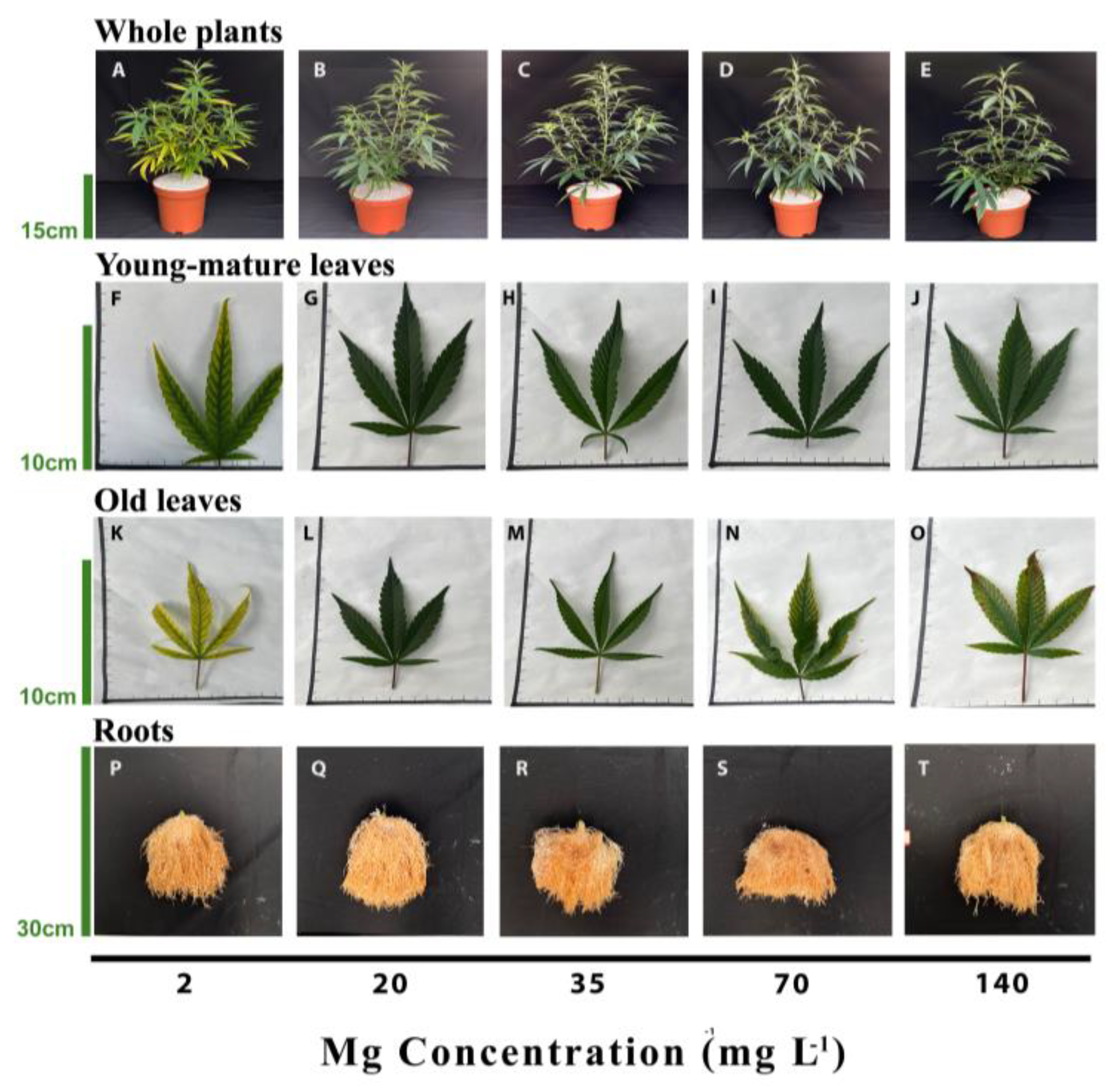
3.1. Plant Growth and Development and Visual Appearance
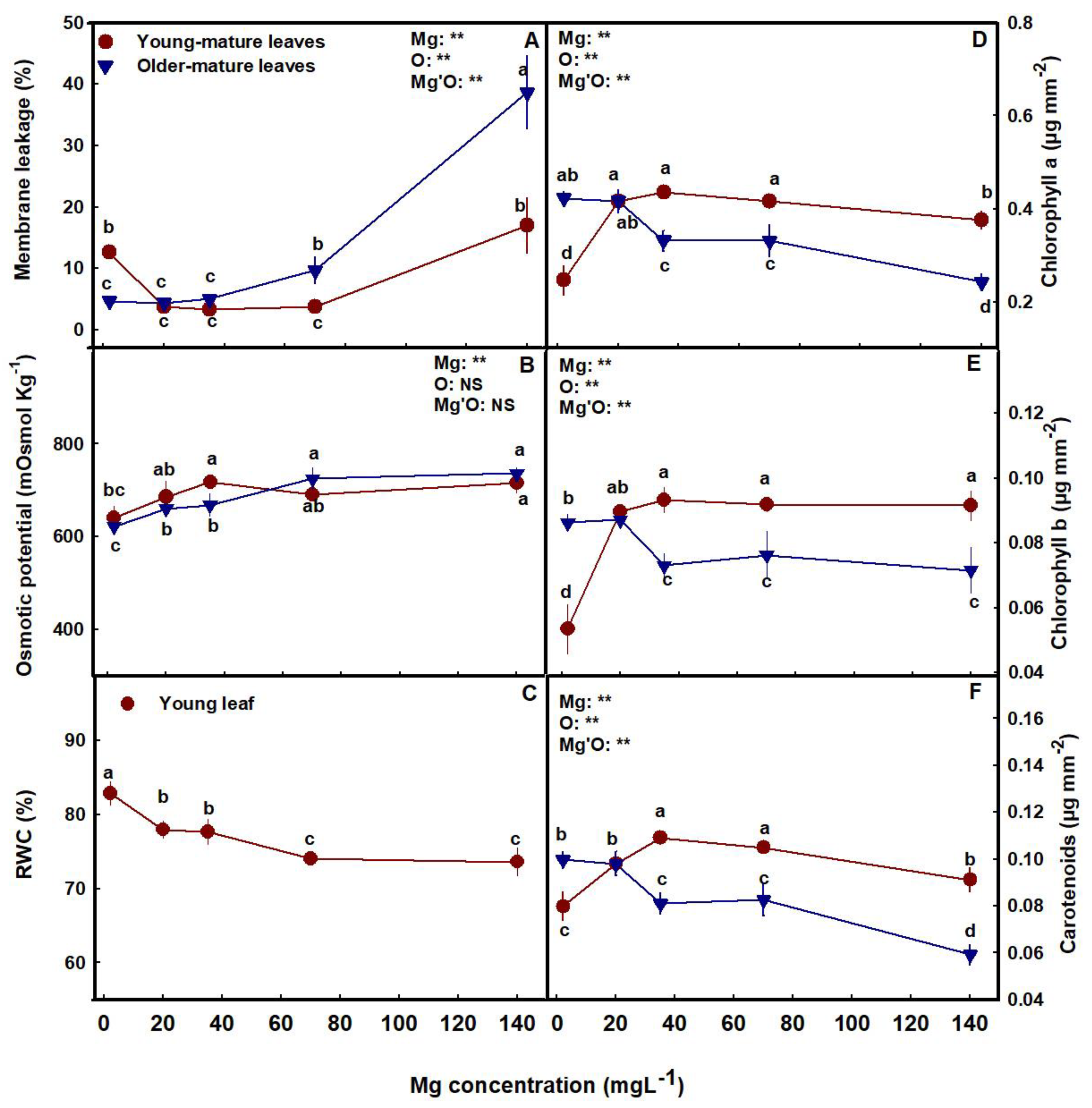
3.2. Physiological Parameters
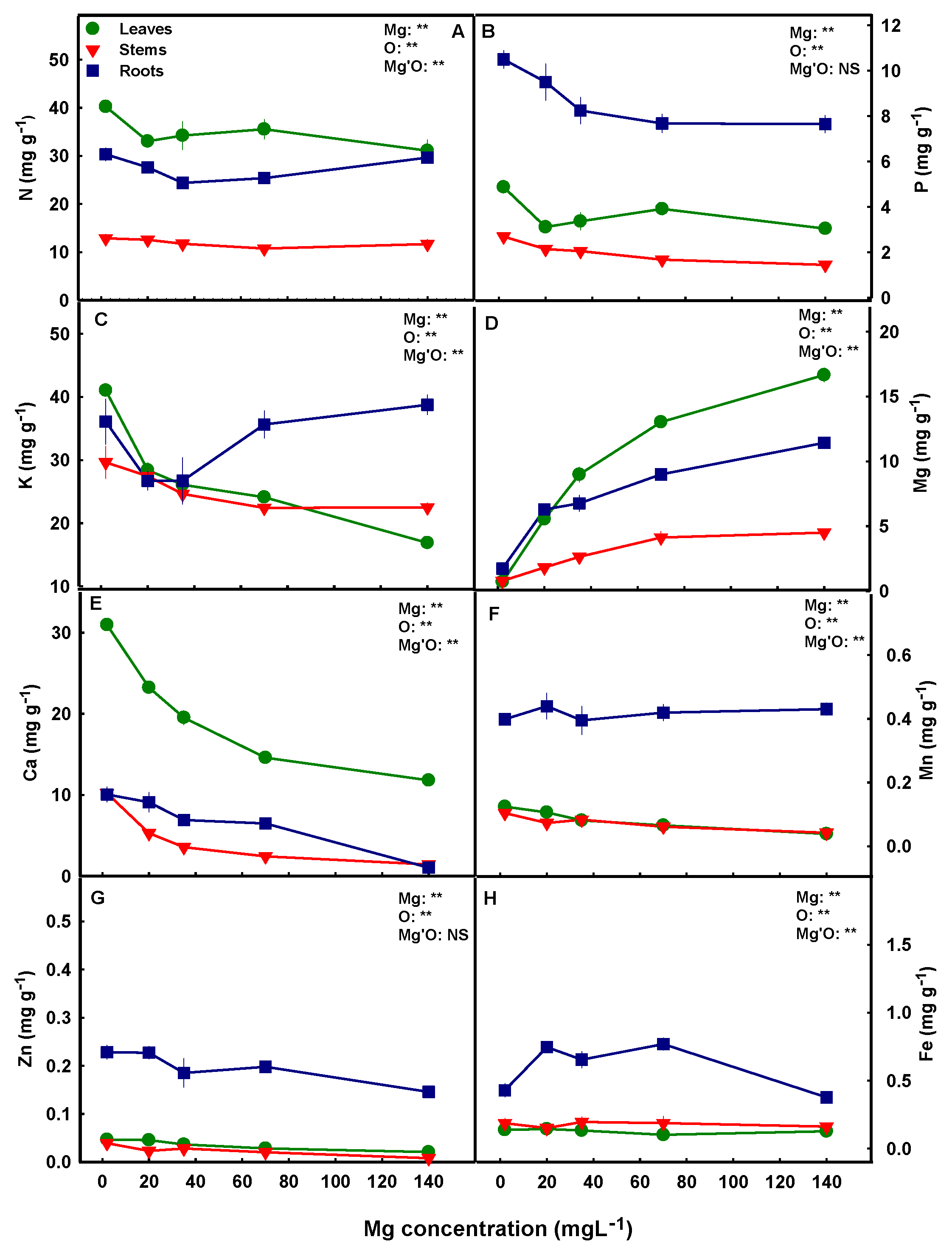
3.3. Macro- and Micronutrient Concentrations

4. Discussion
4.1. Plant Visual Appearance, Development, and Function
4.2. Interrelations between Mg Supply and the Cannabis Plant Ionome
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Russo, E.B. History of cannabis and its preparations in saga, science, and sobriquet. Chem. Biodivers. 2007, 4, 1614–1648. [Google Scholar] [CrossRef] [PubMed]
- Mishchenko, S.; Mokher, J.; Laiko, I.; Burbulis, N.; Kyrychenko, H.; Dudukova, S. Phenological growth stages of hemp (Cannabis sativa L.): Codification and description according to the BBCH scale. Žemės ūkio Moksl. 2017, 24, 31–36. [Google Scholar] [CrossRef]
- Zuardi, A.W. History of cannabis as a medicine: A review. Rev. Bras. Psiquiatr. 2006, 28, 153–157. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, N.; Gorelick, J.; Koch, S. Interplay between chemistry and morphology in medical cannabis (Cannabis sativa L.). Ind. Crops Prod. 2019, 129, 185–194. [Google Scholar] [CrossRef]
- Chandra, S.; Lata, H.; ElSohly, M.A. Cannabis sativa L.—Botany and Biotechnology; Chandra, S., Lata, H., ElSohly, M.A., Eds.; Springer International Publishing: Cham, Switzerland, 2017; ISBN 978-3-319-54563-9. [Google Scholar]
- Caplan, D.; Stemeroff, J.; Dixon, M.; Zheng, Y. Vegetative propagation of cannabis by stem cuttings: Eeffects of leaf number, cutting position, rooting hormone, and leaf tip removal. Can. J. Plant Sci. 2018, 98, 1126–1132. [Google Scholar] [CrossRef]
- Shiponi, S.; Bernstein, N. The highs and lows of P supply in medical cannabis: Effects on cannabinoids, the ionome, and morpho-physiology. Front. Plant Sci. 2021, 12, 657323. [Google Scholar] [CrossRef]
- Shiponi, S.; Bernstein, N. Response of medical cannabis (Cannabis sativa L.) genotypes to P supply under long photoperiod: Functional phenotyping and the ionome. Ind. Crops Prod. 2021, 161, 113154. [Google Scholar] [CrossRef]
- Kirkby, E. Introduction, definition and classification of nutrients. In Marschner’s Mineral Nutrition of Higher Plants; Elsevier: Amsterdam, The Netherlands, 2012; pp. 3–5. ISBN 9780123849052. [Google Scholar]
- Hawkesford, M.; Horst, W.; Kichey, T.; Lambers, H.; Schjoerring, J.; Møller, I.S.; White, P. Functions of macronutrients. In Marschner’s Mineral Nutrition of Higher Plants; Elsevier: Amsterdam, The Netherlands, 2012; pp. 135–189. [Google Scholar]
- Aibara, I.; Miwa, K. Strategies for optimization of mineral nutrient transport in plants: Multilevel regulation of nutrient-dependent dynamics of root architecture and transporter activity. Plant Cell Physiol. 2014, 55, 2027–2036. [Google Scholar] [CrossRef]
- Fageria, N.K.; Baligar, V.C.; Li, Y.C. The role of nutrient efficient plants in improving crop yields in the twenty first century. J. Plant Nutr. 2008, 31, 1121–1157. [Google Scholar] [CrossRef]
- Saloner, A.; Sacks, M.M.; Bernstein, N. Response of medical cannabis (Cannabis sativa L.) genotypes to K supply under long photoperiod. Front. Plant Sci. 2019, 10, 1369. [Google Scholar] [CrossRef]
