Modeling of the Spatial Distribution of Forest Carbon Storage in a Tropical/Subtropical Island with Multiple Ecozones
Abstract
1. Introduction
2. Results
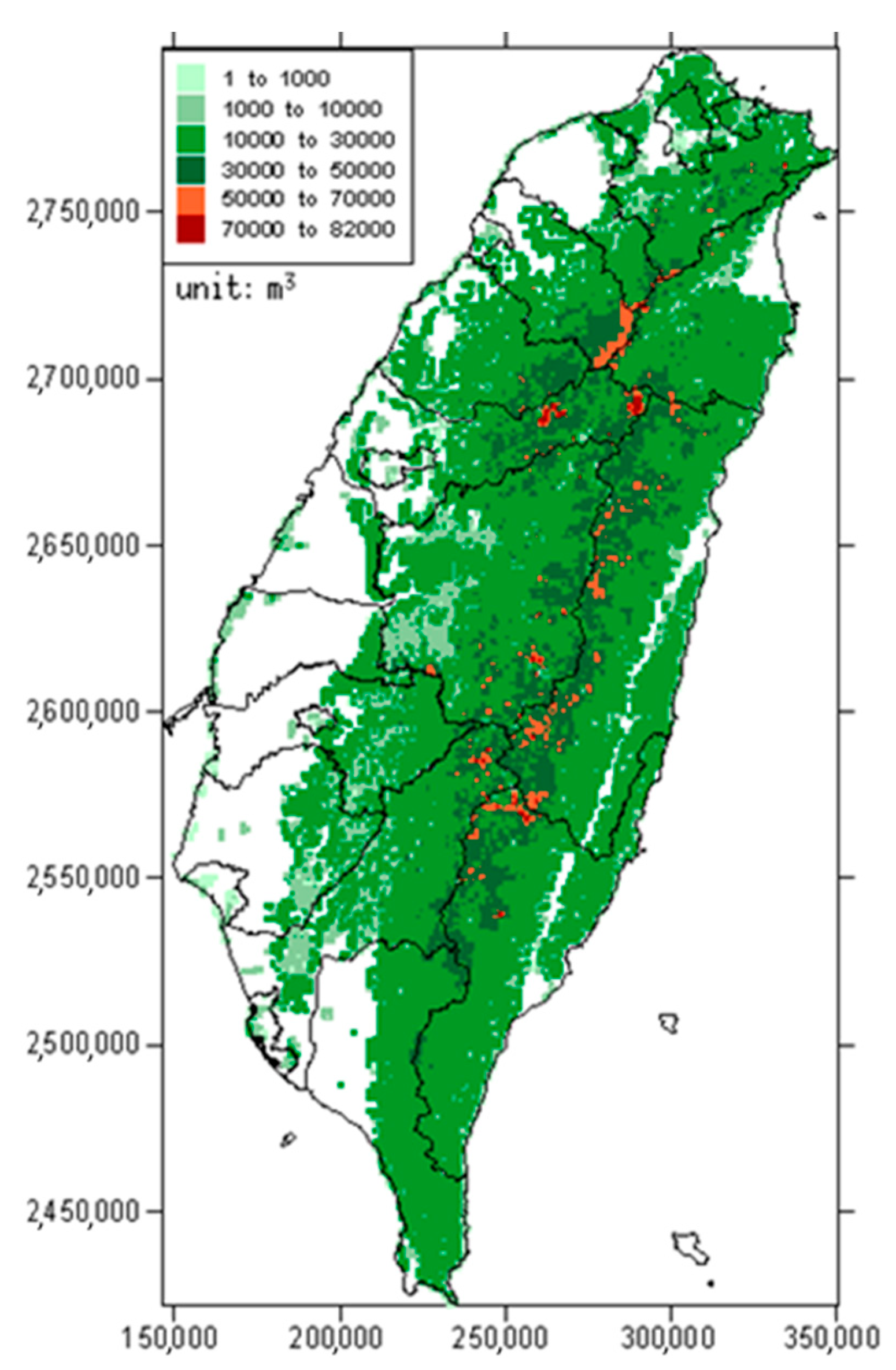
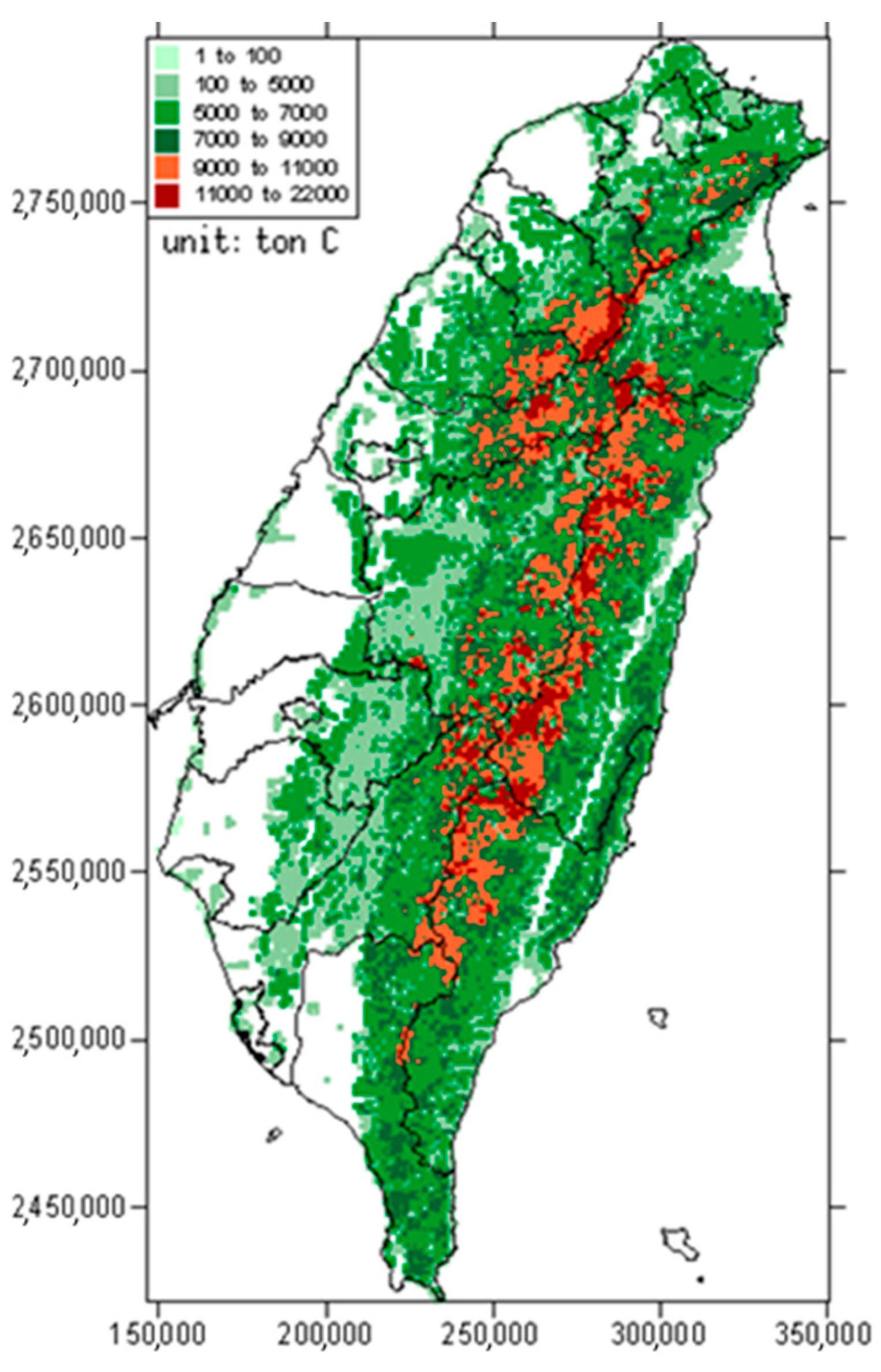
2.1. Estimation of Forest Carbon Storage
2.2. Estimation of Annual Carbon Sequestration from 2006 to 2007
3. Discussion
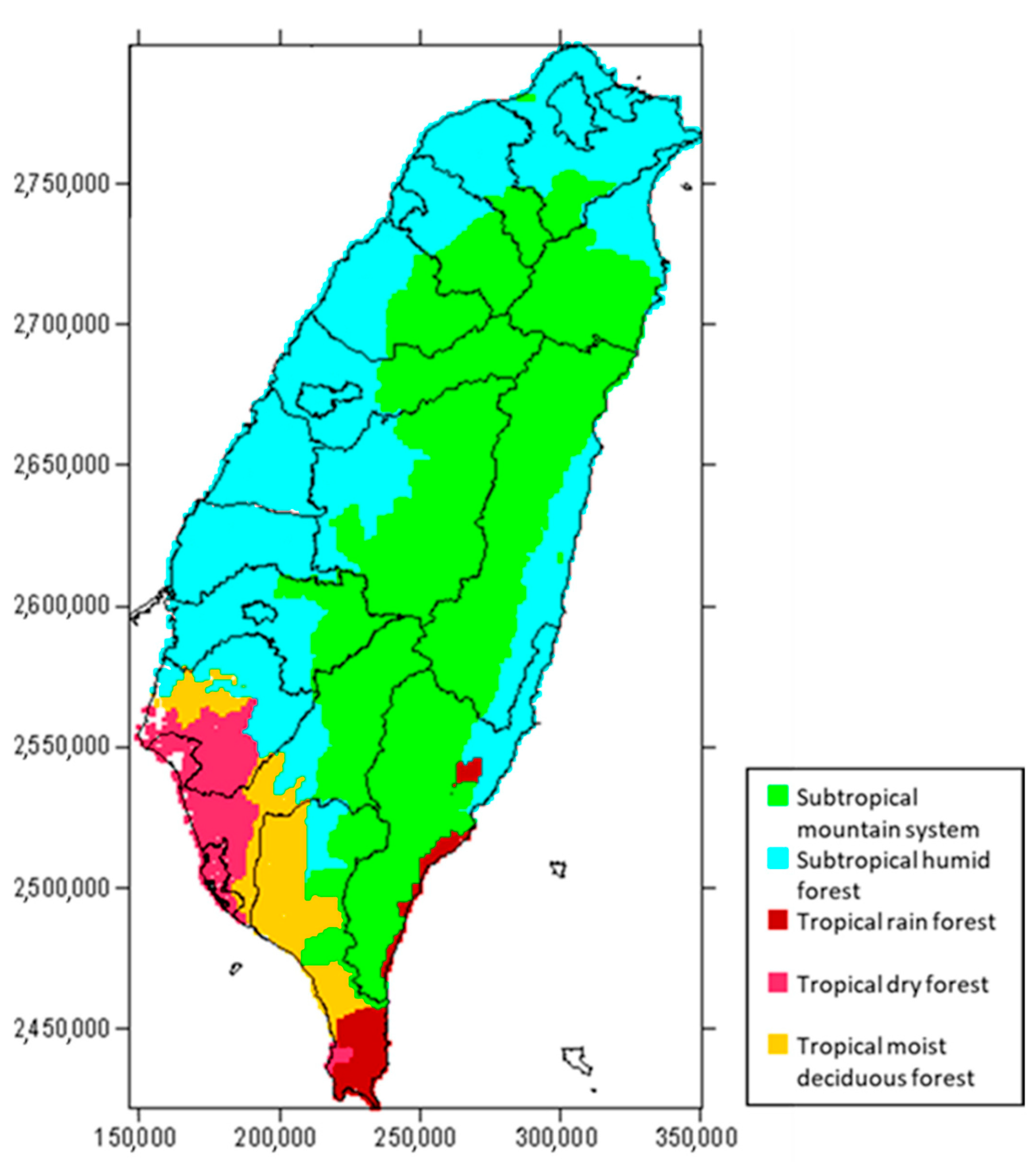
3.1. Estimation of Forest Carbon Distribution in Taiwan
3.2. Carbon Sequestration Simulation Results
4. Methodology
4.1. Specification of the Grid Carbon Storage and Carbon Sequestration Modeling of Taiwan
4.2. Algorithms and Parameters
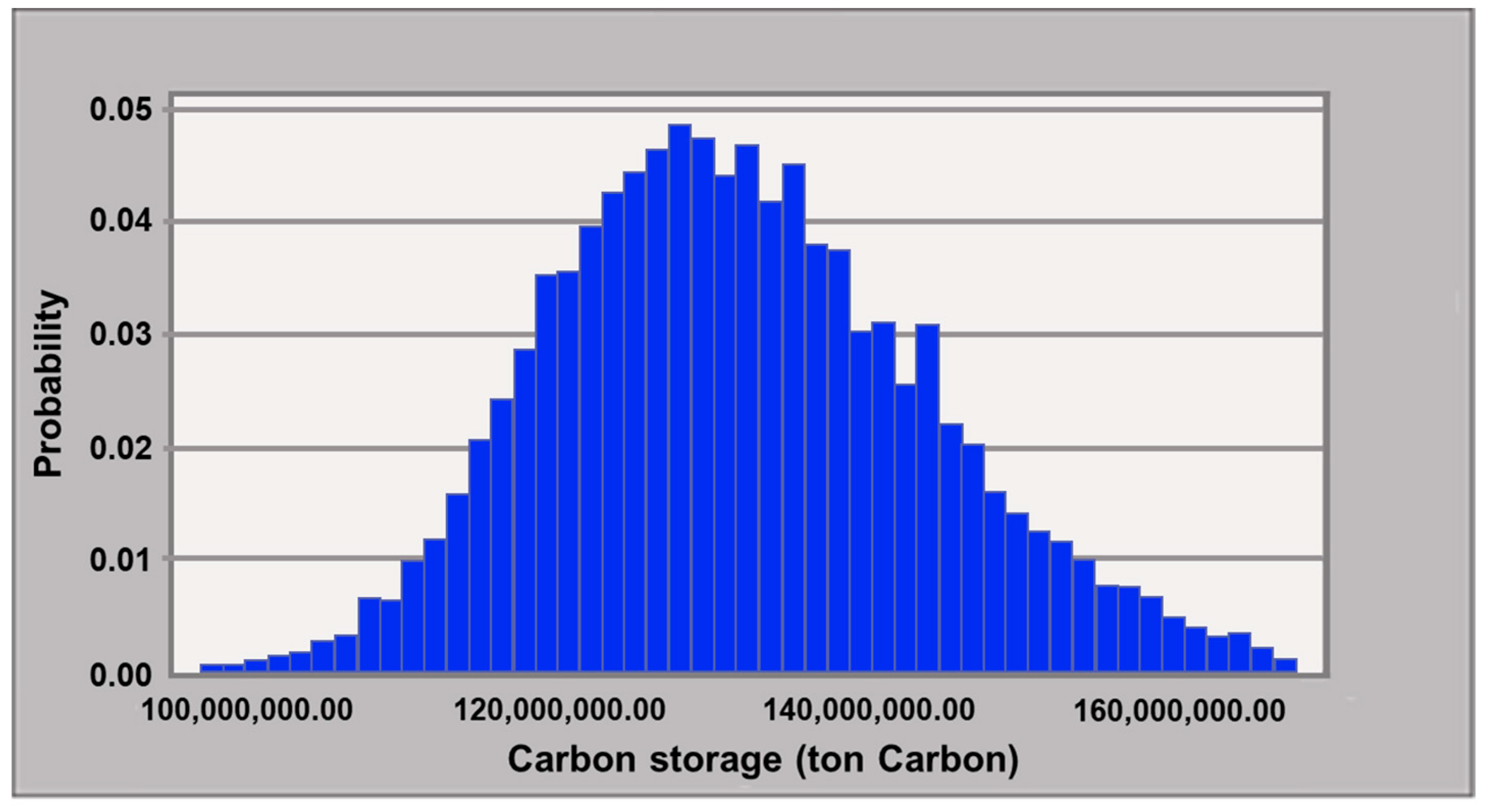
4.3. Model Uncertainty Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Myhre, G.; Shindell, D.; Bréon, F.-M.; Collins, W.; Fuglestvedt, J.; Huang, J.; Koch, D.; Lamarque, J.-F.; Lee, D.; Mendoza, B.; et al. Anthropogenic and Natural Radiative Forcing. In Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Stocker, T.F., Qin, D., Plattner, G.-K., Tignor, M., Allen, S.K., Boschung, J., Nauels, A., Xia, Y., Bex, V., Midgley, P.M., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2013. [Google Scholar]
- Friedlingstein, P.; Jones, M.W.; O’Sullivan, M.; Andrew, R.M.; Bakker, D.C.E.; Hauck, J.; Le Quéré, C.; Peters, G.P.; Peters, W.; Pongratz, J.; et al. Global Carbon Budget 2021. Earth Syst. Sci. Data 2022, 14, 1917–2005. [Google Scholar] [CrossRef]
- Allen, M.R.; Dube, O.P.; Solecki, W.; Aragón-Durand, F.; Cramer, W.; Humphreys, S.; Kainuma, M.; Kala, J.; Mahowald, N.; Mulugetta, Y.; et al. Framing and Context. In Global Warming of 1.5 °C. An IPCC Special Report on the Impacts of Global Warming of 1.5 °C above Pre-Industrial Levels and Related Global Greenhouse Gas Emission Pathways, in the Context of Strengthening the Global Response to the Threat of Climate Change, Sustainable Development, and Efforts to Eradicate Poverty; Masson-Delmotte, V., Zhai, P., Pörtner, H.