Short-Term Impact of Recycling-Derived Fertilizers on Their P Supply for Perennial Ryegrass (Lolium perenne)
Abstract
1. Introduction
2. Results
2.1. Shoot Biomass Yield, Agronomic Efficiency and Elemental Analysis of Ryegrass Biomass
2.2. RDF Influence on P-Mineralizing and P-Solubilizing Microbial Community
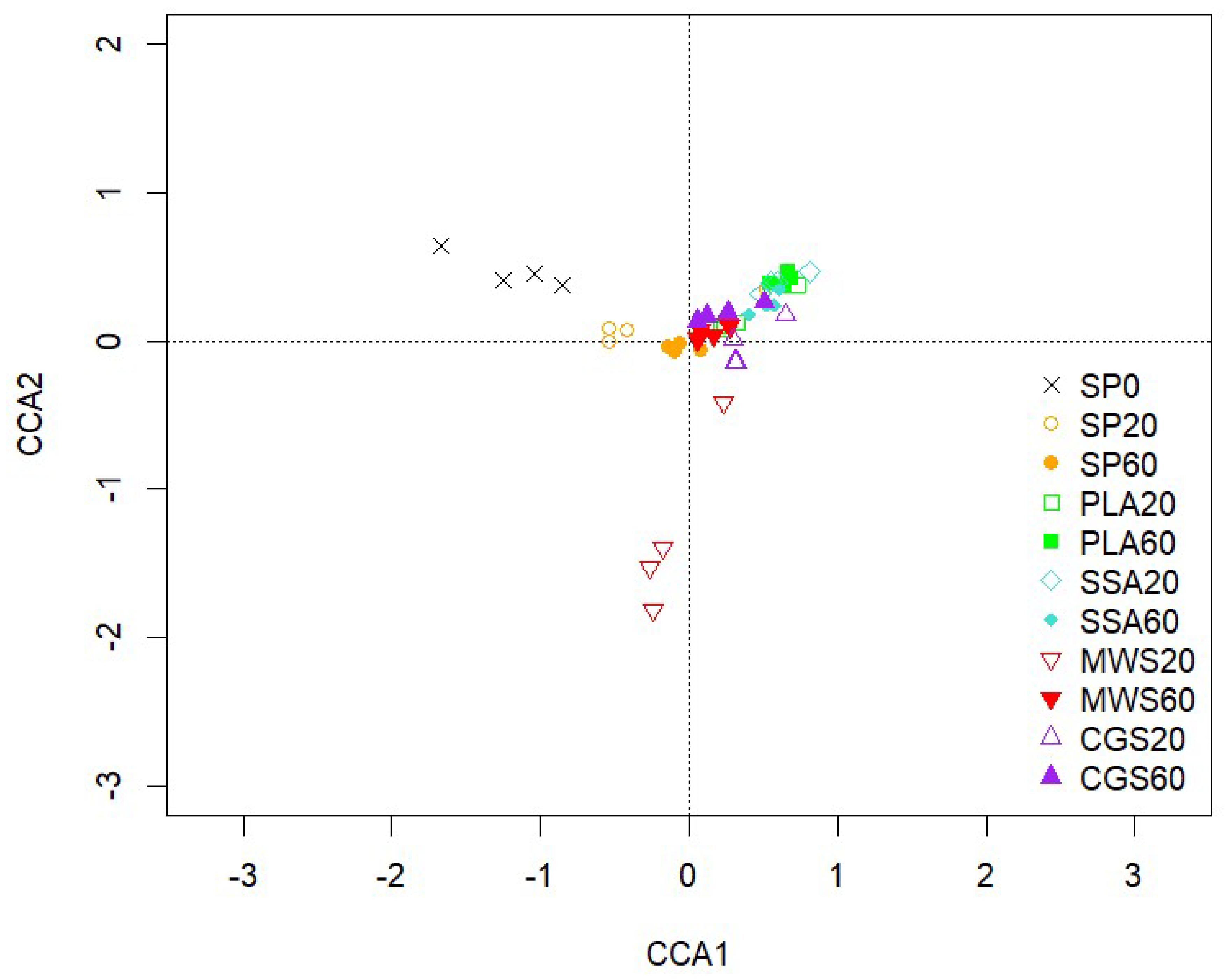
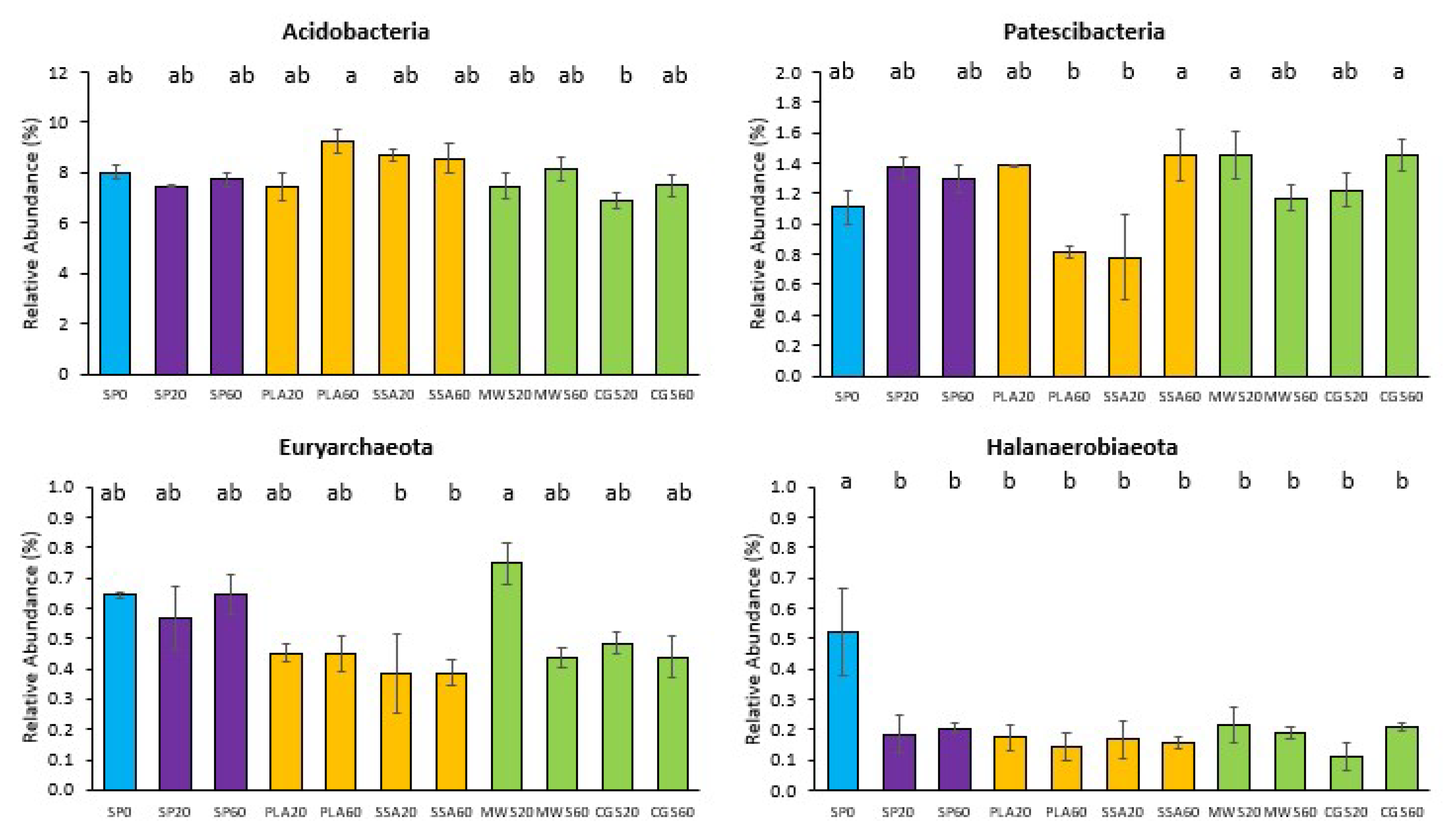
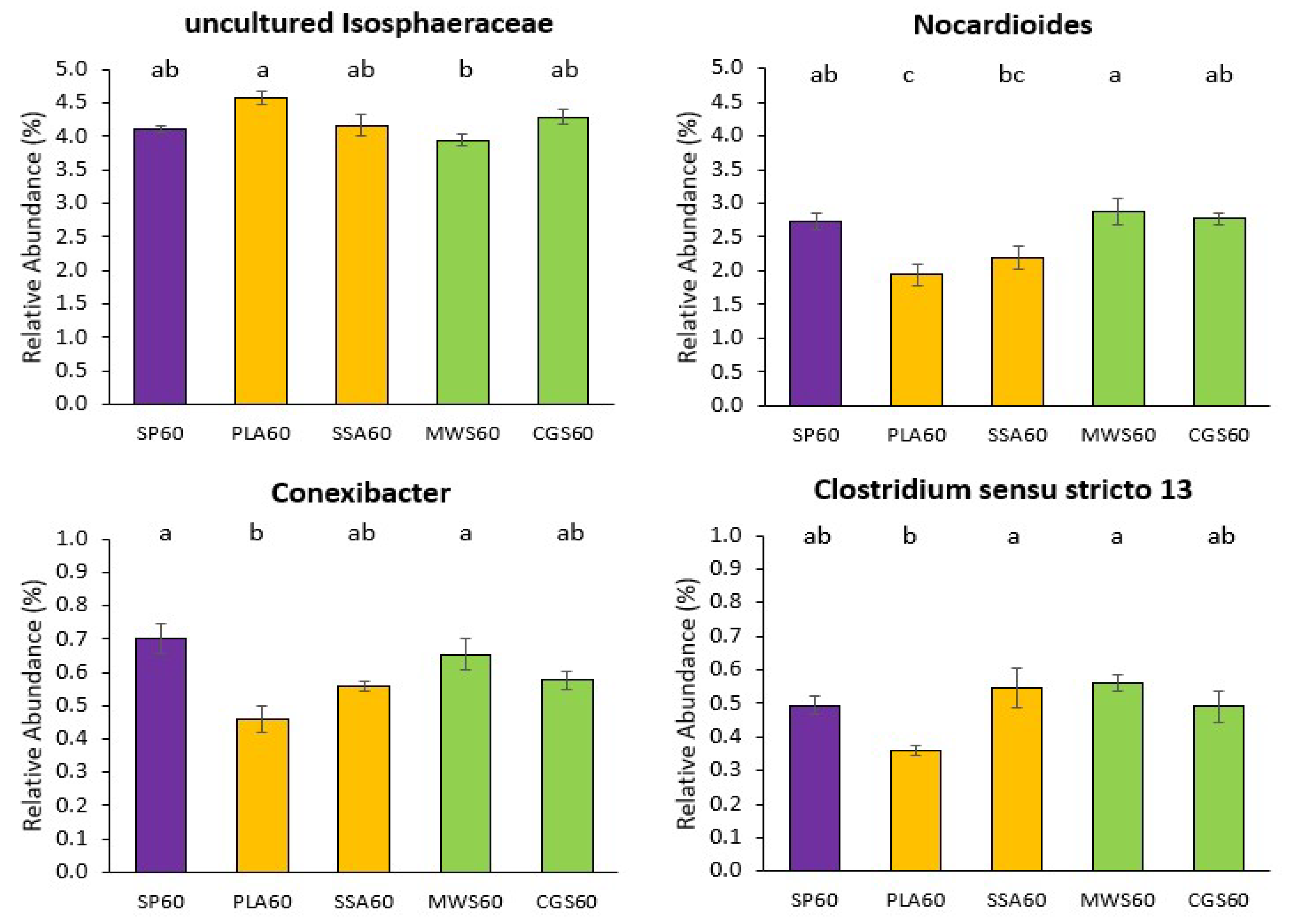
2.3. DNA Fingerprint Analysis of Bacterial Community Structure and Next-Generation Sequencing of Bacterial 16S rRNA Gene
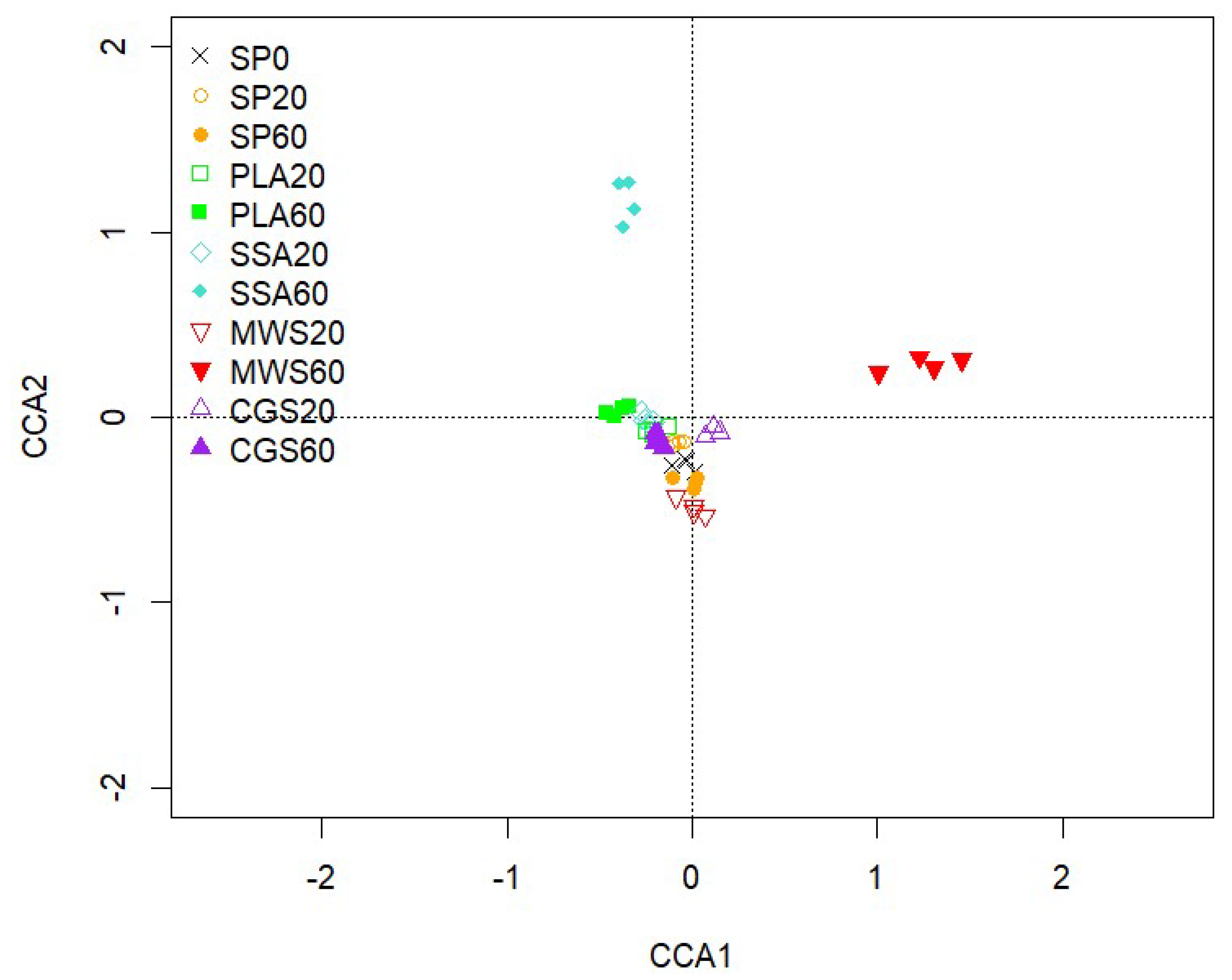
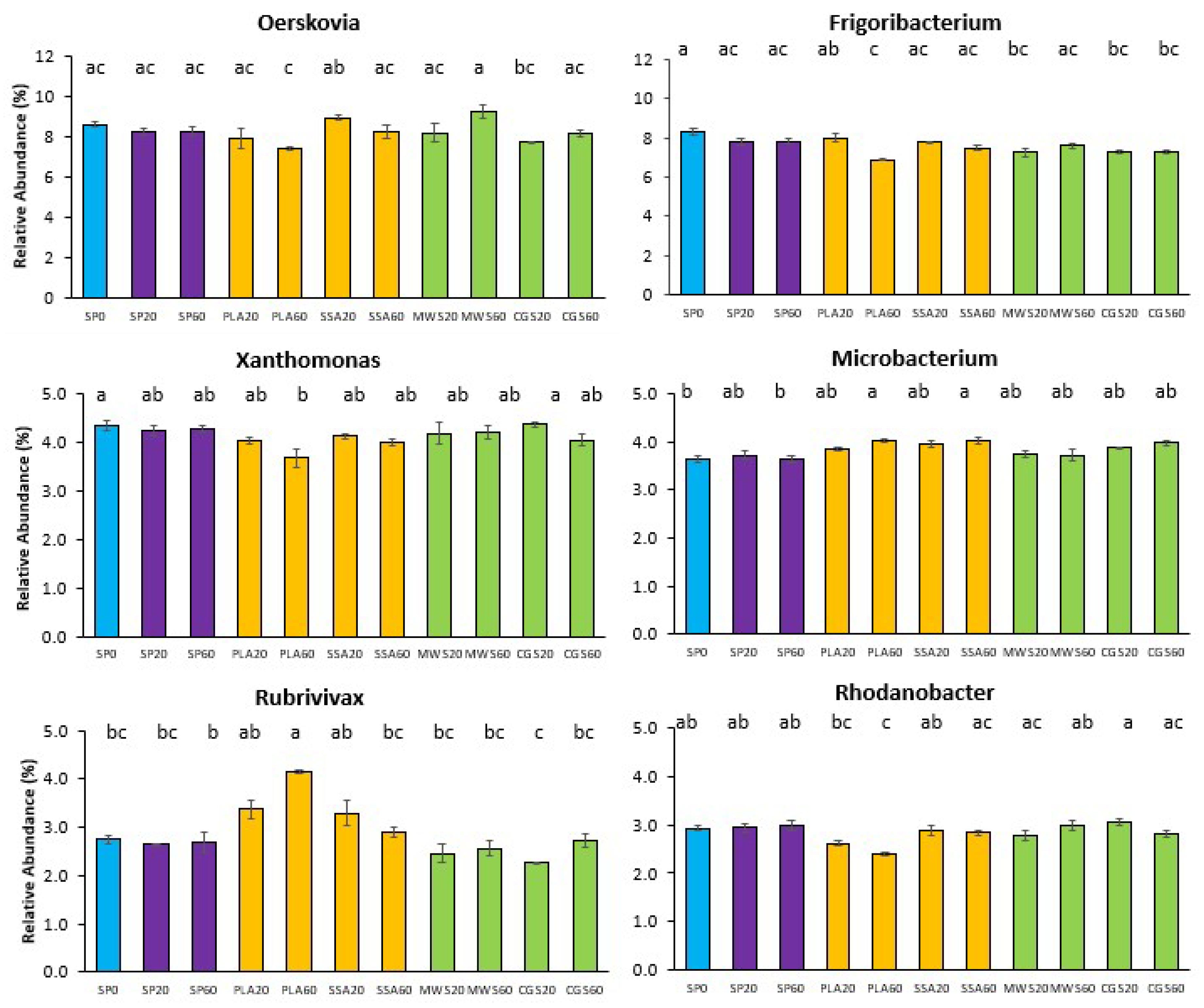
2.4. Next-Generation Sequencing Analysis of the phoD-Harboring Bacterial Community
3. Discussion
4. Materials and Methods
4.1. Pot Trial Setup and Fertilizer Preparation
4.2. Harvest Procedure of Plants and Soil
4.3. Post-Harvest Experiments
4.4. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Elser, J.J. Phosphorus: A Limiting Nutrient for Humanity? Curr. Opin. Biotechnol. 2012, 23, 833–838. [Google Scholar] [CrossRef] [PubMed]
- Roy, E.D.; Richards, P.D.; Martinelli, L.A.; Coletta, L.D.; Lins, S.R.M.; Vazquez, F.F.; Willig, E.; Spera, S.A.; VanWey, L.K.; Porder, S. The Phosphorus Cost of Agricultural Intensification in the Tropics. Nat. Plants 2016, 2, 16043. [Google Scholar] [CrossRef] [PubMed]
- Bindraban, P.S.; Dimkpa, C.O.; Pandey, R. Exploring Phosphorus Fertilizers and Fertilization Strategies for Improved Human and Environmental Health. Biol. Fertil. Soils 2020, 56, 299–317. [Google Scholar] [CrossRef]
- European Commission. European Communities—Directive Concerning Urban Waste Water Treatment (91/271/EEC); European Commission: Brussels, Belgium, 1991; Volume 135, p. 30. [Google Scholar]
- Carpenter, S.R.; Bennett, E.M. Reconsideration of the Planetary Boundary for Phosphorus. Environ. Res. Lett. 2011, 6, 014009. [Google Scholar] [CrossRef]
- Cordell, D.; Rosemarin, A.; Schröder, J.J.; Smit, A.L. Towards Global Phosphorus Security: A Systems Framework for Phosphorus Recovery and Reuse Options. Chemosphere 2011, 84, 747–758. [Google Scholar] [CrossRef]
- van Kauwenbergh, S. World Phosphate Rock Reserves and Resources; The International Fertilizer Development Center (IFDC): Muscle Shoals, AL, USA, 2010. [Google Scholar]
- Bano, S.A.; Iqbal, S.M. Biological Nitrogen Fixation to Improve Plant Growth and Productivity. Int. J. Agric. Innov. Res. 2016, 4, 2319-1473. [Google Scholar]
- Richardson, A.E.; Simpson, R.J. Soil Microorganisms Mediating Phosphorus Availability Update on Microbial Phosphorus. Plant Physiol. 2011, 156, 989–996. [Google Scholar] [CrossRef]
- Custódio, V.; Gonin, M.; Stabl, G.; Bakhoum, N.; Oliveira, M.M.; Gutjahr, C.; Castrillo, G. Sculpting the Soil Microbiota. Plant J. 2022, 109, 508–522. [Google Scholar] [CrossRef]
- Mukerji, K.G.; Manoharachary, C.; Singh, J. Microbial Activity in the Rhizosphere; Springer: Berlin, Germany; New York, NY, USA, 2006. [Google Scholar]
- Borrelli, P.; Robinson, D.A.; Panagos, P.; Lugato, E.; Yang, J.E.; Alewell, C.; Wuepper, D.; Montanarella, L.; Ballabio, C. Land Use and Climate Change Impacts on Global Soil Erosion by Water (2015–2070). Proc. Natl. Acad. Sci. USA 2020, 117, 21994–22001. [Google Scholar] [CrossRef]
