Mitigation of Acetamiprid Residue Disruption on Pea Seed Germination by Selenium Nanoparticles and Lentinans
Abstract
:1. Introduction
2. Results
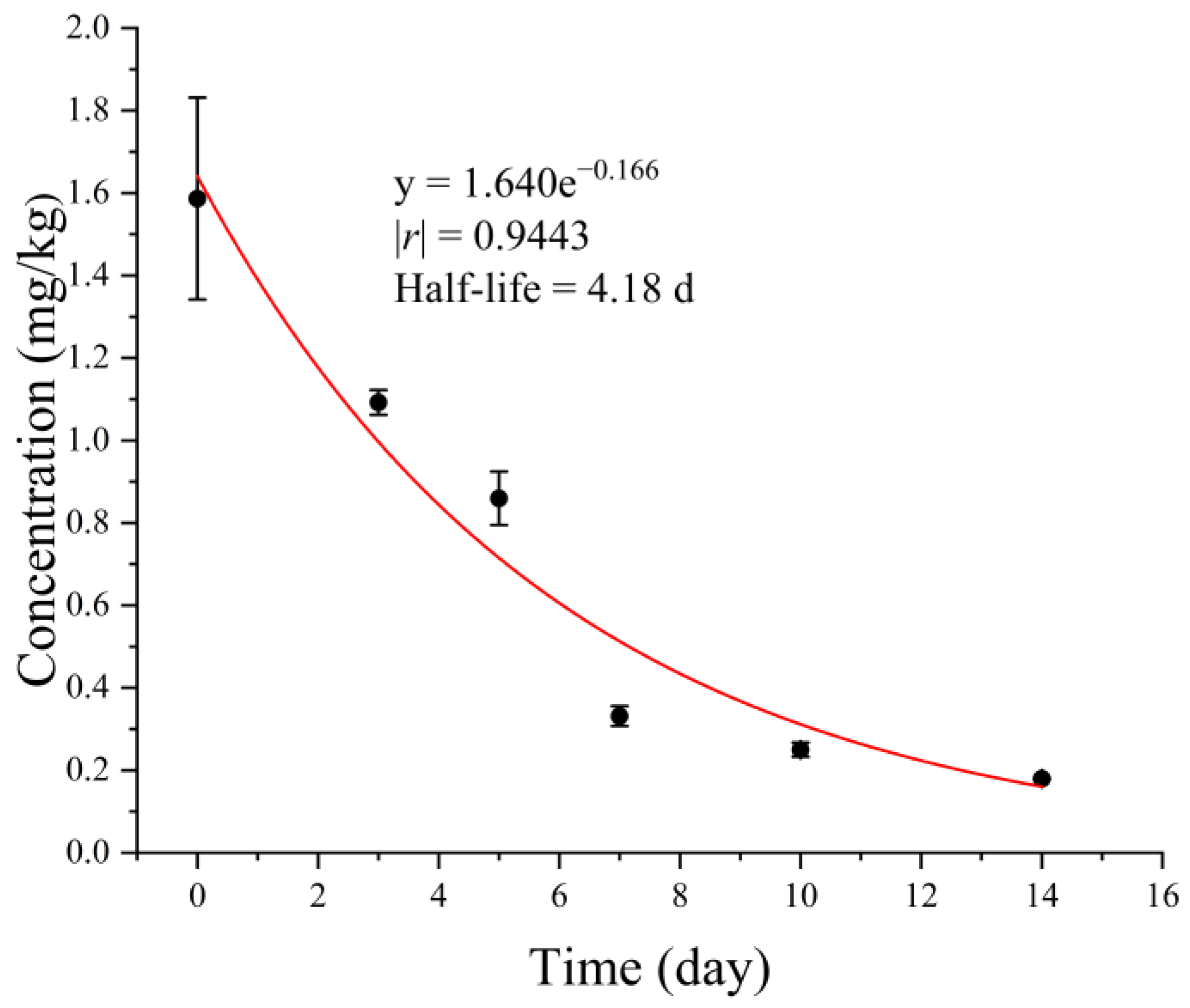
2.1. Degradation and Residue of Acetamiprid on Pea Seeds
2.2. Growth and Morphometric Parameters
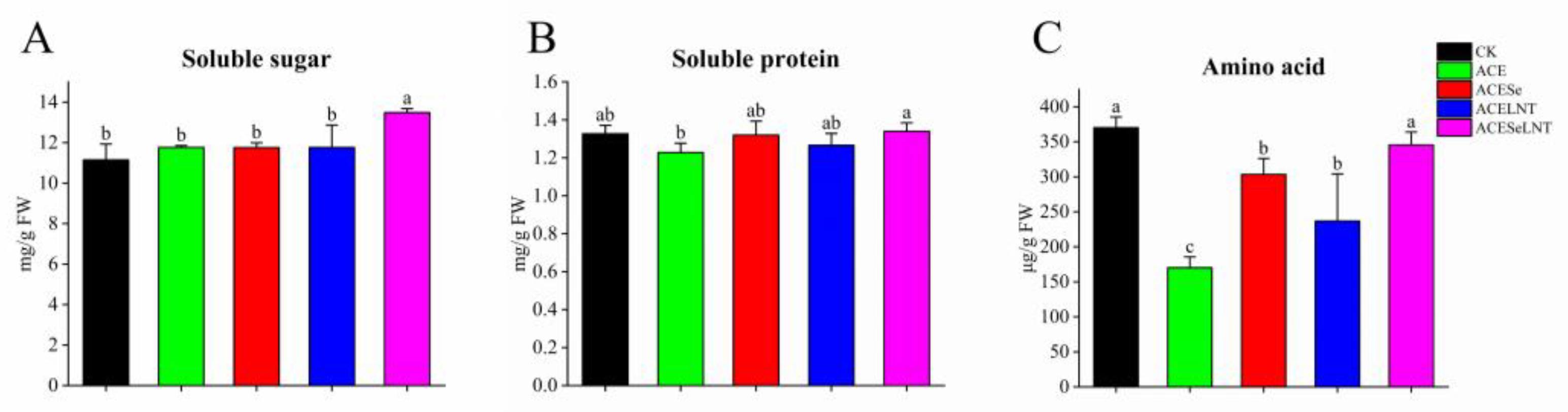
2.3. Nutrient Contents
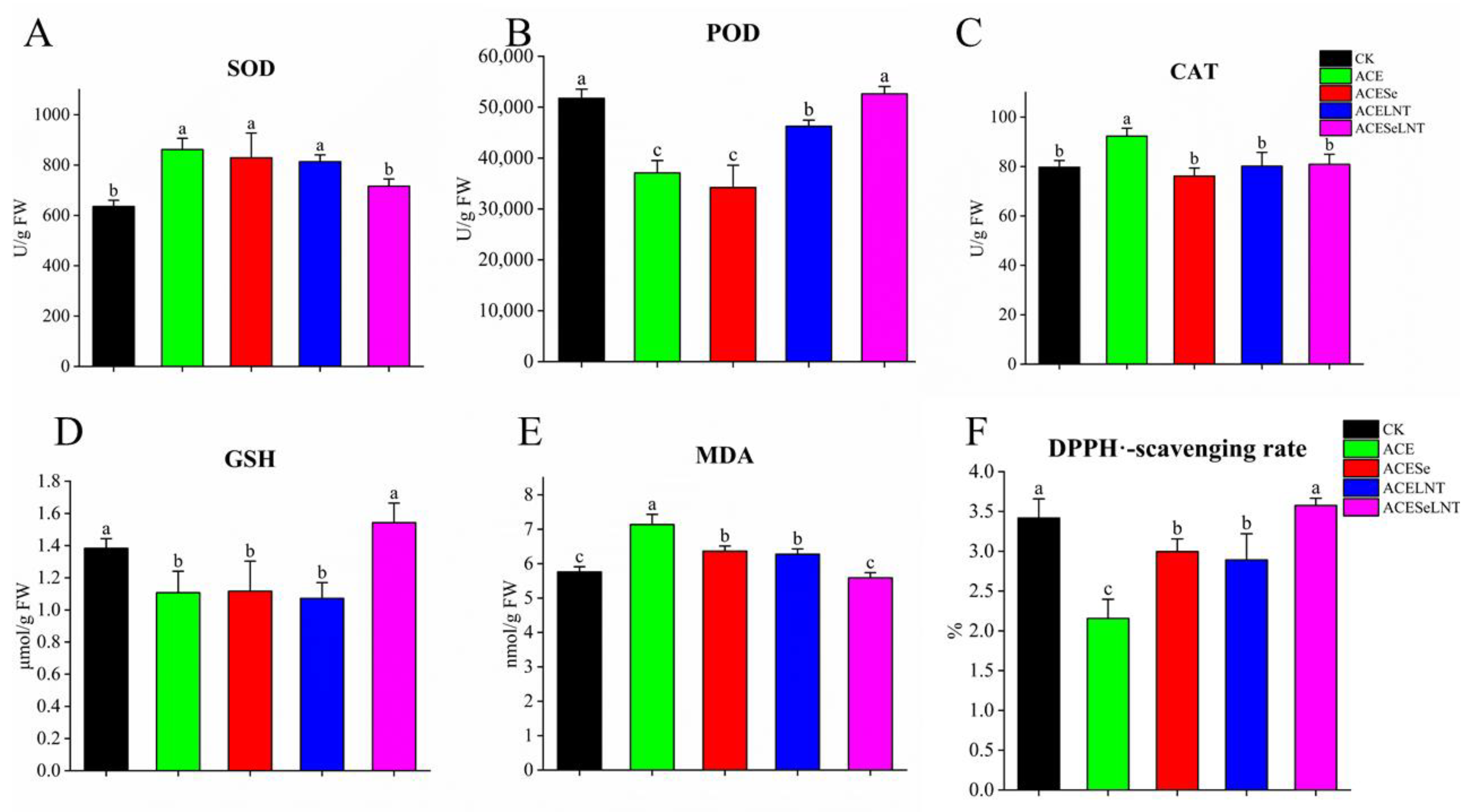
2.4. Antioxidant Indexes
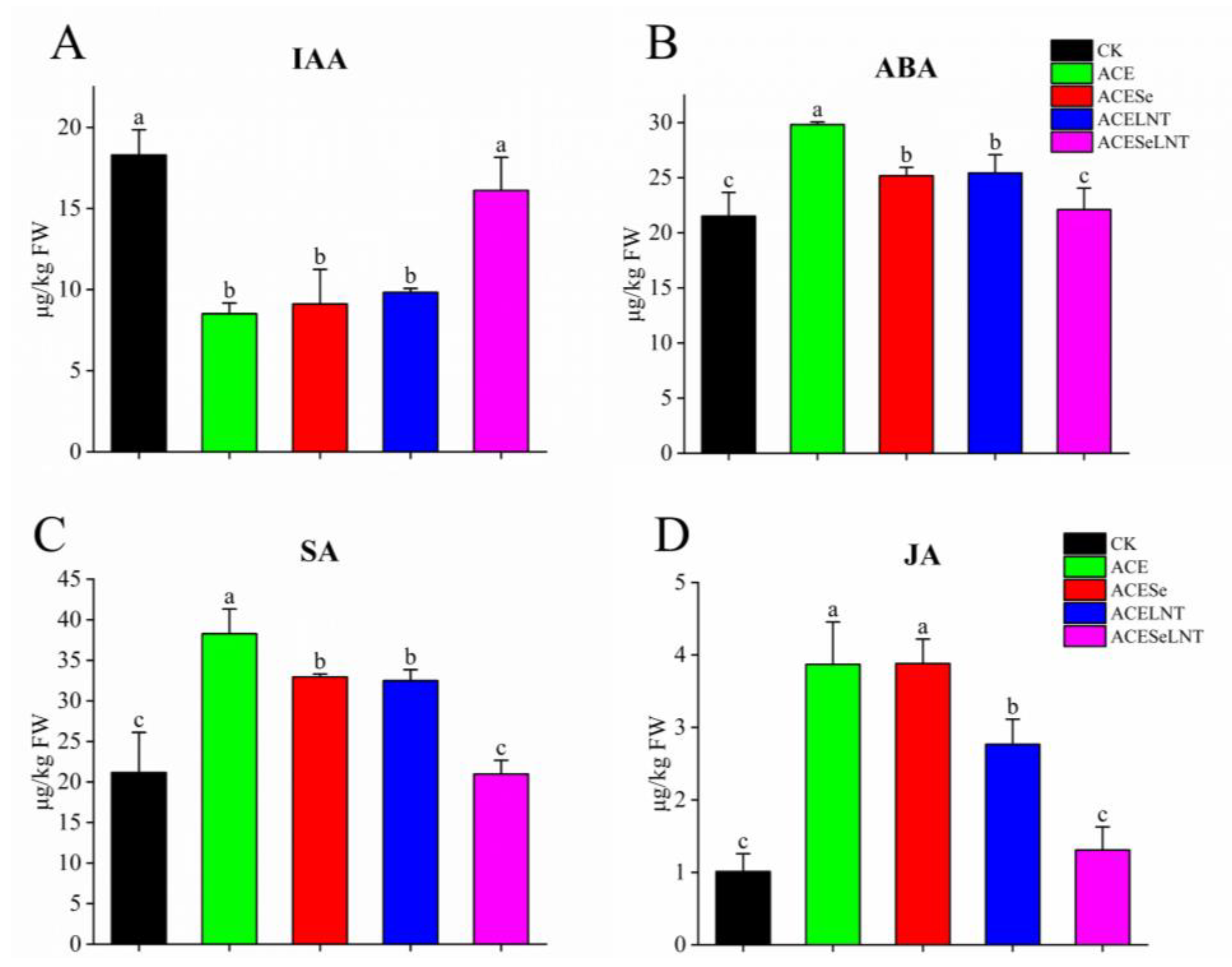
2.5. Plant Hormones
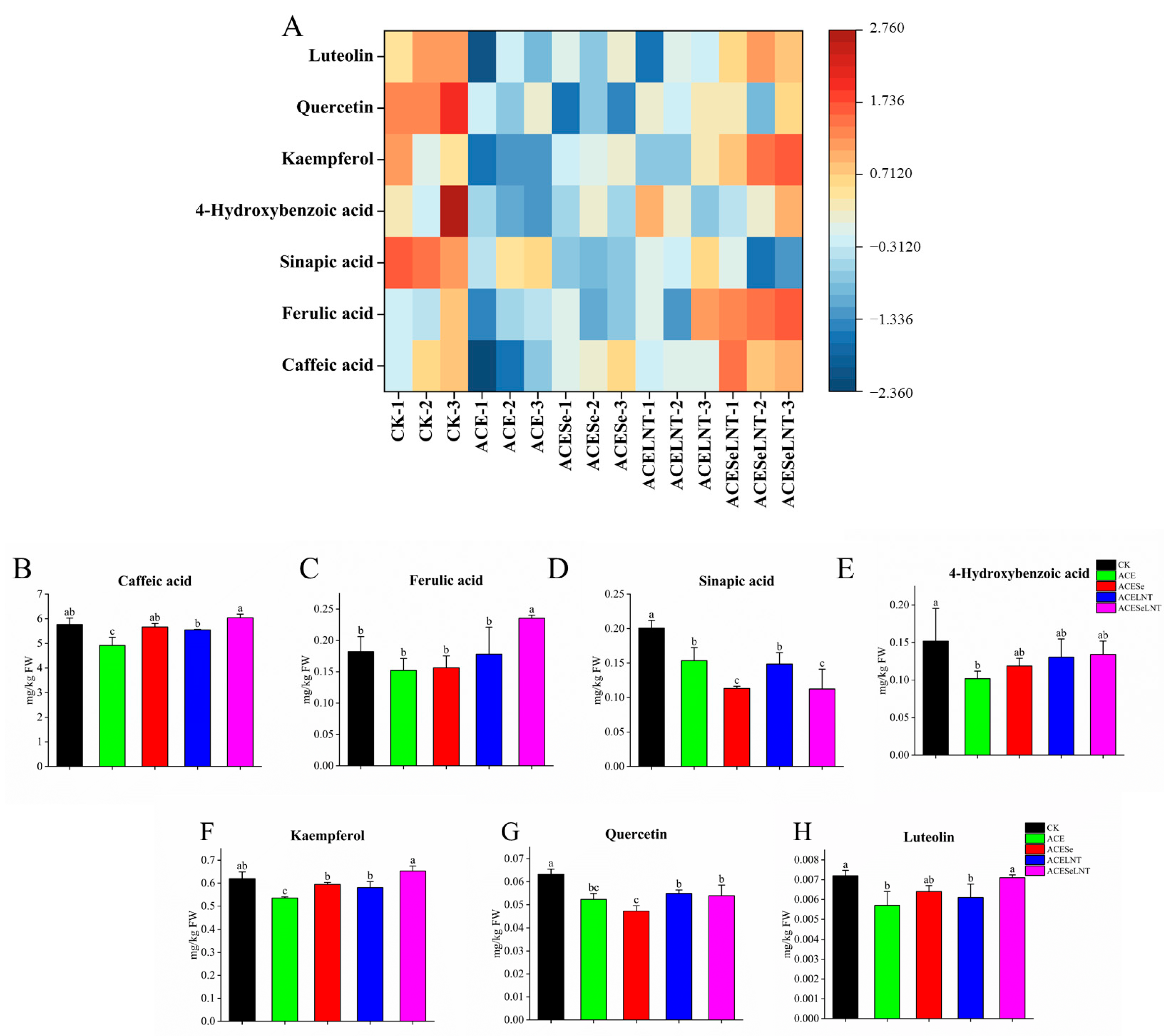
2.6. Phenols
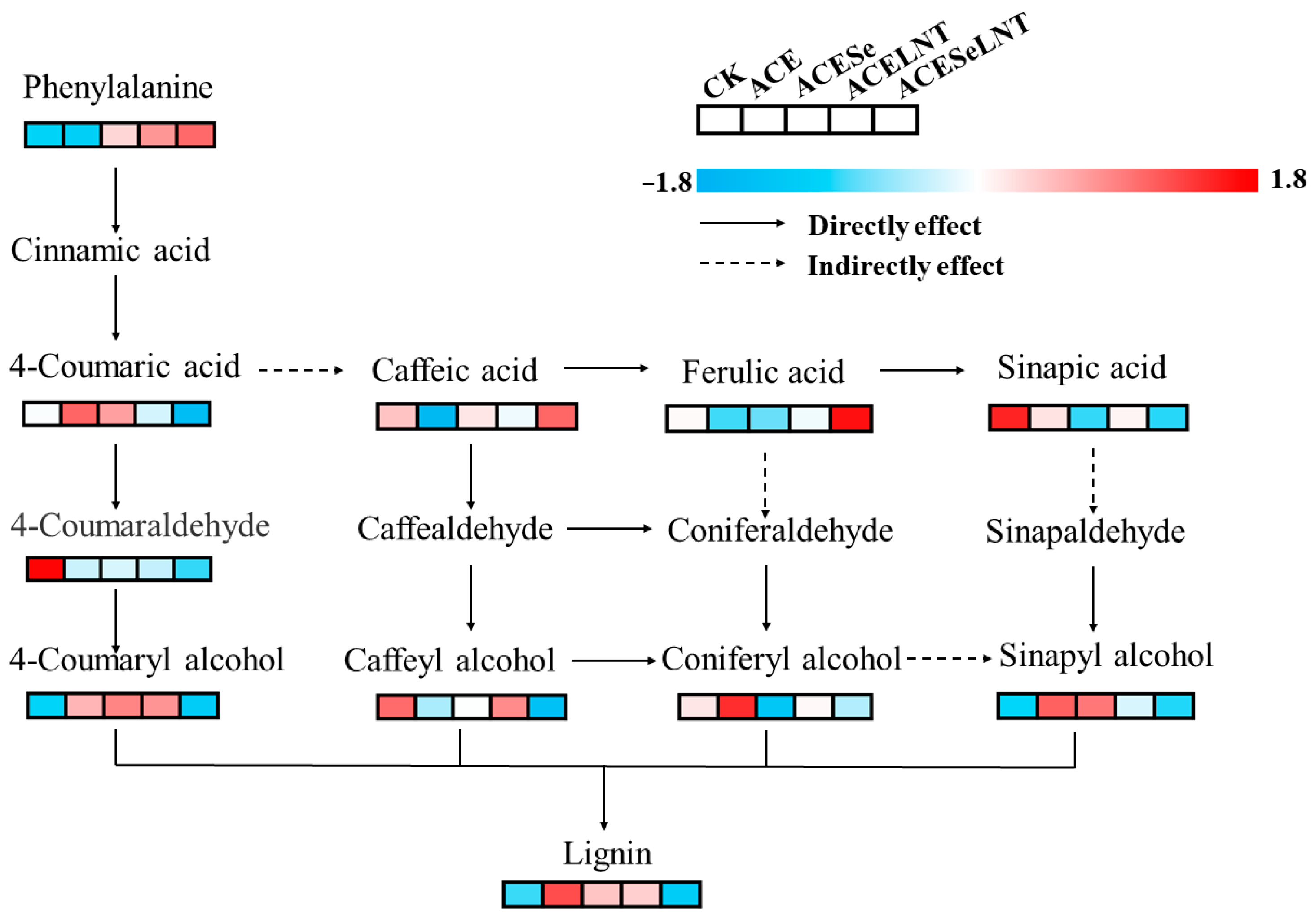
2.7. Lignin Biosynthesis Pathway
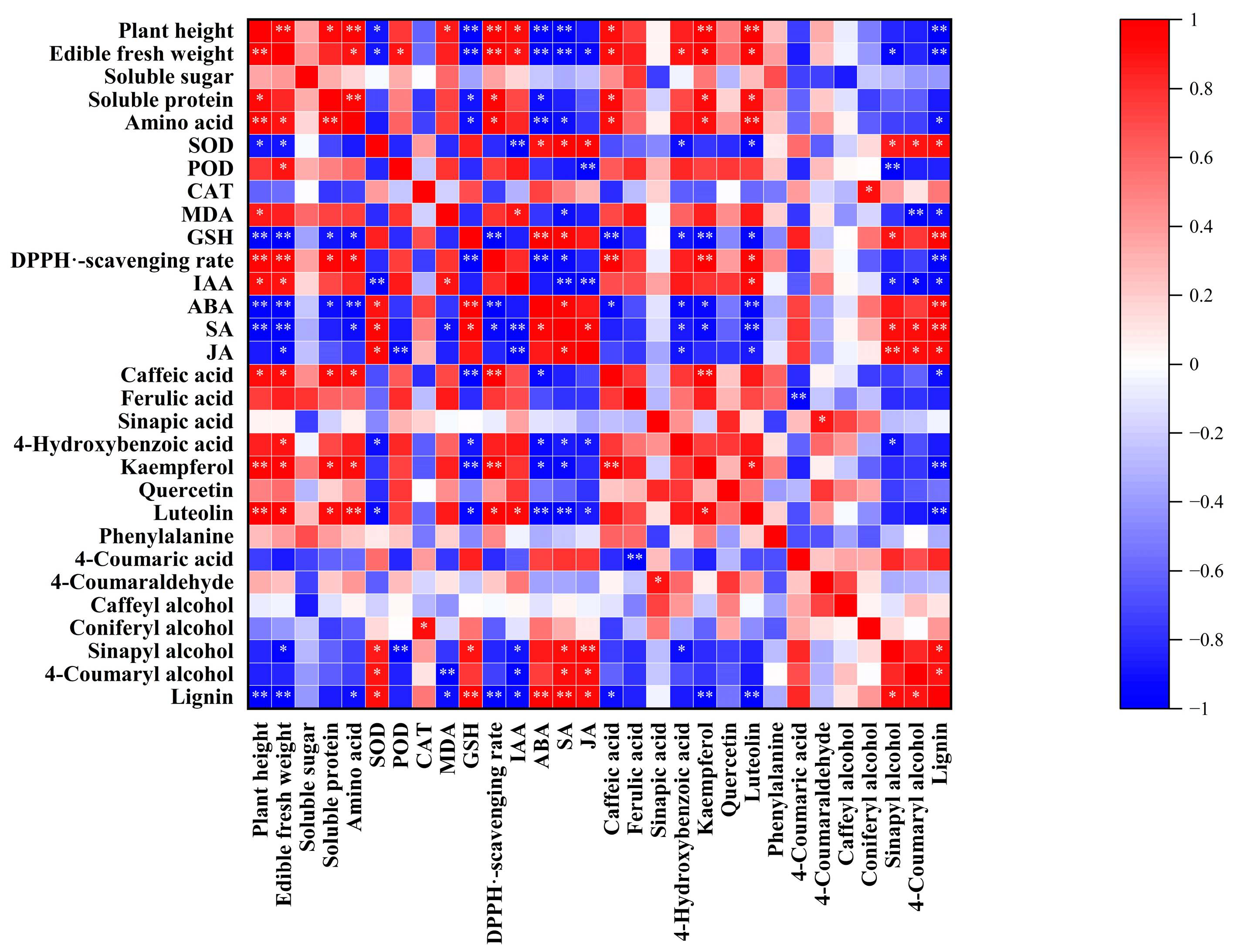
2.8. Correlation Analysis
3. Discussion
4. Materials and Methods
4.1. Matericals and Chemicals
4.2. Plant Materials and Culture
4.3. Analysis of Acetamiprid Residues on Pea Seeds
4.4. Nutrients and Antioxidant Indicators
4.5. Plant Hormone Analysis
4.6. Phenolic Compound Analysis
4.7. Metabolites in Lignin Biosynthesis Analysis
4.8. Data Process
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Xiao, Z.L.; Lester, G.E.; Luo, Y.G.; Wang, Q. Assessment of vitamin and carotenoid concentrations of emerging food products: Edible microgreens. J. Agric. Food Chem. 2012, 60, 7644–7651. [Google Scholar] [CrossRef]
- Xiao, Z.L.; Lester, G.E.; Park, E.; Saftner, R.A.; Luo, Y.G.; Wang, Q. Evaluation and correlation of sensory attributes and chemical compositions of emerging fresh produce: Microgreens. Postharvest Biol. Technol. 2015, 110, 140–148. [Google Scholar] [CrossRef]
- Ebert, A.W. Sprouts and microgreens-novel food sources for healthy diets. Plants 2022, 11, 571. [Google Scholar] [CrossRef] [PubMed]
- Suarez-Estrella, D.; Bresciani, A.; Iametti, S.; Marengo, M.; Pagani, M.A.; Marti, A. Effect of sprouting on proteins and starch in quinoa (Chenopodium quinoa Willd.). Plant Foods Hum. Nutr. 2020, 75, 635–641. [Google Scholar] [CrossRef] [PubMed]
- Aloo, S.O.; Ofosu, F.K.; Kilonzi, S.M.; Shabbir, U.; Oh, D.H. Edible plant sprouts: Health benefits, trends, and opportunities for novel exploration. Nutrients 2021, 13, 2882. [Google Scholar] [CrossRef]
- Ikram, A.; Saeed, F.; Afzaal, M.; Imran, A.; Niaz, B.; Tufail, T.; Hussain, M.; Anjum, F.M. Nutritional and end-use perspectives of sprouted grains: A comprehensive review. Food Sci. Nutr. 2021, 9, 4617–4628. [Google Scholar] [CrossRef]
