Remnants from the Past: From an 18th Century Manuscript to 21st Century Ethnobotany in Valle Imagna (Bergamo, Italy)
Abstract
:1. Introduction
2. Results and Discussion
2.1. Current Ethnobotanical Survey
2.2. Diachronic Analysis
2.3. Comparison among Similar Preparations in 18th and 21st Century in Valle Imagna
3. Materials and Methods
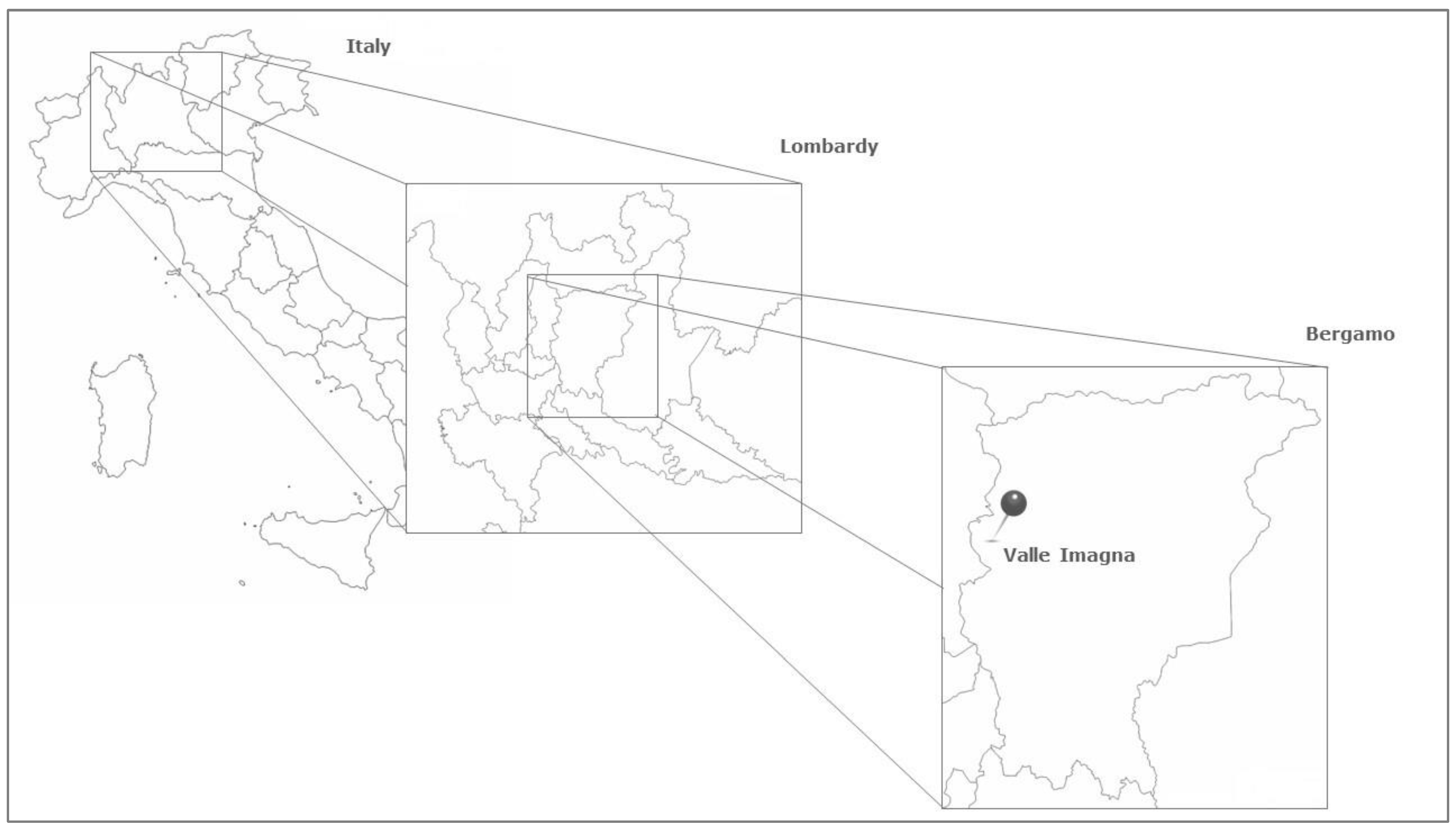
3.1. Area of Investigation
3.2. Ethnobotanical Survey, Data Archiving and Processing
3.3. Data Analysis
3.4. Bibliographic Research
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- da Silva, T.C.; Medeiros, P.M.; Balcazár, A.L.; de Sousa Araújo, T.A.; Pirondo, A.; Medeiros, M.F.T. Historical ethnobotany: An overview of selected studies. Ethnobiol. Conserv. 2014, 3, 4. [Google Scholar] [CrossRef] [Green Version]
- Fontefrancesco, M.F.; Pieroni, A. Renegotiating situativity: Transformations of local herbal knowledge in a Western Alpine valley during the past 40 years. J. Ethnobiol. Ethnomed. 2020, 16, 58. [Google Scholar] [CrossRef]
- Mattalia, G.; Graetz, F.; Harms, M.; Segor, A.; Tomarelli, A.; Kieser, V.; Zerbe, S.; Pieroni, A. Temporal Changes in the Use of Wild Medicinal Plants in Tyrol, South Trentino. Plants 2023, 12, 2372. [Google Scholar] [CrossRef] [PubMed]
- Leonti, M.; Casu, L.; Sanna, F.; Bonsignore, L. A comparison of medicinal plant use in Sardinia and Sicily-De Materia Medica revisited? J. Ethnopharmacol. 2009, 121, 255–267. [Google Scholar] [CrossRef] [PubMed]
- Leonti, M.; Cabras, S.; Weckerle, C.S.; Solinas, M.N.; Casu, L. The causal dependence of present plant knowledge on herbals-Contemporary medicinal plant use in Campania (Italy) compared to Matthioli (1568). J. Ethnopharmacol. 2010, 130, 379–391. [Google Scholar] [CrossRef] [PubMed]
- Dal Cero, M.; Saller, R.; Leonti, M.; Weckerle, C.S. Trends of Medicinal Plant Use over the Last 2000 Years in Central Europe. Plants 2023, 12, 135. [Google Scholar] [CrossRef]
- Stepp, J.R.; Moerman, D.E. The importance of weeds in ethnopharmacology. J. Ethnopharmacol. 2001, 75, 19–23. [Google Scholar] [CrossRef]
- Bruschi, P.; Sugni, M.; Moretti, A.; Signorini, M.A.; Fico, G. Children’s versus adult’s knowledge of medicinal plants: An ethnobotanical study in Tremezzina (Como, Lombardy, Italy). Brazilian J. Pharmacogn. 2019, 29, 644–655. [Google Scholar] [CrossRef]
- Aziz, M.A.; Volpato, G.; Fontefrancesco, M.F.; Pieroni, A. Perceptions and Revitalization of Local Ecological Knowledge in Four Schools in Yasin Valley, North Pakistan. Mt. Res. Dev. 2022, 42, R1–R9. [Google Scholar] [CrossRef]
- Arjona-García, C.; Blancas, J.; Beltrán-Rodríguez, L.; López Binnqüist, C.; Colín Bahena, H.; Moreno-Calles, A.I.; Sierra-Huelsz, J.A.; López-Medellín, X. How does urbanization affect perceptions and traditional knowledge of medicinal plants? J. Ethnobiol. Ethnomed. 2021, 17, 48. [Google Scholar] [CrossRef]
- Applequist, W.L.; Brinckmann, J.A.; Cunningham, A.B.; Hart, R.E.; Heinrich, M.; Katerere, D.R.; Van Andel, T. Scientists warning on climate change and medicinal plants. Planta Med. 2020, 86, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, U.B.; Lamsal, P.; Ghimire, S.K.; Shrestha, B.B.; Dhakal, S.; Shrestha, S.; Atreya, K. Climate change-induced distributional change of medicinal and aromatic plants in the Nepal Himalaya. Ecol. Evol. 2022, 12, e9204. [Google Scholar] [CrossRef] [PubMed]
- Mattalia, G.; Sõukand, R.; Corvo, P.; Pieroni, A. “We Became Rich and We Lost Everything”: Ethnobotany of Remote Mountain Villages of Abruzzo and Molise, Central Italy. Hum. Ecol. 2021, 49, 217–224. [Google Scholar] [CrossRef]
- Mattalia, G.; Stryamets, N.; Balázsi, Á.; Molnár, G.; Gliga, A.; Pieroni, A.; Sõukand, R.; Reyes-García, V. Hutsuls’ Perceptions of Forests and Uses of Forest Resource in Ukrainian and Romanian Bukovina. Int. For. Rev. 2022, 24, 393–410. [Google Scholar] [CrossRef]
- Leonti, M. The future is written: Impact of scripts on the cognition, selection, knowledge and transmission of medicinal plant use and its implications for ethnobotany and ethnopharmacology. J. Ethnopharmacol. 2011, 134, 542–555. [Google Scholar] [CrossRef]
- Prakofjewa, J.; Kalle, R.; Belichenko, O.; Kolosova, V.; Sõukand, R. Re-written narrative: Transformation of the image of Ivan-chaj in Eastern Europe. Heliyon 2020, 6, e04632. [Google Scholar] [CrossRef]
- Milani, F.; Fico, G. Raccolta di Diversi Rimedj a Varj Mali. Studio Etnobotanico di un Manoscritto Lombardo del Diciottesimo Secolo; Centro Sudi Valle Imagna: Bergamo, Italy, 2021; ISBN 978-88-6417-106-7. [Google Scholar]
- Colonna, R.; Piscitelli, A.; Iadevaia, V. Una breve storia della farmacologia occidentale. G. Ital. di Farmacol. Clin. 2019, 33, 86–106. [Google Scholar] [CrossRef]
- Maconi, G. La Medicina Popolare in Valle Imagna. Componenti Magiche, Religiose ed Empiriche Tradizionali tra L’Ottocento e il Novecento; Centro Studi Valle Imagna: Bergamo, Italy, 2006. [Google Scholar]
- Mangili, C. GEV Valle Imagna. In Piccola Flora della Valle Imagna; Comunità Montana Valle Imagna: Bergamo, Italy, 2016. [Google Scholar]
- Flora Alpina Bergamasca. Parco delle Orobie Bergamasche. In Fiori Delle Orobie 2—Gli Alberi; EQUA Editrice: Bergamo, Italy, 2015. [Google Scholar]
- Flora Alpina Bergamasca. Parco delle Orobie Bergamasche. In Fiori delle Orobie 1—Collina e Bassa Montagna; EQUA Editrice: Bergamo, Italy, 2014. [Google Scholar]
- Flora Alpina Bergamasca. Parco delle Orobie Bergamasche. In Fiori delle Orobie 3—Media e Alta Montagna; EQUA Editrice: Bergamo, Italy, 2016. [Google Scholar]
- Sgalippa, G.; Silva, M. Gente e Terra d’Imagna. Atti del Convegno di Sant’Omobono Imagna, 15 Aprile—13 maggio 1993; Il Pomerio Srl di Lodi: Lodi, Italy, 1996. [Google Scholar]
- Vandebroek, I.; Balick, M.J. Globalization and loss of plant knowledge: Challenging the paradigm. PLoS ONE 2012, 7, e37643. [Google Scholar] [CrossRef]
- Petelka, J.; Plagg, B.; Säumel, I.; Zerbe, S. Traditional medicinal plants in South Tyrol (northern Italy, southern Alps): Biodiversity and use. J. Ethnobiol. Ethnomed. 2020, 16, 74. [Google Scholar] [CrossRef]
- Sõukand, R.; Kalle, R.; Pieroni, A. Homogenisation of Biocultural Diversity: Plant Ethnomedicine and Its Diachronic Change in Setomaa and Võromaa, Estonia, in the Last Century. Biology 2022, 11, 192. [Google Scholar] [CrossRef]
- Guarrera, P.M. Usi e Tradizioni della Flora Italiana. Medicina Popolare ed Etnobotanica; Aracne: Rome, Italy, 2006. [Google Scholar]
- Schütz, K.; Carle, R.; Schieber, A. Taraxacum-A review on its phytochemical and pharmacological profile. J. Ethnopharmacol. 2006, 107, 313–323. [Google Scholar] [CrossRef] [PubMed]
- Lis, B.; Olas, B. Pro-health activity of dandelion (Taraxacum officinale L.) and its food products—History and present. J. Funct. Foods 2019, 59, 40–48. [Google Scholar] [CrossRef]
- Dal Cero, M.; Saller, R.; Weckerle, C.S. The use of the local flora in Switzerland: A comparison of past and recent medicinal plant knowledge. J. Ethnopharmacol. 2014, 151, 253–264. [Google Scholar] [CrossRef]
- Vitalini, S.; Tomè, F.; Fico, G. Traditional uses of medicinal plants in Valvestino (Italy). J. Ethnopharmacol. 2009, 121, 106–116. [Google Scholar] [CrossRef]
- Vitalini, S.; Puricelli, C.; Mikerezi, I.; Iriti, M. Plants, people and traditions: Ethnobotanical survey in the Lombard Stelvio National Park and neighbouring areas (Central Alps, Italy). J. Ethnopharmacol. 2015, 173, 435–458. [Google Scholar] [CrossRef]
- Bottoni, M.; Milani, F.; Colombo, L.; Nallio, K.; Colombo, P.S.; Giuliani, C.; Bruschi, P.; Fico, G. Using Medicinal Plants in Valmalenco (Italian Alps): From Tradition to Scientific Approaches. Molecules 2020, 25, 4144. [Google Scholar] [CrossRef]
- Bottoni, M.; Colombo, L.; Gianoli, C.; Milani, F.; Colombo, P.S.; Bruschi, P.; Giuliani, C.; Fico, G. Alpine ethnobotanical knowledge in Sondalo (SO, Lombardy, Italy). Ethnobot. Res. Appl. 2022, 24, 1–63. [Google Scholar] [CrossRef]
- Dei Cas, L.; Pugni, F.; Fico, G. Tradition of use on medicinal species in Valfurva (Sondrio, Italy). J. Ethnopharmacol. 2015, 163, 113–134. [Google Scholar] [CrossRef]
- Vitalini, S.; Iriti, M.; Puricelli, C.; Ciuchi, D.; Segale, A.; Fico, G. Traditional knowledge on medicinal and food plants used in Val San Giacomo (Sondrio, Italy)—An alpine ethnobotanical study. J. Ethnopharmacol. 2013, 145, 517–529. [Google Scholar] [CrossRef]