- Saloner, A.; Bernstein, N. Response of medical cannabis (Cannabis sativa L.) to nitrogen supply under long photoperiod. Front. Plant Sci. 2020, 11, 572293. [Google Scholar] [CrossRef]
- Saloner, A.; Bernstein, N. Effect of potassium (K) supply on cannabinoids, terpenoids and plant function in medical cannabis. Agronomy 2022, 12, 1242. [Google Scholar] [CrossRef]
- Saloner, A.; Bernstein, N. Nitrogen supply affects cannabinoid and terpenoid profile in medical cannabis (Cannabis sativa L.). Ind. Crops Prod. 2021, 167, 113516. [Google Scholar] [CrossRef]
- Bernstein, N.; Gorelick, J.; Zerahia, R.; Koch, S. Impact of N, P, K, and humic acid supplementation on the chemical profile of medical cannabis (Cannabis sativa L.). Front. Plant Sci. 2019, 10, 736. [Google Scholar] [CrossRef] [PubMed]
- Bevan, L.; Jones, M.; Zheng, Y. Optimisation of nitrogen, phosphorus, and potassium for soilless production of Cannabis sativa in the flowering stage using response surface analysis. Front. Plant Sci. 2021, 12, 2587. [Google Scholar] [CrossRef]
- Chen, Z.C.; Peng, W.T.; Li, J.; Liao, H. Functional dissection and transport mechanism of magnesium in plants. Semin. Cell Dev. Biol. 2018, 74, 142–152. [Google Scholar] [CrossRef]
- Li, L. A Novel family of magnesium transport genes in arabidopsis. Plant Cell Online 2001, 13, 2761–2775. [Google Scholar] [CrossRef]
- Lorimer, G.H.; Badger, M.R.; Andrews, T.J. The activation of ribulose-1,5-bisphosphate carboxylase by carbon dioxide and magnesium ions. Equilibria, kinetics, a suggested mechanism, and physiological implications. Biochemistry 1976, 15, 529–536. [Google Scholar] [CrossRef]
- Verbruggen, N.; Hermans, C. Physiological and molecular responses to magnesium nutritional imbalance in plants. Plant Soil 2013, 368, 87–99. [Google Scholar] [CrossRef]
- Chen, C.-T.; Lee, C.-L.; Yeh, D.-M. Effects of nitrogen, phosphorus, potassium, calcium, or magnesium deficiency on growth and photosynthesis of eustoma. HortScience 2018, 53, 795–798. [Google Scholar] [CrossRef]
- Shaul, O. Magnesium transport and function in plants: The tip of the iceberg. BioMetals 2002, 15, 309–323. [Google Scholar] [CrossRef] [PubMed]
- Cowan, J.A. Structural and catalytic chemistry of magnesium-dependent enzymes. BioMetals 2002, 15, 225–235. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Nazim, H.; Liang, Z.; Yang, D. Magnesium deficiency in plants: An urgent problem. Crop J. 2016, 4, 83–91. [Google Scholar] [CrossRef]
- Tränkner, M.; Jákli, B.; Tavakol, E.; Geilfus, C.-M.; Cakmak, I.; Dittert, K.; Senbayram, M. Magnesium deficiency decreases biomass water-use efficiency and increases leaf water-use efficiency and oxidative stress in barley plants. Plant Soil 2016, 406, 409–423. [Google Scholar] [CrossRef]
- Hermans, C.; Vuylsteke, M.; Coppens, F.; Cristescu, S.M.; Harren, F.J.M.; Inzé, D.; Verbruggen, N. Systems analysis of the responses to long-term magnesium deficiency and restoration in Arabidopsis thaliana. New Phytol. 2010, 187, 132–144. [Google Scholar] [CrossRef]
- Hermans, C.; Verbruggen, N. Physiological characterization of Mg deficiency in Arabidopsis thaliana. J. Exp. Bot. 2005, 56, 2153–2161. [Google Scholar] [CrossRef]