-O., Roberts, D., Skea, J., Shukla, P.R., Pirani, A., Moufouma-Okia, W., Péan, C., Pidcock, R., et al., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2018; pp. 49–92. [Google Scholar] [CrossRef]
- Greene, C.H.; Pershing, A.J. Climate Drives Sea Change. Science 2007, 315, 1084–1085. [Google Scholar] [CrossRef] [PubMed]
- Weiskopf, S.R.; Rubenstein, M.A.; Crozier, L.G.; Gaichas, S.; Griffis, R.; Halofsky, J.E.; Hyde, K.J.W.; Morelli, T.; Morisette, J.T.; Muñoz, R.C.; et al. Climate change effects on biodiversity, ecosystems, ecosystem services, and natural resource management in the United States. Sci. Total Environ. 2020, 733, 137782. [Google Scholar] [CrossRef] [PubMed]
- Manes, S.; Costello, M.J.; Beckett, H.; Debnath, A.; Devenish-Nelson, E.; Grey, K.-A.; Jenkins, R.; Khan, T.M.; Kiessling, W.; Krause, C.; et al. Endemism increases species’ climate change risk in areas of global biodiversity importance. Biol. Conserv. 2021, 257, 109070. [Google Scholar] [CrossRef]
- Macinnis-Ng, C.; Mcintosh, A.R.; Monks, J.M.; Waipara, N.; White, R.S.A.; Boudjelas, S.; Clark, C.D.; Clearwater, M.J.; Curran, T.J.; Dickinson, K.J.M.; et al. Climate-change impacts exacerbate conservation threats in island systems: New Zealand as a case study. Front. Ecol. Environ. 2021, 19, 216–224. [Google Scholar] [CrossRef]
- Halofsky, J.E.; Peterson, D.L.; Harvey, B.J. Changing wildfire, changing forests: The effects of climate change on fire regimes and vegetation in the Pacific Northwest, USA. Fire Ecol. 2020, 16, 4–16. [Google Scholar] [CrossRef]