- Pignatti, S.; Guorino, R.; La Rosa, M. Flora d’Italia, 2nd ed.; Edagricole-New Business Media: Bologna, Italy, 2018. [Google Scholar]
- World Flora Online. Available online: http://www.worldfloraonline.org/ (accessed on 29 June 2023).
- Pitman, N.C.A.; Cerón, C.E.; Reyes, C.I.; Thurber, M.; Arellano, J. Catastrophic natural origin of a species-poor tree community in the world’s richest forest. J. Trop. Ecol. 2005, 21, 559–568. [Google Scholar] [CrossRef] [Green Version]
- Cook, F.E. Economic Botanic Data Collection Standard; Prendergast, H., Ed.; Royal Botanic Gardens, Kew: London, UK, 1995; ISBN 0947643710. [Google Scholar]
- González, J.A.; García-Barriuso, M.; Amich, F. Ethnobotanical study of medicinal plants traditionally used in the Arribes del Duero, western Spain. J. Ethnopharmacol. 2010, 131, 343–355. [Google Scholar] [CrossRef]
- Staub, P.O.; Geck, M.S.; Weckerle, C.S.; Casu, L.; Leonti, M. Classifying diseases and remedies in ethnomedicine and ethnopharmacology. J. Ethnopharmacol. 2015, 174, 514–519. [Google Scholar] [CrossRef] [Green Version]
- Lucchetti, L.; Zitti, S.; Taffetani, F. Ethnobotanical uses in the Ancona district (Marche region, Central Italy). J. Ethnobiol. Ethnomed. 2019, 15, 9. [Google Scholar] [CrossRef] [Green Version]
- Guarrera, P.M. Traditional phytotherapy in Central Italy (Marche, Abruzzo, and Latium). Fitoterapia 2005, 76, 1–25. [Google Scholar] [CrossRef]
- Cornara, L.; La Rocca, A.; Terrizzano, L.; Dente, F.; Mariotti, M.G. Ethnobotanical and phytomedical knowledge in the North-Western Ligurian Alps. J. Ethnopharmacol. 2014, 155, 463–484. [Google Scholar] [CrossRef]
- Pagano, C.; Marinozzi, M.; Baiocchi, C.; Beccari, T.; Calarco, P.; Ceccarini, M.R.; Chielli, M.; Orabona, C.; Orecchini, E.; Ortenzi, R.; et al. Bioadhesive polymeric films based on red onion skins extract for wound treatment: An innovative and eco-friendly formulation. Molecules 2020, 25, 318. [Google Scholar] [CrossRef] [Green Version]
- Dorsch, W.; Ring, J. Suppression of Immediate and Late Anti-IgE-Induced Skin Reactions by Topically Applied Alcohol/Onion Extract. Allergy 1984, 39, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Batiha, G.E.S.; Beshbishy, A.M.; El-Mleeh, A.; Abdel-Daim, M.M.; Devkota, H.P. Traditional uses, bioactive chemical constituents, and pharmacological and toxicological activities of Glycyrrhiza glabra L. (fabaceae). Biomolecules 2020, 10, 352. [Google Scholar] [CrossRef] [Green Version]
- Shang, A.; Cao, S.Y.; Xu, X.Y.; Gan, R.Y.; Tang, G.Y.; Corke, H.; Mavumengwana, V.; Li, H. Bin Bioactive compounds and biological functions of garlic (Allium sativum L.). Foods 2019, 8, 246. [Google Scholar] [CrossRef] [Green Version]
- Sarker, S.; Nahar, L. Natural Medicine:The Genus Angelica. Curr. Med. Chem. 2004, 11, 1479–1500. [Google Scholar] [CrossRef] [PubMed]
- Facino, R.M.; Carini, M.; Stefani, R.; Aldini, G.; Saibene, L. Anti-Elastase and Anti-Hyaluronidase Activities of Saponins and Sapogenins from Hedera helix, Aesculus hippocastanum, and Ruscus aculeatus: Factors Contributing to their Efficacy in the Treatment of Venous Insufficiency. Arch. Pharm. 1995, 328, 720–724. [Google Scholar] [CrossRef]
- Vázquez-Castilla, S.; De la Puerta, R.; García Giménez, M.D.; Fernández-Arche, M.A.; Guillén-Bejarano, R. Bioactive constituents from “triguero” asparagus improve the plasma lipid profile and liver antioxidant status in hypercholesterolemic rats. Int. J. Mol. Sci. 2013, 14, 21227–21239. [Google Scholar] [CrossRef] [Green Version]
- World Health Organization (Ed.) WHO Monographs on Selected Medicinal Plants; WHO Library Cataloguing in Publication Data: Geneva, Switzerland, 2002; Volume 2. [Google Scholar]
- Applequist, W.L.; Moerman, D.E. Yarrow (Achillea millefolium L.): A Neglected Panacea? A Review of Ethnobotany, Bioactivity, and Biomedical Research1. Econ. Bot. 2011, 65, 209–225. [Google Scholar] [CrossRef]
- Ali, S.I.; Gopalakrishnan, B.; Venkatesalu, V. Pharmacognosy, Phytochemistry and Pharmacological Properties of Achillea millefolium L.: A Review. Phyther. Res. 2017, 31, 1140–1161. [Google Scholar] [CrossRef] [PubMed]
- Saeidnia, S.; Gohari, A.R.; Mokhber-Dezfuli, N.; Kiuchi, F. A review on phytochemistry and medicinal properties of the genus Achillea. DARU, J. Pharm. Sci. 2011, 19, 173–186. [Google Scholar]
- Mattalia, G.; Quave, C.L.; Pieroni, A. Traditional uses of wild food and medicinal plants among Brigasc, Kyé, and Provençal communities on the Western Italian Alps. Genet Resour Crop Evol. 2013, 60, 587–603. [Google Scholar] [CrossRef]
- Pieroni, A.; Giusti, M.E. Alpine ethnobotany in Italy: Traditional knowledge of gastronomic and medicinal plants among the Occitans of the upper Varaita valley, Piedmont. J. Ethnobiol. Ethnomed. 2009, 5, 32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bellia, G.; Pieroni, A. Isolated, but Transnational: The Glocal Nature of Waldensian Ethnobotany, Western Alps, NW Italy. J. Ethnobiol. Ethnomed. 2015, 11. [Google Scholar] [CrossRef] [Green Version]
- Kriplani, P.; Guarve, K.; Baghael, U.S. Arnica montana L.—A plant of healing: Review. J. Pharm. Pharmacol. 2017, 69, 925–945. [Google Scholar] [CrossRef] [Green Version]
- Lyss, G.; Schmidt, T.J.; Merfort, I.; Pahl, H.L. Helenalin, an anti-inflammatory sesquiterpene lactone from Arnica, selectively inhibits transcription factor NF-κB. Biol. Chem. 1997, 378, 951–961. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Arif, M.; Nirala, R.K.; Gupta, R.; Thakur, S.C. Cumulative therapeutic effects of phytochemicals in Arnica montana flower extract alleviated collagen-induced arthritis: Inhibition of both pro-inflammatory mediators and oxidative stress. J. Sci. Food Agric. 2015, 96, 1500–1510. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (Ed.) WHO Monographs on Selected Medicinal Plants; WHO Library Cataloguing in Publication Data: Geneva, Switzerland, 2007; Volume 3. [Google Scholar]
- Hall, I.H.; Lee, K.H.; Starenes, C.O.; Sumida, Y.; Wu, R.Y.; Waddell, T.G.; Cochran, J.W.; Gerhart, K.G. Anti-inflammatory activity of sesquiterpene lactones and related compounds. J. Pharm. Sci. 1979, 68, 537–542. [Google Scholar] [CrossRef] [PubMed]
- Hall, I.H.; Starenes, C.O.; Lee, K.H.; Waddell, T.G. Mode of action of sesquiterpene lactones as anti-inflammatory agents. J. Pharm. Sci. 1980, 69, 537–543. [Google Scholar] [CrossRef]
- Capasso, F.; Grandolini, G.; Izzo, A.A. Fitoterapia. Impiego Razionale delle Droghe Vegetali; Springer: Berlin/Heidelberg, Germany, 2006. [Google Scholar]
- McMullen, M.K.; Whitehouse, J.M.; Whitton, P.A.; Towell, A. Bitter tastants alter gastric-phase postprandial haemodynamics. J. Ethnopharmacol. 2014, 154, 719–727. [Google Scholar] [CrossRef] [PubMed]
- McMullen, M.K.; Whitehouse, J.M.; Towell, A. Bitters: Time for a new paradigm. Evidence-Based Complement. Altern. Med. 2015, 2015, 670504. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Batiha, G.E.; Olatunde, A.; El-mleeh, A.; Hetta, H.F.; Al-rejaie, S.; Alghamdi, S.; Zahoor, M.; Beshbishy, A.M. Pharmacokinetics of Wormwood (Artemisia absinthium). Antibiotics 2020, 9, 353. [Google Scholar] [CrossRef]
- Brockhoff, A.; Behrens, M.; Massarotti, A.; Appending, G.; Meyerhof, W. Broad tuning of the human bitter taste receptor hTAS2R46 to various sesquiterpene lactones, clerodane and labdane diterpenoids, strychnine, and denatonium. J. Agric. Food Chem. 2007, 55, 6236–6243. [Google Scholar] [CrossRef]
- Gras, A.; Parada, M.; Vallès, J.; Garnatje, T. The Role of Traditional Plant Knowledge in the Fight Against Infectious Diseases: A Meta-Analytic Study in the Catalan Linguistic Area. Front. Pharmacol. 2021, 12, 744616. [Google Scholar] [CrossRef]
- Khattak, S.G.; Gilani, S.N.; Ikram, M. Antipyretic studies on some indigenous Pakistani medicinal plants. J. Ethnopharmacol. 1985, 14, 45–51. [Google Scholar] [CrossRef]
- Rakhshandeh, H.; Heidari, A.; Pourbagher-Shahri, A.M.; Rashidi, R.; Forouzanfar, F. Hypnotic Effect of A. absinthium Hydroalcoholic Extract in Pentobarbital-Treated Mice. Neurol. Res. Int. 2021, 2021, 5521019. [Google Scholar] [CrossRef]
