Stability of Phenols, Antioxidant Capacity and Grain Yield of Six Rice Genotypes
Abstract
:1. Introduction
2. Results
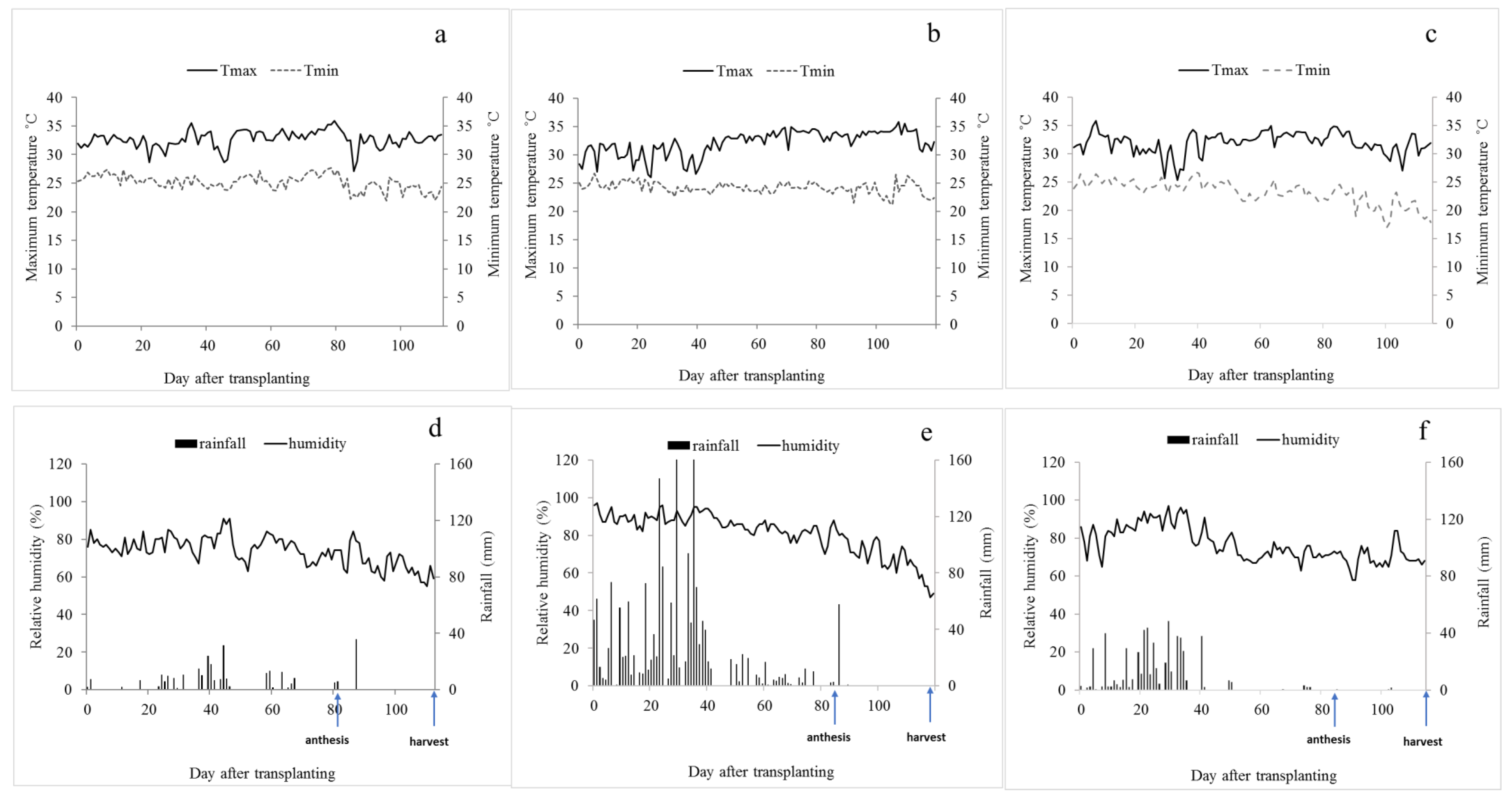
2.1. Effects of Environment, Genotype and Their Interaction on Traits
2.2. Yield and Yield Components
2.3. Total Phenolic Content (TPC), Ferulic Acid and Antioxidant Capacity
2.4. Stability Analysis
2.5. Correlation between Grain Yield, Yield Components and Antioxidant Compounds
3. Discussion
3.1. Effects of Genotype and Genotype–Environment Interactions on Traits
3.2. Stability of Grain Yield, Phenols and Antioxidant Capacity
3.3. Correlation between Grain Yield, Yield Components and Antioxidant Compounds
4. Materials and Methods
4.1. Plant Materials and Experimental Design
4.2. Yield and Yield Components
4.3. Total Phenolic Content (TPC)
4.4. Ferulic Acid
4.5. Antioxidant Capacity (2,2-Diphenyl-1-Picrylhydrazyl, DPPH)
4.6. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fukagawa, N.K.; Ziaka, L.H. Rice: Importance for global nutrition. J. Nutr. Sci. Vitaminol. 2019, 56, s2–s3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muttagi, G.C.; Ravindra, U. Chemical and nutritional composition of traditional rice varieties of Karnataka. J. Pharmacogn. Phytochem. 2020, 9, 2300–2309. [Google Scholar] [CrossRef]
- Ma, Y.; Zhang, S.; Rong, L.; Wu, Z.; Sun, W. Polyphenol composition and antioxidant activity of Japonica rice cultivars and intake status. Foods 2022, 11, 3788. [Google Scholar] [CrossRef]
