Enhancing Maize Transformation and Targeted Mutagenesis through the Assistance of Non-Integrating Wus2 Vector
Abstract
:1. Introduction
2. Results
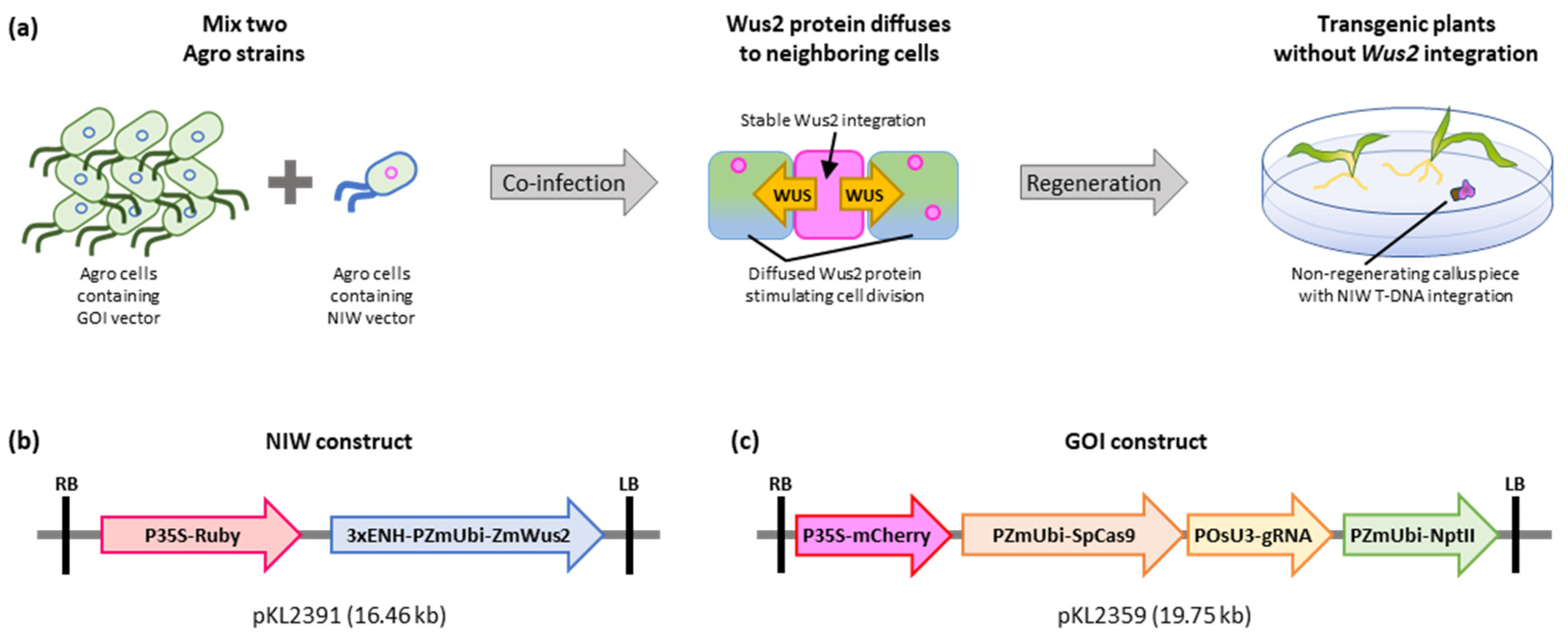
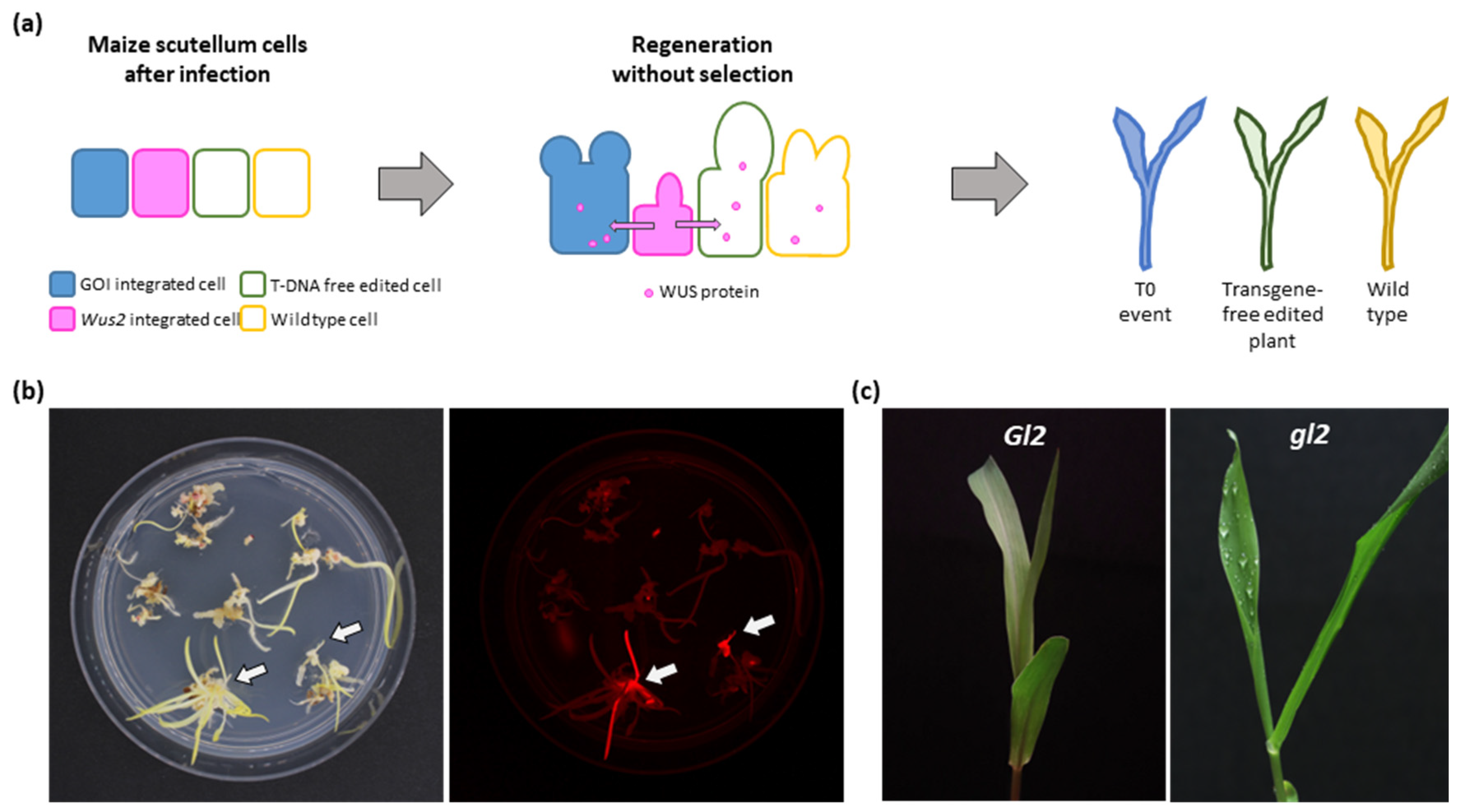
2.1. NIW-Assisted Transformation of Maize B104
2.2. Transgene-Free Gene Editing in Maize B73
3. Discussion
4. Materials and Methods
4.1. NIW Vector Construction
4.2. Agrobacterium Transformation
4.3. NIW-Assisted Transformation of Maize B104 and B73
4.4. Genotyping of Regenerated Plants
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zsögön, A.; Čermák, T.; Naves, E.R.; Notini, M.M.; Edel, K.H.; Weinl, S.; Freschi, L.; Voytas, D.F.; Kudla, J.; Peres, L.E.P. De novo domestication of wild tomato using genome editing. Nat. Biotechnol. 2018, 36, 1211–1216. [Google Scholar] [CrossRef] [Green Version]
- Fernie, A.R.; Yan, J. De Novo Domestication: An Alternative Route toward New Crops for the Future. Mol. Plant 2019, 12, 615–631. [Google Scholar] [CrossRef] [Green Version]
- Curtin, S.; Qi, Y.; Peres, L.E.P.; Fernie, A.R.; Zsögön, A. Pathways to de novo domestication of crop wild relatives. Plant Physiol. 2021, 188, 1746–1756. [Google Scholar] [CrossRef]
- Zhang, Y.; Pribil, M.; Palmgren, M.; Gao, C. A CRISPR way for accelerating improvement of food crops. Nat. Food 2020, 1, 200–205. [Google Scholar] [CrossRef]
- Zhu, H.; Li, C.; Gao, C. Applications of CRISPR–Cas in agriculture and plant biotechnology. Nat. Rev. Mol. Cell Biol. 2020, 21, 661–677. [Google Scholar] [CrossRef]
- Beying, N.; Schmidt, C.; Pacher, M.; Houben, A.; Puchta, H. CRISPR–Cas9-mediated induction of heritable chromosomal translocations in Arabidopsis. Nat. Plants 2020, 6, 638–645. [Google Scholar] [CrossRef]
- Schwartz, C.; Lenderts, B.; Feigenbutz, L.; Barone, P.; Llaca, V.; Fengler, K.; Svitashev, S. CRISPR-Cas9-mediated 75.5-Mb inversion in maize. Nat. Plants 2020, 6, 1427–1431. [Google Scholar] [CrossRef]
- Wang, J.Y.; Doudna, J.A. CRISPR technology: A decade of genome editing is only the beginning. Science 2023, 379, eadd8643. [Google Scholar] [CrossRef]
