Enhancing the Adaptability of Tea Plants (Camellia sinensis L.) to High-Temperature Stress with Small Peptides and Biosurfactants
Abstract
:1. Introduction
2. Results
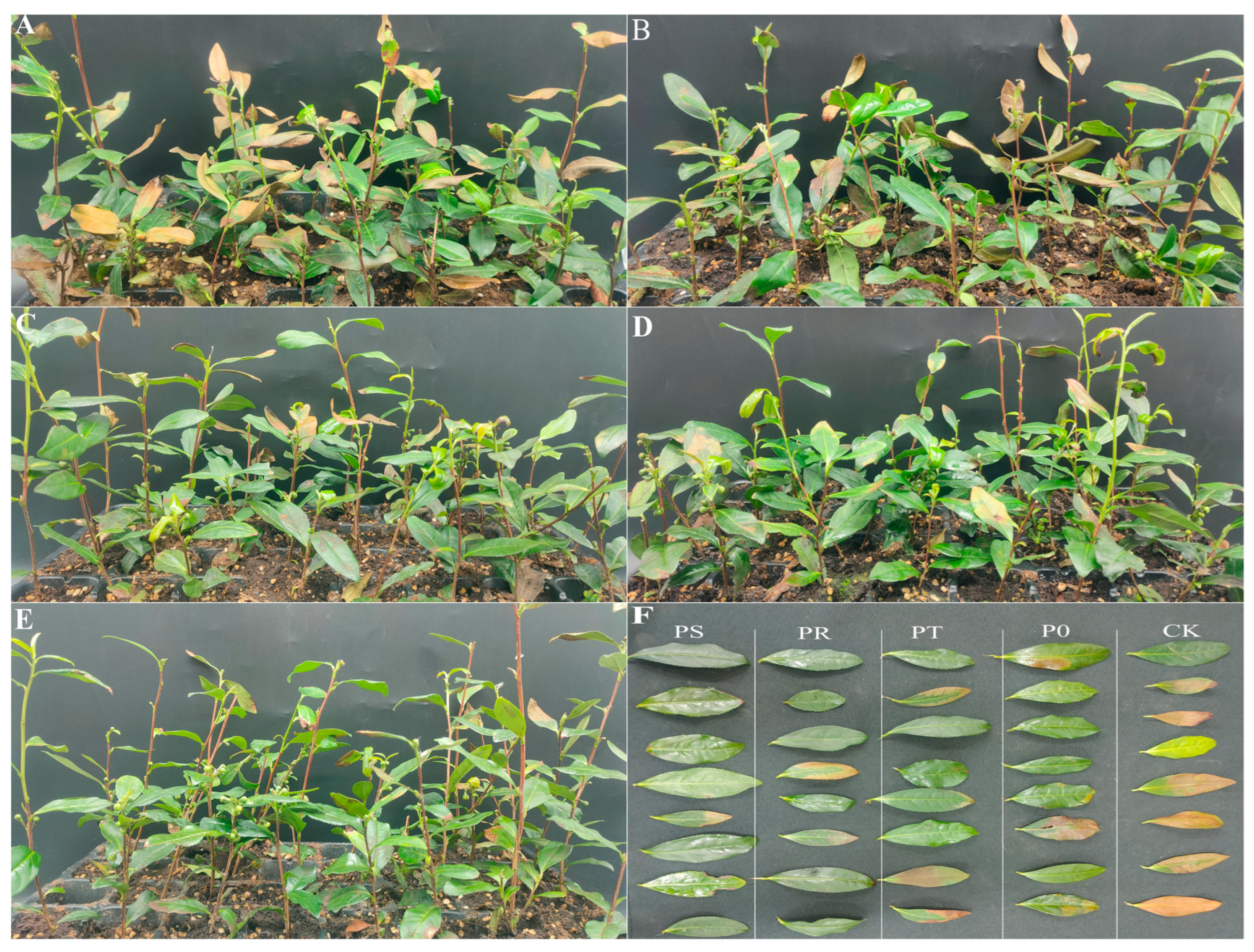
2.1. Effects of Small Peptides and Surfactants on Stress-Resistance-Related Indexes of Tea Leaves under High-Temperature Stress
2.2. Effects of Small Peptides and Surfactants on Photosynthetic Indexes of Tea Leaves under High-Temperature Stress
2.3. Effects of Small Peptides and Surfactants on Phytohormones Content in Tea Leaves under High-Temperature Stress
2.4. Effects of Small Peptides and Surfactants on Amino Acids in Tea Leaves under High-Temperature Stress
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Experimental Treatments
4.2. Determination of Antioxidant Enzyme Activity, MDA and SS Content, and Survival Rate
4.3. Determination of Photosynthetic Pigment and Fv/Fm
4.4. Determination of Phytohormones and Amino Acids
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Mathur, S.; Agrawal, D.; Jajoo, A. Photosynthesis: Response to High Temperature Stress. J. Photochem. Photobiol. B Biol. 2014, 137, 116–126. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.H.; Ma, Y.; Wu, Y.; Akbar, A.; Shaban, M.; Ullah, A.; Deng, J.; Khan, A.S.; Chi, H.; Zhu, L.; et al. High-Temperature Stress Suppresses Allene Oxide Cyclase 2 and Causes Male Sterility in Cotton by Disrupting Jasmonic Acid Signaling. Crop J. 2023, 11, 33–45. [Google Scholar] [CrossRef]
- Lü, G.; Wu, Y.; Bai, W.; Ma, B.; Wang, C.; Song, J. Influence of High Temperature Stress on Net Photosynthesis, Dry Matter Partitioning and Rice Grain Yield at Flowering and Grain Filling Stages. J. Integr. Agric. 2013, 12, 603–609. [Google Scholar] [CrossRef]
- Ullah, F.; Haq, I.; Gul, H.; Hafeez, M.; Guncan, A.; Tariq, K.; Desneux, N.; Zhao, Z.; Li, Z. Impact of Temperature Stress on Demographic Traits and Population Projection of Bactrocera dorsalis. Entomol. Gen. 2022, 42, 949–957. [Google Scholar] [CrossRef]
- Shi, W.; Zhang, X.; Yang, J.; Impa, S.M.; Wang, D.; Lai, Y.; Yang, Z.; Xu, H.; Wu, J.; Zhang, J.; et al. Irrigating with Cooler Water Does Not Reverse High Temperature Impact on Grain Yield and Quality in Hybrid Rice. Crop J. 2022, 11, 904–913. [Google Scholar] [CrossRef]
- Yan, Y.; Jeong, S.; Park, C.-E.; Mueller, N.D.; Piao, S.; Park, H.; Joo, J.; Chen, X.; Wang, X.; Liu, J.; et al. Effects of Extreme Temperature on China’s Tea Production. Environ. Res. Lett. 2021, 16, 044040. [Google Scholar] [CrossRef]