- Amat, N.; Upur, H.; Blažeković, B. In vivo hepatoprotective activity of the aqueous extract of Artemisia absinthium L. against chemically and immunologically induced liver injuries in mice. J. Ethnopharmacol. 2010, 131, 478–484. [Google Scholar] [CrossRef]
- World Health Organization (Ed.) WHO Monographs on Selected Medicinal Plants; WHO Library Cataloguing in Publication Data: Geneva, Switzerland, 1999; Volume 1. [Google Scholar]
- Dinda, M.; Mazumdar, S.; Das, S.; Ganguly, D.; Dasgupta, U.B.; Dutta, A.; Jana, K.; Karmakar, P. The Water Fraction of Calendula officinalis Hydroethanol Extract Stimulates In Vitro and In Vivo Proliferation of Dermal Fibroblasts in Wound Healing. Phyther. Res. 2016, 30, 1696–1707. [Google Scholar] [CrossRef]
- Fonseca, Y.M.; Catini, C.D.; Vicentini, F.T.M.C.; Nomizo, A.; Gerlach, R.F.; Fonseca, M.J.V. Protective effect of Calendula officinalis extract against UVB-induced oxidative stress in skin: Evaluation of reduced glutathione levels and matrix metalloproteinase secretion. J. Ethnopharmacol. 2010, 127, 596–601. [Google Scholar] [CrossRef]
- Jahdi, F.; Khabbaz, A.; Kashian, M.; Taghizadeh, M.; Haghani, H. The impact of Calendula ointment on cesarean wound healing: A randomized controlled clinical trial. J. Fam. Med. Prim. Care 2018, 7, 893. [Google Scholar] [CrossRef]
- Nicolaus, C.; Junghanns, S.; Hartmann, A.; Murillo, R.; Ganzera, M.; Merfort, I. In vitro studies to evaluate the wound healing properties of Calendula officinalis extracts. J. Ethnopharmacol. 2017, 196, 94–103. [Google Scholar] [CrossRef]
- Givol, O.; Kornhaber, R.; Visentin, D.; Cleary, M.; Haik, J.; Harats, M. A systematic review of Calendula officinalis extract for wound healing. Wound Repair Regen. 2019, 27, 548–561. [Google Scholar] [CrossRef]
- Okada, N.; Kobayashi, S.; Moriyama, K.; Miyataka, K.; Abe, S.; Sato, C.; Kawazoe, K. Helianthus tuberosus (Jerusalem artichoke) tubers improve glucose tolerance and hepatic lipid profile in rats fed a high-fat diet. Asian Pac. J. Trop. Med. 2017, 10, 439–443. [Google Scholar] [CrossRef] [PubMed]
- McKay, D.L.; Blumberg, J.B. A Review of the bioactivity and potential health benefits of chamomile tea (Matricaria recutita L.). Phyther. Res. 2006, 20, 519–530. [Google Scholar] [CrossRef]
- Miraj, S.; Alesaeidi, S. A systematic review study of therapeutic effects of Matricaria recutita chamomile (chamomile). Electron. Physician 2016, 8, 3024–3031. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Keefe, J.R.; Mao, J.J.; Soeller, I.; Li, Q.S.; Amsterdam, J.D. Short-term open-label chamomile (Matricaria chamomilla L.) therapy of moderate to severe generalized anxiety disorder. Phytomedicine 2016, 23, 1699–1705. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El Mihyaoui, A.; Esteves da Silva, J.C.G.; Charfi, S.; Candela Castillo, M.E.; Lamarti, A.; Arnao, M.B. Chamomile (Matricaria chamomilla L.): A Review of Ethnomedicinal Use, Phytochemistry and Pharmacological Uses. Life 2022, 12, 479. [Google Scholar] [CrossRef]
- Marmouzi, I.; Bouyahya, A.; Ezzat, S.M.; El Jemli, M.; Kharbach, M. The food plant Silybum marianum (L.) Gaertn.: Phytochemistry, Ethnopharmacology and clinical evidence. J. Ethnopharmacol. 2021, 265, 113303. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Chen, Y.; Sun-Waterhouse, D. The potential of dandelion in the fight against gastrointestinal diseases: A review. J. Ethnopharmacol. 2022, 293, 115272. [Google Scholar] [CrossRef]
- González-Castejón, M.; Visioli, F.; Rodriguez-Casado, A. Diverse biological activities of dandelion. Nutr. Rev. 2012, 70, 534–547. [Google Scholar] [CrossRef]
- Guarrera, P.M. Food medicine and minor nourishment in the folk traditions of Central Italy (Marche, Abruzzo and Latium). Fitoterapia 2003, 74, 515–544. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Wu, H.; Wang, L.; Luo, H.; Lu, Y.; Zhang, Q.; Tang, L.; Wang, Z. Farfarae Flos: A review of botany, traditional uses, phytochemistry, pharmacology, and toxicology. J. Ethnopharmacol. 2020, 260, 113038. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.Z.; Zhao, D.X.; Xiang, J.; Zhang, M.; Zhang, C.F.; Xu, X.H. Antitussive, expectorant, and anti-inflammatory activities of four caffeoylquinic acids isolated from Tussilago farfara. Pharm. Biol. 2016, 54, 1117–1124. [Google Scholar] [CrossRef] [Green Version]
- Welna, M.; Szymczycha-Madeja, A.; Pohl, P. Simplified ICP OES-based method for determination of 12 elements in commercial bottled birch saps: Validation and bioaccessibility study. Molecules 2020, 25, 1256. [Google Scholar] [CrossRef] [Green Version]
- Cameron, M.; Chrubasik, S. Topical herbal therapies for treating osteoarthritis. Cochrane Database Syst. Rev. 2013, 5, CD010538. [Google Scholar] [CrossRef] [Green Version]
- Frost, R.; MacPherson, H.; O’Meara, S. A critical scoping review of external uses of comfrey (Symphytum spp.). Complement. Ther. Med. 2013, 21, 724–745. [Google Scholar] [CrossRef]
- Rakhecha, B.; Agnihotri, P.; Dakal, T.C.; Saquib, M.; Monu; Biswas, S. Anti-inflammatory activity of nicotine isolated from Brassica oleracea in rheumatoid arthritis. Biosci. Rep. 2022, 42, BSR20211392. [Google Scholar] [CrossRef]
- Kazimierski, M.; Regula, J.; Molska, M. Cornelian cherry (Cornus mas L.)—characteristics, nutritional and pro-health properties. Acta Sci. Pol. Technol. Aliment. 2019, 18, 5–12. [Google Scholar] [CrossRef]
- Carneiro, D.M.; Freire, R.C.; Honório, T.C.D.D.; Zoghaib, I.; Cardoso, F.F.D.S.; Tresvenzol, L.M.F.; de Paula, J.R.; Sousa, A.L.L.; Jardim, P.C.B.V.; Cunha, L.C.; et al. Randomized, Double-Blind Clinical Trial to Assess the Acute Diuretic Effect of Equisetum arvense (Field Horsetail) in Healthy Volunteers. Evidence-Based Complement. Altern. Med. 2014, 2014, 760683. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Snafi, A.E. The pharmacology of Equisetum arvense—A review. IOSR J. Pharm. 2017, 7, 31–42. [Google Scholar] [CrossRef]
- Asgarpanah, J. Phytochemistry and pharmacological properties of Equisetum arvense L. J. Med. Plants Res. 2012, 6, 3689–3693. [Google Scholar] [CrossRef] [Green Version]
- World Health Organization (Ed.) WHO Monographs on Selected Medicinal Plants; WHO Library Cataloguing in Publication Data: Geneva, Switzerland, 2009; Volume 4. [Google Scholar]
- Deng, H.W.; Tian, Y.; Zhou, X.J.; Zhang, X.M.; Meng, J. Effect of Bilberry Extract on Development of Form-Deprivation Myopia in the Guinea Pig. J. Ocul. Pharmacol. Ther. 2016, 32, 196–202. [Google Scholar] [CrossRef] [PubMed]
- Gizzi, C.; Belcaro, G.; Gizzi, G.; Feragalli, B.; Dugall, M.; Luzzi, R.; Cornelli, U. Bilberry extracts are not created equal: The role of non anthocyanin fraction. Discovering the “dark side of the force” in a preliminary study. Eur. Rev. Med. Pharmacol. Sci. 2016, 20, 2418–2424. [Google Scholar]
- Ogawa, K.; Tsuruma, K.; Tanaka, J.; Kakino, M.; Kobayashi, S.; Shimazawa, M.; Hara, H. The Protective Effects of Bilberry and Lingonberry Extracts against UV Light-Induced Retinal Photoreceptor Cell Damage in Vitro. J. Agric. Food Chem. 2013, 61, 10345–10353. [Google Scholar] [CrossRef]
- Ogawa, K.; Kuse, Y.; Tsuruma, K.; Kobayashi, S.; Shimazawa, M.; Hara, H. Protective effects of bilberry and lingonberry extracts against blue light-emitting diode light-induced retinal photoreceptor cell damage in vitro. BMC Complement. Altern. Med. 2014, 14, 120. [Google Scholar] [CrossRef] [Green Version]
- Ozawa, Y.; Kawashima, M.; Inoue, S.; Inagaki, E.; Suzuki, A.; Ooe, E.; Kobayashi, S.; Tsubota, K. Bilberry extract supplementation for preventing eye fatigue in video display terminal workers. J. Nutr. Health Aging 2015, 19, 548–554. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, L.; Lu, F.; Yang, X.; Deng, Q.; Ji, B.; Huang, F.; Kitts, D.D. Retinoprotective effects of bilberry anthocyanins via antioxidant, anti-inflammatory, and anti-apoptotic mechanisms in a visible light-induced retinal degeneration model in pigmented rabbits. Molecules 2015, 20, 22395–22410. [Google Scholar] [CrossRef] [Green Version]
- Canter, P.H.; Ernst, E. Anthocyanosides of Vaccinium myrtillus (bilberry) for night vision—A systematic review of placebo-controlled trials. Surv. Ophthalmol. 2004, 49, 38–50. [Google Scholar] [CrossRef]
- Szajdek, A.; Borowska, E.J. Bioactive Compounds and Health-Promoting Properties of Berry Fruits: A Review. Plant Foods Hum. Nutr. 2008, 63, 147–156. [Google Scholar] [CrossRef]