- Shahidi, F.; Danielski, R.; Rhein, S.O.; Meisel, L.A.; Fuentes, J.; Speisky, H.; Schwember, A.R.; Camargo, A.C. Wheat and rice beyond phenolic acids: Genetics, identification database, antioxidant properties, and potential health effects. Plants 2022, 11, 3283. [Google Scholar] [CrossRef] [PubMed]
- Šamec, D.; Karalija, E.; Šola, I.; Bok, V.V.; Salopek-Sondi, B. The role of polyphenols in abiotic stress response: The influence of molecular structure. Plants 2021, 10, 118. [Google Scholar] [CrossRef]
- Zhou, Z.; Robards, K.; Helliwell, S.; Blanchard, C. The distribution of phenolic acids in rice. Food Chem. 2004, 87, 401–406. [Google Scholar] [CrossRef]
- Sing, S.X.; Lee, H.H.; Wong, S.C.; Bong, C.F.J.; You, P.H. Ferulic acid, gamma oryzanol and GABA content in whole grain rice and their variation with bran colour. Emir. J. Food Agric. 2015, 27, 706–711. [Google Scholar] [CrossRef] [Green Version]
- Shen, Y.; Jin, L.; Xiao, P.; Lu, Y.; Bao, J. Total phenolics, flavonoids, antioxidant capacity in rice grain and their relations to grain color, size and weight. J. Cereal Sci. 2009, 49, 106–111. [Google Scholar] [CrossRef]
- Martirosyan, D.M.; Singharaj, B. Health claims and functional food: The future of functional foods under FDA and EFSA regulation. In Functional Foods for Chronic Diseases; Food Science Publisher: Dallas, TX, USA, 2016; pp. 410–424. [Google Scholar]
- World Health Organization. Noncommunicable Diseases. 2023. Available online: https://www.who.int/health-topics/noncommunicable-diseases#tab=tab_1 (accessed on 24 May 2023).