- Abram, N.J.; Henley, B.J.; Sen Gupta, A.; Lippmann, T.J.R.; Clarke, H.; Dowdy, A.J.; Sharples, J.J.; Nolan, R.H.; Zhang, T.; Wooster, M.J.; et al. Connections of climate change and variability to large and extreme forest fires in southeast Australia. Commun. Earth Environ. 2021, 2, 8. [Google Scholar] [CrossRef]
- Kahiluoto, H.; Kaseva, J.; Balek, J.; Olesen, J.E.; Ruiz-Ramos, M.; Gobin, A.; Kersebaum, K.C.; Takáč, J.; Ruget, F.; Ferrise, R.; et al. Decline in climate resilience of European wheat. Proc. Natl. Acad. Sci. USA 2019, 116, 123–128. [Google Scholar] [CrossRef]
- Mukhopadhyay, R.; Sarkar, B.; Jat, H.S.; Sharma, P.C.; Bolan, N.S. Soil salinity under climate change: Challenges for sustainable agriculture and food security. J. Environ. Manag. 2021, 280, 111736. [Google Scholar] [CrossRef]
- Schrag, D.P. Preparing to Capture Carbon. Science 2007, 315, 812–813. [Google Scholar] [CrossRef]
- Bonan, G.B. Forests and Climate Change: Forcings, Feedbacks, and the Climate Benefits of Forests. Science 2008, 320, 1444–1449. [Google Scholar] [CrossRef]
- Pan, Y.; Birdsey, R.A.; Fang, J.; Houghton, R.; Kauppi, P.E.; Kurz, W.A.; Phillips, O.L.; Shvidenko, A.; Lewis, S.L.; Canadell, J.G.; et al. A Large and Persistent Carbon Sink in the World’s Forests. Science 2011, 333, 988–993. [Google Scholar] [CrossRef]
- Pugh, T.A.M.; Lindeskog, M.; Smith, B.; Poulter, B.; Arneth, A.; Haverd, V.; Calle, L. Role of forest regrowth in global carbon sink dynamics. Proc. Natl. Acad. Sci. USA 2019, 116, 4382–4387. [Google Scholar] [CrossRef] [PubMed]
- Chiou, C.R.; Yang, S.I. Estimation of the Stand Carrying Capacity of Chinese Fir Plantations In Taiwan. Taiwan J. For. Sci. 2019, 34, 305–315. [Google Scholar]