- Tunaru, S.; Althoff, T.F.; Nüsing, R.M.; Diener, M.; Offermanns, S. Castor oil induces laxation and uterus contraction via ricinoleic acid activating prostaglandin EP3 receptors. Proc. Natl. Acad. Sci. USA 2012, 109, 9179–9184. [Google Scholar] [CrossRef]
- Chávez-Santoscoy, R.A.; Gutiérrez-Uribe, J.A.; Serna-Saldívar, S.O. Effect of Flavonoids and Saponins Extracted from Black Bean (Phaseolus vulgaris L.) Seed Coats as Cholesterol Micelle Disruptors. Plant Foods Hum. Nutr. 2013, 68, 416–423. [Google Scholar] [CrossRef] [PubMed]
- Wölfle, U.; Seelinger, G.; Schempp, C.M. Topical application of St John’s wort (Hypericum perforatum). Planta Med. 2014, 80, 109–120. [Google Scholar]
- Uslusoy, F.; Nazıroğlu, M.; Övey, İ.S.; Sönmez, T.T. Hypericum perforatum L. supplementation protects sciatic nerve injury-induced apoptotic, inflammatory and oxidative damage to muscle, blood and brain in rats. J. Pharm. Pharmacol. 2019, 71, 83–92. [Google Scholar] [CrossRef] [Green Version]
- Khiljee, S.; Rehman, N.U.; Khiljee, T.; Loebenberg, R.; Ahmad, R.S. Formulation and clinical evaluation of topical dosage forms of Indian Penny Wort, walnut and turmeric in eczema. Pak. J. Pharm. Sci. 2015, 28, 2001–2007. [Google Scholar] [PubMed]
- Cavanagh, H.M.A.; Wilkinson, J.M. Biological activities of lavender essential oil. Phyther. Res. 2002, 16, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Recalde, M.; Ruiz Arias, I.E.; Hermida, É.B. Could essential oils enhance biopolymers performance for wound healing? A systematic review. Phytomedicine 2018, 38, 57–65. [Google Scholar] [CrossRef]
- Rai, V.K.; Sinha, P.; Yadav, K.S.; Shukla, A.; Saxena, A.; Bawankule, D.U.; Tandon, S.; Khan, F.; Chanotiya, C.S.; Yadav, N.P. Anti-psoriatic effect of Lavandula angustifolia essential oil and its major components linalool and linalyl acetate. J. Ethnopharmacol. 2020, 261, 113127. [Google Scholar] [CrossRef]
- Luo, J.; Jiang, W. A critical review on clinical evidence of the efficacy of lavender in sleep disorders. Phyther. Res. 2022, 36, 2342–2351. [Google Scholar] [CrossRef]
- Nawrot, J.; Gornowicz-Porowska, J.; Budzianowski, J.; Nowak, G.; Schroeder, G.; Kurczewska, J. Medicinal Herbs in the Relief of Neurological, Cardiovascular, and Respiratory Symptoms after COVID-19 Infection a Literature Review. Cells 2022, 11, 1897. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, M.; Yamanaka, A.; Asanuma, C.; Asano, H.; Satou, T.; Koike, K. Anxiolytic-like effect of inhalation of essential oil from Lavandula officinalis: Investigation of changes in 5-HT turnover and involvement of olfactory stimulation. Nat. Prod. Commun. 2014, 9, 1023–1026. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brown, M.E.; Browning, J.C. A case of psoriasis replaced by allergic contact dermatitis in a 12-year-old boy. Pediatr. Dermatol. 2016, 33, e125–e126. [Google Scholar] [CrossRef]
- Dobros, N.; Zawada, K.D.; Paradowska, K. Phytochemical Profiling, Antioxidant and Anti-Inflammatory Activity of Plants Belonging to the Lavandula Genus. Molecules 2023, 28, 256. [Google Scholar] [CrossRef] [PubMed]
- Sasannejad, P.; Saeedi, M.; Shoeibi, A.; Gorji, A.; Abbasi, M.; Foroughipour, M. Lavender essential oil in the treatment of migraine headache: A placebo-controlled clinical trial. Eur. Neurol. 2012, 67, 288–291. [Google Scholar] [CrossRef]
- Shamabadi, A.; Akhondzadeh, S. Efficacy and tolerability of Lavandula angustifolia in treating patients with the diagnosis of depression: A systematic review of randomized controlled trials. J. Complement. Integr. Med. 2021, 20, 81–91. [Google Scholar] [CrossRef]
- Rauf, A.; Akram, M.; Semwal, P.; Mujawah, A.A.H.; Muhammad, N.; Riaz, Z.; Munir, N.; Piotrovsky, D.; Vdovina, I.; Bouyahya, A.; et al. Antispasmodic Potential of Medicinal Plants: A Comprehensive Review. Oxid. Med. Cell. Longev. 2021, 2021, 4889719. [Google Scholar] [CrossRef]
- Naghdi, F.; Gholamnezhad, Z.; Boskabady, M.H.; Bakhshesh, M. Muscarinic receptors, nitric oxide formation and cyclooxygenase pathway involved in tracheal smooth muscle relaxant effect of hydro-ethanolic extract of Lavandula angustifolia flowers. Biomed. Pharmacother. 2018, 102, 1221–1228. [Google Scholar] [CrossRef]
- Ueno-Iio, T.; Shibakura, M.; Yokota, K.; Aoe, M.; Hyoda, T.; Shinohata, R.; Kanehiro, A.; Tanimoto, M.; Kataoka, M. Lavender essential oil inhalation suppresses allergic airway inflammation and mucous cell hyperplasia in a murine model of asthma. Life Sci. 2014, 108, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Haybar, H.; Javid, A.Z.; Haghighizadeh, M.H.; Valizadeh, E.; Mohaghegh, S.M.; Mohammadzadeh, A. The effects of Melissa officinalis supplementation on depression, anxiety, stress, and sleep disorder in patients with chronic stable angina. Clin. Nutr. ESPEN 2018, 26, 47–52. [Google Scholar] [CrossRef]
- Bardot, V.; Escalon, A.; Ripoche, I.; Denis, S.; Alric, M.; Chalancon, S.; Chalard, P.; Cotte, C.; Berthomier, L.; Leremboure, M.; et al. Benefits of the ipowder® extraction process applied to: Melissa officinalis L.: Improvement of antioxidant activity and in vitro gastro-intestinal release profile of rosmarinic acid. Food Funct. 2020, 11, 722–729. [Google Scholar] [CrossRef] [Green Version]
- Aubert, P.; Guinobert, I.; Blondeau, C.; Bardot, V.; Ripoche, I.; Chalard, P.; Neunlist, M. Basal and spasmolytic effects of a hydroethanolic leaf extract of Melissa officinalis L. on intestinal motility: An ex vivo study. J. Med. Food 2019, 22, 653–662. [Google Scholar] [CrossRef] [Green Version]
- Farzaei, M.H.; Bahramsoltani, R.; Ghobadi, A.; Farzaei, F.; Najafi, F. Pharmacological activity of Mentha longifolia and its phytoconstituents. J. Tradit. Chin. Med. 2017, 37, 710–720. [Google Scholar] [CrossRef]
- Caro, D.C.; Rivera, D.E.; Ocampo, Y.; Franco, L.A.; Salas, R.D. Pharmacological Evaluation of Mentha spicata L. and Plantago major L., Medicinal Plants Used to Treat Anxiety and Insomnia in Colombian Caribbean Coast. Evidence-Based Complement. Altern. Med. 2018, 2018, 5921514. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ben-Arye, E.; Dudai, N.; Eini, A.; Torem, M.; Schiff, E.; Rakover, Y. Treatment of upper respiratory tract infections in primary care: A randomized study using aromatic herbs. Evidence-Based Complement. Altern. Med. 2011, 2011, 690346. [Google Scholar] [CrossRef] [Green Version]
- Sun, Z.; Wang, H.; Wang, J.; Zhou, L.; Yang, P. Chemical Composition and Anti-Inflammatory, Cytotoxic and Antioxidant Activities of Essential Oil from Leaves of Mentha piperita Grown in China. PLoS ONE 2014, 9, e114767. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.X.; Liu, Y.B.; Ma, A.Q.; Bao, Y.; Wang, M.; Sun, Z.L. In vitro antiviral, anti-inflammatory, and antioxidant activities of the ethanol extract of Mentha piperita L. Food Sci. Biotechnol. 2017, 26, 1675–1683. [Google Scholar] [CrossRef]
- Verma, S.M.; Arora, H.; Dubey, R. Anti—inflammatory and sedative—hypnotic activity of the methanolic extract of the leaves of Mentha arvensis. Anc. Sci. Life 2003, 23, 95–99. [Google Scholar]
- Atta, A.H.; Alkofahi, A. Anti-nociceptive and anti-inflammatory effects of some Jordanian medicinal plant extracts. J. Ethnopharmacol. 1998, 60, 117–124. [Google Scholar] [CrossRef]
- Mahendran, G.; Verma, S.K.; Rahman, L.U. The traditional uses, phytochemistry and pharmacology of spearmint (Mentha spicata L.): A review. J. Ethnopharmacol. 2021, 278, 114266. [Google Scholar] [CrossRef]
- Chen, H.P.; Yang, K.; You, C.X.; Lei, N.; Sun, R.Q.; Geng, Z.F.; Ma, P.; Cai, Q.; Du, S.S.; Deng, Z.W. Chemical constituents and insecticidal activities of the essential oil of Cinnamomum camphora leaves against Lasioderma serricorne. J. Chem. 2014, 2014, 963729. [Google Scholar] [CrossRef] [Green Version]
- Arumugam, G.; Swamy, M.K.; Sinniah, U.R. Plectranthus amboinicus (Lour.) Spreng: Botanical, Phytochemical, Pharmacological and Nutritional Significance. Molecules 2016, 21, 369. [Google Scholar] [CrossRef] [Green Version]