- Shao, Y.; Xu, F.; Chen, Y.; Huang, Y.; Beta, T.; Bao, J. Analysis of genotype, environment, and their interaction effects on the phytochemicals and antioxidant capacities of red rice (Oryza sativa L.). Cereal Chem. 2015, 92, 204–210. [Google Scholar] [CrossRef]
- Nurmi, T.; Lampi, A.; Nystro, M.L.; Piironen, V. Effects of environment and genotype on phytosterols in wheat in the HEALTHGRAIN diversity screen. J. Agric. Food Chem. 2010, 58, 9314–9323. [Google Scholar] [CrossRef]
- Zewdu, Z.; Abebe, T.; Mitiku, T.; Worede, F.; Dessie, A.; Berie, A.; Atna, M. Performance evaluation and yield stability of upland rice (Oryza sativa L.) varieties in Ethiopia. Cogent Food Agric. 2020, 6, 1842679. [Google Scholar] [CrossRef]
- Jaruchai, W.; Monkham, T.; Chankaew, S.; Suriharn, B.; Sanitchon, J. Evaluation of stability and yield potential of upland rice genotypes in North and Northeast Thailand. J. Integr. Agric. 2018, 17, 28–36. [Google Scholar] [CrossRef]
- Shrestha, J.; Kushwaha, U.K.S.; Maharjan, B.; Kandel, M.; Gurung, S.B.; Poudel, A.P.; Karna, M.K.L.; Acharya, R. Grain yield stability of rice genotypes. Indones. J. Agric. Res. 2020, 3, 116–126. [Google Scholar] [CrossRef]
- Kouke, R.Y.S.; Djihinto, C.A.; Zavinon, F.; Djehoungo, P.G.; Chougourou, D. Genotype x environment interaction and stability analysis of agronomic performance in aromatic rice accessions in Benin. J. Appl. Biosci. 2022, 177, 18353–18363. [Google Scholar]
- Haryanto, T.A.D.; Suwarto, S.; Yoshida, T. Yield stability of aromatic upland rice with high yielding ability in Indonesia. Plant Prod. Sci. 2008, 11, 96–103. [Google Scholar] [CrossRef]
- Yamuangmorn, S.; Jumrus, S.; Jamjod, S.; Yimyam, N.; Prom-u-Thai, C. Stabilizing grain yield and nutrition quality in purple rice varieties by management of planting elevation and storage conditions. Agronomy 2021, 11, 83. [Google Scholar] [CrossRef]
- Eberhart, S.A.; Russell, W.A. Stability parameters for comparing varieties. Crop Sci. 1996, 6, 36–40. [Google Scholar] [CrossRef] [Green Version]
- Ulzen, O.O.; Buri, M.M.; Sekyi-Annan, E.; Devkota, K.P.; Dossou-Yovo, E.R.; Ayamba, B.E.; Adjei, E.O. Yield potentials of improved rice varieties for increased lowland rice production within the mankran watershed in Ghana. Plant Prod. Sci. 2023, 26, 17–27. [Google Scholar] [CrossRef]
- Mitsuya, S.; Murakami, N.; Sato, T.; Kazama, T.; Toriyama, K.; Skoulding, N.S.; Kano-Nakata, M.; Yamauchi, A. Evaluation of rice grain yield and yield components of Nona Bokra chromosome segment substitution lines with the genetic background of Koshihikari, in a saline paddy field. AoB Plants 2019, 11, plz040. [Google Scholar] [CrossRef]
- Huang, M.; Zou, Y.B.; Jiang, P.; Xia, B.; Md, I.; Ao, H.J. Relationship between grain yield and yield components in super hybrid rice. Agr. Sci. China 2011, 10, 1537–1544. [Google Scholar] [CrossRef]
- Khalil, I.H.; Bari, A.; Khan, S.; Zada, I. Genetic variation for yield and yield components in rice. J. Agric. Biol. Sci. 2009, 4, 60–64. [Google Scholar]