- Chiou, C.-R.; Lin, J.-C.; Liu, W.-Y. The Carbon Benefit of Thinned Wood for Bioenergy in Taiwan. Forests 2019, 10, 255. [Google Scholar] [CrossRef]
- Fawzy, S.; Osman, A.I.; Doran, J.; Rooney, D.W. Strategies for mitigation of climate change: A review. Environ. Chem. Lett. 2020, 18, 2069–2094. [Google Scholar] [CrossRef]
- Wang, L.J. Impact and Adaptation of Climate Change to Taiwan Forestry (In Chinese). In Proceedings of the Symposium on Climate Change Impact Assessment and Coping Strategies, Symposium on Climate Change Impact Assessment and Coping Strategies, Taipei, Taiwan, 1 April 1996. [Google Scholar]
- Yang, S.S. Estimation of the capacity of forest carbon dioxide capturing in Taiwan (In Chinese). J. Biomass Energy Soc. China 1997, 16, 1–10. [Google Scholar]
- Lin, Y.; Lee, K.; Lin, J. Forest Carbon Sink Estimation for Taiwan by Biomass-Volume Relationship Method. J. Exp. For. Natl. Taiwan Univ. 2002, 16, 71–79. [Google Scholar]
- Chiou, C.R.; Wang, R.M.; Liang, Y.C. Aspects of Forest Carbon Evaluation in Taiwan (In Chinese). In Proceedings of the Conference on Forest Carbon Sequestration, Taipei, Taiwan, 7 December 2006. [Google Scholar]
- Lin, J.C. Estimation of Forest Carbon in Taiwan and Discussion on Sources of Uncertainty (In Chinese). In Proceedings of the Conference of Carbon Cycling in Taiwan, Taipei, Taiwan, 27 November 2007. [Google Scholar]
- Wang, R. A Study on the Estimative Approach of Forest Carbon Sequestration for National Forest Land in Taiwan. Master’s Thesis, National Taiwan University, Taipei, Taiwan, 2007. [Google Scholar] [CrossRef]
- Taiwan Forest Bureau. Report of the Forth Forest Resources Survey. 2015. Available online: https://www.forest.gov.tw/File.aspx?fno=66716 (accessed on 6 April 2022).