- Altpeter, F.; Springer, N.M.; Bartley, L.E.; Blechl, A.E.; Brutnell, T.P.; Citovsky, V.; Conrad, L.J.; Gelvin, S.B.; Jackson, D.P.; Kausch, A.P.; et al. Advancing Crop Transformation in the Era of Genome Editing. Plant Cell 2016, 28, 1510–1520. [Google Scholar] [CrossRef] [Green Version]
- Andorf, C.; Beavis, W.D.; Hufford, M.; Smith, S.; Suza, W.P.; Wang, K.; Woodhouse, M.; Yu, J.; Lübberstedt, T. Technological advances in maize breeding: Past, present and future. Theor. Appl. Genet. 2019, 132, 817–849. [Google Scholar] [CrossRef] [Green Version]
- Kang, M.; Lee, K.; Finley, T.; Chappell, H.; Veena, V.; Wang, K. An Improved Agrobacterium-Mediated Transformation and Genome-Editing Method for Maize Inbred B104 Using a Ternary Vector System and Immature Embryos. Front. Plant Sci. 2022, 13, 860971. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Kang, M.; Ji, Q.; Grosic, S.; Wang, K. New T-DNA binary vectors with NptII selection and RUBY reporter for efficient maize transformation and targeted mutagenesis. Plant Physiol. 2023, kiad231. [Google Scholar] [CrossRef] [PubMed]
- Hoerster, G.; Wang, N.; Ryan, L.; Wu, E.; Anand, A.; McBride, K.; Lowe, K.; Jones, T.; Gordon-Kamm, B. Use of non-integrating Zm-Wus2 vectors to enhance maize transformation: Non-integrating WUS2 enhances transformation. In Vitro Cell. Dev. Biol.-Plant 2020, 56, 265–279. [Google Scholar] [CrossRef] [Green Version]
- Lowe, K.; Wu, E.; Wang, N.; Hoerster, G.; Hastings, C.; Cho, M.-J.; Scelonge, C.; Lenderts, B.; Chamberlin, M.; Cushatt, J.; et al. Morphogenic Regulators Baby boom and Wuschel Improve Monocot Transformation. Plant Cell 2016, 28, 1998–2015. [Google Scholar] [CrossRef] [Green Version]
- Lowe, K.; La Rota, M.; Hoerster, G.; Hastings, C.; Wang, N.; Chamberlin, M.; Wu, E.; Jones, T.; Gordon-Kamm, W. Rapid genotype “independent” Zea mays L. (maize) transformation via direct somatic embryogenesis. In Vitro Cell Dev. Biol.-Plant 2018, 54, 240–252. [Google Scholar] [CrossRef] [Green Version]
- Lee, K.; Wang, K. Strategies for genotype-flexible plant transformation. Curr. Opin. Biotechnol. 2023, 79, 102848. [Google Scholar] [CrossRef]