- Xiao, H.T.; Wen, B.; Shen, X.C.; Bian, Z.X. Potential of plant-sourced phenols for nflammatory bowel disease. Curr. Med. Chem. 2018, 25, 5191–5217. [Google Scholar] [CrossRef] [PubMed]
- Stejskal, V.; Aulicky, R.; Kucerova, Z. Pest control strategies and damage potential of seed-infesting pests in the Czech stores—A Review. Plant Prot. Sci. 2014, 50, 165–173. [Google Scholar] [CrossRef]
- Mendesil, E.; Shumeta, Z.; Anderson, P.; Ramert, B. Smallholder farmers’ knowledge, perceptions and management of pea weevil in north and north-western Ethiopia. Crop Protect. 2016, 81, 30–37. [Google Scholar] [CrossRef]
- Benbrook, C.M.; Davis, D.R. The dietary risk index system: A tool to track pesticide dietary risks. Environ. Health 2020, 19, 103. [Google Scholar] [CrossRef]
- Parween, T.; Jan, S.; Mahmooduzzafar, S.; Fatma, T.; Siddiqui, Z.H. Selective effect of pesticides on plant-a review. Crit. Rev. Food Sci. Nutr. 2016, 56, 160–179. [Google Scholar] [CrossRef] [PubMed]
- Kyriacou, M.C.; Rouphael, Y.; Di Gioia, F.; Kyratzis, A.; Serio, F.; Renna, M.; De Pascale, S.; Santamaria, P. Micro-scale vegetable production and the rise of microgreens. Trends Food Sci. Technol. 2016, 57, 103–115. [Google Scholar] [CrossRef]
- Zhang, S.J.; Hu, T.T.; Liu, H.K.; Chen, Y.Y.; Pang, X.J.; Zheng, L.L.; Chang, S.M.; Kang, Y.F. Moderate vacuum packing and low temperature effects on qualities of harvested mung bean (Vigna radiata L.) sprouts. Postharvest Biol. Technol. 2018, 145, 83–92. [Google Scholar] [CrossRef]
- Singh, R.; Rastogi, S.; Dwivedi, U.N. Phenylpropanoid metabolism in ripening fruits. Compr. Rev. Food. Sci. Food Saf. 2010, 9, 398–416. [Google Scholar] [CrossRef]
- Shi, Y.N.; Li, B.J.; Su, G.Q.; Zhang, M.X.; Grierson, D.; Chen, K.S. Transcriptional regulation of fleshy fruit texture. J. Integr. Plant Biol. 2022, 64, 1649–1672. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, J.; Yang, H.S. Comparison of the metabolic responses of eight Escherichia coli strains including the "big six" in pea sprouts to low concentration electrolysed water by NMR spectroscopy. Food Control 2022, 131, 108458. [Google Scholar] [CrossRef]
- Wang, Y.; Gao, X.F.; Yang, H.S. Integrated metabolomics of "big six" Escherichia coli on pea sprouts to organic acid treatments. Food Res. Int. 2022, 157, 111354. [Google Scholar] [CrossRef]
- Wongmaneepratip, W.; Yang, H.S. Investigating the migration of pyrethroid residues between mung bean sprouts and growth media. Food Chem. 2021, 343, 128480. [Google Scholar] [CrossRef]