- Taiwan Forest Bureau. The Third Forest Resources and Land Use Inventory in Taiwan; Taiwan Forest Bureau: Taipei, Taiwan, 1995. [Google Scholar]
- FAO. Global Forest Resources Assessment 2020: Main Report, FAO: Rome, Italy, 2020. [CrossRef]
- Lin, C.; Li, C.; Zelený, D.; Chytrý, M.; Nakamura, Y.; Chen, M.; Chen, T.; Hsia, Y.; Hsieh, C.; Liu, H.; et al. Classification of the High-Mountain Coniferous Forests in Taiwan. Folia Geobot. 2012, 47, 373–401. [Google Scholar] [CrossRef]
- Chiu, C.; Tzeng, H.; Lin, C.; Chang, K.; Liao, M. Spatial Distribution and Climate Warming Impact on Abies kawakamii Forest on a Subtropical Island. Plants 2022, 11, 1346. [Google Scholar] [CrossRef]
- Hsu, R.C.-C.; Tamis, W.L.M.; Raes, N.; de Snoo, G.R.; Wolf, J.H.D.; Oostermeijer, G.; Lin, S. Simulating climate change impacts on forests and associated vascular epiphytes in a subtropical island of East Asia. Divers. Distrib. 2012, 18, 334–347. [Google Scholar] [CrossRef]
- Takano, K.T.; Hibino, K.; Numata, A.; Oguro, M.; Aiba, M.; Shiogama, H.; Takayabu, I.; Nakashizuka, T. Detecting latitudinal and altitudinal expansion of invasive bamboo Phyllostachys edulis and Phyllostachys bambusoides (Poaceae) in Japan to project potential habitats under 1.5 °C–4.0 °C global warming. Ecol Evol. 2017, 7, 9848–9859. [Google Scholar] [CrossRef]
- McClure, F.A. The Bamboos: A Fresh Perspective; Harvard University Press: Cambridge, MA, USA; London, UK, 1966. [Google Scholar] [CrossRef]
- Yen, T.-M.; Ji, Y.-J.; Lee, J.-S. Estimating biomass production and carbon storage for a fast-growing makino bamboo (Phyllostachys makinoi) plant based on the diameter distribution model. For. Ecol. Manag. 2010, 260, 339–344. [Google Scholar] [CrossRef]
- Chen, Y.-Y.; Huang, W.; Wang, W.-H.; Juang, J.-Y.; Hong, J.-S.; Kato, T.; Luyssaert, S. Reconstructing Taiwan’s land cover changes between 1904 and 2015 from historical maps and satellite images. Sci. Rep. 2019, 9, 3643. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-C.; Mai, G.-S. Applying Open-Source Software to Update a Taiwanese Forest Fire Database. Taiwan J. For. Sci. 2014, 29, 1–11. [Google Scholar]
- IPCC. Revised 1996 IPCC Guidelines for National Greenhouse Gas Inventories; UK Meteorological Office: Bracknell, UK, 1996. [Google Scholar]
- Wang, Y.-C.; Liu, W.-Y.; Ko, S.-H.; Lin, J.-C. Tree Species Diversity and Carbon Storage in Air Quality Enhancement Zones in Taiwan. Aerosol Air Qual. Res. 2015, 15, 1291–1299. [Google Scholar] [CrossRef]
- CSRSR. Satellite Telemetry Data of Taiwan in 2006. Retrieved by request from Center for Space and Remote Sensing Research, National Central University, 2007 (retrieved on 1 March 2010).