- Kaminsky, L.M.; Thompson, G.L.; Trexler, R.V.; Bell, T.H.; Kao-Kniffin, J. Medicago sativa has Reduced Biomass and Nodulation When Grown with Soil Microbiomes Conditioned to High Phosphorus Inputs. Phytobiomes J. 2018, 2, 237–248. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, J.; Li, D.; Xu, C.; Xiang, X. Differential Responses of Arbuscular Mycorrhizal Fungal Communities to Mineral and Organic Fertilization. MicrobiologyOpen 2020, 9, e00920. [Google Scholar] [CrossRef]
- McLaughlin, A.; Mineau, P. The Impact of Agricultural Practices on Biodiversity. Agric. Ecosyst. Environ. 1995, 55, 201–212. [Google Scholar] [CrossRef]
- Mozumder, P.; Berrens, R.P. Inorganic Fertilizer Use and Biodiversity Risk: An Empirical Investigation. Ecol. Econ. 2007, 62, 538–543. [Google Scholar] [CrossRef]
- Cornel, P.; Schaum, C. Phosphorus Recovery from Wastewater: Needs, Technologies and Costs. Water Sci. Technol. 2009, 59, 1069–1076. [Google Scholar] [CrossRef]
- Roy, E.D. Phosphorus Recovery and Recycling with Ecological Engineering: A Review. Ecol. Eng. 2017, 98, 213–227. [Google Scholar] [CrossRef]
- Chojnacka, K.; Moustakas, K.; Witek-Krowiak, A. Bio-Based Fertilizers: A Practical Approach Towards Circular Economy. Bioresour. Technol. 2020, 295, 122223. [Google Scholar] [CrossRef]
- Postma, R.; Saju, A.; Egene, C.E.; Meers, E.; Sigurnjak, I.; Bouthier, A.; Lagrange, H.; Murphy, J.; Forrestal, P. WPT2, Activity 3 Deliverable 3.1: Protocols for the Evaluation of the Agronomic Value of Recycling-Derived Fertilisers; Interreg North West Europe: Wageningen, The Netherlands; Brussels, Belgium, 2020. [Google Scholar] [CrossRef]
- El Wali, M.; Golroudbary, S.R.; Kraslawski, A. Impact of Recycling Improvement on the Life cycle of Phosphorus. Chin. J. Chem. Eng. 2019, 27, 1219–1229. [Google Scholar] [CrossRef]
- Mogollón, J.M.; Beusen, A.H.W.; van Grinsven, H.J.M.; Westhoek, H.; Bouwman, A.F. Future Agricultural Phosphorus Demand According to the Shared Socioeconomic Pathways. Glob. Environ. Change 2018, 50, 149–163. [Google Scholar] [CrossRef]
- Brown, S.; Beecher, N.; Carpenter, A. Calculator Tool for Determining Greenhouse Gas Emissions for Biosolids Processing and End Use. Environ. Sci. Technol. 2010, 44, 9509–9515. [Google Scholar] [CrossRef]
- Gherghel, A.; Teodosiu, C.; De Gisi, S. A Review on Wastewater Sludge Valorisation and Its Challenges in the Context of Circular Economy. J. Clean. Prod. 2019, 228, 244–263. [Google Scholar] [CrossRef]
- Ramankutty, N.; Evan, A.T.; Monfreda, C.; Foley, J.A. Farming the Planet: 1. Geographic Distribution of Global Agricultural Lands in the Year 2000: Global Agricultural Lands in 2000. Glob. Biogeochem. Cycles 2008, 22, GB1003. [Google Scholar] [CrossRef]
- Virto, I.; Imaz, M.; Fernández-Ugalde, O.; Gartzia-Bengoetxea, N.; Enrique, A.; Bescansa, P. Soil Degradation and Soil Quality in Western Europe: Current Situation and Future Perspectives. Sustainability 2014, 7, 313–365. [Google Scholar] [CrossRef]