- Keneni, G.; Bekele, E.; Getu, E.; Imtiaz, M.; Damte, T.; Mulatu, B.; Dagne, K. Breeding food legumes for resistance to storage insect pests: Potential and limitations. Sustainability 2011, 3, 1399–1415. [Google Scholar] [CrossRef] [Green Version]
- Hu, J.; Xianyu, Y.L. When nano meets plants: A review on the interplay between nanoparticles and plants. Nano Today 2021, 38, 101143. [Google Scholar] [CrossRef]
- Kumar, D.; Dhankher, O.P.; Tripathi, R.D.; Seth, C.S. Titanium dioxide nanoparticles potentially regulate the mechanism(s) for photosynthetic attributes, genotoxicity, antioxidants defense machinery, and phytochelatins synthesis in relation to hexavalent chromium toxicity in Helianthus annuus L. J. Hazard. Mater. 2023, 454, 131418. [Google Scholar] [CrossRef]
- Chanda, M.J.; Merghoub, N.; El Arroussi, H. Microalgae polysaccharides: The new sustainable bioactive products for the development of plant bio-stimulants? World J. Microbiol. Biotechnol. 2019, 35, 177. [Google Scholar] [CrossRef] [PubMed]
- Reddy, G.V.P.; Sharma, A.; Gadi, R.L. Biology, ecology, and management of the pea weevil (Coleoptera: Chrysomelidae). Ann. Entomol. Soc. Am. 2018, 111, 161–171. [Google Scholar] [CrossRef]
- Fu, D.H.; Zhang, S.Y.; Wang, M.; Liang, X.Y.; Xie, Y.L.; Zhang, Y.; Zhang, C.H. Dissipation behavior, residue distribution and dietary risk assessment of cyromazine, acetamiprid and their mixture in cowpea and cowpea field soil. J. Sci. Food Agric. 2020, 100, 4540–4548. [Google Scholar] [CrossRef]
- Wu, J.X.; Wang, K.; Zhang, H.Y. Dissipation and residue of acetamiprid in watermelon and soil in the open field. Bull. Environ. Contam. Toxicol. 2012, 89, 644–648. [Google Scholar] [CrossRef]
- Badawy, M.E.I.; Ismail, A.M.E.; Ibrahim, A.I.H. Quantitative analysis of acetamiprid and imidacloprid residues in tomato fruits under greenhouse conditions. J. Environ. Sci. Health Part B-Pestic. Contam. Agric. Wastes 2019, 54, 898–905. [Google Scholar] [CrossRef]
- Park, J.Y.; Choi, J.H.; Kim, B.M.; Park, J.H.; Cho, S.K.; Ghafar, M.W.; Abd El-Aty, A.M.; Shim, J.H. Determination of acetamiprid residues in zucchini grown under greenhouse conditions: Application to behavioral dynamics. Biomed. Chromatogr. 2011, 25, 136–146. [Google Scholar] [CrossRef]