- Fageria, N.K. Ionic interactions in rice plants from dilute solutions. Plant Soil 1983, 70, 309–316. [Google Scholar] [CrossRef]
- Omar, M.A.; Kobbia, T. EL Some observations on the interrelationships of potassium and magnesium. Soil Sci. 1966, 101, 437–440. [Google Scholar] [CrossRef]
- Fageria, V.D. Nutrient interactions in crop plants. J. Plant Nutr. 2001, 24, 1269–1290. [Google Scholar] [CrossRef]
- Bernstein, N.; Ioffe, M.; Bruner, M.; Nishri, Y.; Luria, G.; Dori, I.; Matan, E.; Philosoph-Hadas, S.; Umiel, N.; Hagiladi, A. Effects of Supplied Nitrogen Form and Quantity on Growth and Postharvest Quality of Ranunculus asiaticus Flowers. Hortscience 2005, 40, 1879–1886. [Google Scholar] [CrossRef]
- Bernstein, N.; Shoresh, M.; Xu, Y.; Huang, B. Free radical biology & medicine involvement of the plant antioxidative response in the differential growth sensitivity to salinity of leaves vs roots during cell development. Free Radic. Biol. Med. 2010, 49, 1161–1171. [Google Scholar] [CrossRef] [PubMed]
- Lichtenthaler, H.K.; Wellburn, A.R. Determinations of total carotenoids and chlorophylls a and b of leaf extracts in different solvents. Biochem. Soc. Trans. 1983, 11, 591–592. [Google Scholar] [CrossRef]
- Bhatla, S.C.; Lal, M.A. Plant Physiology, Development and Metabhatla; Springer: Berlin/Heidelberg, Germany, 2018; ISBN 9789811320224. [Google Scholar]
- Senbayram, M.; Gransee, A.; Wahle, V.; Thiel, H. Role of magnesium rertilisers in agriculture: Plant-soil continuum. Crop Pasture Sci. 2015, 66, 1219–1229. [Google Scholar] [CrossRef]
- Xie, K.; Cakmak, I.; Wang, S.; Zhang, F.; Guo, S. Synergistic and antagonistic interactions between potassium and magnesium in higher plants. Crop J. 2021, 9, 249–256. [Google Scholar] [CrossRef]
- Guo, W.; Chen, S.; Hussain, N.; Cong, Y.; Liang, Z.; Chen, K. Magnesium stress signaling in plant: Just a beginning. Plant Signal. Behav. 2015, 10, e992287. [Google Scholar] [CrossRef]
- Saloner, A.; Bernstein, N. Nitrogen source matters: High NH4/NO3 ratio reduces cannabinoids, terpenoids, and yield in medical cannabis. Front. Plant Sci. 2022, 13, 830224. [Google Scholar] [CrossRef] [PubMed]
- Song, C.; Saloner, A.; Fait, A.; Bernstein, N. Nitrogen deficiency stimulates cannabinoid biosynthesis in medical cannabis plants by inducing a metabolic shift towards production of low-N metabolites. Ind. Crop. Prod. 2023, 202, 116969. [Google Scholar] [CrossRef]
- Westmoreland, F.M.; Bugbee, B. Sustainable Cannabis Nutrition: Elevated root-zone phosphorus significantly increases leachate P and does not improve yield or quality. Front. Plant Sci. 2022, 13. [Google Scholar] [CrossRef]
- Wong, M. Visual symptoms of plant nutrient deficiencies in nursery and landscape plants. Soil Crop Manag. 2005, 10, 1–4. [Google Scholar]
- McCauley, A.; Jones, C.; Jacobsen, J. Plant nutrient functions and deficiency and toxicity symptoms. Nutr. Manag. Modul. 2011, 9, 1–16. [Google Scholar]
