Nitrogen Use Efficiency in Oil Palm Seedlings: Unraveling the Untapped Potential of Elevated External Ammonium Supply
Abstract
:1. Introduction
2. Results
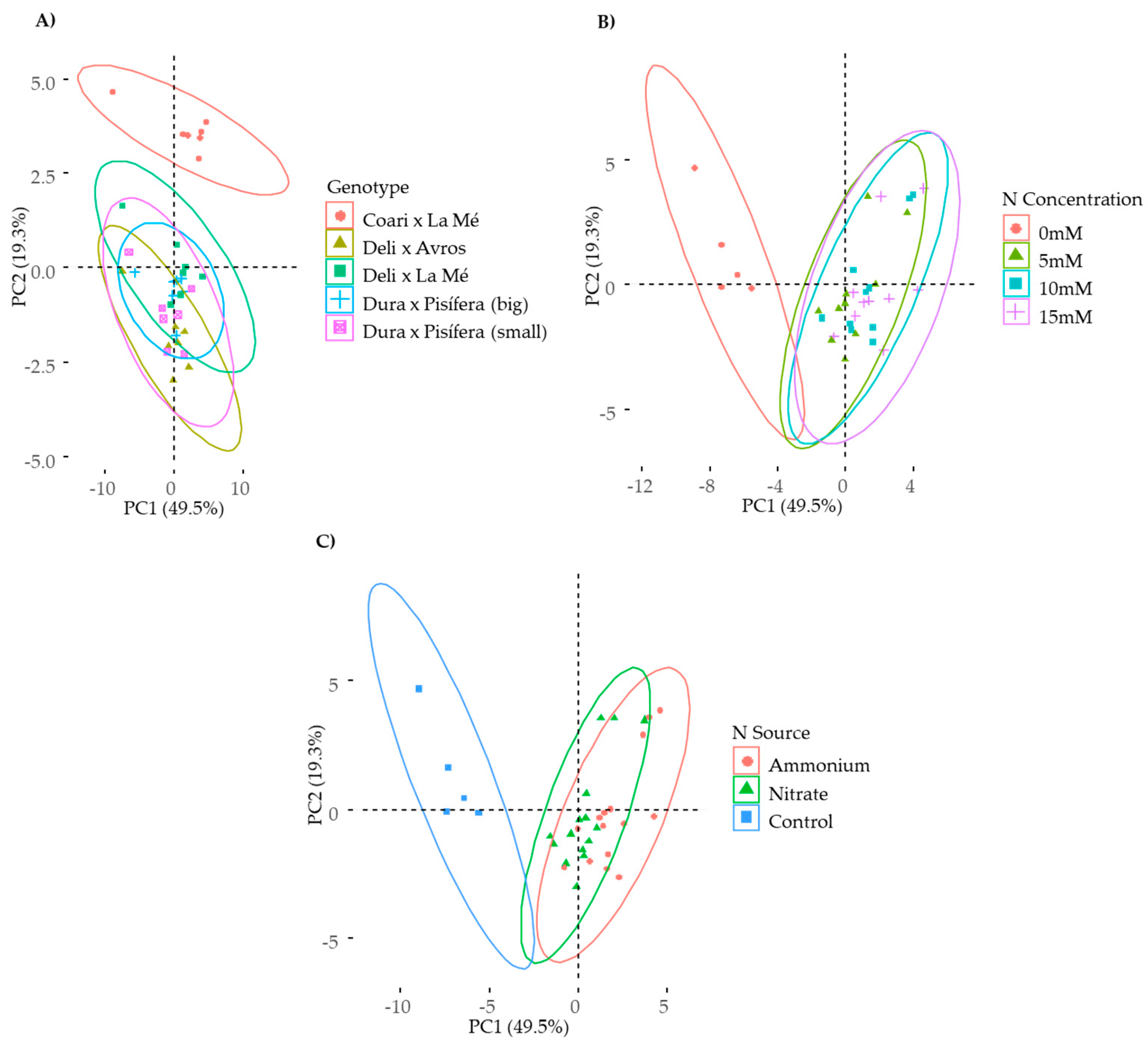
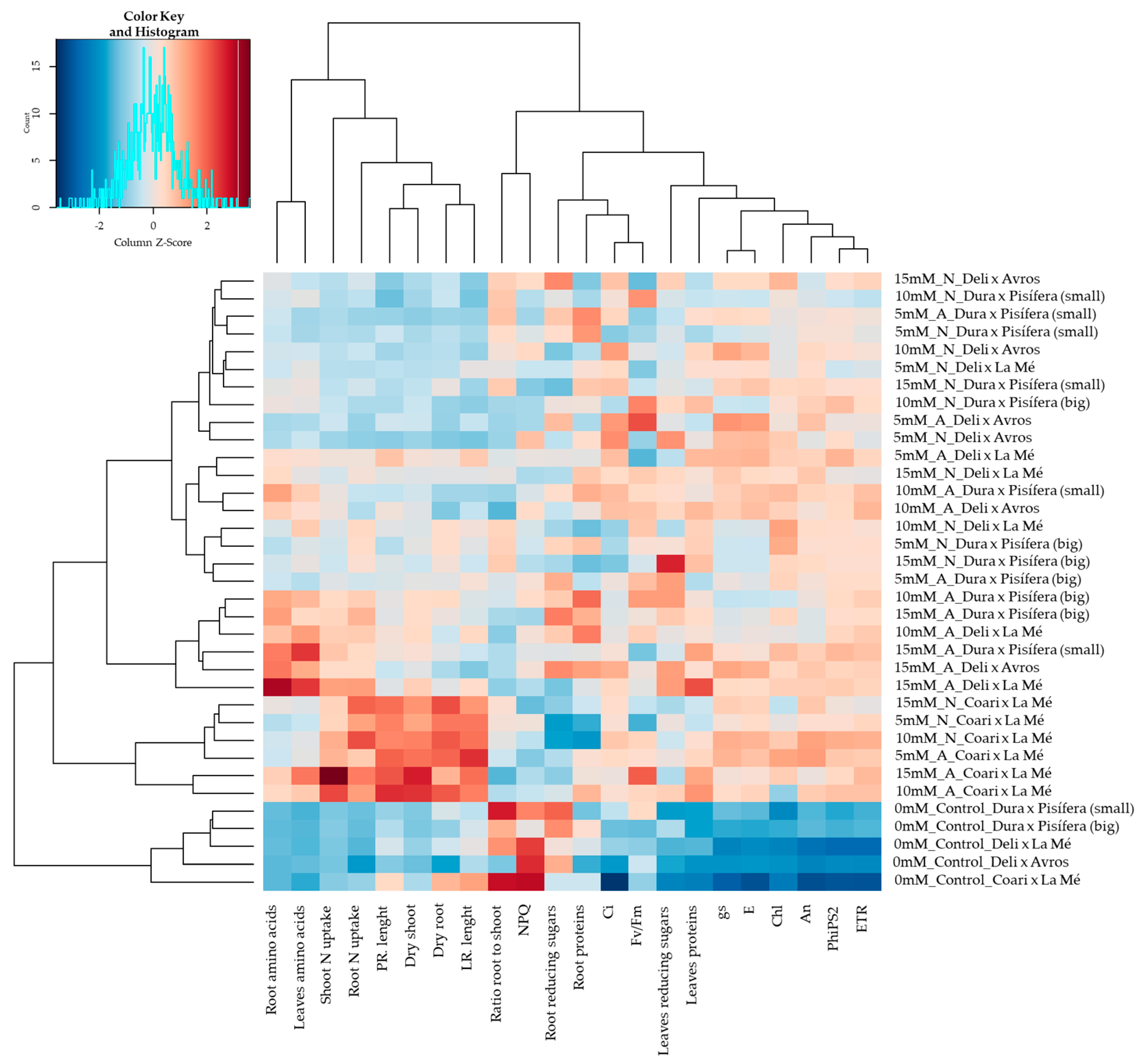
2.1. Multivariate Analysis
2.2. Growth and Development
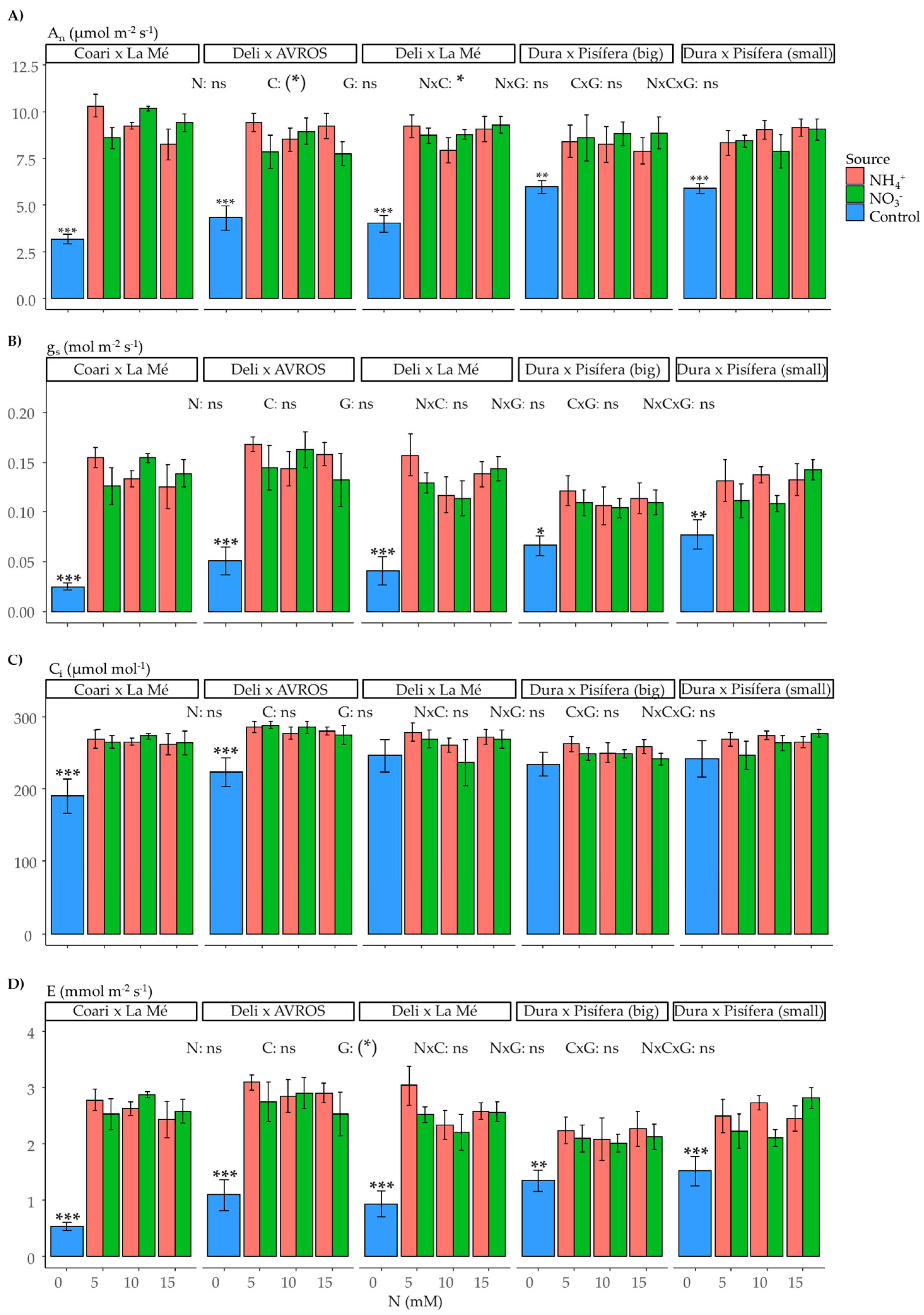
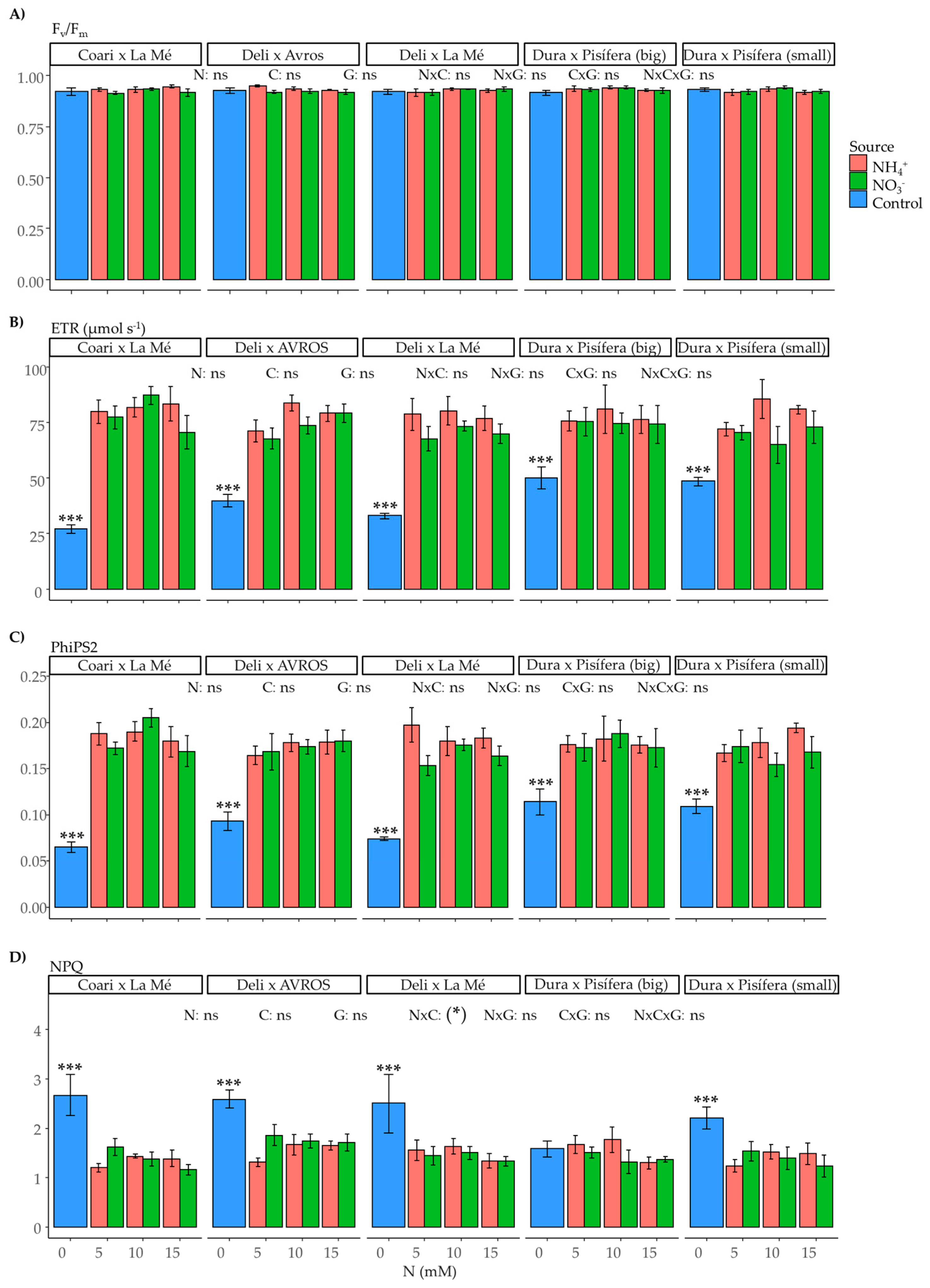
2.3. Leaf Gas Exchange and Fluorescence Parameters
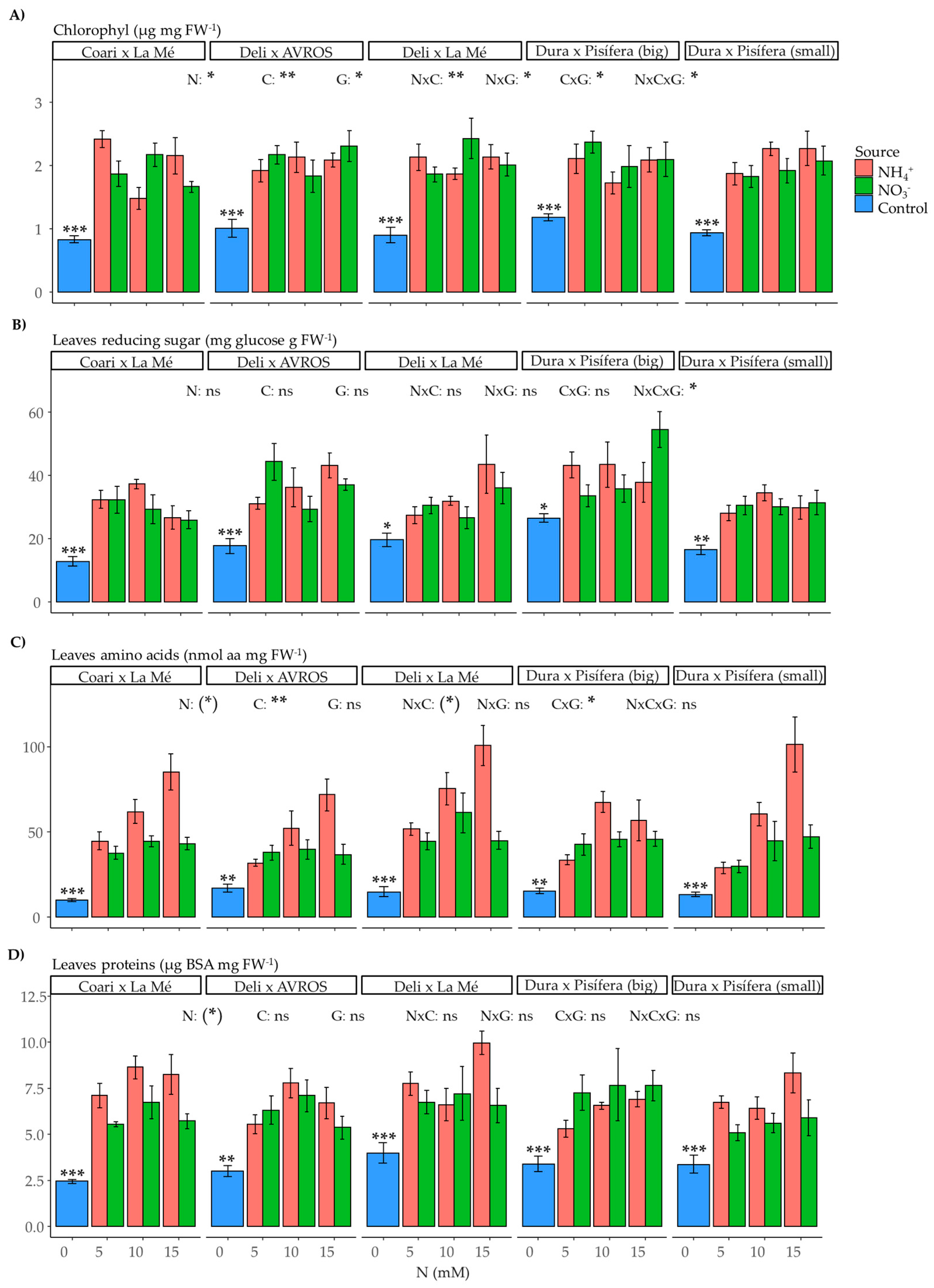
2.4. Metabolite Content
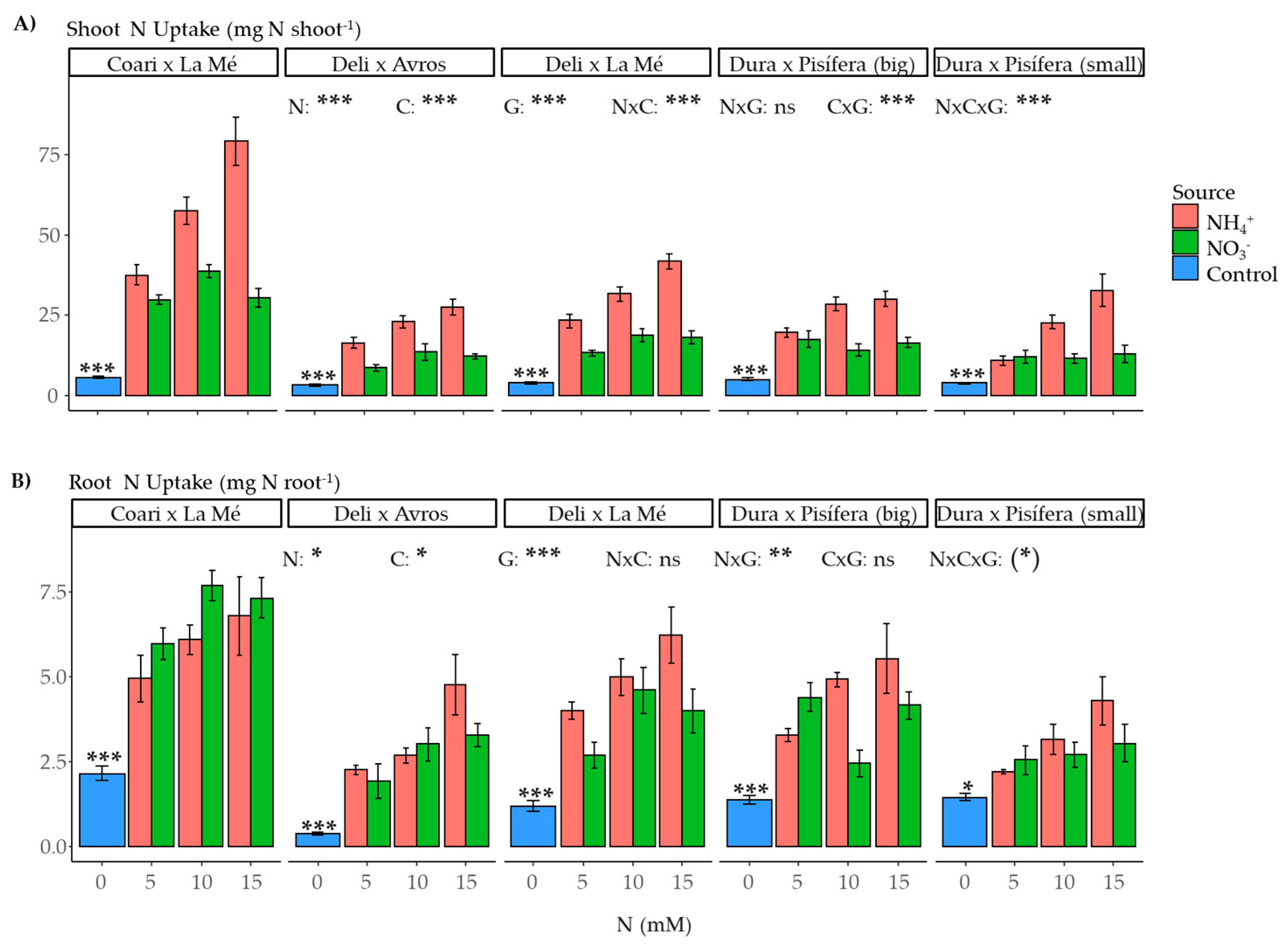
2.5. Nitrogen Uptake
3. Discussion
4. Materials and Methods
4.1. Growth Conditions and Experimental Design
4.2. Gas Exchange, Biomass Content, Root Morphology, and Tissue Metabolite Analysis
4.3. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pardon, L.; Bessou, C.; Nelson, P.N.; Dubos, B.; Ollivier, J.; Marichal, R.; Caliman, J.; Gabrielle, B. Key Unknowns in Nitrogen Budget for Oil Palm Plantations. A Review. Agron. Sustain. Dev. 2016, 36, 20. [Google Scholar] [CrossRef] [Green Version]