- Olsson, V.; Joos, L.; Zhu, S.; Gevaert, K.; Butenko, M.A.; De Smet, I. Look Closely, the Beautiful May Be Small: Precursor-Derived Peptides in Plants. Annu. Rev. Plant Biol. 2018, 70, 153–186. [Google Scholar] [CrossRef] [Green Version]
- Kang, J.; Wang, X.; Ishida, T.; Grienenberger, E.; Zheng, Q.; Wang, J.; Zhang, Y.; Chen, W.; Chen, M.; Song, X.-F.; et al. A Group of CLE Peptides Regulates de Novo Shoot Regeneration in Arabidopsis thaliana. New Phytol. 2022, 235, 2300–2312. [Google Scholar] [CrossRef]
- Zhang, Y.; Tan, S.; Gao, Y.; Kan, C.; Wang, H.; Yang, Q.; Xia, X.; Ishida, T.; Sawa, S.; Guo, H.; et al. CLE42 Delays Leaf Senescence by Antagonizing Ethylene Pathway in Arabidopsis. New Phytol. 2022, 235, 550–562. [Google Scholar] [CrossRef]
- Liu, Z.; Hou, S.; Rodrigues, O.; Wang, P.; Luo, D.; Munemasa, S.; Lei, J.; Liu, J.; Ortiz-Morea, F.A.; Wang, X. Phytocytokine Signalling Reopens Stomata in Plant Immunity and Water Loss. Nature 2022, 605, 332–339. [Google Scholar] [CrossRef]
- Takahashi, F.; Suzuki, T.; Osakabe, Y.; Betsuyaku, S.; Kondo, Y.; Dohmae, N.; Fukuda, H.; Yamaguchi-Shinozaki, K.; Shinozaki, K. A Small Peptide Modulates Stomatal Control via Abscisic Acid in Long-Distance Signalling. Nature 2018, 556, 235–238. [Google Scholar] [CrossRef] [PubMed]
- Christmann, A.; Grill, E. Peptide Signal Alerts Plants to Drought. Nature 2018, 556, 178–179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ogawa-Ohnishi, M.; Yamashita, T.; Kakita, M.; Nakayama, T.; Ohkubo, Y.; Hayashi, Y.; Yamashita, Y.; Nomura, T.; Noda, S.; Shinohara, H.; et al. Peptide Ligand-Mediated Trade-off between Plant Growth and Stress Response. Science 2022, 378, 175–180. [Google Scholar] [CrossRef] [PubMed]
- Ormancey, M.; Guillotin, B.; Clemente, H.S.; Thuleau, P.; Plaza, S.; Combier, J.P. Use of MicroRNA-Encoded Peptides to Improve Agronomic Traits. Plant Biotechnol. J. 2021, 19, 1687–1689. [Google Scholar] [CrossRef]
- Appah, S.; Jia, W.; Ou, M.; Wang, P.; Asante, E.A. Analysis of Potential Impaction and Phytotoxicity of Surfactant-Plant Surface Interaction in Pesticide Application. Crop Prot. 2020, 127, 104961. [Google Scholar] [CrossRef]
- Sachdev, D.P.; Cameotra, S.S. Biosurfactants in Agriculture. Appl. Microbiol. Biotechnol. 2013, 97, 1005–1016. [Google Scholar] [CrossRef] [Green Version]
- Guo, B.-F.; Guo, Y.; Wang, J.; Zhang, L.-J.; Jin, L.-G.; Hong, H.-L.; Chang, R.-Z.; Qiu, L.-J. Co-Treatment with Surfactant and Sonication Significantly Improves Agrobacterium-Mediated Resistant Bud Formation and Transient Expression Efficiency in Soybean. J. Integr. Agric. 2015, 14, 1242–1250. [Google Scholar] [CrossRef]