- Wang, Z.; Hassan, M.U.; Nadeem, F.; Wu, L. Magnesium fertilization improves crop yield in most production systems: A meta-analysis. Front. Plant Sci. 2020, 10, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Ceylan, Y.; Kutman, U.B.; Mengutay, M.; Cakmak, I. Magnesium applications to growth medium and foliage affect the starch distribution, increase the grain size and improve the seed germination in wheat. Plant Soil 2016, 406, 145–156. [Google Scholar] [CrossRef]
- Tian, X.-Y.; He, D.-D.; Bai, S.; Zeng, W.-Z.; Wang, Z.; Wang, M.; Wu, L.-Q.; Chen, Z.-C. Physiological and molecular advances in magnesium nutrition of plants. Plant Soil 2021, 468, 1–17. [Google Scholar] [CrossRef]
- Cakmak, I.; Yazici, A.M. Magnesium: A forgotten element in crop production. Better Crop. 2010, 94, 23–25. [Google Scholar]
- Gerendás, J.; Führs, H. The significance of magnesium for crop quality. Plant Soil 2013, 368, 101–128. [Google Scholar] [CrossRef]
- Hauer-Jákli, M.; Tränkner, M. Critical leaf magnesium thresholds and the impact of magnesium on plant growth and photo-oxidative defense: A systematic review and meta-analysis from 70 years of research. Front. Plant Sci. 2019, 10, 766. [Google Scholar] [CrossRef]
- Zhou, M.; Gong, X.; Ying, W.; Chao, L.; Hong, M.; Wang, L.; Fashui, H. Cerium relieves the inhibition of chlorophyll biosynthesis of maize caused by magnesium deficiency. Biol. Trace Elem. Res. 2011, 143, 468–477. [Google Scholar] [CrossRef]
- Laing, W.; Greer, D.; Sun, O.; Beets, P.; Lowe, A.; Payn, T. Physiological impacts of Mg deficiency in Pinus radiata: Growth and photosynthesis. New Phytol. 2000, 146, 47–57. [Google Scholar] [CrossRef]
- Tewari, R.K.; Kumar, P.; Sharma, P.N. Magnesium deficiency induced oxidative stress and antioxidant responses in mulberry plants. Sci. Hortic. 2006, 108, 7–14. [Google Scholar] [CrossRef]
- Da Silva, D.; Brandão, I.R.; Alves, J.D.; de Santos, M.O.; de Souza, K.R.D.; de Silveira, H.R.O. Physiological and biochemical impacts of magnesium-deficiency in two cultivars of coffee. Plant Soil 2014, 382, 133–150. [Google Scholar] [CrossRef]
- Puthiyaveetil, S.; Van Oort, B.; Kirchhoff, H. Surface charge dynamics in photosynthetic membranes and the structural consequences. Nat. Plants 2017, 3, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Masuda, T. Recent overview of the Mg branch of the tetrapyrrole biosynthesis leading to chlorophylls. Photosynth. Res. 2008, 96, 121–143. [Google Scholar] [CrossRef] [PubMed]
- Portis, A.R. Rubisco Activase—Rubisco’s catalytic chaperone. Photosynth. Res. 2003, 75, 11–27. [Google Scholar] [CrossRef]
- Yin, S.; Ze, Y.; Liu, C.; Li, N.; Zhou, M.; Duan, Y.; Hong, F. Cerium relieves the inhibition of nitrogen metabolism of spinach caused by magnesium deficiency. Biol. Trace Elem. Res. 2009, 132, 247–258. [Google Scholar] [CrossRef]
- Sun, O.J.; Gielen, G.J.H.P.; Sands, R.; Smith, C.T.; Thorn, A.J. Growth, Mg nutrition and photosynthetic activity in Pinus radiata: Evidence that NaCl addition counteracts the impact of low Mg supply. Trees 2001, 15, 335–340. [Google Scholar] [CrossRef]
- Tang, N.; Li, Y.; Chen, L. Magnesium deficiency–induced impairment of photosynthesis in leaves of fruiting Citrus reticulata trees accompanied by up-regulation of antioxidant metabolism to avoid photo-oxidative damage. J. Plant Nutr. Soil Sci. 2012, 175, 784–793. [Google Scholar] [CrossRef]