- Recio, J.; Vallejo, A.; Le-Noë, J.; Garnier, J.; García-Marco, S.; Álvarez, J.M.; Sanz-Cobena, A. The Effect of Nitrification Inhibitors on NH3 and N2O Emissions in Highly N Fertilized Irrigated Mediterranean Cropping Systems. Sci. Total Environ. 2018, 636, 427–436. [Google Scholar] [CrossRef] [PubMed]
- Edenhofer, O.; Pichs-Madruga, R.; Sokona, Y.; Farahani, E.; Kadner, S.; Seyboth, K.; Adler, A.; Baum, I.; Brunner, S.; Eickemeier, P.; et al. Summary for Policymakers. In Climate Change 2014: Mitigation of Climate Change; Contribution of Working Group III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; IPCC: Geneva, Switzerland, 2014; pp. 1–30. [Google Scholar] [CrossRef]
- Corrochano-Monsalve, M.; Bozal-Leorri, A.; Sánchez, C.; González-Murua, C.; Estavillo, J.M. Joint Application of Urease and Nitrification Inhibitors to Diminish Gaseous Nitrogen Losses under Different Tillage Systems. J. Clean. Prod. 2021, 289, 125701. [Google Scholar] [CrossRef]
- González-Moro, M.B.; González-Moro, I.; de la Peña, M.; Estavillo, J.M.; Aparicio-Tejo, P.M.; Marino, D.; González-Murua, C.; Vega-Mas, I. A Multi-Species Analysis Defines Anaplerotic Enzymes and Amides as Metabolic Markers for Ammonium Nutrition. Front. Plant Sci. 2021, 11, 632285. [Google Scholar] [CrossRef] [PubMed]
- Agamuthu, P.; Broughton, W.J. Factors Affecting the Development of the Rooting System in Young Oil Palms (Elaeis guineensis Jacq.). Agric. Ecosyst. Environ. 1986, 17, 173–179. [Google Scholar] [CrossRef]
- Aluko, O.O.; Li, C.; Yuan, G.; Nong, T.; Xiang, H.; Wang, Q.; Li, X.; Liu, H. Differential Effects of Ammonium (NH4+) and Potassium (K+) Nutrition on Photoassimilate Partitioning and Growth of Tobacco Seedlings. Plants 2022, 11, 3295. [Google Scholar] [CrossRef]