- Xi, N.N.; Li, Y.; Xia, X.H. A review of pesticide phototransformation on the leaf surface: Models, mechanism, and influencing factors. Chemosphere 2022, 308, 136260. [Google Scholar] [CrossRef]
- Shakir, S.K.; Kanwal, M.; Murad, W.; ur Rehman, Z.; ur Rehman, S.; Daud, M.K.; Azizullah, A. Effect of some commonly used pesticides on seed germination, biomass production and photosynthetic pigments in tomato (Lycopersicon esculentum). Ecotoxicology 2016, 25, 329–341. [Google Scholar] [CrossRef]
- Braganca, I.; Lemos, P.C.; Barros, P.; Delerue-Matos, C.; Domingues, V.F. Phytotoxicity of pyrethroid pesticides and its metabolite towards Cucumis sativus. Sci. Total Environ. 2018, 619, 685–691. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hernandez, A.A.R.; Zavala, F.A.; Sandez, O.R.; Frias, J.; Astiazaran-Garcia, H.; Sanchez, R.M.R. Consumption of sprouts and perceptions of their health properties in a region of northwestern Mexico. Foods 2021, 10, 3098. [Google Scholar] [CrossRef]
- Lemmens, E.; Moroni, A.V.; Pagand, J.; Heirbaut, P.; Ritala, A.; Karlen, Y.; Le, K.A.; Van den Broeck, H.C.; Brouns, F.; De Brier, N.; et al. Impact of cereal seed sprouting on its nutritional and technological properties: A critical review. Compr. Rev. Food. Sci. Food Saf. 2019, 18, 305–328. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.Y.; Yin, F.M.; Li, J.H.; Ren, S.H.; Liang, X.Y.; Zhang, Y.; Wang, L.F.; Wang, M.; Zhang, C.H. Transcriptomic and metabolomic investigation of metabolic disruption in Vigna unguiculata L. triggered by acetamiprid and cyromazine. Ecotoxicol. Environ. Saf. 2022, 239, 113675. [Google Scholar] [CrossRef] [PubMed]
- Selim, S.; Akhtar, N.; El Azab, E.; Warrad, M.; Alhassan, H.H.; Abdel-Mawgoud, M.; Al Jaouni, S.K.; Abdelgawad, H. Innovating the synergistic assets of beta-amino butyric acid (BABA) and selenium nanoparticles (SeNPs) in improving the growth, nitrogen metabolism, biological activities, and nutritive value of Medicago interexta sprouts. Plants 2022, 11, 306. [Google Scholar] [CrossRef]
- Singh, P.; Prasad, S.M. Antioxidant enzyme responses to the oxidative stress due to chlorpyrifos, dimethoate and dieldrin stress in palak (Spinacia oleracea L.) and their toxicity alleviation by soil amendments in tropical croplands. Sci. Total Environ. 2018, 630, 839–848. [Google Scholar] [CrossRef]