- Jezek, M.; Geilfus, C.M.; Bayer, A.; Muhling, K.H. Photosynthetic capacity, nutrient status, and growth of maize (Zea mays L.) upon MgSO4 leaf-application. Front. Plant Sci. 2015, 5, 781. [Google Scholar] [CrossRef]
- Pego, J.V.; Kortstee, A.J.; Huijser, C.; Smeekens, S.C.M. Photosynthesis, sugars and the regulation of gene expression. J. Exp. Bot. 2000, 51, 407–416. [Google Scholar] [CrossRef]
- Hermans, C.; Bourgis, F.; Faucher, M.; Strasser, R.J.; Delrot, S.; Verbruggen, N. Magnesium deficiency in sugar beets alters sugar partitioning and phloem loading in young mature leaves. Planta 2005, 220, 541–549. [Google Scholar] [CrossRef]
- Cakmak, I.; Hengeler, C.; Marschner, H. Partitioning of shoot and root dry natter and carbohydrates in bean plants suffering from phosphorus, potassium and magnesium deficiency. J. Exp. Bot. 1994, 45, 1245–1250. [Google Scholar] [CrossRef]
- Ayre, B.G. Membrane-transport systems for sucrose in relation to whole-plant carbon partitioning. Mol. Plant 2011, 4, 377–394. [Google Scholar] [CrossRef] [PubMed]
- Ze, Y.; Yin, S.; Ji, Z.; Luo, L.; Liu, C.; Hong, F. Influences of magnesium deficiency and cerium on antioxidant system of spinach chloroplasts. BioMetals 2009, 22, 941–949. [Google Scholar] [CrossRef] [PubMed]
- Tanoi, K.; Kobayashi, N. Leaf senescence by magnesium deficiency. Plants 2015, 4, 756–772. [Google Scholar] [CrossRef] [PubMed]
- Bergmann, W. Ernaehrungsstoerungen Bei Kulturpflanzen; Entstehung und Diagnose; The Food and Agriculture Organization (FAO): Rome, Italy, 1988; pp. 166–178. [Google Scholar]
- Kobayashi, N.I.; Saito, T.; Iwata, N.; Ohmae, Y.; Iwata, R.; Tanoi, K.; Nakanishi, T.M. Leaf senescence in rice due to magnesium deficiency mediated defect in transpiration rate before sugar accumulation and chlorosis. Physiol. Plant. 2013, 148, 490–501. [Google Scholar] [CrossRef]
- Kobayashi, N.; Tanoi, K. Critical issues in the study of magnesium transport systems and magnesium deficiency symptoms in plants. Int. J. Mol. Sci. 2015, 16, 23076–23093. [Google Scholar] [CrossRef]
- Pel, Z.M.; Murata, Y.; Benning, G.; Thomine, S.; Klüsener, B.; Allen, G.J.; Grill, E.; Schroeder, J.I. Calcium channels activated by hydrogen peroxide mediate abscisic acid signalling in guard cells. Nature 2000, 406, 731–734. [Google Scholar] [CrossRef]
- Inoue, S.; Hayashi, M.; Huang, S.; Yokosho, K.; Gotoh, E.; Ikematsu, S.; Okumura, M.; Suzuki, T.; Kamura, T.; Kinoshita, T. A tonoplast-localized magnesium transporter is crucial for stomatal opening in arabidopsis under high Mg2+ Conditions. New Phytol. 2022, 236, 864–877. [Google Scholar] [CrossRef]
- Fischer, E.S.; Lohaus, G.; Heineke, D.; Heldt, H.W. Magnesium deficiency results in accumulation of carbohydrates and amino acids in source and sink leaves of spinach. Physiol. Plant. 1998, 102, 16–20. [Google Scholar] [CrossRef]
- Samborska, I.A.; Kalaji, H.M.; Sieczko, L.; Goltsev, V.; Borucki, W.; Jajoo, A. Structural and functional disorder in the photosynthetic apparatus of radish plants under magnesium deficiency. Funct. Plant Biol. 2018, 45, 668–679. [Google Scholar] [CrossRef]