- Chang, K.H.; Yu, J.Y.; Chen, T.F.; Lin, Y.P. Estimating Taiwan biogenic VOC emission: Leaf energy balance consideration. Atmos. Environ. 2009, 43, 5092–5100. [Google Scholar] [CrossRef]
- Yu, J.Y.; Chang, K.H.; Chen, T.F. Estimation of CO2 Assimilation and Emission Flux of Vegetation in Subtropical Island—Taiwan. Aerosol Air Qual. Res. 2016, 16, 3302–3311. [Google Scholar] [CrossRef]
- IPCC. 2006 IPCC Guidelines for National Greenhouse Gas Inventories; IGES: Kanagawa, Japan, 2006. [Google Scholar]
- Lee, K.J.; Lin, J.; Chen, L. The Potential of Carbon Sequestration and Its Cost-benefit Analysis for Taiwania Plantations. Taiwan J. For. Sci. 2000, 15, 115–123. [Google Scholar] [CrossRef]
- Lin, Y.; Liu, C.; Lin, J. Measurement of Specific Gravity and Carbon Content of Important Timber Species in Taiwan. Taiwan J. For. Sci. 2002, 17, 291–299. [Google Scholar] [CrossRef]
- Ji, Y. A Biomass and Carbon Storage of Phyllostachys makinoi Plantation in Central Taiwan. Master’s Thesis, National Chung Hsing University, Taichung, Taiwan, 2008. [Google Scholar]
- Chang, I.-S.; Lin, C.-T. Report of Continuous Investigation on Taiwan’s Forest Resources—Growth and Mortality of Taiwan’s Forest Resources; Taiwan Forest Bureau: Taipei, Taiwan, 1982. [Google Scholar]
| Wood Type | Regeneration | Forest Type | CA (t ha−1) | C (Mt C) | Proportion to Total (%) |
|---|---|---|---|---|---|
| Coniferous | Nature | FIR-NF | 47.17 | 1.01 | 0.6 |
| TSU-NF | 98.94 | 5.34 | 3.2 | ||
| CYP-NF | 149.94 | 7.39 | 4.5 | ||
| PIN-NF | 107.41 | 7.55 | 4.6 | ||
| SPR-NF | 221.48 | 1.57 | 0.9 | ||
| O-C-NF | 210.12 | 4.87 | 2.9 | ||
| Average or Total | 123.03 | 27.73 | 16.7 | ||
| Plantation | CYP-P | 34.97 | 0.88 | 0.5 | |
| PIN-P | 67.28 | 3.11 | 1.9 | ||
| LF-P | 74.08 | 2.24 | 1.4 | ||
| TAI-P | 43.56 | 0.22 | 0.1 | ||
| JC-P | 37.79 | 1.78 | 1.1 | ||
| TIC-P | 78.38 | 0.10 | 0.1 | ||
| O-C-P | 115.97 | 0.20 | 0.1 | ||
| MC-P | 67.85 | 3.05 | 1.8 | ||
| P-C-P | 79.64 | 0.32 | 0.2 | ||
| NEW-C-P | 78.52 | 0.02 | <0.05 | ||
| Average or Total | 57.87 | 11.92 | 7.2 | ||
| Average or Total | 91.92 | 39.65 | 23.9 | ||
| Broadleaved | Nature | B-NF | 51.78 | 0.81 | 0.5 |
| MB-NF | 71.80 | 51.79 | 31.3 | ||
| Average or Total | 71.38 | 52.60 | 31.8 | ||
| Plantation | ACA-P | 81.06 | 2.97 | 1.8 | |
| SG-P | 35.61 | 0.15 | 0.1 | ||
| CAM-P | 34.42 | 0.20 | 0.1 | ||
| ASH-P | 54.04 | 0.72 | 0.4 | ||
| JE-P | 7.10 | 0.04 | <0.05 | ||
| SDT-P | 53.88 | 0.45 | 0.3 | ||
| O-B-P | 66.31 | 0.92 | 0.6 | ||
| MB-P | 59.31 | 1.55 | 0.9 | ||
| P-B-P | 53.88 | 26.98 | 16.3 | ||
| NEW-B-P | 50.53 | 0.17 | 0.1 | ||
| Average or Total | 55.26 | 34.15 | 20.6 | ||
| Average or Total | 64.02 | 86.75 | 52.4 | ||
| Bamboo | Nature | MAK-BAM-NF | 18.82 | 0.19 | 0.1 |
| MOS-BAM-NF | 26.77 | 0.05 | <0.05 | ||
| TG-BAM-NF | 69.59 | 0.72 | 0.4 | ||
| THO-BAM-NF | 18.67 | 0.04 | 0.0 | ||
| BAM-BAM-NF | 20.36 | <0.005 | <0.05 | ||
| O-BAM-NF | 12.96 | <0.005 | <0.05 | ||
| FAR-BAM-NF | 18.73 | 0.34 | 0.2 | ||
| Average or Total | 31.33 | 1.36 | 0.8 | ||
| Plantation | MAK-BAM-P | 18.85 | 0.15 | 0.1 | |
| MOS-BAM-P | 26.29 | 0.08 | <0.05 | ||
| TG-BAM-P | 69.99 | 0.52 | 0.3 | ||
| THO-BAM-P | 18.70 | 0.22 | 0.1 | ||
| BAM-BAM-P | 19.66 | 0.02 | <0.05 | ||
| O-BAM-P | 16.52 | 0.01 | <0.05 | ||
| P-BAM-P | 18.72 | 0.41 | 0.2 | ||
| Average or Total | 26.24 | 1.41 | 0.8 | ||
| Average or Total | 28.51 | 2.76 | 1.7 | ||
| Mixed | Nature | M-CB-NF | 100.20 | 29.47 | 17.8 |
| M-BAMC-NF | 126.17 | 0.01 | <0.05 | ||
| M-BAMB-NF | 45.86 | 2.92 | 1.8 | ||
| M-BAMCB-NF | 74.86 | 0.06 | <0.05 | ||
| Average or Total | 90.50 | 32.46 | 19.6 | ||
| Plantation | M-CB-P | 62.99 | 2.49 | 1.5 | |
| M-BAMC-P | 48.04 | 0.10 | 0.1 | ||
| M-BAMB-P | 44.55 | 1.13 | 0.7 | ||