- Lemos, I.C.S.; De Araújo Delmondes, G.; Ferreira Dos Santos, A.D.; Santos, E.S.; De Oliveira, D.R.; De Figueiredo, P.R.L.; De Araújo Alves, D.; Barbosa, R.; De Menezes, I.R.A.; Coutinho, H.D.; et al. Ethnobiological survey of plants and animals used for the treatment of acute respiratory infections in children of a traditional community in the municipality of barbalha, Ceará, Brazil. African J. Tradit. Complement. Altern. Med. 2016, 13, 166–175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmed, H.M.; Babakir-Mina, M. Investigation of rosemary herbal extracts (Rosmarinus officinalis) and their potential effects on immunity. Phyther. Res. 2020, 34, 1829–1837. [Google Scholar] [CrossRef]
- Machado, D.G.; Bettio, L.E.B.; Cunha, M.P.; Capra, J.C.; Dalmarco, J.B.; Pizzolatti, M.G.; Rodrigues, A.L.S. Antidepressant-like effect of the extract of Rosmarinus officinalis in mice: Involvement of the monoaminergic system. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2009, 33, 642–650. [Google Scholar] [CrossRef]
- Malvezzi, L.; Mendes, É.; Militao, L.; Lacalendola, L.; Artem, J.; Barbosa, E.; Gava, P. Rosemary (Rosmarinus officinalis L., syn Salvia rosmarinus Spenn.) and Its Topical Applications: A Review. Plants 2020, 9, 651. [Google Scholar]
- Shin, H.B.; Choi, M.S.; Ryu, B.; Lee, N.R.; Kim, H.I.; Choi, H.E.; Chang, J.; Lee, K.T.; Jang, D.S.; Inn, K.S. Antiviral activity of carnosic acid against respiratory syncytial virus. Virol. J. 2013, 10, 303. [Google Scholar] [CrossRef] [Green Version]
- Bieski, I.G.C.; Leonti, M.; Arnason, J.T.; Ferrier, J.; Rapinski, M.; Violante, I.M.P.; Balogun, S.O.; Pereira, J.F.C.A.; Figueiredo, R.D.C.F.; Lopes, C.R.A.S.; et al. Ethnobotanical study of medicinal plants by population of Valley of Juruena Region, Legal Amazon, Mato Grosso, Brazil. J. Ethnopharmacol. 2015, 173, 383–423. [Google Scholar] [CrossRef] [PubMed]
- Jamila, F.; Mostafa, E. Ethnobotanical survey of medicinal plants used by people in Oriental Morocco to manage various ailments. J. Ethnopharmacol. 2014, 154, 76–87. [Google Scholar] [CrossRef]
- Noureddine, B.; Mostafa, E.; Mandal, S.C. Ethnobotanical, pharmacological, phytochemical, and clinical investigations on Moroccan medicinal plants traditionally used for the management of renal dysfunctions. J. Ethnopharmacol. 2022, 292, 115178. [Google Scholar] [CrossRef] [PubMed]
- Haloui, M.; Louedec, L.; Michel, J.B.; Lyoussi, B. Experimental diuretic effects of Rosmarinus officinalis and Centaurium erythraea. J. Ethnopharmacol. 2000, 71, 465–472. [Google Scholar] [CrossRef] [PubMed]
- Ghorbani, A.; Esmaeilizadeh, M. Pharmacological properties of Salvia officinalis and its components. J. Tradit. Complement. Med. 2017, 7, 433–440. [Google Scholar] [CrossRef]
- Lemle, K.L. Salvia officinalis used in pharmaceutics. IOP Conf. Ser. Mater. Sci. Eng. 2018, 294, 012037. [Google Scholar] [CrossRef]
- Kerkoub, N.; Panda, S.K.; Yang, M.-R.; Lu, J.-G.; Jiang, Z.-H.; Nasri, H.; Luyten, W. Bioassay-Guided Isolation of Anti-Candida Biofilm Compounds from Methanol Extracts of the Aerial Parts of Salvia officinalis (Annaba, Algeria). Front. Pharmacol. 2018, 9, 1418. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karpiński, T.M.; Ożarowski, M.; Seremak-Mrozikiewicz, A.; Wolski, H. Anti-Candida and Antibiofilm Activity of Selected Lamiaceae Essential Oils. Front. Biosci. 2023, 28, 28. [Google Scholar] [CrossRef]
- Jakovljević, M.; Jokić, S.; Molnar, M.; Jašić, M.; Babić, J.; Jukić, H.; Banjari, I. Bioactive Profile of Various Salvia officinalis L. Preparations. Plants 2019, 8, 55. [Google Scholar] [CrossRef] [Green Version]
- Ehrnhöfer-Ressler, M.M.; Fricke, K.; Pignitter, M.; Walker, J.M.; Walker, J.; Rychlik, M.; Somoza, V. Identification of 1,8-Cineole, Borneol, Camphor, and Thujone as Anti-inflammatory Compounds in a Salvia officinalis L. Infusion Using Human Gingival Fibroblasts. J. Agric. Food Chem. 2013, 61, 3451–3459. [Google Scholar] [CrossRef]
- Karimzadeh, S.; Farahpour, M.R. Topical application of Salvia officinalis hydroethanolic leaf extract improves wound healing process. Indian J. Exp. Biol. 2017, 55, 98–106. [Google Scholar] [PubMed]
- Ayrle, H.; Mevissen, M.; Kaske, M.; Nathues, H.; Gruetzner, N.; Melzig, M.; Walkenhorst, M. Medicinal plants—Prophylactic and therapeutic options for gastrointestinal and respiratory diseases in calves and piglets? A systematic review. BMC Vet. Res. 2016, 12, 89. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jarić, S.; Mitrović, M.; Pavlović, P. Review of Ethnobotanical, Phytochemical, and Pharmacological Study of Thymus serpyllum L. Evidence-Based Complement. Altern. Med. 2015, 2015, 101978. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salehi, B.; Prakash Mishra, A.; Shukla, I.; Sharifi-Rad, M.; Del Mar Contreras, M.; Segura-Carretero, A.; Fathi, H.; Nasri Nasrabasi, N.; Kobarfard, F.; Sharifi-Rad, J. Thymol, Thyme, and Other Plant Sources: Health and Potential Uses. Phyther. Res. 2018, 32, 1688–1706. [Google Scholar] [CrossRef]
- Shakeri, F.; Ghorani, V.; Saadat, S.; Gholamnezhad, Z.; Boskabady, M.H. The stimulatory effects of medicinal plants on β2-adrenoceptors of tracheal smooth muscle. Iran. J. Allergy Asthma Immunol. 2019, 18, 12–26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sakkas, H.; Papadopoulou, C. Antimicrobial Activity of Basil, Oregano, and Thyme Essential Oils. J. Microbiol. Biotechnol. 2017, 27, 429–438. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saleem, A.; Afzal, M.; Naveed, M.; Makhdoom, S.I.; Mazhar, M.; Aziz, T.; Khan, A.A.; Kamal, Z.; Shahzad, M.; Alharbi, M.; et al. HPLC, FTIR and GC-MS Analyses of Thymus vulgaris Phytochemicals Executing In Vitro and In Vivo Biological Activities and Effects on COX-1, COX-2 and Gastric Cancer Genes Computationally. Molecules 2022, 27, 8512. [Google Scholar] [CrossRef]
- El Yaagoubi, M.; Mechqoq, H.; El Hamdaoui, A.; Jrv Mukku, V.; El Mousadik, A.; Msanda, F.; El Aouad, N. A review on Moroccan Thymus species: Traditional uses, essential oils chemical composition and biological effects. J. Ethnopharmacol. 2021, 278, 114205. [Google Scholar] [CrossRef]
- Mustafa, B.; Hajdari, A.; Pieroni, A.; Pulaj, B.; Koro, X.; Quave, C.L. A cross-cultural comparison of folk plant uses among Albanians, Bosniaks, Gorani and Turks living in south Kosovo. J. Ethnobiol. Ethnomed. 2015, 11, 39. [Google Scholar] [CrossRef] [Green Version]
- Silva, P.T.M.; Silva, M.A.F.; Silva, L.; Seca, A.M.L. Ethnobotanical knowledge in sete cidades, azores archipelago: First ethnomedicinal report. Plants 2019, 8, 256. [Google Scholar] [CrossRef] [Green Version]
- Aziz, M.A.; Adnan, M.; Khan, A.H.; Shahat, A.A.; Al-Said, M.S.; Ullah, R. Traditional uses of medicinal plants practiced by the indigenous communities at Mohmand Agency, FATA, Pakistan. J. Ethnobiol. Ethnomed. 2018, 14, 2. [Google Scholar] [CrossRef] [Green Version]
- Micucci, M.; Protti, M.; Aldini, R.; Frosini, M.; Corazza, I.; Marzetti, C.; Mattioli, L.B.; Tocci, G.; Chiarini, A.; Mercolini, L.; et al. Thymus vulgaris L. Essential oil solid formulation: Chemical profile and spasmolytic and antimicrobial effects. Biomolecules 2020, 10, 860. [Google Scholar] [CrossRef]
- Rivera, D.; Alcaraz, F.; Obón, C. Wild and cultivated plants used as food and medicine by the Cimbrian ethnic minority in the Alps. I Int. Symp. Med. Aromat. Nutraceutical Plants Mt. Areas 2011, 955, 31–39. [Google Scholar] [CrossRef]
- Alsakhawy, S.A.; Baghdadi, H.H.; El-Shenawy, M.A.; Sabra, S.A.; El-Hosseiny, L.S. Encapsulation of thymus vulgaris essential oil in caseinate/gelatin nanocomposite hydrogel: In vitro antibacterial activity and in vivo wound healing potential. Int. J. Pharm. 2022, 628, 122280. [Google Scholar] [CrossRef] [PubMed]
- Al-Mijalli, S.H.; Mrabti, H.N.; Ouassou, H.; Flouchi, R.; Abdallah, E.M.; Sheikh, R.A.; Alshahrani, M.M.; Awadh, A.A.A.; Harhar, H.; Omari, N.E.; et al. Chemical Composition, Antioxidant, Anti-Diabetic, Anti-Acetylcholinesterase, Anti-Inflammatory, and Antimicrobial Properties of Arbutus unedo L. and Laurus nobilis L. Essential Oils. Life 2022, 12, 1876. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.H.; Shin, J.H.; Kim, S.S.; Lee, H.; Yang, S.R.; Seo, S.R. Laurus nobilis leaf extract controls inflammation by suppressing NLRP3 inflammasome activation. J. Cell. Physiol. 2019, 234, 6854–6864. [Google Scholar] [CrossRef]
- Bouadid, I.; Amssayef, A.; Eddouks, M. Study of the Antihypertensive Effect of Laurus nobilis in Rats. Cardiovasc. Hematol. Agents Med. Chem. 2022, 21, 42–54. [Google Scholar] [CrossRef]