- Aninbon, C.; Srihanoo, C.; Phakamas, N. Genotypic variations in ferulic acid, antioxidant capacity and yield components of Thai landrace rice genotypes. Agrivita 2022, 44, 55–64. [Google Scholar] [CrossRef]
- Maina, S.; Ryu, D.H.; Bakari, G.; Misinzo, G.; Nho, C.W.; Kim, H.Y. Variation in phenolic compounds and antioxidant activity of various organs of African cabbage (Cleome gynandra L.) accessions at different growth stages. Antioxidants 2021, 10, 1952. [Google Scholar] [CrossRef] [PubMed]
- Muntana, N.; Prasong, S. Study on total phenolic contents and their antioxidant activities of Thai white, red and black rice bran extracts. Pak. J. Biol. Sci. 2010, 13, 170–174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kanfany, G.; Ayenan, M.A.T.; Zoclanclounon, Y.A.B.; Kane, T.; Ndiaye, M.; Diatta, C.; Diatta, J.; Gueye, T.; Fofana, A. Analysis of genotype-environment interaction and yield stability of introduced upland rice in the Groundnut Basin Agroclimatic Zone of Senegal. Adv. Agric. 2021, 2021, 4156167. [Google Scholar] [CrossRef]
- Harakotr, B.; Prompoh, K.; Suriharn, K.; Lertrat, K. Genotype by environment interaction effects on nutraceutical lipid compounds of pigmented rice (Oryza sativa L. ssp. indica). Int. J. Agron. 2021, 2021, 8880487. [Google Scholar] [CrossRef]
- Guo, X.; Ma, Y.; Parry, J.; Gao, J.; Yu, L.; Wang, M. Phenolics content and antioxidant activity of tartary buckwheat from different locations. Molecules 2011, 16, 9850–9867. [Google Scholar] [CrossRef] [Green Version]
- Soil Resources Survey and Research Division. Eastern and Southern Soil Series; Agriculture Fundamentals. 2017. Available online: http://oss101.ldd.go.th/soilr/SoilGrp25k48_53/pdf/E_Sseries_2557.pdf (accessed on 5 April 2023).
- Mishra, J.S.; Kumar, R.; Saurabh, K.; Bhatt, B.P. Conservation Agriculture for Climate Resilient Farming & Doubling Farmers’ Income; ICAR Research Complex for Eastern Region: Patna, India, 2019; 246p. [Google Scholar]
- Punithavathi, M.; Vasanthakumar, R.; Mariappan, V.N. Studies on drought tolerant and high yielding groundnut varieties in Perambalur district. Int. J. Bio-Resour. Stress Manag. 2021, 12, 064–067. [Google Scholar] [CrossRef]
- Simtowe, F.; Amondo, E.; Marenya, P.; Rahut, D.; Sonder, K.; Erenstein, O. Impacts of drought-tolerant maize varieties on productivity, risk, and resource use: Evidence from Uganda. Land Use Policy 2019, 88, 2–11. [Google Scholar] [CrossRef]
- Yan, C.; Song, S.; Wang, W.; Wang, C.; Li, H.; Wang, F.; Li, S.; Sun, X. Screening diverse soybean genotypes for drought tolerance by membership function value based on multiple traits and drought tolerant coefficient of yield. BMC Plant Biol. 2020, 20, 321. [Google Scholar] [CrossRef]