| M-BAMCB-P | 55.43 | 0.30 | 0.2 | ||
| P-M-CB-P | 62.93 | 0.01 | <0.05 | ||
| Average or Total | 55.53 | 4.03 | 2.4 | ||
| Average or Total | 84.62 | 36.49 | 22.0 | ||
| Average or Total | 54.86 | 165.65 | 100.0 |
| Forest Type | Carbon Storage (Mt C) | Relative Carbon Storage (%) | ||
|---|---|---|---|---|
| 97.5th | 2.5th | 97.5th | 2.5th | |
| FIR-NF | 0.26 | 1.79 | −74 | 78 |
| TSU-NF | 2.33 | 9.62 | −56 | 80 |
| CYP-NF | 6.01 | 9.60 | −19 | 30 |
| PIN-NF | 5.22 | 10.75 | −31 | 42 |
| SPR-NF | 0.73 | 2.69 | −54 | 72 |
| O-C-NF | 3.20 | 7.19 | −34 | 48 |
| CYP-P | 0.60 | 1.26 | −32 | 43 |
| PIN-P | 2.06 | 4.58 | −34 | 47 |
| LF-P | 1.47 | 3.34 | −34 | 49 |
| TAI-P | 0.14 | 0.32 | −35 | 48 |
| JC-P | 1.15 | 2.64 | −35 | 48 |
| TIC-P | 0.07 | 0.15 | −33 | 51 |
| O-C-P | 0.13 | 0.29 | −35 | 45 |
| MC-P | 1.63 | 3.70 | −46 | 21 |
| B-NF | 0.52 | 1.21 | −35 | 50 |
| MB-NF | 33.18 | 77.95 | −36 | 51 |
| ACA-P | 2.11 | 4.04 | −29 | 36 |
| SG-P | 0.10 | 0.21 | −36 | 43 |
| CAM-P | 0.13 | 0.29 | −34 | 47 |
| ASH-P | 0.48 | 1.06 | −34 | 47 |
| JE-P | 0.03 | 0.06 | −34 | 47 |
| SDT-P | 0.29 | 0.67 | −36 | 49 |
| O-B-P | 0.59 | 1.39 | −35 | 51 |
| MB-P | 1.00 | 2.34 | −35 | 51 |
| M-CB-NF | 22.31 | 40.10 | −24 | 36 |
| M-CB-P | 1.63 | 3.70 | −34 | 49 |
| Total | 110.07 | 159.59 | −16 | 22 |
| Wood Type | Regeneration | Forest Type | ΔCA (t C ha−1 yr−1) | ΔC (Mt C yr−1) | Proportion to Total (%) |
|---|---|---|---|---|---|
| Coniferous | Nature | FIR-NF | 0.40 | 0.01 | 0.2 |
| TSU-NF | 0.84 | 0.05 | 0.9 | ||
| CYP-NF | 1.33 | 0.07 | 1.3 | ||
| PIN-NF | 1.28 | 0.09 | 1.7 | ||
| SPR-NF | 1.88 | 0.01 | 0.3 | ||
| O-C-NF | 4.59 | 0.11 | 2.0 | ||
| Average or Total | 0.68 | 0.33 | 6.3 | ||
| Plantation | CYP-P | 1.85 | 0.05 | 0.9 | |
| PIN-P | 3.56 | 0.16 | 3.2 | ||
| LF-P | 3.91 | 0.12 | 2.3 | ||
| TAI-P | 2.33 | 0.01 | 0.2 | ||
| JC-P | 2.00 | 0.09 | 1.8 | ||
| TIC-P | 4.07 | 0.01 | 0.1 | ||
| O-C-P | 6.22 | 0.01 | 0.2 | ||
| MC-P | 3.59 | 0.16 | 3.1 | ||
| P-C-P | 4.23 | 0.02 | 0.3 | ||
| NEW-C-P | 4.15 | <0.005 | <0.05 | ||
| Average or Total | 0.33 | 0.63 | 12.1 | ||
| Average or Total | 0.45 | 0.96 | 18.4 | ||
| Broadleaved | Nature | B-NF | 1.22 | 0.02 | 0.4 |
| MB-NF | 1.74 | 1.26 | 24.1 | ||
| Average or Total | 0.58 | 1.27 | 24.5 | ||
| Plantation | ACA-P | 5.19 | 0.19 | 3.6 | |
| SG-P | 2.34 | 0.01 | 0.2 | ||
| CAM-P | 2.20 | 0.01 | 0.2 | ||
| ASH-P | 3.46 | 0.05 | 0.9 | ||
| JE-P | 0.46 | <0.005 | <0.05 | ||
| SDT-P | 3.47 | 0.03 | 0.6 | ||
| O-B-P | 4.23 | 0.06 | 1.1 | ||
| MB-P | 3.78 | 0.10 | 1.9 | ||
| P-B-P | 3.44 | 1.72 | 33.1 | ||
| NEW-B-P | 3.23 | 0.01 | 0.2 | ||
| Average or Total | 0.28 | 2.18 | 41.9 | ||
| Average or Total | 0.39 | 3.46 | 66.4 | ||
| Bamboo | Nature | MAK-BAM-NF | 0.98 | 0.01 | 0.2 |
| MOS-BAM-NF | 1.39 | 0.00 | 0.1 | ||
| TG-BAM-NF | 3.62 | 0.04 | 0.7 | ||
| THO-BAM-NF | 0.97 | <0.005 | <0.05 | ||
| BAM-BAM-NF | 1.04 | <0.005 | <0.05 | ||
| O-BAM-NF | 0.68 | <0.005 | <0.05 | ||
| FAR-BAM-NF | 0.97 | 0.02 | 0.3 | ||
| Average or Total | 0.61 | 0.07 | 1.4 | ||
| Plantation | MAK-BAM-P | 0.98 | 0.01 | 0.2 | |
| MOS-BAM-P | 1.37 | 0.00 | 0.1 | ||
| TG-BAM-P | 3.64 | 0.03 | 0.5 | ||
| THO-BAM-P | 0.97 | 0.01 | 0.2 | ||
| BAM-BAM-P | 1.02 | <0.005 | <0.05 | ||
| O-BAM-P | 0.86 | <0.005 | <0.05 | ||
| P-BAM-P | 0.97 | 0.02 | 0.4 | ||
| Average or Total | 0.73 | 0.07 | 1.4 | ||
| Average or Total | 0.68 | 0.14 | 2.8 | ||
| Mixed | Nature | M-CB-NF | 1.42 | 0.42 | 8.0 |
| M-BAMC-NF | 5.45 | <0.005 | <0.05 | ||
| M-BAMB-NF | 1.98 | 0.13 | 2.4 | ||
| M-BAMCB-NF | 3.24 | <0.005 | <0.05 | ||
| Average or Total | 0.66 | 0.55 | 10.5 | ||
| Plantation | M-CB-P | 0.89 | 0.04 | 0.7 | |
| M-BAMC-P | 2.08 | <0.005 | 0.1 | ||
| M-BAMB-P | 1.93 | 0.05 | 0.9 | ||