- Shakir, S.K.; Irfan, S.; Akhtar, B.; Rehman, S.U.; Daud, M.K.; Taimur, N.; Azizullah, A. Pesticide-induced oxidative stress and antioxidant responses in tomato (Solanum lycopersicum) seedlings. Ecotoxicology 2018, 27, 919–935. [Google Scholar] [CrossRef]
- Liu, T.T.; Li, T.T.; Zhang, L.Y.; Li, H.L.; Liu, S.K.; Yang, S.; An, Q.S.; Pan, C.P.; Zou, N. Exogenous salicylic acid alleviates the accumulation of pesticides and mitigates pesticide-induced oxidative stress in cucumber plants (Cucumis sativus). Ecotoxicol. Environ. Saf. 2021, 208, 111654. [Google Scholar] [CrossRef]
- Meng, Y.J.; Shuai, H.W.; Luo, X.F.; Chen, F.; Zhou, W.G.; Yang, W.Y.; Shu, K. Karrikins: Regulators involved in phytohormone signaling networks during seed germination and seedling development. Front. Plant Sci. 2017, 7, 2021. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verma, V.; Ravindran, P.; Kumar, P.P. Plant hormone-mediated regulation of stress responses. BMC Plant Biol. 2016, 16, 86. [Google Scholar] [CrossRef] [Green Version]
- Agnihotri, A.; Gupta, P.; Dwivedi, A.; Seth, C.S. Counteractive mechanism (s) of salicylic acid in response to lead toxicity in Brassica juncea (L.) Czern. cv. Varuna. Planta 2018, 248, 49–68. [Google Scholar] [CrossRef]
- Shohag, M.J.I.; Wei, Y.Y.; Yang, X.E. Changes of folate and other potential health-promoting phytochemicals in legume seeds as affected by germination. J. Agric. Food Chem. 2012, 60, 9137–9143. [Google Scholar] [CrossRef] [PubMed]
- Tang, D.Y.; Dong, Y.M.; Guo, N.; Li, L.; Ren, H.K. Metabolomic analysis of the polyphenols in germinating mung beans (Vigna radiata) seeds and sprouts. J. Sci. Food Agric. 2014, 94, 1639–1647. [Google Scholar] [CrossRef] [PubMed]
- Williams, D.J.; Edwards, D.; Hamernig, I.; Jian, L.; James, A.P.; Johnson, S.K.; Tapsell, L.C. Vegetables containing phytochemicals with potential anti-obesity properties: A review. Food Res. Int. 2013, 52, 323–333. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.Y.; Pu, Y.J.; Xu, Y.; He, X.; Cao, J.K.; Ma, Y.X.; Jiang, W.B. Anti-diabetic and anti-obesity: Efficacy evaluation and exploitation of polyphenols in fruits and vegetables. Food Res. Int. 2022, 151, 111202. [Google Scholar] [CrossRef] [PubMed]
- Kehinde, B.A.; Majid, I.; Hussain, S. Isolation of bioactive peptides and multiple nutraceuticals of antidiabetic and antioxidant functionalities through sprouting: Recent advances. J. Food Biochem. 2022, 46, e14317. [Google Scholar] [CrossRef]