- Deinert, L.; Egeter, B.; Ikoyi, I.; Forrestal, P.; Schmalenberger, A. Short Term Impact of Recycling-Derived Fertilizers on Their P Supply for Perennial Ryegrass (Lolium perenne). bioRxiv 2023. [Google Scholar] [CrossRef]
- Schütz, L.; Gattinger, A.; Meier, M.; Müller, A.; Boller, T.; Mäder, P.; Mathimaran, N. Improving Crop Yield and Nutrient Use Efficiency via Biofertilization—A Global Meta-Analysis. Front. Plant Sci. 2018, 8, 2204. [Google Scholar] [CrossRef] [PubMed]
- González, C.; Fernández, B.; Molina, F.; Camargo-Valero, M.A.; Peláez, C. The Determination of Fertiliser Quality of the Formed Struvite from a WWTP. Water Sci. Technol. 2021, 83, 3041–3053. [Google Scholar] [CrossRef]
- Degryse, F.; Baird, R.; da Silva, R.C.; McLaughlin, M.J. Dissolution Rate and Agronomic Effectiveness of Struvite Fertilizers—Effect of Soil pH, Granulation and Base Excess. Plant Soil 2017, 410, 139–152. [Google Scholar] [CrossRef]
- Fox, A.; Kwapinski, W.; Griffiths, B.S.; Schmalenberger, A. The Role of Sulfur- and Phosphorus-Mobilizing Bacteria in Biochar-Induced Growth Promotion of Lolium perenne. FEMS Microbiol. Ecol. 2014, 90, 78–91. [Google Scholar] [CrossRef]
- Pang, P.C.K.; Kolenko, H. Phosphomonoesterase Activity in Forest Soils. Soil Biol. Biochem. 1986, 18, 35–39. [Google Scholar] [CrossRef]
- Herbien, S.A.; Neal, J.L. Soil pH and Phosphatase Activity. Commun. Soil Sci. Plant Anal. 1990, 21, 439–456. [Google Scholar] [CrossRef]
- Nannipieri, P.; Giagnoni, L.; Landi, L.; Renella, G. Role of Phosphatase Enzymes in Soil. In Phosphorus in Action; Bünemann, E., Oberson, A., Frossard, E., Eds.; Springer: Berlin/Heidelberg, Germany, 2011; Volume 26, pp. 215–243. [Google Scholar]
- Harrison, A.F. Relationship Between Intensity of Phosphatase Activity and Physico-Chemical Properties in Woodland Soils. Soil Biol. Biochem. 1983, 15, 93–99. [Google Scholar] [CrossRef]
- Saha, S.; Mina, B.L.; Gopinath, K.A.; Kundu, S.; Gupta, H.S. Relative Changes in Phosphatase Activities as Influenced by Source and Application Rate of Organic Composts in Field Crops. Bioresour. Technol. 2008, 99, 1750–1757. [Google Scholar] [CrossRef]
- Robinson, W.D.; Park, J.; Tran, H.T.; Del Vecchio, H.A.; Ying, S.; Zins, J.L.; Patel, K.; McKnight, T.D.; Plaxton, W.C. The Secreted Purple Acid Phosphatase Isozymes AtPAP12 and AtPAP26 Play a Pivotal Role in Extracellular Phosphate-Scavenging by Arabidopsis thaliana. J. Exp. Bot. 2012, 63, 6531–6542. [Google Scholar] [CrossRef]
- Kielak, A.M.; Barreto, C.C.; Kowalchuk, G.A.; van Veen, J.A.; Kuramae, E.E. The Ecology of Acidobacteria: Moving beyond Genes and Genomes. Front. Microbiol. 2016, 7, 744. [Google Scholar] [CrossRef]
- Jones, R.T.; Robeson, M.S.; Lauber, C.L.; Hamady, M.; Knight, R.; Fierer, N. A Comprehensive Survey of Soil Acidobacterial Diversity Using Pyrosequencing and Clone Library Analyses. ISME J. 2009, 3, 442–453. [Google Scholar] [CrossRef]
- Navarrete, A.A.; Kuramae, E.E.; de Hollander, M.; Pijl, A.S.; van Veen, J.A.; Tsai, S.M. Acidobacterial community Responses to Agricultural Management of Soybean in Amazon Forest Soils. FEMS Microbiol. Ecol. 2013, 83, 607–621. [Google Scholar] [CrossRef]