- Pimratch, S.; Jogloy, S.; Vorasoot, N.; Toomsan, B.; Patanothai, A.; Holbrook, C.C. Relationship between biomass production and nitrogen fixation under drought-stress conditions in peanut genotypes with different levels of drought resistance. J. Agron. Crop Sci. 2007, 194, 15–25. [Google Scholar] [CrossRef]
- Shao, Y.; Xu, F.; Sun, X.; Bao, J.; Beta, T. Phenolic acids, anthocyanins, and antioxidant capacity in rice (Oryza sativa L.) grains at four stages of development after flowering. Food Chem. 2014, 143, 90–96. [Google Scholar] [CrossRef] [PubMed]
- Ghasemzadeh, A.; Karbalaii, M.T.; Jaafar, H.Z.E.; Rahmat, A. Phytochemical constituents, antioxidant activity, and antiproliferative properties of black, red, and brown rice bran. Chem. Cent. J. 2018, 12, 17. [Google Scholar] [CrossRef] [Green Version]
- Zhang, M.W.; Zhang, R.F.; Zhang, F.X.; Liu, R.H. Phenolic profiles and antioxidant activity of black rice bran of different commercially available varieties. J. Agric. Food Chem. 2010, 58, 7580–7587. [Google Scholar] [CrossRef]
- Hu, Z.; Tang, X.; Liu, J.; Zhu, Z.; Shao, Y. Effect of parboiling on phytochemical content, antioxidant activity and physicochemical properties of germinated red rice. Food Chem. 2017, 214, 285–292. [Google Scholar] [CrossRef]
- Thaworn, S.; Chadchawan, S.; Paliyavuth, C.; Kasettranan, W. Clustering of white, red and purple rice cultivars according to their total phenolic content, total flavonoid content and antioxidant capacity in their grains. Agr. Nat. Resour. 2021, 55, 89–97. [Google Scholar]
- Mangmee, K.; Homthawornchoo, W. Antioxidant activity and physicochemical properties of rice starchchitosan-based films containing green tea extract. FAB J. 2016, 4, 126–137. [Google Scholar]
- Muflihah, Y.M.; Gollavelli, G.; Ling, Y.C. Correlation study of antioxidant activity with phenolic and flavonoid compounds in 12 Indonesian indigenous herbs. Antioxidants 2021, 10, 1530. [Google Scholar] [CrossRef]
- Karamać, M.; Koleva, L.; Kancheva, V.D.; Amarowicz, R. The structure–antioxidant activity relationship of ferulates. Molecules 2017, 22, 527. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodnuch, N.; Phongsri, S.; Aninbon, C. Responses of landrace rice to organic fertilizer for physiological traits, grain yield and total phenolic content. Curr. Appl. Sci. Technol. 2019, 19, 297–305. [Google Scholar]
- Sawetavong, C.; Lilitcham, S.; Pavadhgul, P.; Ayusuk, K.; Krinangkura, K. Partial extraction method for quantifying ferulic acid in rice bran. In Proceedings of the 48th Kasetsart University Annual Conference: Agro-Industry, Kasetsart, Thailand, 3–5 March 2010; pp. 530–537. [Google Scholar]
- Kaur, M.; Asthir, B.; Mahajan, G. Variation in antioxidants, bioactive compounds and antioxidant capacity in germinated and ungerminated grains of ten rice cultivars. Rice Sci. 2017, 24, 349–359. [Google Scholar] [CrossRef]
- Voltas, J.; López-Córcoles, H.; Borrás, G. Use of biplot analysis and factorial regression for the investigation of superior genotypes in multi-environment trials. Eur. J. Agron. 2005, 22, 309–324. [Google Scholar] [CrossRef]