- Hermans, C.; Johnson, G.N.; Strasser, R.J.; Verbruggen, N. Physiological characterisation of magnesium deficiency in sugar beet: Acclimation to low magnesium differentially affects photosystems I and II. Planta 2004, 220, 344–355. [Google Scholar] [CrossRef]
- Yang, G.-H.; Yang, L.-T.; Jiang, H.-X.; Li, Y.; Wang, P.; Chen, L.-S. Physiological impacts of magnesium-deficiency in citrus seedlings: Photosynthesis, antioxidant system and carbohydrates. Trees 2012, 26, 1237–1250. [Google Scholar] [CrossRef]
- Li, Z.; Phillip, D.; Neuhäuser, B.; Schulze, W.X.; Ludewig, U. Protein dynamics in young maize root hairs in response to macro- and micronutrient deprivation. J. Proteome Res. 2015, 14, 3362–3371. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Xu, G. Low magnesium with high potassium supply changes sugar partitioning and root growth pattern prior to visible magnesium deficiency in leaves of rice (Oryza sativa L.). Am. J. Plant Sci. 2011, 2, 601–608. [Google Scholar] [CrossRef]
- Tanoi, K.; Kobayashi, N.I.; Saito, T.; Iwata, N.; Kamada, R.; Iwata, R.; Suzuki, H.; Hirose, A.; Ohmae, Y.; Sugita, R.; et al. Effects of magnesium deficiency on magnesium uptake activity of rice root, evaluated using 28 Mg as a tracer. Plant Soil 2014, 384, 69–77. [Google Scholar] [CrossRef]
- Ogura, T.; Kobayashi, N.I.; Suzuki, H.; Iwata, R.; Nakanishi, T.M.; Tanoi, K. Magnesium uptake characteristics in arabidopsis revealed by 28Mg tracer studies. Planta 2018, 248, 745–750. [Google Scholar] [CrossRef] [PubMed]
- Yan, B.; Hou, Y. Effect of soil magnesium on plants: A review. IOP Conf. Ser. Earth Environ. Sci. 2018, 170, 022168. [Google Scholar] [CrossRef]
- Lasa, B.; Frechilla, S.; Aleu, M.; González-Moro, B.; Lamsfus, C.; Aparicio-Tejo, P.M. Effects of low and high levels of magnesium on the response of sunflower plants grown with ammonium and nitrate. Plant Soil 2000, 225, 167–174. [Google Scholar] [CrossRef]
- Kleiber, T.; Golcz, A.; Krzesiński, W. Effect of magnesium nutrition of onion (Allium cepa L.). Part I. Yielding and nutrient status. Ecol. Chem. Eng. S 2012, 19, 97–105. [Google Scholar] [CrossRef]
- He, H.; Khan, S.; Deng, Y.; Jin, X.; Ma, H.; Li, X.; Yin, L.; Huang, J. Physiological response to short-term magnesium deficiency in banana cultivars. J. Soil Sci. Plant Nutr. 2021, 21, 2826–2836. [Google Scholar] [CrossRef]
- Ding, Y.-C.; Chang, C.-R.; Luo, W.; Wu, Y.-S.; Ren, X.-L.; Wang, P.; Xu, G.-H. High potassium aggravates the oxidative stress inducedy by magnesium deflciency in rice leaves. Pedosphere 2008, 18, 316–327. [Google Scholar] [CrossRef]
- Kobayashi, H.; Masaoka, Y.; Sato, S. Effects of excess magnesium on the growth and mineral content of rice and echinochloa. Plant Prod. Sci. 2005, 8, 38–43. [Google Scholar] [CrossRef]
- Pathak, A.N.; Kalra, Y.P. Antagonism between potassium, calcium and magnesium in several varieties of hybrid corn. Zeitschrift Pflanzenernährung Bodenkd. 1971, 130, 118–124. [Google Scholar] [CrossRef]
- Ohno, T.; Grunes, D.L. Potassium-magnesium interactions affecting nutrient uptake by wheat forage. Soil Sci. Soc. Am. J. 1985, 49, 685–690. [Google Scholar] [CrossRef]
