Investigation of the Relationship between Genetic and Breeding Characteristics of WBPH Behavior according to Resistant Materials in Rice
Abstract
:1. Introduction
2. Results
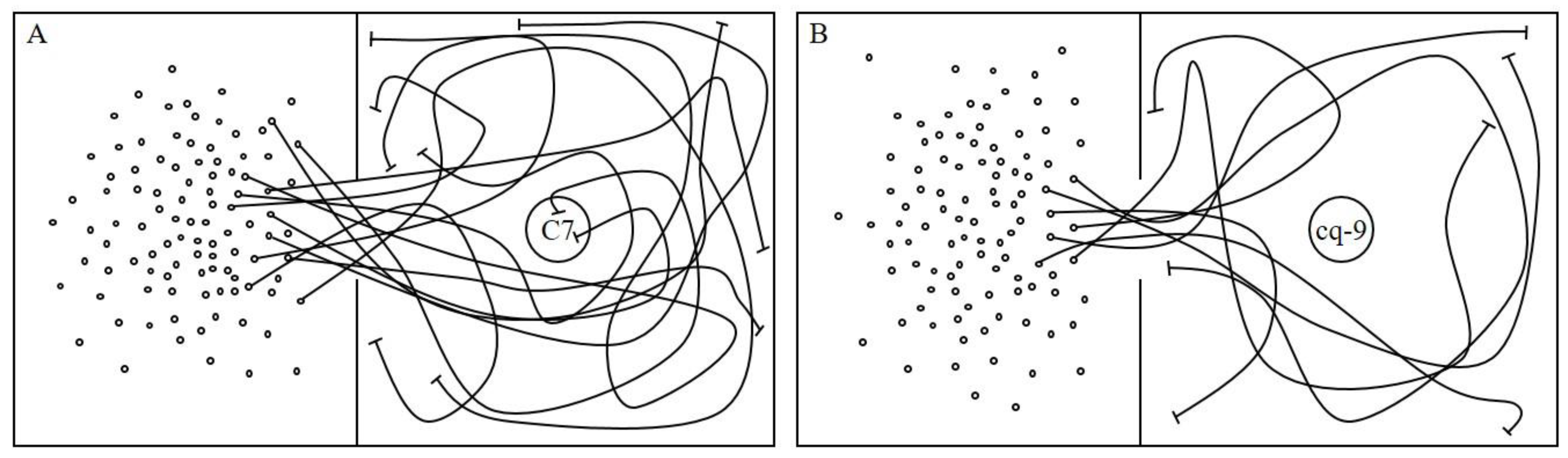
2.1. Behavioral Changes in WBPH in Response to C7