| Source of Variation | df | Number of Tillers/Plant | Number of Panicles/Plant | Number of Grains/Panicle | 1000-Grain Weight | Grain Yield | Total Phenolic Content | Ferulic Acid | DPPH (%) |
|---|---|---|---|---|---|---|---|---|---|
| Environment | 2 | 100.1 ** | 58.6 ** | 1911.5 ns | 21.2 ** | 1,591,754 ** | 44.0 ns | 100.0 ** | 321.6 * |
| (43.10) | (68.38) | (29.54) | (49.19) | (44.98) | (3.21) | (35.54) | (11.63) | ||
| Rep. within site | 6 | 5.0 | 6.2 | 313.4 | 1.6 | 74,698 | 37.9 | 8.3 | 113.6 |
| (2.15) | (7.23) | (4.84) | (3.71) | (2.11) | (2.77) | (2.95) | (4.11) | ||
| Genotype (G) | 5 | 73.8 ** | 9.6 ** | 1177.0 ns | 14.1 ** | 1,032,721 ** | 1199.1 ** | 160.3 ** | 1965.6 ** |
| (31.78) | (11.20) | (18.19) | (32.26) | (29.18) | (87.05) | (56.96) | (71.10) | ||
| G × E | 10 | 48.2 ns | 9.7 ** | 2307.4 ** | 3.0 ns | 763,938 ** | 16.1 ns | 9.3 * | 301.6 ** |
| (20.76) | (11.32) | (35.65) | (6.96) | (21.59) | (1.18) | (3.30) | (10.91) | ||
| Error (G*Rep*E) | 30 | 5.1 | 1.6 | 762.4 | 3.2 | 75,233 | 71.2 | 3.5 | 62.0 |
| (2.19) | (1.87) | (11.78) | (7.42) | (7.15) | (5.20) | (1.24) | (2.24) | ||
| Total | 53 | ||||||||
| C.V. (%) | 17.68 | 10.99 | 15.81 | 7.86 | 10.25 | 27.73 | 10.14 | 24.31 |
| Genotype | Bangkok | Trat | Sakon Nakhon | Mean | Genotype | Bangkok | Trat | Sakon Nakhon | Mean |
|---|---|---|---|---|---|---|---|---|---|
| Number of tillers per plant | Number of panicles per plant | ||||||||
| Hom Nang Nual 1 | 9.05 cd1/ | 14.07 bc | 13.17 b | 12.09 B2/ | Hom Nang Nual 1 | 8.95 b | 13.51 ab | 14.67 a | 12.37 A |
| Lhueang Thong | 11.29 ab | 12.06 c | 10.50 b | 11.28 B | Lhueang Thong | 10.38 b | 11.25 c | 9.42 b | 10.34 B |
| KDML105 | 9.57 bc | 18.46 a | 27.55 a | 18.53 A | KDML105 | 9.00 b | 12.12 bc | 14.75 a | 11.95 AB |
| Mali Nil Boran | 11.53 a | 14.20 b | 12.83 b | 12.85 B | Mali Nil Boran | 10.38 b | 13.20 abc | 12.83 a | 12.13 AB |
| Mali Nil Surin | 7.36 d | 14.84 b | 9.58 b | 10.59 B | Mali Nil Surin | 7.21 c | 14.47 a | 9.58 b | 10.42 B |
| Riceberry | 12.57 a | 14.63 b | 8.83 b | 12.01 B | Riceberry | 11.99 a | 14.23 ab | 12.17 ab | 12.79 A |
| Mean | 10.23 B | 14.71 A | 13.74 A | Mean | 9.65 B | 13.12 A | 12.23 A | ||
| F-test | ** | ** | ** | F-test | ** | * | ** | ||
| C.V. (%) | 10.16 | 7.68 | 26.48 | C.V. (%) | 8.81 | 8.96 | 13.75 | ||
| Number of grains per panicle | 1000-grain weight (g) | ||||||||
| Hom Nang Nual 1 | 178.33 | 155.53 | 153.33 cd | 162.40 | Hom Nang Nual 1 | 24.37 | 23.51 a | 25.30 a | 24.39 A |
| Lhueang Thong | 189.67 | 162.60 | 215.58 a | 189.28 | Lhueang Thong | 26.26 | 23.37 a | 24.0 b | 24.54 A |
| KDML105 | 162.20 | 174.47 | 180.67 bc | 172.45 | KDML105 | 23.67 | 23.28 a | 22.57 c | 23.17 AB |
| Mali Nil Boran | 202.87 | 174.67 | 146.50 d | 174.68 | Mali Nil Boran | 23.17 | 21.27 b | 19.90 c | 21.45 C |
| Mali Nil Surin | 159.50 | 141.33 | 187.00 ab | 162.61 | Mali Nil Surin | 24.80 | 21.12 b | 21.13 d | 22.36 BC |