- Li, Y.; Chi, J.; Ao, J.; Gao, X.; Liu, X.; Sun, Y.; Zhu, W. Effects of Different Continuous Cropping Years on Bacterial Community and Diversity of Cucumber Rhizosphere Soil in Solar-Greenhouse. Curr. Microbiol. 2021, 78, 2380–2390. [Google Scholar] [CrossRef]
- Song, Q.; Song, X.; Deng, X.; Luo, J.; Wang, J.; Min, K.; Song, R. Effects of Plant Growth Promoting Rhizobacteria Microbial on the Growth, Rhizosphere Soil Properties, and Bacterial Community of Pinus sylvestris var. mongolica Seedlings. Scand. J. For. Res. 2021, 36, 249–262. [Google Scholar] [CrossRef]
- Shrivastava, N.; Mahajan, S.; Varma, A. Symbiotic Soil Microorganisms: Biology and Applications; Springer: Cham, Switzerland, 2021; p. 489. [Google Scholar]
- Verma, P.; Yadav, A.N.; Kumar, V.; Singh, D.P.; Saxena, A.K. Beneficial Plant-Microbes Interactions: Biodiversity of Microbes from Diverse Extreme Environments and Its Impact for Crop Improvement. In Plant-Microbe Interactions in Agro-Ecological Perspectives; Singh, D.P., Singh, H.B., Prabha, R., Eds.; Springer: Singapore, 2017; pp. 543–580. [Google Scholar]
- Yadav, A.N.; Verma, P.; Kaushik, R.; Dhaliwal, H.; Saxena, A. Archaea Endowed with Plant Growth Promoting Attributes. EC Microbiol. 2017, 8, 294–298. [Google Scholar]
- Li, Y.; Ma, J.; Yong, X.; Luo, L.; Wong, J.W.C.; Zhang, Y.; Wu, H.; Zhou, J. Effect of Biochar Combined With A Biotrickling Filter on Deodorization, Nitrogen Retention, and Microbial Community Succession During Chicken Manure Composting. Bioresour. Technol. 2022, 343, 126137. [Google Scholar] [CrossRef]
- Xie, G.; Kong, X.; Kang, J.; Su, N.; Luo, G.; Fei, J. Community-Level Dormancy Potential Regulates Bacterial Beta-Diversity Succession During the Co-Composting of Manure and Crop Residues. Sci. Total Environ. 2021, 772, 145506. [Google Scholar] [CrossRef]
- Heitkamp, K.; Latorre-Pérez, A.; Nefigmann, S.; Gimeno-Valero, H.; Vilanova, C.; Jahmad, E.; Abendroth, C. Monitoring of Seven Industrial Anaerobic Digesters Supplied with Biochar. Biotechnol. Biofuels 2021, 14, 185. [Google Scholar] [CrossRef] [PubMed]
- Deng, Q.; Zhang, T.; Xie, D.; Yang, Y. Rhizosphere Microbial Communities Are Significantly Affected by Optimized Phosphorus Management in a Slope Farming System. Front. Microbiol. 2021, 12, 739844. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Yang, L.; Liu, W.; Zhang, Y.; Zhou, Q.; Wu, Z.; He, F. Effects of Root Exudates on Rhizosphere Bacteria and Nutrient Removal in Pond-Ditch Circulation Systems (PDCSs) for Rural Wastewater Treatment. Sci. Total Environ. 2021, 785, 147282. [Google Scholar] [CrossRef]
- Wang, Y.; Huang, Q.; Gao, H.; Zhang, R.; Yang, L.; Guo, Y.; Li, H.; Awasthi, M.K.; Li, G. Long-Term Cover Crops Improved Soil Phosphorus Availability in a Rain-Fed Apple Orchard. Chemosphere 2021, 275, 130093. [Google Scholar] [CrossRef] [PubMed]
- Robles-Aguilar, A.A.; Grunert, O.; Hernandez-Sanabria, E.; Mysara, M.; Meers, E.; Boon, N.; Jablonowski, N.D. Effect of Applying Struvite and Organic N as Recovered Fertilizers on the Rhizosphere Dynamics and Cultivation of Lupine (Lupinus angustifolius). Front. Plant Sci. 2020, 11, 572741. [Google Scholar] [CrossRef] [PubMed]