- Wu, S.-S.; Tseng, C.-T.; Yang, Y.-H.; Liu, Y.-C.; Chang, J.-C.; Gyawali, P.; Li, Y.-H.; Yang, T.-H.; Tsai, Y.-F.; Tang, L.-C.; et al. Potential of a Combination of Entomopathogenic Fungal Strains and a Non-Ionic Surfactant to Control the Fall Armyworm (Spodoptera frugiperda). J. Asia-Pac. Entomol. 2022, 25, 102001. [Google Scholar] [CrossRef]
- Barman, D.; Ghimire, O.P.; Chinnusamy, V.; Kumar, R.R.; Arora, A. Amelioration of Heat Stress during Reproductive Stage in Rice by Melatonin. Indian J. Agric. Sci. 2019, 89, 91–96. [Google Scholar] [CrossRef]
- Wilson, R.A.; Gupta, S.; Sangha, M.K.; Kaur, G. Effect of Heat Stress on Enzymatic and Non-Enzymatic Antioxidants in Brassica rapa. J. Environ. Biol. 2019, 40, 119–124. [Google Scholar] [CrossRef]
- Gong, H.-L.; Chen, Q.-Q. Exogenous Sucrose Protects Potato Seedlings Against Heat Stress by Enhancing the Antioxidant Defense System. J. Soil Sci. Plant Nutr. 2021, 21, 1511–1519. [Google Scholar] [CrossRef]
- Yuan, X.K.; Yang, Z.Q.; Li, Y.X.; Liu, Q.; Han, W. Effects of Different Levels of Water Stress on Leaf Photosynthetic Characteristics and Antioxidant Enzyme Activities of Greenhouse Tomato. Photosynthetica 2016, 54, 28–39. [Google Scholar] [CrossRef]
- Chen, J.-H.; Tang, M.; Jin, X.-Q.; Li, H.; Chen, L.-S.; Wang, Q.-L.; Sun, A.-Z.; Yi, Y.; Guo, F.-Q. Regulation of Calvin–Benson Cycle Enzymes under High Temperature Stress. aBIOTECH 2022, 3, 65–77. [Google Scholar] [CrossRef] [PubMed]
- Ru, C.; Hu, X.; Chen, D.; Wang, W.; Song, T. Heat and Drought Priming Induce Tolerance to Subsequent Heat and Drought Stress by Regulating Leaf Photosynthesis, Root Morphology, and Antioxidant Defense in Maize Seedlings. Environ. Exp. Bot. 2022, 202, 105010. [Google Scholar] [CrossRef]
- Sonjaroon, W.; Jutamanee, K.; Khamsuk, O.; Thussagunpanit, J.; Kaveeta, L.; Suksamrarn, A. Impact of Brassinosteroid Mimic on Photosynthesis, Carbohydrate Content and Rice Seed Set at Reproductive Stage under Heat Stress. Agric. Nat. Resour. 2018, 52, 234–240. [Google Scholar] [CrossRef]
- Ru, C.; Hu, X.; Chen, D.; Wang, W.; Zhen, J. Photosynthetic, Antioxidant Activities, and Osmoregulatory Responses in Winter Wheat Differ during the Stress and Recovery Periods under Heat, Drought, and Combined Stress. Plant Sci. 2023, 327, 111557. [Google Scholar] [CrossRef]
- Zahra, N.; Hafeez, M.B.; Ghaffar, A.; Kausar, A.; Zeidi, M.A.; Siddique, K.H.M.; Farooq, M. Plant Photosynthesis under Heat Stress: Effects and Management. Environ. Exp. Bot. 2023, 206, 105178. [Google Scholar] [CrossRef]