| Riceberry | 226.40 | 197.87 | 135.17 d | 186.48 | Riceberry | 23.22 | 20.87 b | 22.43 c | 22.17 BC |
| Mean | 186.49 | 167.75 | 169.71 | Mean | 24.26 A | 22.24 B | 22.56 B | ||
| F-test | ns | ns | ** | F-test | ns | ** | ** | ||
| C.V. (%) | 21.50 | 11.16 | 10.68 | C.V. (%) | 12.17 | 3.87 | 2.68 | ||
| Genotype | Bangkok | Trat | Sakon Nakhon | Mean |
|---|---|---|---|---|
| Grain yield (kg ha−1) | ||||
| Hom Nang Nual 1 | 3136.5 bc1/ | 2428.7 bc | 3156.7 a | 2907.3 A2/ |
| Lhueang Thong | 3063.0 bc | 2170.4 bc | 2616.3 b | 2616.5 B |
| KDML105 | 2736.7 c | 2157.4 bc | 3209.1 a | 2701.1 AB |
| Mali Nil Boran | 3809.0 a | 2690.4 ab | 2360.2 bc | 2953.2 A |
| Mali Nil Surin | 2013.5 d | 2075.5 c | 2012.3 c | 2033.7 C |
| Riceberry | 3326.6 b | 3228.0 a | 1963.9 c | 2839.5 AB |
| Mean | 3014.2 A | 2458.4 B | 2553.1 B | |
| F-test | ** | ** | ** | |
| C.V. (%) | 17.92 | 12.11 | 11.08 | |
| Total phenolic content (mg/100 g seeds) | ||||
| Hom Nang Nual 1 | 21.27 cd | 20.64 bc | 24.06 b | 21.99 C |
| Lhueang Thong | 24.52 cd | 21.65 bc | 20.41 b | 22.19 C |
| KDML105 | 17.16 d | 16.53 c | 20.64 b | 18.11 C |
| Mali Nil Boran | 30.26 bc | 35.60 ab | 33.43 b | 33.09 B |
| Mali Nil Surin | 44.29 a | 45.53 a | 50.64 a | 46.82 A |
| Riceberry | 37.93 ab | 39.25 a | 44.05 a | 40.41 A |
| Mean | 29.24 | 29.86 | 32.01 | |
| F-test | ** | * | * | |
| C.V. (%) | 21.42 | 29.64 | 30.43 | |
| Ferulic acid (mg/100 g seeds) | ||||
| Hom Nang Nual 1 | 16.25 b | 15.78 b | 14.13 c | 15.39 C |
| Lhueang Thong | 18.13 b | 15.35 b | 13.27 c | 15.58 C |
| KDML105 | 15.02 b | 14.07 b | 12.44 c | 13.84 C |
| Mali Nil Boran | 25.28 a | 20.10 a | 20.49 ab | 21.95 AB |
| Mali Nil Surin | 26.28 a | 21.49 a | 23.79 a | 23.85 A |
| Riceberry | 27.27 a | 19.37 a | 17.75 b | 21.46 B |
| Mean | 21.37 A | 17.69 B | 16.98 B | |
| F-test | ** | ** | ** | |
| C.V. (%) | 10.57 | 8.73 | 10.66 | |
| Antioxidant activity (%) | ||||
| Hom Nang Nual 1 | 20.09 c | 19.55 c | 17.05 cd | 18.90 C |
| Lhueang Thong | 22.56 c | 20.52 c | 17.64 cd | 20.24 C |
| KDML105 | 21.75 c | 20.55 c | 16.05 d | 19.45 C |
| Mali Nil Boran | 39.65 b | 27.64 bc | 53.49 ab | 40.26 B |
| Mali Nil Surin | 44.89 b | 42.65 a | 71.68 a | 53.08 A |
| Riceberry | 57.15 a | 34.30 ab | 35.73 bc | 42.39 B |
| Mean | 34.35 A | 27.53 B | 35.27 A | |
| F-test | ** | ** | ** | |
| C.V. (%) | 17.23 | 22.09 | 30.26 | |
| Genotype | Bangkok | Trat | Sakon Nakhon | Mean |
|---|---|---|---|---|
| Grain yield (kg ha−1) | ||||
| White rice | 2978.7 | 2664.6 | 2994.0 a1/ | 2741.6 |
| Black rice | 3094.7 | 2252.2 | 2112.1 b | 2608.8 |
| Mean | 3014.2 A2/ | 2458.4 B | 2553.1 B | |
| Total phenolic content (mg/100 g seeds) | ||||
| White rice | 20.98 b | 19.61 b | 21.70 b | 20.76 B |
| Black rice | 37.49 a | 40.12 a | 42.71 a | 40.10 A |
| Mean | 29.23 | 29.86 | 32.20 | |
| Ferulic acid (mg/100 g seeds) | ||||
| White rice | 16.46 b | 15.07 b | 13.27 b | 14.93 B |
| Black rice | 26.78 a | 20.32 a | 20.67 a | 22.42 A |