- Plunkett, M.; Wall, D.; Forrestal, P. Spring Fertilizer Advice—Grassland; Teagasc Johnstown Castle: Wexford, Ireland, 2016. [Google Scholar]
- Plunkett, M.; Wall, D.P. Major & Micro Nutrient Advice for Productive Agricultural Crops; Teagasc Soils, Environment and Land Use Research Centre, Johnstown Castle: Wexford, Ireland, 2016. [Google Scholar]
- Reasoner, D.J.; Geldreich, E.E. A new medium for the enumeration and subculture of bacteria from potable water. Appl. Environ. Microbiol. 1985, 49, 1–7. [Google Scholar] [CrossRef]
- Tabatabai, M.A.; Bremner, J.M. Use of p-Nitrophenyl Phosphate for Assay of Soil Phosphatase Activity. Soil Biol. Biochem. 1969, 1, 301–307. [Google Scholar] [CrossRef]
- Daly, K.; Casey, A. Evaluating Morgan’s Phosphorus Test as an Environmental Indicator; Johnstown Castle Research Centre: Wexford, Ireland, 2005. [Google Scholar]
- Peech, M.; English, L. Rapid Microbiological Soil Tests. Soil Sci. 1944, 57, 167–195. [Google Scholar] [CrossRef]
- Murphy, J.; Riley, J.P. A Modified Single Solution Method for the Determination of Phosphate in Natural Waters. Anal. Chim. 1962, 27, 31–36. [Google Scholar] [CrossRef]
- Roberts, T.L.; Johnston, A.E. Phosphorus Use Efficiency and Management in Agriculture. Resour. Conserv. Recycl. 2015, 105, 275–281. [Google Scholar] [CrossRef]
- Muyzer, G.; de Waal, E.C.; Uitterlinden, A.G. Profiling of Complex Microbial Populations by Denaturing Gradient Gel Electrophoresis Analysis of Polymerase Chain Reaction-Amplified Genes Coding for 16S rRNA. Appl. Environ. Microbiol. 1993, 59, 695–700. [Google Scholar] [CrossRef]
- Schmalenberger, A.; O’Sullivan, O.; Gahan, J.; Cotter, P.D.; Courtney, R. Bacterial Communities Established in Bauxite Residues with Different Restoration Histories. Environ. Sci. Technol. 2013, 47, 7110–7119. [Google Scholar] [CrossRef]
- Ragot, S.A.; Kertesz, M.A.; Bünemann, E.K. phoD Alkaline Phosphatase Gene Diversity in Soil. Appl. Environ. Microbiol. 2015, 81, 7281–7289. [Google Scholar] [CrossRef]
- Fraser, T.D.; Lynch, D.H.; Gaiero, J.; Khosla, K.; Dunfield, K.E. Quantification of Bacterial Non-Specific Acid (phoC) and Alkaline (phoD) Phosphatase Genes in Bulk and Rhizosphere Soil from Organically Managed Soybean Fields. Appl. Soil Ecol. 2017, 111, 48–56. [Google Scholar] [CrossRef]
- Sakurai, M.; Wasaki, J.; Tomizawa, Y.; Shinano, T.; Osaki, M. Analysis of Bacterial Communities on Alkaline Phosphatase Genes in Soil Supplied with Organic Matter. Soil Sci. Plant Nutr. 2008, 54, 62–71. [Google Scholar] [CrossRef]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, Interactive, Scalable and Extensible Microbiome Data Science Using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-Resolution Sample Inference from Illumina Amplicon Data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef]
- Katoh, K.; Standley, D.M. MAFFT Multiple Sequence Alignment Software Version 7: Improvements in Performance and Usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef]