- Ge, C.; Yu, X.; Kan, M.; Qu, C. Adaption of Ulva pertusa to Multiple-Contamination of Heavy Metals and Nutrients: Biological Mechanism of Outbreak of Ulva Sp. Green Tide. Mar. Pollut. Bull. 2017, 125, 250–253. [Google Scholar] [CrossRef]
- Liu, J.; Zhao, Y.; Song, H.; Chen, J.; Long, Y. Antagonism or Synergism? Combined Effects of Enhanced UV-B Radiation and Acid Rain on Photosynthesis in Seedlings of Two C4 Plants. Acta Ecol. Sin. 2020, 40, 72–80. [Google Scholar] [CrossRef]
- Bouargalne, Y.; Raguénès-Nicol, C.; Guilbaud, F.; Cheron, A.; Clouet, V.; Deleu, C.; Le Cahérec, F. New Insights into Chlorophyll-WSCP (Water-Soluble Chlorophyll Proteins) Interactions: The Case Study of BnD22 (Brassica napus Drought-Induced 22 KDa). Plant Physiol. Biochem. 2022, 181, 71–80. [Google Scholar] [CrossRef]
- Sun, M.; Zhao, C.; Shang, H.; Hao, Y.; Han, L.; Qian, K.; White, J.C.; Ma, C.; Xing, B. ZnO Quantum Dots Outperform Nanoscale and Bulk Particles for Enhancing Tomato (Solanum lycopersicum) Growth and Nutritional Values. Sci. Total Environ. 2023, 857, 159330. [Google Scholar] [CrossRef] [PubMed]
- Poor, P.; Nawaz, K.; Gupta, R.; Ashfaque, F.; Khan, M.I.R. Ethylene Involvement in the Regulation of Heat Stress Tolerance in Plants. Plant Cell Rep. 2022, 41, 675–698. [Google Scholar] [CrossRef] [PubMed]
- Prerostova, S.; Dobrev, P.I.; Kramna, B.; Gaudinova, A.; Knirsch, V.; Spichal, L.; Zatloukal, M.; Vankova, R. Heat Acclimation and Inhibition of Cytokinin Degradation Positively Affect Heat Stress Tolerance of Arabidopsis. Front. Plant Sci. 2020, 11, 87. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, T.; Gull, S.; Ali, M.M.; Yousef, A.F.; Ercisli, S.; Kalaji, H.M.; Telesinski, A.; Auriga, A.; Wrobel, J.; Radwan, N.S.; et al. Heat Stress Mitigation in Tomato (Solanum lycopersicum L.) through Foliar Application of Gibberellic Acid. Sci. Rep. 2022, 12, 11324. [Google Scholar] [CrossRef]
- Li, N.; Euring, D.; Cha, J.Y.; Lin, Z.; Lu, M.; Huang, L.-J.; Kim, W.Y. Plant Hormone-Mediated Regulation of Heat Tolerance in Response to Global Climate Change. Front. Plant Sci. 2021, 11, 627969. [Google Scholar] [CrossRef]
- Jha, U.C.; Nayyar, H.; Siddique, K.H.M. Role of Phytohormones in Regulating Heat Stress Acclimation in Agricultural Crops. J. Plant Growth Regul. 2022, 41, 1041–1064. [Google Scholar] [CrossRef]
- Da Costa, M.V.J.; Ramegowda, V.; Sreeman, S.; Nataraja, K.N. Targeted Phytohormone Profiling Identifies Potential Regulators of Spikelet Sterility in Rice under Combined Drought and Heat Stress. Int. J. Mol. Sci. 2021, 22, 11690. [Google Scholar] [CrossRef]