| M-BAMCB-P | 2.40 | 0.01 | 0.2 | ||
| P-M-CB-P | 0.89 | <0.005 | <0.005 | ||
| Average or Total | 0.71 | 0.10 | 2.0 | ||
| Average or Total | 0.66 | 0.65 | 12.5 | ||
| Average or Total | 0.44 | 5.21 | 100.0 |
| Wood Type | Regeneration | Forest Type | Abbreviation |
|---|---|---|---|
| Coniferous | Nature | Natural Fir Forest | FIR-NF |
| Natural Tsuga Forest | TSU-NF | ||
| Natural Cypress Forest | CYP-NF | ||
| Natural Pine Forest | PIN-NF | ||
| Natural Spruce Forest | SPR-NF | ||
| Other Natural Coniferous Forest | O-C-NF | ||
| Plantation | Cypress Plantation | CYP-P | |
| Pine Plantation | PIN-P | ||
| Luanta Fir Plantation | LF-P | ||
| Taiwania Plantation | TAI-P | ||
| Japanese Cedar Plantation | JC-P | ||
| Taiwan Incense Cedar Plantation | TIC-P | ||
| Other Conifer Plantation | O-C-P | ||
| Mixed Conifer Plantation | MC-P | ||
| Private Coniferous Plantation | P-C-P | ||
| Newly added Coniferous Plantation | NEW-C-P | ||
| Broadleaved | Nature | Natural Broadleaved Forest | B-NF |
| Natural Mixed Broadleaved Forest | MB-NF | ||
| Plantation | Acacia Plantation | ACA-P | |
| Sweet Gum Plantation | SG-P | ||
| Camphor Plantation | CAM-P | ||
| Ash Plantation | ASH-P | ||
| Japanese elm Plantation | JE-P | ||
| Sapphire Dragon Tree Plantation | SDT-P | ||
| Other Broadleaved Plantation | O-B-P | ||
| Mixed Broadleaved Plantation | MB-P | ||
| Private Broadleaved Plantation | P-B-P | ||
| Newly added Broadleaved Plantation | NEW-B-P | ||
| Bamboo | Nature | Natural Makino Bamboo Forest | MAK-BAM-NF |
| Natural Moso Bamboo Forest | MOS-BAM-NF | ||
| Natural Taiwan Giant Bamboo Forest | TG-BAM-NF | ||
| Natural Thorny Bamboo Forest | THO-BAM-NF | ||
| Natural Bambusa Forest | BAM-BAM-NF | ||
| Natural Fargesia Forest | FAR-BAM-NF | ||
| Other Natural Bamboo Forest | O-BAM-NF | ||
| Plantation | Makino Bamboo Plantation | MAK-BAM-P | |
| Moso Bamboo Plantation | MOS-BAM-P | ||
| Taiwan Giant Bamboo Plantation | TG-BAM-P | ||
| Thorny Bamboo Plantation | THO-BAM-P | ||
| Bambusa Plantation | BAM-BAM-P | ||
| Other Bamboo Plantation | O-BAM-P | ||
| Private Bamboo Plantation | P-BAM-P | ||
| Mixed | Nature | Natural Coniferous and Broadleaved Mixed Forest | M-CB-NF |
| Natural Bamboo and Coniferous Mixed Forest | M-BAMC-NF | ||
| Natural Bamboo and Broadleaved Mixed Forest | M-BAMB-NF | ||
| Natural Bamboo, Coniferous and Broadleaved Mixed Forest | M-BAMCB-NF | ||
| Plantation | Coniferous and Broadleaved Mixed Plantation | M-CB-P | |
| Bamboo and Coniferous Mixed Plantation | M-BAMC-P | ||
| Bamboo and Broadleaved Mixed Plantation | M-BAMB-P | ||
| Bamboo, Coniferous and Broadleaved Mixed Plantation | M-BAMCB-P | ||
| Private Coniferous and Broadleaved Mixed Plantation | P-M-CB-P |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, T.-W.; Chen, G.-F.; Chang, K.-H. Modeling of the Spatial Distribution of Forest Carbon Storage in a Tropical/Subtropical Island with Multiple Ecozones. Plants 2023, 12, 2777. https://doi.org/10.3390/plants12152777
Chang T-W, Chen G-F, Chang K-H. Modeling of the Spatial Distribution of Forest Carbon Storage in a Tropical/Subtropical Island with Multiple Ecozones. Plants. 2023; 12(15):2777. https://doi.org/10.3390/plants12152777
Chicago/Turabian StyleChang, Ting-Wei, Guan-Fu Chen, and Ken-Hui Chang. 2023. "Modeling of the Spatial Distribution of Forest Carbon Storage in a Tropical/Subtropical Island with Multiple Ecozones" Plants 12, no. 15: 2777. https://doi.org/10.3390/plants12152777
APA StyleChang, T.-W., Chen, G.-F., & Chang, K.-H. (2023). Modeling of the Spatial Distribution of Forest Carbon Storage in a Tropical/Subtropical Island with Multiple Ecozones. Plants, 12(15), 2777. https://doi.org/10.3390/plants12152777