- Da Silva, G.B.; de Mello Prado, R.; Silva, S.L.O.; Campos, C.N.S.; Castellanos, L.G.; dos Santos, L.C.N.; Barreto, R.F.; Teodoro, P.E. Nitrogen Concentrations and Proportions of Ammonium and Nitrate in the Nutrition and Growth of Yellow Passion Fruit Seedlings. J. Plant Nutr. 2020, 43, 2533–2547. [Google Scholar] [CrossRef]
- Chen, H.; Jia, Y.; Xu, H.; Wang, Y.; Zhou, Y.; Huang, Z.; Yang, L.; Li, Y.; Chen, L.S.; Guo, J. Ammonium Nutrition Inhibits Plant Growth and Nitrogen Uptake in Citrus Seedlings. Sci. Hortic. 2020, 272, 109526. [Google Scholar] [CrossRef]
- Coleto, I.; Bejarano, I.; Marín-Peña, A.J.; Medina, J.; Rioja, C.; Burow, M.; Marino, D. Arabidopsis Thaliana Transcription Factors MYB28 and MYB29 Shape Ammonium Stress Responses by Regulating Fe Homeostasis. New Phytol. 2021, 229, 1021–1035. [Google Scholar] [CrossRef] [PubMed]
- Poucet, T.; González-Moro, M.B.; Cabasson, C.; Beauvoit, B.; Gibon, Y.; Dieuaide-Noubhani, M.; Marino, D. Ammonium Supply Induces Differential Metabolic Adaptive Responses in Tomato According to Leaf Phenological Stage. J. Exp. Bot. 2021, 72, 3185–3199. [Google Scholar] [CrossRef]
- Marino, D.; Ariz, I.; Lasa, B.; Santamaría, E.; Fernández-Irigoyen, J.; González-Murua, C.; Tejo, P.M.A. Quantitative Proteomics Reveals the Importance of Nitrogen Source to Control Glucosinolate Metabolism in Arabidopsis thaliana and Brassica oleracea. J. Exp. Bot. 2016, 67, 3313–3323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, P.; Xu, J.; Zhang, Z.; Nie, T. “Preferential” Ammonium Uptake by Rice Does Not Always Turn into Higher N Recovery of Fertilizer Sources under Water-Saving Irrigation. Agric. Water Manag. 2022, 272, 107867. [Google Scholar] [CrossRef]
- Chen, G.; Guo, S.; Kronzucker, H.J.; Shi, W. Nitrogen Use Efficiency (NUE) in Rice Links to NH4+ Toxicity and Futile NH4+ Cycling in Roots. Plant Soil 2013, 369, 351–363. [Google Scholar] [CrossRef] [Green Version]