- Elkhouni, A.; Zorrig, W.; Smaoui, A. Effects of magnesium deficiency on photosynthesis and carbohydrate partitioning. Acta Physiol. Plant. 2016, 38, 145. [Google Scholar] [CrossRef]
- White, P.J. Ion Uptake Mechanisms of individual cells and roots. In Marschner’s Mineral Nutrition of Higher Plants; Elsevier: Amsterdam, The Netherlands, 2012; pp. 7–47. ISBN 9780123849052. [Google Scholar]
- Tapia, M.L.; Gutierrez, V. Distribution pattern of dry weight, nitrogen, phosphorus, and potassium through tomato ontogenesis. J. Plant Nutr. 1997, 20, 783–791. [Google Scholar] [CrossRef]
- Omirou, M.D.; Papadopoulou, K.K.; Papastylianou, I.; Constantinou, M.; Karpouzas, D.G.; Asimakopoulos, I.; Ehaliotis, C. Impact of nitrogen and sulfur fertilization on the composition of glucosinolates in relation to sulfur assimilation in different plant organs of broccoli. J. Agric. Food Chem. 2009, 57, 9408–9417. [Google Scholar] [CrossRef]
- Yu, K.-Q.; Zharo, Y.-R.; Li, X.-L.; Shao, Y.-N.; Liu, F.; He, Y. Hyperspectral imaging for mapping of total nitrogen spatial distribution in pepper plant. PLoS ONE 2014, 9, e116205. [Google Scholar] [CrossRef]
- Hansson, M.; Lundqvist, J.; Sirijovski, N.; Al-Karadaghi, S. Magnesium chelatase: The molecular motor of chlorophyll biosynthesis. In Handbook of Porphyrin Science; World Scientific: Singapore, 2013; Volume 28, pp. 41–84. ISBN 9789814407755. [Google Scholar]
- Huang, Z.A.; Jiang, D.A.; Yang, Y.; Sun, J.W.; Jin, S.H. Effects of nitrogen deficiency on gas exchange, chlorophyll fluorescence, and antioxidant enzymes in leaves of rice plants. Photosynthetica 2004, 42, 357–364. [Google Scholar] [CrossRef]
- Peng, W.T.; Qi, W.L.; Nie, M.M.; Xiao, Y.B.; Liao, H.; Chen, Z.C. Magnesium supports nitrogen uptake through regulating NRT2.1/2.2 in soybean. Plant Soil 2020, 457, 97–111. [Google Scholar] [CrossRef]
- Liu, T.-Y.; Huang, T.-K.; Yang, S.-Y.; Hong, Y.-T.; Huang, S.-M.; Wang, F.-N.; Chiang, S.-F.; Tsai, S.-Y.; Lu, W.-C.; Chiou, T.-J. Identification of plant vacuolar transporters mediating phosphate storage. Nat. Commun. 2016, 7, 11095. [Google Scholar] [CrossRef] [PubMed]
- Webb, J.R.; Ohlrogge, A.J.; Barber, S.A. The effect of magnesium upon the growth and the phosphorus content of soybean Plants. Soil Sci. Soc. Am. J. 1954, 18, 458–462. [Google Scholar] [CrossRef]
- Weih, M.; Liu, H.; Colombi, T.; Keller, T.; Jäck, O.; Vallenback, P.; Westerbergh, A. Evidence for magnesium-phosphorus synergism and Co-limitation of grain yield in wheat agriculture. Sci. Rep. 2021, 11, 9012. [Google Scholar] [CrossRef] [PubMed]
- Broadley, M.; Brown, P.; Cakmak, I.; Rengel, Z.; Zhao, F. Function of nutrients. In Marschner’s Mineral Nutrition of Higher Plants; Elsevier: Amsterdam, The Netherlands, 2012; pp. 191–248. ISBN 9780123849052. [Google Scholar]
- Kaur Sidhu, M.; Chandra Raturi, H.; Singh Kachwaya, D.; Sharma, A.; Harish Chandra Raturi, C. Role of micronutrients in vegetable production: A review. J. Pharmacogn. Phytochem. 2019, 8, 332–340. [Google Scholar]
- Shabala, S.; Hariadi, Y. Effects of magnesium availability on the activity of plasma membrane ion transporters and light-induced responses from broad bean leaf mesophyll. Planta 2005, 221, 56–65. [Google Scholar] [CrossRef]