- Benso, B.; Rosalen, P.L.; Alencar, S.M.; Murata, R.M. Malva sylvestris Inhibits Inflammatory Response in Oral Human Cells. An In Vitro Infection Model. PLoS ONE 2015, 10, e0140331. [Google Scholar] [CrossRef] [Green Version]
- Braga, A.S.; Pires, J.G.; Magalhães, A.C. Effect of a mouthrinse containing Malva sylvestris on the viability and activity of microcosm biofilm and on enamel demineralization compared to known antimicrobials mouthrinses. Biofouling 2018, 34, 252–261. [Google Scholar] [CrossRef]
- Gasparetto, J.C.; Martins, C.A.F.; Hayashi, S.S.; Otuky, M.F.; Pontarolo, R. Ethnobotanical and scientific aspects of Malva sylvestris L.: A millennial herbal medicine: Scientific evidences of Malva sylvestris. J. Pharm. Pharmacol. 2012, 64, 172–189. [Google Scholar] [CrossRef]
- Martins, C.; Campos, M.; Irioda, A.; Stremel, D.; Trindade, A.; Pontarolo, R. Anti-Inflammatory Effect of Malva sylvestris, Sida cordifolia, and Pelargonium graveolens Is Related to Inhibition of Prostanoid Production. Molecules 2017, 22, 1883. [Google Scholar] [CrossRef] [Green Version]
- Jeambey, Z.; Johns, T.; Talhouk, S.; Batal, M. Perceived health and medicinal properties of six species of wild edible plants in north-east Lebanon. Public Health Nutr. 2009, 12, 1902–1911. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prudente, A.S.; Loddi, A.M.V.; Duarte, M.R.; Santos, A.R.S.; Pochapski, M.T.; Pizzolatti, M.G.; Hayashi, S.S.; Campos, F.R.; Pontarolo, R.; Santos, F.A.; et al. Pre-clinical Anti-Inflammatory Aspects of a Cuisine and Medicinal Millennial Herb: Malva sylvestris L. Food Chem. Toxicol. 2013, 58, 324–331. [Google Scholar] [CrossRef] [PubMed]
- Fahimi, S.; Abdollahi, M.; Mortazavi, S.A.; Hajimehdipoor, H.; Abdolghaffari, A.H.; Rezvanfar, M.A. Wound Healing Activity of a Traditionally Used Poly Herbal Product in a Burn Wound Model in Rats. Iran. Red Crescent Med. J. 2015, 17, e19960. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pirbalouti, A.G.; Shahrzad, A.; Abed, K.; Hamedi, B. Wound healing activity of Malva sylvestris and Punica granatum in alloxan-induced diabetic rats. Acta Pol. Pharm.—Drug Res. 2010, 67, 511–516. [Google Scholar]
- Prudente, A.S.; Sponchiado, G.; Mendes, D.A.G.B.; Soley, B.S.; Cabrini, D.A.; Otuki, M.F. Pre-clinical efficacy assessment of Malva sylvestris on chronic skin inflammation. Biomed. Pharmacother. 2017, 93, 852–860. [Google Scholar] [CrossRef]
- Mohamadi Yarijani, Z.; Najafi, H.; Shackebaei, D.; Madani, S.H.; Modarresi, M.; Jassemi, S.V. Amelioration of renal and hepatic function, oxidative stress, inflammation and histopathologic damages by Malva sylvestris extract in gentamicin induced renal toxicity. Biomed. Pharmacother. 2019, 112, 108635. [Google Scholar] [CrossRef]
- Javid, A.; Motevalli Haghi, N.; Emami, S.A.; Ansari, A.; Zojaji, S.A.; Khoshkhui, M.; Ahanchian, H. Short-course administration of a traditional herbal mixture ameliorates asthma symptoms of the common cold in children. Avicenna J. Phytomed. 2019, 9, 126–133. [Google Scholar]
- Bohlooli, S.; Mohebipoor, A.; Mohammadi, S.; Kouhnavard, M.; Pashapoor, S. Comparative study of fig tree efficacy in the treatment of common warts (Verruca vulgaris) vs. cryotherapy. Int. J. Dermatol. 2007, 46, 524–526. [Google Scholar] [CrossRef] [Green Version]
- Juergens, U.R. Anti-inflammatory properties of the monoterpene 18-cineole: Current evidence for co-medication in inflammatory airway diseases. Drug Res. 2014, 64, 638–646. [Google Scholar] [CrossRef]
- Zougagh, S.; Belghiti, A.; Rochd, T.; Zerdani, I.; Mouslim, J. Medicinal and Aromatic Plants Used in Traditional Treatment of the Oral Pathology: The Ethnobotanical Survey in the Economic Capital Casablanca, Morocco (North Africa). Nat. Products Bioprospect. 2019, 9, 35–48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosas-Piñón, Y.; Mejía, A.; Díaz-Ruiz, G.; Aguilar, M.I.; Sánchez-Nieto, S.; Rivero-Cruz, J.F. Ethnobotanical survey and antibacterial activity of plants used in the Altiplane region of Mexico for the treatment of oral cavity infections. J. Ethnopharmacol. 2012, 141, 860–865. [Google Scholar] [CrossRef]
- Marchese, A.; Barbieri, R.; Coppo, E.; Orhan, I.E.; Daglia, M.; Nabavi, S.F.; Izadi, M.; Abdollahi, M.; Nabavi, S.M.; Ajami, M. Antimicrobial activity of eugenol and essential oils containing eugenol: A mechanistic viewpoint. Crit. Rev. Microbiol. 2017, 43, 668–689. [Google Scholar] [CrossRef]
- Elmaksoud, H.A.A.; Motawea, M.H.; Desoky, A.A.; Elharrif, M.G.; Ibrahimi, A. Hydroxytyrosol alleviate intestinal inflammation, oxidative stress and apoptosis resulted in ulcerative colitis. Biomed. Pharmacother. 2021, 142, 112073. [Google Scholar] [CrossRef]
- Fernández-Aparicio, Á.; Correa-Rodríguez, M.; Castellano, J.M.; Schmidt-RioValle, J.; Perona, J.S.; González-Jiménez, E. Potential Molecular Targets of Oleanolic Acid in Insulin Resistance and Underlying Oxidative Stress: A Systematic Review. Antioxidants 2022, 11, 1517. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.K.; Zhong, L.; Santiago, J.L. Anti-inflammatory and skin barrier repair effects of topical application of some plant oils. Int. J. Mol. Sci. 2018, 19, 70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Melguizo-rodríguez, L.; de Luna-Bertos, E.; Ramos-torrecillas, J.; Illescas-montesa, R.; Costela-ruiz, V.J.; García-martínez, O. Potential effects of phenolic compounds that can be found in olive oil on wound healing. Foods 2021, 10, 1642. [Google Scholar] [CrossRef] [PubMed]
- Foxlee, R.; Johansson, A.C.; Wejfalk, J.; Dooley, L.; Del Mar, C.B. Topical analgesia for acute otitis media. Cochrane Database Syst. Rev. 2006, 2011, CD005657. [Google Scholar] [CrossRef]
- Nawrot, J.; Wilk-jędrusik, M.; Nawrot, S.; Nawrot, K.; Wilk, B.; Dawid-Pać, R.; Urbańska, M.; Micek, I.; Nowak, G.; Gornowicz-porowska, J. Milky sap of greater celandine (Chelidonium majus L.) and anti-viral properties. Int. J. Environ. Res. Public Health 2020, 17, 1540. [Google Scholar] [CrossRef] [Green Version]
- Nawrot, R. Defense-related Proteins from Chelidonium majus L. as Important Components of its Latex. Curr. Protein Pept. Sci. 2017, 18, 864–880. [Google Scholar] [CrossRef]
- Rautio, M.; Sipponen, A.; Peltola, R.; Lohi, J.; Jokinen, J.J.; Papp, A.; Carlson, P.; Sipponen, P. Antibacterial effects of home-made resin salve from Norway spruce (Picea abies). Apmis 2007, 115, 335–340. [Google Scholar] [CrossRef] [PubMed]
- Jokinen, J.J.; Sipponen, A. Refined Spruce Resin to Treat Chronic Wounds: Rebirth of an Old Folkloristic Therapy. Adv. Wound Care 2016, 5, 198–207. [Google Scholar] [CrossRef] [Green Version]
- Goels, T.; Eichenauer, E.; Tahir, A.; Glasl, S.; Prochaska, P.; Hoeller, F.; Heiß, E.H. Exudates of Picea abies, Pinus nigra, and Larix decidua: Chromatographic Comparison and Pro-Migratory Effects on Keratinocytes In Vitro. Plants. 2023, 11, 599. [Google Scholar] [CrossRef]
- Mitić, Z.S.; Jovanović, B.; Jovanović, S.Č.; Mihajilov-Krstev, T.; Stojanović-Radić, Z.Z.; Cvetković, V.J.; Mitrović, T.L.; Marin, P.D.; Zlatković, B.K.; Stojanović, G.S. Comparative study of the essential oils of four Pinus species: Chemical composition, antimicrobial and insect larvicidal activity. Ind. Crops Prod. 2018, 111, 55–62. [Google Scholar] [CrossRef]
- Ciuman, R.R. Phytotherapeutic and naturopathic adjuvant therapies in otorhinolaryngology. Eur. Arch. Oto-Rhino-Laryngol. 2012, 269, 389–397. [Google Scholar] [CrossRef] [Green Version]
- Majkić, T.; Bekvalac, K.; Beara, I. Plantain (Plantago L.) species as modulators of prostaglandin E2 and thromboxane A2 production in inflammation. J. Ethnopharmacol. 2020, 262. [Google Scholar] [CrossRef]
- Kováč, I.; ɰurkáč, J.; Hollý, M.; Jakubčová, K.; Perže̘ová, V.; Mučaji, P.; Švajdlenka, E.; Sabol, F.; Legáth, J.; Belák, J.; et al. Plantago lanceolata L. water extract induces transition of fibroblasts into myofibroblasts and increases tensile strength of healing skin wounds. J. Pharm. Pharmacol. 2015, 67, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Kurt, B.; Bilge, N.; Sözmen, M.; Aydın, U.; Önyay, T. Özaydın Effects of Plantago lanceolata L. extract on full-thickness excisional wound healing in a mouse model. Biotech. Histochem. 2018, 93, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Miraj, S. A review study of pharmacological properties of Plantago major L. Der Pharma Chem. 2016, 8, 21–25. [Google Scholar]