- Waliat, S.; Arshad, M.S.; Hanif, H.; Ejaz, A.; Khalid, W.; Kauser, S.; Al-Farga, A. A review on bioactive compounds in sprouts: Extraction techniques, food application and health functionality. Int. J. Food Prop. 2023, 26, 647–665. [Google Scholar] [CrossRef]
- Geng, J.Z.; Li, J.X.; Zhu, F.M.; Chen, X.N.; Du, B.; Tian, H.L.; Li, J. Plant sprout foods: Biological activities, health benefits, and bioavailability. J. Food Biochem. 2022, 46, e13777. [Google Scholar] [CrossRef]
- Wulff, J.A.; Kiani, M.; Regan, K.; Eubanks, M.D.; Szczepaniec, A. Neonicotinoid insecticides alter the transcriptome of soybean and decrease plant resistance. Int. J. Mol. Sci. 2019, 20, 783. [Google Scholar] [CrossRef] [Green Version]
- Li, D.; Zhou, C.R.; Zou, N.; Wu, Y.L.; Zhang, J.B.; An, Q.S.; Li, J.Q.; Pan, C.P. Nanoselenium foliar application enhances biosynthesis of tea leaves in metabolic cycles and associated responsive pathways. Environ. Pollut. 2021, 273, 116503. [Google Scholar] [CrossRef]
- Wang, J.; Yu, G.H.; Li, Y.H.; Shen, L.L.; Qian, Y.M.; Yang, J.G.; Wang, F.L. Inhibitory effects of sulfated lentinan with different degree of sulfation against tobacco mosaic virus (TMV) in tobacco seedlings. Pestic. Biochem. Physiol. 2015, 122, 38–43. [Google Scholar] [CrossRef]
- Socha, R.; Juszczak, L.; Pietrzyk, S.; Fortuna, T. Antioxidant activity and phenolic composition of herbhoneys. Food Chem. 2009, 113, 568–574. [Google Scholar] [CrossRef]
- Zugic, A.; Dordevic, S.; Arsic, I.; Markovic, G.; Zivkovic, J.; Jovanovic, S.; Tadic, V. Antioxidant activity and phenolic compounds in 10 selected herbs from Vrujci Spa, Serbia. Ind. Crop. Prod. 2014, 52, 519–527. [Google Scholar] [CrossRef]
- Dong, N.Q.; Lin, H.X. Contribution of phenylpropanoid metabolism to plant development and plant-environment interactions. J. Integr. Plant Biol. 2021, 63, 180–209. [Google Scholar] [CrossRef]
- Toivonen, P.M.A.; Brummell, D.A. Biochemical bases of appearance and texture changes in fresh-cut fruit and vegetables. Postharvest Biol. Technol. 2008, 48, 1–14. [Google Scholar] [CrossRef]
- Liu, C.J. Deciphering the enigma of lignification: Precursor transport, oxidation, and the topochemistry of lignin assembly. Mol. Plant 2012, 5, 304–317. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Zhang, X.F.; Yang, S.L.; Yuan, Y.B. Lignin involvement in programmed changes in peach-fruit texture indicated by metabolite and transcriptome analyses. J. Agric. Food Chem. 2018, 66, 12627–12640. [Google Scholar] [CrossRef]