- Saleem, M.; Asghar, H.N.; Khan, M.Y.; Zahir, Z.A. Gibberellic Acid in Combination with Pressmud Enhances the Growth of Sunflower and Stabilizes Chromium(VI)-Contaminated Soil. Environ. Sci. Pollut. Res. 2015, 22, 10610–10617. [Google Scholar] [CrossRef]
- Hedden, P.; Thomas, S.G. Gibberellin Biosynthesis and Its Regulation. Biochem. J. 2012, 444, 11–25. [Google Scholar] [CrossRef] [Green Version]
- Vanstraelen, M.; Benková, E. Hormonal Interactions in the Regulation of Plant Development. Annu. Rev. Cell Dev. Biol. 2012, 28, 463–487. [Google Scholar] [CrossRef]
- Richards, D.E.; King, K.E.; Aitali, A.T.; Harberd, N.P. HOW GIBBERELLIN REGULATES PLANT GROWTH AND DEVELOPMENT: A Molecular Genetic Analysis of Gibberellin Signaling. Annu. Rev. Plant Physiol. Plant Mol. Biol. 2001, 52, 67–88. [Google Scholar] [CrossRef] [Green Version]
- Achard, P.; Gong, F.; Cheminant, S.; Alioua, M.; Hedden, P.; Genschik, P. The Cold-Inducible CBF1 Factor-Dependent Signaling Pathway Modulates the Accumulation of the Growth-Repressing DELLA Proteins via Its Effect on Gibberellin Metabolism. Plant Cell 2008, 20, 2117–2129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Magome, H.; Yamaguchi, S.; Hanada, A.; Kamiya, Y.; Oda, K. The DDF1 Transcriptional Activator Upregulates Expression of a Gibberellin-Deactivating Gene, GA2ox7, under High-Salinity Stress in Arabidopsis. Plant J. 2008, 56, 613–626. [Google Scholar] [CrossRef] [PubMed]
- Sun, T. The Molecular Mechanism and Evolution of the GA–GID1–DELLA Signaling Module in Plants. Curr. Biol. 2011, 21, R338–R345. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, B.; Jaleel, H.; Shabbir, A.; Khan, M.; Haroon, Y.S. Concomitant Application of Depolymerized Chitosan and GA3 Modulates Photosynthesis, Essential Oil and Menthol Production in Peppermint (Mentha piperita L.). Sci. Hortic. 2019, 246, 371–379. [Google Scholar] [CrossRef]
- Pirbalouti, A.G.; Sajjadi, S.E.; Parang, K. A Review (Research and Patents) on Jasmonic Acid and Its Derivatives. Arch. Pharm. 2014, 347, 229–239. [Google Scholar] [CrossRef]
- Jang, G.; Yoon, Y.; Choi, Y.D. Crosstalk with Jasmonic Acid Integrates Multiple Responses in Plant Development. Int. J. Mol. Sci. 2020, 21, 305. [Google Scholar] [CrossRef] [Green Version]
- Kaya, A.; Doganlar, Z.B. Exogenous Jasmonic Acid Induces Stress Tolerance in Tobacco (Nicotiana tabacum) Exposed to Imazapic. Ecotox. Environ. Safe. 2016, 124, 470–479. [Google Scholar] [CrossRef]
- Ilyas, N.; Gull, R.; Mazhar, R.; Saeed, M.; Kanwal, S.; Shabir, S.; Bibi, F. Influence of Salicylic Acid and Jasmonic Acid on Wheat Under Drought Stress. Commun. Soil Sci. Plant Anal. 2017, 48, 2715–2723. [Google Scholar] [CrossRef]