- Adom, M.B.; Taher, M.; Mutalabisin, M.F.; Amri, M.S.; Abdul Kudos, M.B.; Wan Sulaiman, M.W.A.; Sengupta, P.; Susanti, D. Chemical constituents and medical benefits of Plantago major. Biomed. Pharmacother. 2017, 96, 348–360. [Google Scholar] [CrossRef]
- Najafian, Y.; Hamedi, S.S.; Kaboli Farshchi, M.; Feyzabadi, Z. Plantago major in Traditional Persian Medicine and modern phytotherapy: A narrative review. Electron. Physician 2018, 10, 6390–6399. [Google Scholar] [CrossRef] [Green Version]
- Bhangale, J.; Acharya, S. Antiarthritic activity of Cynodon dactylon (L.) Pers. Indian J. Exp. Biol. 2014, 52, 215–222. [Google Scholar] [PubMed]
- Sadki, C.; Hacht, B.; Souliman, A.; Atmani, F. Acute diuretic activity of aqueous Erica multiflora flowers and Cynodon dactylon rhizomes extracts in rats. J. Ethnopharmacol. 2010, 128, 352–356. [Google Scholar] [CrossRef]
- Golshan, A.; Hayatdavoudi, P.; Hadjzadeh, M.A.-R.; Khajavi Rad, A.; Mohamadian Roshan, N.; Abbasnezhad, A.; Mousavi, S.M.; Pakdel, R.; Zarei, B.; Aghaee, A. Kidney stone formation and antioxidant effects of Cynodon dactylon decoction in male Wistar rats. Avicenna J. Phytomed. 2017, 7, 180–190. [Google Scholar]
- Zhang, Y.; Liu, J.; Guan, L.; Fan, D.; Xia, F.; Wang, A.; Bao, Y.; Xu, Y. By-Products of Zea mays L.: A Promising Source of Medicinal Properties with Phytochemistry and Pharmacological Activities: A Comprehensive Review. Chem. Biodivers. 2023, 20, e202200940. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.X.; Xin, H.L.; Rahman, K.; Wang, S.J.; Peng, C.; Zhang, H. Portulaca oleracea L.: A review of phytochemistry and pharmacological effects. BioMed Res. Int. 2015, 2015, 925631. [Google Scholar] [CrossRef] [Green Version]
- Vasilenko, T.; Kovac, I.; Slezak, M.; Durkac, J.; Perzelova, V.; Coma, M.; Kanuchova, M.; Urban, L.; Szabo, P.; Dvorankova, B.; et al. Agrimonia eupatoria L. Aqueous Extract Improves Skin Wound Healing: An In Vitro Study in Fibroblasts and Keratinocytes and In Vivo Study in Rats. In Vivo (Brooklyn). 2022, 36, 1236–1244. [Google Scholar] [CrossRef]
- Mouro, C.; Dunne, C.P.; Gouveia, I.C. Designing New Antibacterial Wound Dressings: Development of a Dual Layer Cotton Material Coated with Poly(Vinyl Alcohol)_Chitosan Nanofibers Incorporating Agrimonia eupatoria L. Extract. Molecules 2021, 26, 83. [Google Scholar] [CrossRef]
- Qian, H.; Wen-Jun, H.; Hao-Jie, H.; Qing-Wang, H.; Li, C.; Liang, D.; Jie-Jie, L.; Xiang, L.; Ya-Jing, Z.; Ying-Zhi, M.; et al. The four-herb chinese medicine ANBP enhances wound healing and inhibits scar formation via bidirectional regulation of transformation growth factor pathway. PLoS ONE 2014, 9, e112274. [Google Scholar] [CrossRef] [Green Version]
- Malheiros, J.; Simões, D.M.; Figueirinha, A.; Cotrim, M.D.; Fonseca, D.A. Agrimonia eupatoria L.: An integrative perspective on ethnomedicinal use, phenolic composition and pharmacological activity. J. Ethnopharmacol. 2022, 296, 115498. [Google Scholar] [CrossRef]
- Watkins, F.; Pendry, B.; Sanchez-Medina, A.; Corcoran, O. Antimicrobial assays of three native British plants used in Anglo-Saxon medicine for wound healing formulations in 10th century England. J. Ethnopharmacol. 2012, 144, 408–415. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santos, T.N.; Costa, G.; Ferreira, J.P.; Liberal, J.; Francisco, V.; Paranhos, A.; Cruz, M.T.; Castelo-Branco, M.; Figueiredo, I.V.; Batista, M.T. Antioxidant, Anti-Inflammatory, and Analgesic Activities of Agrimonia eupatoria L. Infusion. Evidence-based Complement. Altern. Med. 2017, 2017, 8309894. [Google Scholar] [CrossRef] [Green Version]
- Martino, E.; Collina, S.; Rossi, D.; Bazzoni, D.; Gaggeri, R.; Bracco, F.; Azzolina, O. Influence of the extraction mode on the yield of hyperoside, vitexin and vitexin-2-O-rhamnoside from Crataegus monogyna Jacq. (Hawthorn). Phytochem. Anal. 2008, 19, 534–540. [Google Scholar] [CrossRef]
- Hooman, N.; Mojab, F.; Nickavar, B.; Pouryousefi-Kermani, P. Diuretic effect of powdered Cerasus avium (cherry) tails on healthy volunteers. Pak. J. Pharm. Sci. 2009, 22, 381–383. [Google Scholar] [PubMed]
- Negrean, O.R.; Farcas, A.C.; Pop, O.L.; Socaci, S.A. Blackthorn—A Valuable Source of Phenolic Antioxidants with Potential Health Benefits. Molecules 2023, 28, 3456. [Google Scholar] [CrossRef]
- Tiboni, M.; Coppari, S.; Casettari, L.; Guescini, M.; Colomba, M.; Fraternale, D.; Gorassini, A.; Verardo, G.; Ramakrishna, S.; Guidi, L.; et al. Prunus spinosa extract loaded in biomimetic nanoparticles evokes in vitro anti-inflammatory and wound healing activities. Nanomaterials 2021, 11, 36. [Google Scholar] [CrossRef]
- Coppari, S.; Colomba, M.; Fraternale, D.; Brinkmann, V.; Romeo, M.; Rocchi, M.B.L.; Di Giacomo, B.; Mari, M.; Guidi, L.; Ramakrishna, S.; et al. Antioxidant and anti-inflammaging ability of prune (Prunus spinosa L.) extract result in improved wound healing efficacy. Antioxidants 2021, 10, 374. [Google Scholar] [CrossRef]
- Ayati, Z.; Amiri, M.S.; Ramezani, M.; Delshad, E.; Sahebkar, A.; Emami, S.A. Phytochemistry, Traditional Uses and Pharmacological Profile of Rose Hip: A Review. Curr. Pharm. Des. 2018, 24, 4101–4124. [Google Scholar] [CrossRef] [PubMed]
- van Wietmarschen, H.; van Steenbergen, N.; van der Werf, E.; Baars, E. Effectiveness of herbal medicines to prevent and control symptoms of urinary tract infections and to reduce antibiotic use: A literature review. Integr. Med. Res. 2022, 11, 100892. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, Y.; Liu, X.; Li, J.; Zhang, J.; Liu, D. Chemical constituents and pharmacological activities of medicinal plants from Rosa genus. Chinese Herb. Med. 2022, 14, 187–209. [Google Scholar] [CrossRef] [PubMed]
- Malanga, G.A.; Yan, N.; Stark, J. Mechanisms and efficacy of heat and cold therapies for musculoskeletal injury. Postgrad. Med. 2015, 127, 57–65. [Google Scholar] [CrossRef]
- Mokhtar, M.; Youcefi, F.; Keddari, S.; Saimi, Y.; Otsmane Elhaou, S.; Cacciola, F. Phenolic Content and in Vitro Antioxidant, Anti-Inflammatory and antimicrobial Evaluation of Algerian Ruta graveolens L. Chem. Biodivers. 2022, 19, e202200545. [Google Scholar] [CrossRef]
- Ratheesh, M.; Sindhu, G.; Helen, A. Anti-inflammatory effect of quinoline alkaloid skimmianine isolated from Ruta graveolens L. Inflamm. Res. 2013, 62, 367–376. [Google Scholar] [CrossRef]
- Lans, C. Do recent research studies validate the medicinal plants used in British Columbia, Canada for pet diseases and wild animals taken into temporary care? J. Ethnopharmacol. 2019, 236, 366–392. [Google Scholar] [CrossRef]
- Kenny, O.M.; McCarthy, C.M.; Brunton, N.P.; Hossain, M.B.; Rai, D.K.; Collins, S.G.; Jones, P.W.; Maguire, A.R.; O’Brien, N.M. Anti-inflammatory properties of potato glycoalkaloids in stimulated Jurkat and Raw 264.7 mouse macrophages. Life Sci. 2013, 92, 775–782. [Google Scholar] [CrossRef] [PubMed]
- Visvanathan, R.; Jayathilake, C.; Chaminda Jayawardana, B.; Liyanage, R. Health-beneficial properties of potato and compounds of interest: Health-beneficial properties of potato. J. Sci. Food Agric. 2016, 96, 4850–4860. [Google Scholar] [CrossRef] [PubMed]
- Basilicata, M.G.; Pepe, G.; Rapa, S.F.; Merciai, F.; Ostacolo, C.; Manfra, M.; Di Sarno, V.; Autore, G.; De Vita, D.; Marzocco, S.; et al. Anti-Inflammatory and Antioxidant Properties of Dehydrated Potato-Derived Bioactive Compounds in Intestinal Cells. Int. J. Mol. Sci. 2019, 20, 6087. [Google Scholar] [CrossRef] [Green Version]
- Joshi, B.C.; Mukhija, M.; Kalia, A.N. Pharmacognostical review of Urtica dioica L. Int. J. Green Pharm. 2014, 8, 201–209. [Google Scholar] [CrossRef]
- Asgarpanah, J.; Mohajerani, R. Phytochemistry and pharmacologic properties of Urtica dioica L. J. Med. Plants Res. 2012, 6, 5714–5719. [Google Scholar]
- Bahramsoltani, R.; Rostamiasrabadi, P.; Shahpiri, Z.; Marques, A.M.; Rahimi, R.; Farzaei, M.H. Aloysia citrodora Paláu (Lemon verbena): A review of phytochemistry and pharmacology. J. Ethnopharmacol. 2018, 222, 34–51. [Google Scholar] [CrossRef]
- Picon, P.D.; Picon, R.V.; Costa, A.F.; Sander, G.B.; Amaral, K.M.; Aboy, A.L.; Henriques, A.T. Randomized clinical trial of a phytotherapic compound containing Pimpinella anisum, Foeniculum vulgare, Sambucus nigra, and Cassia augustifolia for chronic constipation. BMC Complement. Altern. Med. 2010, 10, 17. [Google Scholar] [CrossRef] [Green Version]