- Duan, Y.; Yang, H.; Yang, H.; Wei, Z.; Che, J.; Wu, W.; Lyu, L.; Li, W. Physiological and Morphological Responses of Blackberry Seedlings to Different Nitrogen Forms. Plants 2023, 12, 1480. [Google Scholar] [CrossRef]
- Al-Awad, O.A.S.; Prendergast, K.S.; Robson, A.; Rengel, Z. Screening Canola Genotypes for Resistance to Ammonium Toxicity. Agronomy 2023, 13, 1150. [Google Scholar] [CrossRef]
- De Souza Miranda, R.; Gomes-Filho, E.; Prisco, J.T.; Alvarez-Pizarro, J.C. Ammonium Improves Tolerance to Salinity Stress in Sorghum Bicolor Plants. Plant Growth Regul. 2016, 78, 121–131. [Google Scholar] [CrossRef]
- Setién, I.; Fuertes-Mendizabal, T.; González, A.; Aparicio-Tejo, P.M.; González-Murua, C.; González-Moro, M.B.; Estavillo, J.M. High Irradiance Improves Ammonium Tolerance in Wheat Plants by Increasing N Assimilation. J. Plant Physiol. 2013, 170, 758–771. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coleto, I.; de la Peña, M.; Rodríguez-Escalante, J.; Bejarano, I.; Glauser, G.; Aparicio-Tejo, P.M.; González-Moro, M.B.; Marino, D. Leaves Play a Central Role in the Adaptation of Nitrogen and Sulfur Metabolism to Ammonium Nutrition in Oilseed Rape (Brassica napus). BMC Plant Biol. 2017, 17, 157. [Google Scholar] [CrossRef]
- Marino, D.; Moran, J.F. Can Ammonium Stress Be Positive for Plant Performance? Front. Plant Sci. 2019, 10, 1103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seethepalli, A.; Dhakal, K.; Griffiths, M.; Guo, H.; Freschet, G.T.; York, L.M. RhizoVision Explorer: Open-Source Software for Root Image Analysis and Measurement Standardization. AoB Plants 2021, 13, plab056. [Google Scholar] [CrossRef]
- Cross, J.M.; Von Korff, M.; Altmann, T.; Bartzetko, L.; Sulpice, R.; Gibon, Y.; Palacios, N.; Stitt, M. Variation of Enzyme Activities and Metabolite Levels in 24 Arabidopsis Accessions Growing in Carbon-Limited Conditions. Plant Physiol. 2006, 142, 1574–1588. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Somogyi, M. Notes on Sugar Determination. J. Biol. Chem. 1952, 195, 19–23. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De la Peña, M.; Ruiz-Romero, R.; Romero, H.M. Nitrogen Use Efficiency in Oil Palm Seedlings: Unraveling the Untapped Potential of Elevated External Ammonium Supply. Plants 2023, 12, 2819. https://doi.org/10.3390/plants12152819
De la Peña M, Ruiz-Romero R, Romero HM. Nitrogen Use Efficiency in Oil Palm Seedlings: Unraveling the Untapped Potential of Elevated External Ammonium Supply. Plants. 2023; 12(15):2819. https://doi.org/10.3390/plants12152819
Chicago/Turabian StyleDe la Peña, Marlon, Rodrigo Ruiz-Romero, and Hernán Mauricio Romero. 2023. "Nitrogen Use Efficiency in Oil Palm Seedlings: Unraveling the Untapped Potential of Elevated External Ammonium Supply" Plants 12, no. 15: 2819. https://doi.org/10.3390/plants12152819