- Le Bot, J.; Goss, M.J.; Carvalho, M.J.G.P.R.; Van Beusichem, M.L.; Kirkby, E.A. The significance of the magnesium to manganese ratio in plant tissues for growth and alleviation of manganese toxicity in tomato (Lycopersicon esculentum) and wheat (Triticum aestivum) plants. Plant Soil 1990, 124, 205–210. [Google Scholar] [CrossRef]
- Davis, J.G. Soil pH and magnesium effects on manganese toxicity in peanuts. J. Plant Nutr. 1996, 19, 535–550. [Google Scholar] [CrossRef]
- Elamin, O.M.; Wilcox, G.E. Effect of magnesium and manganese nutrition on watermelon growth and manganese toxicity. J. Am. Soc. Hortic. Sci. 1986, 111, 588–593. [Google Scholar] [CrossRef]
- Shi, Q.; Zhu, Z. Effects of exogenous salicylic acid on manganese toxicity, element contents and antioxidative system in cucumber. Environ. Exp. Bot. 2008, 63, 317–326. [Google Scholar] [CrossRef]
- Page, V.; Feller, U. Selective transport of zinc, manganese, nickel, cobalt and cadmium in the root system and transfer to the leaves in young wheat plants. Ann. Bot. 2005, 96, 425–434. [Google Scholar] [CrossRef]
- Hermans, C.; Chen, J.; Coppens, F.; Inzé, D.; Verbruggen, N. Low magnesium status in plants enhances tolerance to cadmium exposure. New Phytol. 2011, 192, 428–436. [Google Scholar] [CrossRef]
- Billard, V.; Maillard, A.; Coquet, L.; Jouenne, T.; Cruz, F.; Garcia-Mina, J.-M.; Yvin, J.-C.; Ourry, A.; Etienne, P. Mg deficiency affects leaf Mg remobilization and the proteome in Brassica napus. Plant Physiol. Biochem. 2016, 107, 337–343. [Google Scholar] [CrossRef] [PubMed]
- Stoyanova, Z.; Doncheva, S. The effect of zinc supply and succinate treatment on plant growth and mineral uptake in pea plant. Brazilian J. Plant Physiol. 2002, 14, 111–116. [Google Scholar] [CrossRef]
- Sagardoy, R.; Morales, F.; López-Millán, A.-F.; Abadía, A.; Abadía, J. Effects of zinc toxicity on sugar beet (Beta vulgaris L.) plants grown in gydroponics. Plant Biol. 2009, 11, 339–350. [Google Scholar] [CrossRef]
- Rietra, R.P.J.J.; Heinen, M.; Dimkpa, C.O.; Bindraban, P.S. Effects of nutrient antagonism and synergism on yield and fertilizer use efficiency. Commun. Soil Sci. Plant Anal. 2017, 48, 1895–1920. [Google Scholar] [CrossRef]
- Shaul, O.; Hilgemann, D.W.; De-Almeida-Engler, J.; Van Montagu, M.; Inze, D.; Galili, G. Cloning and characterization of a novel Mg/H exchanger. Mol. Cell. Neurosci. 1999, 23, 9–23. [Google Scholar]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morad, D.; Bernstein, N. Response of Medical Cannabis to Magnesium (Mg) Supply at the Vegetative Growth Phase. Plants 2023, 12, 2676. https://doi.org/10.3390/plants12142676
Morad D, Bernstein N. Response of Medical Cannabis to Magnesium (Mg) Supply at the Vegetative Growth Phase. Plants. 2023; 12(14):2676. https://doi.org/10.3390/plants12142676
Chicago/Turabian StyleMorad, Dalit, and Nirit Bernstein. 2023. "Response of Medical Cannabis to Magnesium (Mg) Supply at the Vegetative Growth Phase" Plants 12, no. 14: 2676. https://doi.org/10.3390/plants12142676
APA StyleMorad, D., & Bernstein, N. (2023). Response of Medical Cannabis to Magnesium (Mg) Supply at the Vegetative Growth Phase. Plants, 12(14), 2676. https://doi.org/10.3390/plants12142676