- Han, Y.; Yang, R.R.; Zhang, X.J.; Wang, Q.H.; Wang, B.; Zheng, X.Y.; Li, Y.C.; Prusky, D.; Bi, Y. Brassinosteroid accelerates wound healing of potato tubers by activation of reactive oxygen metabolism and phenylpropanoid metabolism. Foods 2022, 11, 906. [Google Scholar] [CrossRef]
- Yan, L.; Li, S.; Cheng, J.; Zhang, Y.R.; Jiang, C.C. Boron-mediated lignin metabolism in response to aluminum toxicity in citrus (Poncirus trifoliata (L.) Raf.) root. Plant Physiol. Biochem. 2022, 185, 1–12. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, Q.; Jiang, H.; Wang, B.; Bi, Y.; Li, Y.C.; Prusky, D. Reactive oxygen species-mediated the accumulation of suberin polyphenolics and lignin at wound sites on muskmelons elicited by benzo (1,2,3)-thiadiazole-7-carbothioic acid S-methyl ester. Postharvest Biol. Technol. 2020, 170, 111325. [Google Scholar] [CrossRef]
- Berni, R.; Luyckx, M.; Xu, X.; Legay, S.; Sergeant, K.; Hausman, J.F.; Lutts, S.; Cai, G.; Guerriero, G. Reactive oxygen species and heavy metal stress in plants: Impact on the cell wall and secondary metabolism. Environ. Exp. Bot. 2019, 161, 98–106. [Google Scholar] [CrossRef]
- Zuo, C.Z.; Hu, Q.H.; Su, A.X.; Xu, H.; Li, X.T.; Mariga, A.M.; Yang, W.J. Nanocomposite packaging delays lignification of Flammulina velutipes by regulating phenylpropanoid pathway and mitochondrial reactive oxygen species metabolisms. Postharvest Biol. Technol. 2021, 171, 111360. [Google Scholar] [CrossRef]
- Wang, Y.; Mo, Y.L.; Li, D.Q.; Xiang, C.Y.; Jiang, Z.D.; Wang, J. The main factors inducing postharvest lignification in king oyster mushrooms (Pleurotus eryngii): Wounding and ROS-mediated senescence. Food Chem. 2019, 301, 125224. [Google Scholar] [CrossRef] [PubMed]
- Chao, N.; Chen, W.Q.; Cao, X.; Jiang, X.N.; Gai, Y.; Liu, L. Plant hormones coordinate monolignol biosynthesis with seasonal changes in Populus tomentosa. Environ. Exp. Bot. 2022, 195, 104784. [Google Scholar] [CrossRef]
- Cecchetti, V.; Altamura, M.M.; Brunetti, P.; Petrocelli, V.; Falasca, G.; Ljung, K.; Costantino, P.; Cardarelli, M. Auxin controls Arabidopsis anther dehiscence by regulating endothecium lignification and jasmonic acid biosynthesis. Plant J. 2013, 74, 411–422. [Google Scholar] [CrossRef] [PubMed]