| Mean | 21.37 A | 17.69 B | 16.97 B | |
| Antioxidant activity (%) | ||||
| White rice | 21.46 b | 20.20 b | 16.91 b | 19.24 B |
| Black rice | 47.23 a | 34.86 a | 53.63 a | 45.24 A |
| Mean | 34.35 A | 27.53 B | 35.27 A | |
| Genotype | Grain Yield (kg ha−1) | b | R2 | Ferulic Acid (mg/100 g) | b | R2 | Total Phenolic Content (mg/100 g) | b | R2 | Antioxidant Activity (%) | b | R2 | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hom Nang Nual 1 | 2907.3 A1/ | 0.0854 | 189,776 ** | 0.9998 | 15.39 C | 0.366 | −0.47 | 0.6030 | 21.99 C | 1.08 | −21.01 | 0.8640 | 18.90 C | −0.17 | −19.32 | 0.1991 |
| Lhueang Thong | 2616.5 B | 1.403 | 25,226 | 0.8600 | 15.58 C | 0.992 | −0.49 | 0.9194 | 22.19 C | −1.15 | −19.50 | 0.7305 | 20.24 C | −0.12 | −11.81 | 0.0426 |
| KDML105 | 2701.1 AB | 0.384 | 503,790 ** | 0.7461 | 13.84 C | 0.48 | −0.60 | 0.7516 | 18.11 C | 1.33 | −20.78 | 0.8850 | 19.45 C | −0.29 | −8.65 | 0.1755 |
| Mali Nil Boran | 2953.2 A | 2.372 | 132,828 * | 0.0853 | 21.95 AB | 1.192 | −0.66 | 0.9514 | 33.09 B | 0.52 | −8.79 | 0.9340 | 40.26 B | 2.75 | 41.12 | 0.8070 |
| Mali Nil Surin | 2033.7 C | −0.075 * | −23,434 | 0.9464 | 23.85 A | 0.808 | 2.79 | 0.6303 | 46.82 A | 2.15 * | −21.89 | 0.9999 | 53.08 A | 2.46 | 280.45 ** | 0.4176 |
| Riceberry | 2839.5 AB | 1.062 | 930,297 ** | 0.9179 | 21.46 B | 2.16 | −1.45 | 0.9991 | 40.41 A | 2.06 ** | −21.89 | 0.9999 | 42.39 B | 1.37 | 237.10 ** | 0.2050 |
| Mean | 2675.2 | 18.68 | 30.44 | 32.39 |
| No. Tiller | No. Panicles | No. Grain | Grain Yield | TPC | Ferulic Content | |
|---|---|---|---|---|---|---|
| No. panicles | 0.6807 ** | |||||
| No. grain | 0.0301 | −0.1725 | ||||
| Grain yield | 0.2108 | 0.1295 | 0.5401 ** | |||
| TPC | −0.2071 | −0.0260 | −0.0409 | −0.2436 | ||
| Ferulic | −0.3737 | −0.2036 | 0.1943 | 0.0484 | 0.6316 ** | |
| DPPH | −0.3226 | −0.2375 | 0.0373 | −0.2097 | 0.6420 ** | 0.7140 ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kunnam, J.; Pinta, W.; Ruttanaprasert, R.; Bunphan, D.; Thabthimtho, T.; Aninbon, C. Stability of Phenols, Antioxidant Capacity and Grain Yield of Six Rice Genotypes. Plants 2023, 12, 2787. https://doi.org/10.3390/plants12152787
Kunnam J, Pinta W, Ruttanaprasert R, Bunphan D, Thabthimtho T, Aninbon C. Stability of Phenols, Antioxidant Capacity and Grain Yield of Six Rice Genotypes. Plants. 2023; 12(15):2787. https://doi.org/10.3390/plants12152787
Chicago/Turabian StyleKunnam, Juthathip, Wanwipa Pinta, Ruttanachira Ruttanaprasert, Darika Bunphan, Thanasin Thabthimtho, and Chorkaew Aninbon. 2023. "Stability of Phenols, Antioxidant Capacity and Grain Yield of Six Rice Genotypes" Plants 12, no. 15: 2787. https://doi.org/10.3390/plants12152787
APA StyleKunnam, J., Pinta, W., Ruttanaprasert, R., Bunphan, D., Thabthimtho, T., & Aninbon, C. (2023). Stability of Phenols, Antioxidant Capacity and Grain Yield of Six Rice Genotypes. Plants, 12(15), 2787. https://doi.org/10.3390/plants12152787