- Kim, H.; Seomun, S.; Yoon, Y.; Jang, G. Jasmonic Acid in Plant Abiotic Stress Tolerance and Interaction with Abscisic Acid. Agronomy 2021, 11, 1886. [Google Scholar] [CrossRef]
- Kamal, A.H.M.; Komatsu, S. Jasmonic Acid Induced Protein Response to Biophoton Emissions and Flooding Stress in Soybean. J. Proteom. 2016, 133, 33–47. [Google Scholar] [CrossRef]
- Liu, H.; Timko, M.P. Jasmonic Acid Signaling and Molecular Crosstalk with Other Phytohormones. Int. J. Mol. Sci. 2021, 22, 2914. [Google Scholar] [CrossRef] [PubMed]
- Ruan, J.; Zhou, Y.; Zhou, M.; Yan, J.; Khurshid, M.; Weng, W.; Cheng, J.; Zhang, K. Jasmonic Acid Signaling Pathway in Plants. Int. J. Mol. Sci. 2019, 20, 2479. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, B.; Zhang, Y.; Wang, S.; Wang, W.; Xu, X.; Wu, J.; Fang, Y.; Ju, Y. Effects of Strigolactone and Abscisic Acid on the Quality and Antioxidant Activity of Grapes (Vitis vinifera L.) and Wines. Food Chem. X 2022, 16, 100496. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Min, Z.; Wu, J.; Liu, B.; Xu, X.; Fang, Y.; Ju, Y. Physiological and Transcriptomic Analysis of Cabernet Sauvginon (Vitis vinifera L.) Reveals the Alleviating Effect of Exogenous Strigolactones on the Response of Grapevine to Drought Stress. Plant Physiol. Biochem. 2021, 167, 400–409. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.-W.; Xi, Z. Strigolactone Agonists/Antagonists for Agricultural Applications: New Opportunities. Adv. Agrochem 2022, 1, 61–72. [Google Scholar] [CrossRef]
- Kaniganti, S.; Bhattacharya, J.; Petla, B.P.; Reddy, P.S. Strigolactone, a Neglected Plant Hormone, with a Great Potential for Crop Improvement: Crosstalk with Other Plant Hormones. Environ. Exp. Bot. 2022, 204, 105072. [Google Scholar] [CrossRef]
- Prandi, C.; Kapulnik, Y.; Koltai, H. Strigolactones: Phytohormones with Promising Biomedical Applications. Eur. J. Org. Chem. 2021, 2021, 4019–4026. [Google Scholar] [CrossRef]
- Chen, X.; Shi, X.; Ai, Q.; Han, J.; Wang, H.; Fu, Q. Transcriptomic and Metabolomic Analyses Reveal that Exogenous Strigolactones Alleviate the Response of Melon Root to Cadmium Stress. Hortic. Plant J. 2022, 8, 637–649. [Google Scholar] [CrossRef]
- Zamljen, T.; Medic, A.; Hudina, M.; Veberic, R.; Slatnar, A. Biostimulative Effect of Amino Acids on the Enzymatic and Metabolic Response of Two Capsicum Annuum L. Cultivars Grown under Salt Stress. Sci. Hortic. 2023, 309, 111713. [Google Scholar] [CrossRef]