- Price, M.N.; Dehal, P.S.; Arkin, A.P. FastTree 2—Approximately Maximum-Likelihood Trees for Large Alignments. PLoS ONE 2010, 5, e9490. [Google Scholar] [CrossRef]
- Faith, D.P. Conservation Evaluation and Phylogenetic Diversity. Biol. Conserv. 1992, 61, 1–10. [Google Scholar] [CrossRef]
- Lozupone, C.; Knight, R. UniFrac: A New Phylogenetic Method for Comparing Microbial Communities. Appl. Environ. Microbiol. 2005, 71, 8228–8235. [Google Scholar] [CrossRef] [PubMed]
- Lozupone, C.A.; Hamady, M.; Kelley, S.T.; Knight, R. Quantitative and Qualitative β Diversity Measures Lead to Different Insights into Factors That Structure Microbial Communities. Appl. Environ. Microbiol. 2007, 73, 1576–1585. [Google Scholar] [CrossRef] [PubMed]
- Bokulich, N.A.; Kaehler, B.D.; Rideout, J.R.; Dillon, M.; Bolyen, E.; Knight, R.; Huttley, G.A.; Gregory Caporaso, J. Optimizing Taxonomic Classification of Marker-Gene Amplicon Sequences with QIIME 2′s q2-feature-classifier Plugin. Microbiome 2018, 6, 90. [Google Scholar] [CrossRef] [PubMed]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glöckner, F.O. The SILVA ribosomal RNA Gene Database Project: Improved Data Processing and Web-Based Tools. Nucleic Acids Res. 2013, 41, D590–D596. [Google Scholar] [CrossRef]
- Yilmaz, P.; Parfrey, L.W.; Yarza, P.; Gerken, J.; Pruesse, E.; Quast, C.; Schweer, T.; Peplies, J.; Ludwig, W.; Glöckner, F.O. The SILVA and “All-species Living Tree Project (LTP)” taxonomic frameworks. Nucleic Acids Res. 2014, 42, D643–D648. [Google Scholar] [CrossRef]
- Martin, M. Cutadapt Removes Adapter Sequences from High-Throughput Sequencing Reads. EMBnet. J. 2011, 17, 10. [Google Scholar] [CrossRef]
- Edgar, R.C. Search and Clustering Orders of Magnitude Faster than BLAST. Bioinformatics 2010, 26, 2460–2461. [Google Scholar] [CrossRef]
- Edgar, R.C. UPARSE: Highly Accurate OTU Sequences from Microbial Amplicon Reads. Nat. Methods 2013, 10, 996–998. [Google Scholar] [CrossRef]
- Fraser, T.D.; Lynch, D.H.; Bent, E.; Entz, M.H.; Dunfield, K.E. Soil Bacterial phoD Gene Abundance and Expression in Response to Applied Phosphorus and Long-Term Management. Soil Biol. Biochem. 2015, 88, 137–147. [Google Scholar] [CrossRef]
- Fish, J.A.; Chai, B.; Wang, Q.; Sun, Y.; Brown, C.T.; Tiedje, J.M.; Cole, J.R. FunGene: The Functional Gene Pipeline and Repository. Front. Microbiol. 2013, 4, 291. [Google Scholar] [CrossRef]
- McMurdie, P.J.; Holmes, S. Waste Not, Want Not: Why Rarefying Microbiome Data Is Inadmissible. PLoS Comput. Biol. 2014, 10, e1003531. [Google Scholar] [CrossRef]
| Treatment | Biomass DW (g) | Shoot Count | DW:Shoot Ratio (mg Shoot−1) | AE | |||
|---|---|---|---|---|---|---|---|
| SP0 | 2.29 c | ±0.06 | 121.25 a | ±1.9 | 18.89 bc | ±0.5 | |
| SP20 | 2.66 ac | ±0.10 | 127.25 a | ±3.6 | 20.88 ac | ±0.5 | 12.50 |
| SP60 | 2.52 ac | ±0.05 | 109.75 a | ±4.1 | 23.04 ab | ±1.0 | 2.73 |
| PLA20 | 2.14 c | ±0.16 | 118.50 a | ±4.7 | 18.19 c | ±1.4 | −8.74 |
| PLA60 | 2.72 ac | ±0.16 | 117.25 a | ±9.1 | 23.40 ab | ±1.3 | 6.60 |
| SSA20 | 2.59 ac | ±0.06 | 125.00 a | ±5.7 | 20.79 ac | ±0.8 | 14.38 |
| SSA60 | 2.75 ac | ±0.17 | 122.00 a | ±4.5 | 22.64 ac | ±1.7 | 7.15 |