- Gordon-Kamm, B.; Sardesai, N.; Arling, M.; Lowe, K.; Hoerster, G.; Betts, S.; Jones, T. Using Morphogenic Genes to Improve Recovery and Regeneration of Transgenic Plants. Plants 2019, 8, 38. [Google Scholar] [CrossRef] [Green Version]
- Yadav, R.K.; Perales, M.; Gruel, J.; Girke, T.; Jönsson, H.; Reddy, G.V. WUSCHEL protein movement mediates stem cell ho-meostasis in the Arabidopsis shoot apex. Genes Dev. 2011, 25, 2025–2030. [Google Scholar] [CrossRef] [Green Version]
- He, Y.; Zhang, T.; Sun, H.; Zhan, H.; Zhao, Y. A reporter for noninvasively monitoring gene expression and plant transformation. Hortic. Res. 2020, 7, 152. [Google Scholar] [CrossRef]
- Ranch, J.P.; Liebergesell, M.; Garnaat, C.W.; Huffman, G.A. Auxotrophic Agrobacterium for Plant Transformation and Methods Thereof. U.S. Patent 8,334,429, 18 December 2012. [Google Scholar]
- Aliu, E.; Lee, K.; Wang, K. CRISPR RNA-guided integrase enables high-efficiency targeted genome engineering in Agrobacterium tumefaciens. Plant Biotechnol. J. 2022, 20, 1916–1927. [Google Scholar] [CrossRef]
- Aliu, E.; Azanu, M.K.; Wang, K.; Lee, K. Generation of thymidine auxotrophic Agrobacterium tumefaciens strains for plant transformation. bioRxiv 2020, 2020-08. [Google Scholar]
- Schnable, P.S.; Ware, D.; Fulton, R.S.; Stein, J.C.; Wei, F.; Pasternak, S.; Liang, C.; Zhang, J.; Fulton, L.; Graves, T.A.; et al. The B73 Maize Genome: Complexity, Diversity, and Dynamics. Science 2009, 326, 1112–1115. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Liang, Z.; Zong, Y.; Wang, Y.; Liu, J.; Chen, K.; Qiu, J.-L.; Gao, C. Efficient and transgene-free genome editing in wheat through transient expression of CRISPR/Cas9 DNA or RNA. Nat. Commun. 2016, 7, 12617. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brinkman, E.K.; van Steensel, B. Rapid quantitative evaluation of CRISPR genome editing by TIDE and TIDER. In CRISPR Gene Ed. Methods Protoc, Edition; Luo, Y., Ed.; Humana Press: New York, NY, USA, 2019; Volume 1961, pp. 29–44. ISBN 978-1-4939-9169-3. [Google Scholar]
- Conant, D.; Hsiau, T.; Rossi, N.; Oki, J.; Maures, T.; Waite, K.; Yang, J.; Joshi, S.; Kelso, R.; Holden, K.; et al. Inference of CRISPR edits from Sanger trace data. CRISPR J. 2022, 5, 123–130. [Google Scholar] [CrossRef]