- Kovacik, J.; Gruz, J.; Backor, M.; Strnad, M.; Repcak, M. Salicylic acid-induced changes to growth and phenolic metabolism in Matricaria chamomilla plants. Plant Cell Rep. 2009, 28, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Orenes, A.; Alba, J.M.; Kant, M.R.; Calderon, A.A.; Ferrer, M.A. OPDA and ABA accumulation in Pb-stressed Zygophyllum fabago can be primed by salicylic acid and coincides with organ-specific differences in accumulation of phenolics. Plant Physiol. Biochem. 2020, 154, 612–621. [Google Scholar] [CrossRef]
- Ministry of Agriculture and Rural Affairs of the People’s Republic of China. Guideline for the Testing of Pesticide Residues in Crops: NY/T 788—2018; China Agriculture Press: Beijing, China, 2018; pp. 5–6.
- Suzhou Comin Biotechnology Co. Ltd. Available online: http://www.cominbio.com/index.html (accessed on 13 July 2023).


| Treatment | Plant Height (cm) n = 12 | Root Length (cm) n = 12 | Edible Fresh Weight (g) n = 6 | Root Fresh Weight (g) n = 6 |
|---|---|---|---|---|
| CK | 7.67 ± 0.28 a | 5.89 ± 1.60 a | 4.39 ± 0.57 a | 0.78 ± 0.16 a |
| ACE | 5.32 ± 0.38 d | 5.24 ± 1.13 a | 2.86 ± 0.22 c | 0.70 ± 0.16 a |
| ACESe | 6.53 ± 0.39 b | 5.90 ± 1.10 a | 3.47 ± 0.25 b | 0.82 ± 0.14 a |
| ACELNT | 6.12 ± 0.24 c | 5.22 ± 1.58 a | 3.73 ± 0.19 b | 0.79 ± 0.09 a |
| ACESeLNT | 7.76 ± 0.32 a | 5.66 ± 1.21 a | 4.59 ± 0.20 a | 0.72 ± 0.16 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, Y.; Zhou, C.; Li, D.; Jia, Y.; Dong, Q.; Yu, H.; Wu, T.; Pan, C. Mitigation of Acetamiprid Residue Disruption on Pea Seed Germination by Selenium Nanoparticles and Lentinans. Plants 2023, 12, 2781. https://doi.org/10.3390/plants12152781
Lin Y, Zhou C, Li D, Jia Y, Dong Q, Yu H, Wu T, Pan C. Mitigation of Acetamiprid Residue Disruption on Pea Seed Germination by Selenium Nanoparticles and Lentinans. Plants. 2023; 12(15):2781. https://doi.org/10.3390/plants12152781
Chicago/Turabian StyleLin, Yongxi, Chunran Zhou, Dong Li, Yujiao Jia, Qinyong Dong, Huan Yu, Tong Wu, and Canping Pan. 2023. "Mitigation of Acetamiprid Residue Disruption on Pea Seed Germination by Selenium Nanoparticles and Lentinans" Plants 12, no. 15: 2781. https://doi.org/10.3390/plants12152781
APA StyleLin, Y., Zhou, C., Li, D., Jia, Y., Dong, Q., Yu, H., Wu, T., & Pan, C. (2023). Mitigation of Acetamiprid Residue Disruption on Pea Seed Germination by Selenium Nanoparticles and Lentinans. Plants, 12(15), 2781. https://doi.org/10.3390/plants12152781