- Ji, Y.; Yue, L.; Cao, X.; Chen, F.; Li, J.; Zhang, J.; Wang, C.; Wang, Z.; Xing, B. Carbon Dots Promoted Soybean Photosynthesis and Amino Acid Biosynthesis under Drought Stress: Reactive Oxygen Species Scavenging and Nitrogen Metabolism. Sci. Total Environ. 2023, 856, 159125. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Zhu, L.; Zhao, X.; Cheng, Y. Effects of Salinity Stress on Osmotic Pressure, Free Amino Acids, and Immune-Associated Parameters of the Juvenile Chinese Mitten Crab, Eriocheir Sinensis. Aquaculture 2022, 549, 737776. [Google Scholar] [CrossRef]
- Zhu, G.; Cheng, D.; Wang, X.; Guo, Q.; Zhang, Q.; Zhang, J.; Tu, Q.; Li, W. Free Amino Acids, Carbon and Nitrogen Isotopic Compositions Responses to Cadmium Stress in Two Castor (Ricinus communis L.) Species. Plant Physiol. Biochem. 2022, 184, 40–46. [Google Scholar] [CrossRef]
- Aydin, A.; Kurt, F.; Hürkan, K. Key Aromatic Amino Acid Players in Soybean (Glycine Max) Genome under Drought and Salt Stresses. Biocatal. Agric. Biotechnol. 2021, 35, 102094. [Google Scholar] [CrossRef]
- Shim, J.S.; Jeong, H.I.; Bang, S.W.; Jung, S.E.; Kim, G.; Kim, Y.S.; Redillas, M.C.F.R.; Oh, S.-J.; Seo, J.S.; Kim, J.-K. Drought-Induced Branched-Chain Amino Acid Aminotransferase Enhances Drought Tolerance in Rice. Plant Physiol. 2022, 191, 1435–1447. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Huang, W.; Zhang, F.; Luo, X.; Hu, B.; Xie, J. Metabolomic Profiling of Dongxiang Wild Rice Under Salinity Demonstrates the Significant Role of Amino Acids in Rice Salt Stress. Front. Plant Sci. 2021, 12, 729004. [Google Scholar] [CrossRef]
- Hamada, A.M.; Jonsson, L.M.V. Thiamine Treatments Alleviate Aphid Infestations in Barley and Pea. Phytochemistry 2013, 94, 135–141. [Google Scholar] [CrossRef]
- Zhou, J.; Sun, A.; Xing, D. Modulation of Cellular Redox Status by Thiamine-Activated NADPH Oxidase Confers Arabidopsis Resistance to Sclerotinia sclerotiorum. J. Exp. Bot. 2013, 64, 3261–3272. [Google Scholar] [CrossRef]
- Minhas, A.P.; Tuli, R.; Puri, S. Pathway Editing Targets for Thiamine Biofortification in Rice Grains. Front. Plant Sci. 2018, 9, 975. [Google Scholar] [CrossRef] [Green Version]
- Thanwisai, L.; Kim Tran, H.T.; Siripornadulsil, W.; Siripornadulsil, S. A Cadmium-Tolerant Endophytic Bacterium Reduces Oxidative Stress and Cd Uptake in KDML105 Rice Seedlings by Inducing Glutathione Reductase-Related Activity and Increasing the Proline Content. Plant Physiol. Biochem. 2022, 192, 72–86. [Google Scholar] [CrossRef]
- Ramzan, M.; Shah, A.A.; Ahmed, M.Z.; Bukhari, M.A.; Ali, L.; Casini, R.; Elansary, H.O. Exogenous Application of Glutathione and Gamma Amino-Butyric Acid Alleviates Salt Stress through Improvement in Antioxidative Defense System and Modulation of CaXTHs Stress-Related Genes. South Afr. J. Bot. 2023, 157, 266–273. [Google Scholar] [CrossRef]