| MWS20 | 3.19 ac | ±0.18 | 117.25 a | ±10.9 | 27.69 a | ±2.1 | 23.89 |
| MWS60 | 2.79 ac | ±0.34 | 107.50 a | ±6.0 | 26.07 ab | ±3.2 | 13.31 |
| CGS20 | 2.27 ac | ±0.15 | 102.25 a | ±7.7 | 22.24 ac | ±0.6 | 2.21 |
| CGS60 | 2.85 ab | ±0.09 | 110.25 a | ±8.1 | 26.36 a | ±2.5 | 8.85 |
| Treatment | Macronutrients (µg Treatment−1) | |||||
|---|---|---|---|---|---|---|
| Calcium | Magnesium | Sulphur | Nitrogen | Phosphorus | Potassium | |
| SP0 | 1619.8 | 371.2 | 360.0 | 4713.2 | 258.7 | 4836.9 |
| SP20 | 1397.2 | 329.3 | 339.3 | 4760.5 | 299.4 | 4471.1 |
| SP60 | 1360.3 | 321.9 | 363.4 | 5077.9 | 415.4 | 4911.7 |
| PLA20 | 1777.8 | 407.4 | 419.8 | 5530.9 | 296.3 | 5370.4 |
| PLA60 | 1245.3 | 290.3 | 318.4 | 4307.1 | 252.8 | 4194.8 |
| SSA20 | 1275.8 | 294.4 | 323.8 | 4367.0 | 235.5 | 4210.0 |
| SSA60 | 1135.7 | 267.8 | 313.9 | 4478.3 | 295.5 | 3942.8 |
| MWS20 | 1194.6 | 280.5 | 298.6 | 4461.5 | 262.4 | 4181.0 |
| MWS60 | 944.0 | 272.0 | 264.0 | 3944.0 | 304.0 | 3576.0 |
| CGS20 | 1507.2 | 363.0 | 374.0 | 5181.5 | 308.0 | 5005.5 |
| CGS60 | 992.0 | 301.2 | 292.3 | 4242.7 | 336.6 | 3950.4 |
| Treatment | Micronutrients | |||||
|---|---|---|---|---|---|---|
| Boron | Copper | Iron | Manganese | Molybdenum | Zinc | |
| SP0 | 0.9 | 5.5 | 217.8 | 36.2 | 0.01 | 6.5 |
| SP20 | 0.6 | 3.3 | 116.8 | 30.7 | 0.02 | 5.8 |
| SP60 | 0.6 | 3.1 | 151.7 | 27.7 | 0.02 | 6.2 |
| PLA20 | 1.0 | 6.1 | 234.0 | 32.8 | 0.02 | 6.6 |
| PLA60 | 0.6 | 2.7 | 146.3 | 18.5 | 0.01 | 5.3 |
| SSA20 | 0.7 | 3.5 | 193.8 | 23.9 | 0.01 | 5.1 |
| SSA60 | 0.6 | 3.0 | 139.8 | 17.1 | 0.01 | 5.4 |
| MWS20 | 0.6 | 2.9 | 144.1 | 24.1 | 0.01 | 4.3 |
| MWS60 | 0.4 | 2.3 | 102.0 | 21.0 | 0.01 | 3.8 |
| CGS20 | 0.7 | 2.9 | 158.0 | 30.6 | 0.01 | 5.5 |
| CGS60 | 0.5 | 2.9 | 122.0 | 23.6 | 0.01 | 4.8 |
| Treatment | PAA (MPN g−1 Soil) | Phy (MPN g−1 Soil) | R2A (MPN g−1 Soil) | TCP (CFU g−1 Soil) | ||||
|---|---|---|---|---|---|---|---|---|
| SP0 | 3.1 × 106 a | ±1.0 × 106 | 18.6 × 106 a | ±5.6 × 106 | 92.5 × 106 a | ±2.5 × 107 | 275.0 × 103 bcd | ±3.1 × 104 |
| SP20 | 7.5 × 106 a | ±3.0 × 106 | 11.5 × 106 a | ±3.5 × 106 | 141.3 × 106 a | ±3.5 × 107 | 150.0 × 103 cd | ±3.2 × 104 |
| SP60 | 6.7 × 106 a | ±1.2 × 105 | 8.3 × 106 a | ±2.9 × 106 | 120.0 × 106 a | ±1.9 × 107 | 138.0 × 103 d | ±3.6 × 104 |
| PLA20 | 9.8 × 106 a | ±2.6 × 106 | 19.8 × 106 a | ±7.0 × 106 | 218.8 × 106 a | ±5.1 × 107 | 250.0 × 103 bcd | ±5.0 × 104 |
| PLA60 | 14.2 × 106 a | ±5.9 × 106 | 11.3 × 106 a | ±1.2 × 105 | 102.5 × 106 a | ±2.4 × 107 | 1.0 × 106 a | ±7.6 × 105 |
| SSA20 | 5.2 × 106 a | ±7.4 × 105 | 31.3 × 106 a | ±1.3 × 107 | 154.5 × 106 a | ±4.9 × 107 | 850.0 × 103 ad | ±1.6 × 105 |
| SSA60 | 10.1 × 106 a | ±4.4 × 106 | 12.8 × 106 a | ±9.4 × 105 | 156.3 × 106 a | ±3.4 × 107 | 488.0 × 103 ad | ±4.4 × 105 |
| MWS20 | 5.4 × 106 a | ±6.5 × 105 | 19.3 × 106 a | ±3.2 × 106 | 156.3 × 106 a | ±2.7 × 107 | 925.0 × 103 ab | ±8.4 × 104 |
| MWS60 | 9.5 × 106 a | ±4.5 × 106 | 16.0 × 106 a | ±2.0 × 105 | 132.5 × 106 a | ±2.0 × 107 | 863.0 × 103 ac | ±9.7 × 104 |
| CGS20 | 6.2 × 106 a | ±2.4 × 105 | 19.8 × 106 a | ±6.7 × 106 | 102.5 × 106 a | ±2.4 × 107 | 575.0 × 103 ad | ±9.4 × 104 |