- Che, P.; Wu, E.; Simon, M.K.; Anand, A.; Lowe, K.; Gao, H.; Sigmund, A.L.; Yang, M.; Albertsen, M.C.; Gordon-Kamm, W.; et al. Wuschel2 enables highly efficient CRISPR/Cas-targeted genome editing during rapid de novo shoot regeneration in sorghum. Commun. Biol. 2022, 5, 344. [Google Scholar] [CrossRef]
- Narasimhulu, S.B.; Deng, X.B.; Sarria, R.; Gelvin, S.B. Early transcription of Agrobacterium T-DNA genes in tobacco and maize. Plant Cell 1996, 8, 873–886. [Google Scholar] [PubMed] [Green Version]
- Mysore, K.S.; Bassuner, B.; Deng, X.-B.; Darbinian, N.S.; Motchoulski, A.; Ream, W.; Gelvin, S.B. Role of the Agrobacterium tumefaciens VirD2 Protein in T-DNA Transfer and Integration. Mol. Plant-Microbe Interact. 1998, 11, 668–683. [Google Scholar] [CrossRef] [Green Version]
- Paz, M.M.; Shou, H.; Guo, Z.; Zhang, Z.; Banerjee, A.K.; Wang, K. Assessment of conditions affecting Agrobacterium-mediated soybean transformation using the cotyledonary node explant. Euphytica 2004, 136, 167–179. [Google Scholar] [CrossRef]
- Wang, N.; Ryan, L.; Sardesai, N.; Wu, E.; Lenderts, B.; Lowe, K.; Che, P.; Anand, A.; Worden, A.; van Dyk, D.; et al. Leaf transformation for efficient random integration and targeted genome modification in maize and sorghum. Nat. Plants 2023, 9, 255–270. [Google Scholar] [CrossRef]
- den Dulk-Ras, A.; Hooykaas, P.J.J. Electroporation of Agrobacterium tumefaciens. Methods Mol. Biol. 1995, 55, 63–72. [Google Scholar] [CrossRef]
- Edwards, K.; Johnstone, C.; Thompson, C. A simple and rapid method for the preparation of plant genomic DNA for PCR analysis. Nucleic Acids Res. 1991, 19, 1349. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Zhang, Y.; Kleinstiver, B.P.; Guo, J.A.; Aryee, M.J.; Miller, J.; Malzahn, A.; Zarecor, S.; Lawrence-Dill, C.J.; Joung, J.K.; et al. Activities and specificities of CRISPR /Cas9 and Cas12a nucleases for targeted mutagenesis in maize. Plant Biotechnol. J. 2019, 17, 362–372. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| #Emb | #Reg Plants | #T0 | TF * | #Co-in | #Wus2 | #Esc | |
|---|---|---|---|---|---|---|---|
| Control | 420 | 45 | 42 | 10.0% a | 0 | 0 | 3 |