- Devnarain, N.; Crampton, B.G.; Olivier, N.; van der Westhuyzen, C.; Becker, J.V.W.; O’Kennedy, M.M. Transcriptomic Analysis of a Sorghum Bicolor Landrace Identifies a Role for Beta-Alanine Betaine Biosynthesis in Drought Tolerance. South Afr. J. Bot. 2019, 127, 244–255. [Google Scholar] [CrossRef]
- Zhao, F.; Yuan, M.; Lei, L.; Li, C.; Xu, X. Enhanced Production of Mono-Rhamnolipid in Pseudomonas Aeruginosa and Application Potential in Agriculture and Petroleum Industry. Bioresour. Technol. 2021, 323, 124605. [Google Scholar] [CrossRef] [PubMed]
- Dierickx, S.; Castelein, M.; Remmery, J.; De Clercq, V.; Lodens, S.; Baccile, N.; De Maeseneire, S.L.; Roelants, S.L.K.W.; Soetaert, W.K. From Bumblebee to Bioeconomy: Recent Developments and Perspectives for Sophorolipid Biosynthesis. Biotechnol. Adv. 2022, 54, 107788. [Google Scholar] [CrossRef]
- Farias, C.B.B.; Almeida, F.C.G.; Silva, I.A.; Souza, T.C.; Meira, H.M.; Soares da Silva, R.d.C.F.; Luna, J.M.; Santos, V.A.; Converti, A.; Banat, I.M.; et al. Production of Green Surfactants: Market Prospects. Electron. J. Biotechnol. 2021, 51, 28–39. [Google Scholar] [CrossRef]
- Qazi, M.A.; Wang, Q.; Dai, Z. Sophorolipids Bioproduction in the Yeast Starmerella bombicola: Current Trends and Perspectives. Bioresour. Technol. 2022, 346, 126593. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, H.; Song, Y.; Li, H.; Zaman, S.; Fan, K.; Ding, Z.; Wang, Y. Enhancing the Adaptability of Tea Plants (Camellia sinensis L.) to High-Temperature Stress with Small Peptides and Biosurfactants. Plants 2023, 12, 2817. https://doi.org/10.3390/plants12152817
Chen H, Song Y, Li H, Zaman S, Fan K, Ding Z, Wang Y. Enhancing the Adaptability of Tea Plants (Camellia sinensis L.) to High-Temperature Stress with Small Peptides and Biosurfactants. Plants. 2023; 12(15):2817. https://doi.org/10.3390/plants12152817
Chicago/Turabian StyleChen, Hao, Yujie Song, He Li, Shah Zaman, Kai Fan, Zhaotang Ding, and Yu Wang. 2023. "Enhancing the Adaptability of Tea Plants (Camellia sinensis L.) to High-Temperature Stress with Small Peptides and Biosurfactants" Plants 12, no. 15: 2817. https://doi.org/10.3390/plants12152817
APA StyleChen, H., Song, Y., Li, H., Zaman, S., Fan, K., Ding, Z., & Wang, Y. (2023). Enhancing the Adaptability of Tea Plants (Camellia sinensis L.) to High-Temperature Stress with Small Peptides and Biosurfactants. Plants, 12(15), 2817. https://doi.org/10.3390/plants12152817