| CGS60 | 12.0 × 106 a | ±1.7 × 106 | 15.3 × 106 a | ±1.3 × 106 | 61.7 × 106 a | ±2.4 × 106 | 763.0 × 103 ad | ±8.7 × 104 |
| Treatment | Soil pH | Morgan’s P (mg P L−1) | ||
|---|---|---|---|---|
| SP0 | 4.75 d | ±0.03 | 0.58 c | ±0.08 |
| SP20 | 4.94 bc | ±0.02 | 1.01 ac | ±0.12 |
| SP60 | 5.05 b | ±0.04 | 2.35 ab | ±0.26 |
| PLA20 | 4.95 bc | ±0.02 | 1.98 ab | ±0.17 |
| PLA60 | 5.22 a | ±0.03 | 2.75 b | ±0.19 |
| SSA20 | 4.96 bc | ±0.02 | 1.57 ab | ±0.11 |
| SSA60 | 5.24 a | ±0.02 | 2.48 ac | ±0.41 |
| MWS20 | 4.87 cd | ±0.04 | 2.00 abc | ±0.33 |
| MWS60 | 5.02 b | ±0.02 | 4.46 abc | ±0.60 |
| CGS20 | 4.90 c | ±0.02 | 2.79 abc | ±0.45 |
| CGS60 | 4.95 bc | ±0.01 | 5.16 abc | ±0.71 |
| Treatment | ACP (µg pNP g−1 Soil h−1) | ALP (µg pNP g−1 Soil h−1) | phoC (phoC Copies g−1 Soil) | phoD (phoD Copies g−1 Soil) | ||||
|---|---|---|---|---|---|---|---|---|
| SP0 | 1653.7 d | ± 77.4 | 218.1 a | ± 10.6 | 131.3 × 103 a | ±6.3 × 104 | 148.2 × 106 ab | ±5.1 × 106 |
| SP20 | 1663.9 d | ± 38.9 | 278.7 a | ± 10.2 | 79.7 × 103 a | ±3.3 × 104 | 140.9 × 106 ab | ±2.9 × 106 |
| SP60 | 2167.0 abc | ± 78.8 | 259.5 a | ± 16.4 | 98.9 × 103 a | ±3.4 × 104 | 140.7 × 106 ab | ±3.1 × 106 |
| PLA20 | 2007.1 bd | ± 45.6 | 208.4 a | ± 20.7 | 75.8 × 103 a | ±3.3 × 104 | 131.7 × 106 b | ±7.0 × 106 |
| PLA60 | 1712.3 cd | ± 81.7 | 217.5 a | ± 7.0 | 119.9 × 103 a | ±5.9 × 104 | 151.7 × 106 ab | ±4.0 × 106 |
| SSA20 | 2183.4 abc | ± 94.3 | 188.0 a | ± 7.1 | 75.9 × 103 a | ±2.9 × 104 | 133.5 × 106 ab | ±6.9 × 106 |
| SSA60 | 2084.2 bd | ± 103.5 | 228.8 a | ± 27.2 | 88.3 × 103 a | ±4.5 × 104 | 138.4 × 106 ab | ±6.6 × 106 |
| MWS20 | 2262.8 bd | ± 137.6 | 233.9 a | ± 33.3 | 100.8 × 103 a | ±3.6 × 104 | 145.5 × 106 ab | ±1.7 × 107 |
| MWS60 | 2262.8 ab | ± 103.1 | 233.9 a | ± 30.4 | 168.6 × 103 a | ±7.5 × 104 | 163.3 × 106 ab | ±5.3 × 106 |
| CGS20 | 2066.9 bd | ± 133.7 | 199.9 a | ± 23.2 | 218.4 × 103 a | ±5.0 × 104 | 168.9 × 106 a | ±9.8 × 106 |
| CGS60 | 2616.4 a | ± 163.7 | 209.0 a | ± 17.9 | 88.2 × 103 a | ±3.3 × 104 | 154.3 × 106 ab | ±4.1 × 106 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deinert, L.; Ikoyi, I.; Egeter, B.; Forrestal, P.; Schmalenberger, A. Short-Term Impact of Recycling-Derived Fertilizers on Their P Supply for Perennial Ryegrass (Lolium perenne). Plants 2023, 12, 2762. https://doi.org/10.3390/plants12152762
Deinert L, Ikoyi I, Egeter B, Forrestal P, Schmalenberger A. Short-Term Impact of Recycling-Derived Fertilizers on Their P Supply for Perennial Ryegrass (Lolium perenne). Plants. 2023; 12(15):2762. https://doi.org/10.3390/plants12152762
Chicago/Turabian StyleDeinert, Lea, Israel Ikoyi, Bastian Egeter, Patrick Forrestal, and Achim Schmalenberger. 2023. "Short-Term Impact of Recycling-Derived Fertilizers on Their P Supply for Perennial Ryegrass (Lolium perenne)" Plants 12, no. 15: 2762. https://doi.org/10.3390/plants12152762
APA StyleDeinert, L., Ikoyi, I., Egeter, B., Forrestal, P., & Schmalenberger, A. (2023). Short-Term Impact of Recycling-Derived Fertilizers on Their P Supply for Perennial Ryegrass (Lolium perenne). Plants, 12(15), 2762. https://doi.org/10.3390/plants12152762