| LBA4404Thy- | 384 | 76 | 72 | 18.8% b | 1 | 0 | 3 |
| EHA105Thy- | 439 | 31 | 30 | 6.8% a | 0 | 0 | 1 |
| EHA105TR | 477 | 85 | 74 | 15.5% ab | 1 | 0 | 10 |
| #Emb | #Reg Plants | #T0 | TF | #Co-in | #Wus2 | #WT | |
|---|---|---|---|---|---|---|---|
| Control | 136 | 0 | 0 | 0.0% | 0 | 0 | 0 |
| LBA4404Thy- | 395 | 79 | 9 | 2.3% | 0 | 0 | 70 |
| Plant ID. | T0 Genotype | Glossy2 Sequence | Indel Mutation | Contribution % | |
|---|---|---|---|---|---|
| WT | Allele 1: | TTGGTCACAGATCACAAACTTCAAATGCGGTGGGCTGGCGCTGGGGTTCAGCT | 0 bp | ||
| Allele 2: | TTGGTCACAGATCACAAACTTCAAATGCGGTGGGCTGGCGCTGGGGTTCAGCT | 0 bp | |||
| B73-R1 | BI | Allele 1: | TTGGTCACAGATCACAAACT----ATGCGGTGGGCTGGCGCTGGGGTTCAGCT | −4 bp | 67 |
| Allele 2: | TTGGTCACAGATCACAAACT---------------------------TCAGCT | −27 bp | 33 | ||
| B73-R2 | HT | Allele 1: | TTGGTCACAGATCACAAACTTCAAAATGCGGTGGGCTGGCGCTGGGGTTCAGCT | +1 bp | 38 |
| Allele 2: | TTGGTCACAGATCACAAACTTCAAATGCGGTGGGCTGGCGCTGGGGTTCAGCT | 0 bp | 38 | ||
| B73-R3 | HT | Allele 1: | TTGGTCACAGATCACAAACTTCA-ATGCGGTGGGCTGGCGCTGGGGTTCAGCT | −1 bp | 40 |
| Allele 2: | TTGGTCACAGATCACAAACTTCAAATGCGGTGGGCTGGCGCTGGGGTTCAGCT | 0 bp | 42 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, M.; Lee, K.; Ji, Q.; Grosic, S.; Wang, K. Enhancing Maize Transformation and Targeted Mutagenesis through the Assistance of Non-Integrating Wus2 Vector. Plants 2023, 12, 2799. https://doi.org/10.3390/plants12152799
Kang M, Lee K, Ji Q, Grosic S, Wang K. Enhancing Maize Transformation and Targeted Mutagenesis through the Assistance of Non-Integrating Wus2 Vector. Plants. 2023; 12(15):2799. https://doi.org/10.3390/plants12152799
Chicago/Turabian StyleKang, Minjeong, Keunsub Lee, Qing Ji, Sehiza Grosic, and Kan Wang. 2023. "Enhancing Maize Transformation and Targeted Mutagenesis through the Assistance of Non-Integrating Wus2 Vector" Plants 12, no. 15: 2799. https://doi.org/10.3390/plants12152799
APA StyleKang, M., Lee, K., Ji, Q., Grosic, S., & Wang, K. (2023). Enhancing Maize Transformation and Targeted Mutagenesis through the Assistance of Non-Integrating Wus2 Vector. Plants, 12(15), 2799. https://doi.org/10.3390/plants12152799