- Porter, R.S.; Bode, R.F. A Review of the Antiviral Properties of Black Elder (Sambucus nigra L.) Products: Antiviral Properties of Black Elder (Sambucus nigra L.). Phyther. Res. 2017, 31, 533–554. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Zuckerman, D.M.; Brantley, S.; Sharpe, M.; Childress, K.; Hoiczyk, E.; Pendleton, A.R. Sambucus nigra extracts inhibit infectious bronchitis virus at an early point during replication. BMC Vet. Res. 2014, 10, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hawkins, J.; Baker, C.; Cherry, L.; Dunne, E. Black elderberry (Sambucus nigra) supplementation effectively treats upper respiratory symptoms: A meta-analysis of randomized, controlled clinical trials. Complement. Ther. Med. 2019, 42, 361–365. [Google Scholar] [CrossRef] [PubMed]
- Harnett, J.; Oakes, K.; Carè, J.; Leach, M.; Brown, D.; Holger, C.; Pinder, T.-A.; Steel, A.; Anheyer, D. The effects of Sambucus nigra berry on acute respiratory viral infections: A rapid review of clinical studies. Adv. Integr. Med. 2020, 7, 240–246. [Google Scholar] [CrossRef]
- Skowrońska, W.; Granica, S.; Czerwińska, M.E.; Osińska, E.; Bazylko, A. Sambucus nigra L. leaves inhibit TNF-α secretion by LPS-stimulated human neutrophils and strongly scavenge reactive oxygen species. J. Ethnopharmacol. 2022, 290, 115116. [Google Scholar] [CrossRef] [PubMed]
- Poles, J.; Karhu, E.; Mcgill, M.; Mcdaniel, H.R.; Lewis, J.E. The effects of twenty-one nutrients and phytonutrients on cognitive function: A narrative review. J. Clin. Transl. Res. 2021, 7, 333–376. [Google Scholar] [CrossRef]
- Mao, Q.Q.; Xu, X.Y.; Cao, S.Y.; Gan, R.Y.; Corke, H.; Beta, T.; Li, H. Bin Bioactive compounds and bioactivities of ginger (Zingiber officinale Roscoe). Foods 2019, 8, 185. [Google Scholar] [CrossRef] [Green Version]
- Karampour, N.S.; Arzi, A.; Rezaie, A.; Pashmforoosh, M.; Kordi, F. Gastroprotective effect of zingerone on ethanol-induced gastric ulcers in rats. Medicina 2019, 55, 64. [Google Scholar] [CrossRef] [Green Version]
- Nikkhah Bodagh, M.; Maleki, I.; Hekmatdoost, A. Ginger in gastrointestinal disorders: A systematic review of clinical trials. Food Sci. Nutr. 2019, 7, 96–108. [Google Scholar] [CrossRef]






| Gender | #Informants | Species | Citations | ||
|---|---|---|---|---|---|
| Mean | ±(sd) | Mean | ±(sd) | ||
| Male | 37 | 3.57 | 4.65 | 5.51 | 8.21 |
| Female | 56 | 5.25 | 4.67 | 8.44 | 9.29 |
| Age | |||||
| 20–29 | 5 | 4.30 | 7.30 | 9.37 | 5.90 |
| 30–39 | 6 | 4.66 | 5.31 | 6.16 | 7.65 |
| 40–49 | 10 | 5.30 | 3.79 | 8.10 | 7.40 |
| 50–59 | 16 | 5.69 | 7.31 | 9.37 | 10.36 |
| 60–69 | 13 | 4.46 | 2.29 | 8.46 | 6.60 |
| 70–79 | 32 | 4.40 | 4.99 | 6.62 | 8.70 |
| 80–89 | 9 | 3.22 | 2.43 | 5.22 | 6.02 |
| 90–96 | 2 | 3.00 | 2.83 | 4.00 | 2.83 |
| All informants | 93 | 4.58 | 4.70 | 7.28 | 8.95 |
| Species | Use Described in the Manuscript [17] | Use Described in Valle Imagna | Activity |
|---|---|---|---|
| Amaryllidaceae | |||
| Allium sativum L. Garlic | Wine decoction with garlic, drunk against hip pain. Eaten raw against gout. | Ointment made of smashed garlic and pork fat to be externally applied against contusions and pain. | Anti-inflammatory for the treatment of musculoskeletal problems. |
| Asteraceae | |||
| Artemisia absinthium L. Absinth | Pills or aqueous preparation with absinth salts (obtained from the ashes of A. absinthium) as diuretic and antipyretic. Powdered absinth mixed with honey. Kept inside the mouth as an anti-inflammatory for the tongue. | Infusion of the leaves or the entire above ground part drunk as an antipyretic, anti-inflammatory, and diuretic. | Anti-inflammatory, antipyretic, and diuretic. |
| Matricaria chamomilla L. Chamomile | Chamomile oil mixed with other ingredients, clysters against hip pain and sciatica. | Macerated chamomile oil, applied externally against muscular pain and inflammation. | Anti-inflammatory for the treatment of musculoskeletal problems. |
| Brassicaceae | |||
| Brassica oleracea L. Cabbage | Cabbage leaves dried in the oven, powdered, and mixed with pork fat. The ointment is applied against hip pain. | Fresh leaves, smashed and applied on contusions and joint pain and inflammations. | Anti-inflammatory for the treatment of musculoskeletal problems. |
| Lamiaceae | |||
| Salvia officinalis L. Sage | Sage leaves, rosemary, and pomegranate boiled in wine. Mouthwashes against painful and loosening teeth. | Sage leaves rubbed on teeth and gums as an anti-inflammatory. Sage infusion used as an anti-inflammatory and disinfectant mouthwash for teeth and gums. | Antibacterial Anti-inflammatory |
| Linaceae | |||
| Linum usitatissimum L. Flax | Flax and fenugreek seeds boiled in water. The seeds are squeezed, and the mucilaginous water is mixed with butter. The ointment is applied on the chest against children cough. | Flax seeds boiled in water until a preparation similar to porridge is obtained. The mucilaginous poultice is applied warm on the chest against cough. | Antitussive Expectorant |
| Oleaceae | |||
| Olea europaea L. Olive | An ointment of olive oil and bee wax or of olive oil and tallow applied on fissured hands and feet and on burns. | An ointment of olive oil and bee wax applied on burns and as soothing agent on inflamed skin. | Anti-inflammatory Soothing Wound healing |
| Papaveraceae | |||
| Chelidonium majus L. Greater celandine | Latex of celandine with latex of parsnip applied on warts. | Latex of celandine applied on warts. | Antiviral Caustic |
| Plantaginaceae | |||
| Plantago spp. Plantago major L. Plantago lanceolata L. Plantain | Poultice of plantain mixed with butter (and other ingredients) for two different ointments applied on inflamed nipples and on wounds. | Leaves applied externally on burns and as a wound healing and anti-inflammatory agent. | Anti-inflammatory Disinfectant Wound healing |
| Rosaceae | |||
| Agrimonia eupatoria L. Agrimony | Complex remedy applied on deep leg ulcers. Above ground parts of agrimony and dried roses are boiled in wine, which is then used to disinfect the ulcers. A mix of powdered herbal and mineral ingredients are then applied on the wounds. Finally, the sediment of cooked agrimony and rose is smeared over the powder, and all is set in place with a gauze. | Fresh leaves are applied externally on deep wounds and set in place with a gauze. Compresses of the infusion of leaves or of above ground parts of agrimony are applied on deep wounds. | Anti-inflammatory Disinfectant Wound healing |
| Rosa canina L. Dog rose | Rose water, obtained from the petals, mixed with other ingredients and drunk in order to ‘refresh the kidneys’. | Infusions of the false fruits drunk as diuretic. | Diuretic |
| Vitaceae | |||
| Vitis vinifera L. Vitis spp. Grapevine | Mouth washes with wine in which sage, rosemary, and pomegranate were boiled. | Mouth washes with grappa. | Anti-inflammatory Disinfectant (Alcohol?) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Milani, F.; Bottoni, M.; Bardelli, L.; Colombo, L.; Colombo, P.S.; Bruschi, P.; Giuliani, C.; Fico, G. Remnants from the Past: From an 18th Century Manuscript to 21st Century Ethnobotany in Valle Imagna (Bergamo, Italy). Plants 2023, 12, 2748. https://doi.org/10.3390/plants12142748
Milani F, Bottoni M, Bardelli L, Colombo L, Colombo PS, Bruschi P, Giuliani C, Fico G. Remnants from the Past: From an 18th Century Manuscript to 21st Century Ethnobotany in Valle Imagna (Bergamo, Italy). Plants. 2023; 12(14):2748. https://doi.org/10.3390/plants12142748
Chicago/Turabian StyleMilani, Fabrizia, Martina Bottoni, Laura Bardelli, Lorenzo Colombo, Paola Sira Colombo, Piero Bruschi, Claudia Giuliani, and Gelsomina Fico. 2023. "Remnants from the Past: From an 18th Century Manuscript to 21st Century Ethnobotany in Valle Imagna (Bergamo, Italy)" Plants 12, no. 14: 2748. https://doi.org/10.3390/plants12142748
APA StyleMilani, F., Bottoni, M., Bardelli, L., Colombo, L., Colombo, P. S., Bruschi, P., Giuliani, C., & Fico, G. (2023). Remnants from the Past: From an 18th Century Manuscript to 21st Century Ethnobotany in Valle Imagna (Bergamo, Italy). Plants, 12(14), 2748. https://doi.org/10.3390/plants12142748