2.2. Behavioral Changes in WBPH in Response to cq-9
2.3. Changes in C7 and cq-9 Concentration caused by WBPH
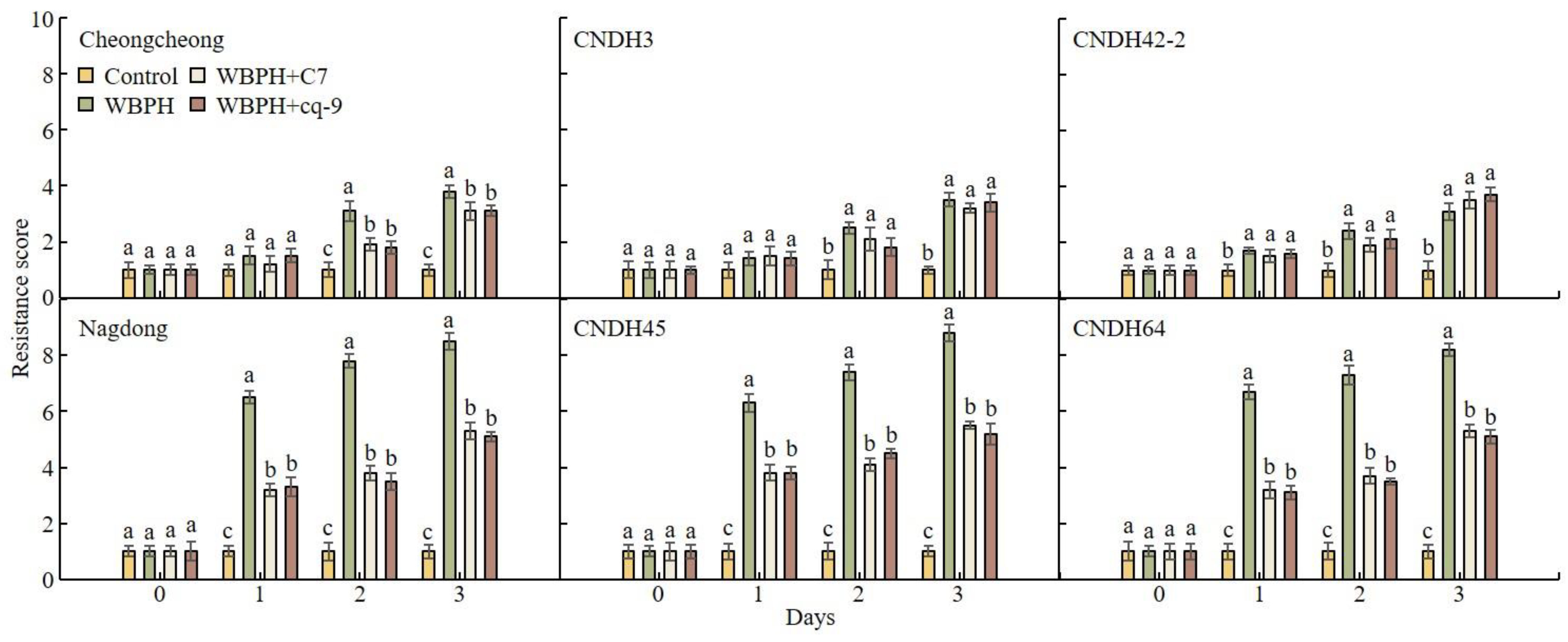
2.4. Changes in Resistance to WBPH Due to Treatment with C7 and cq-9
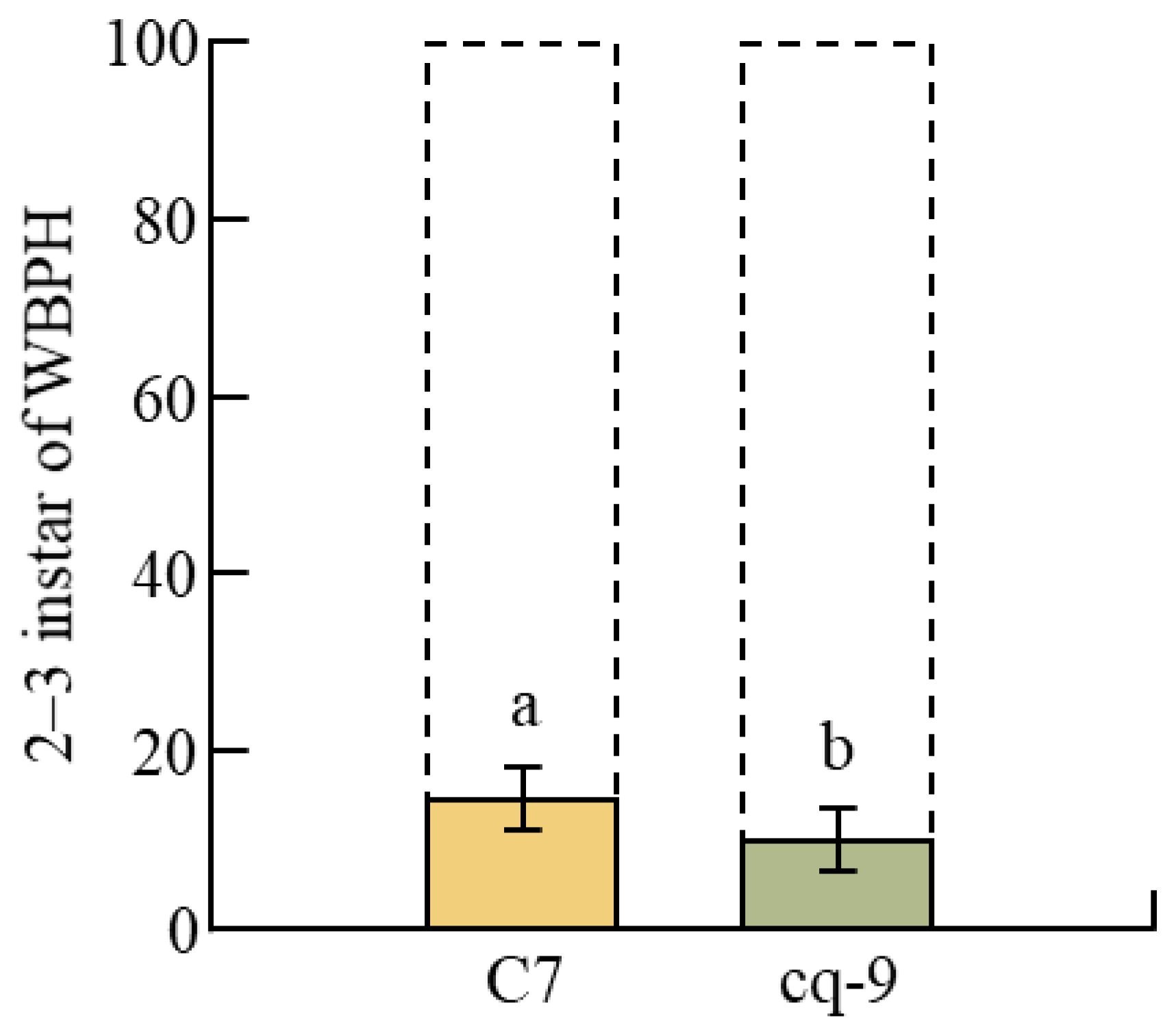
2.5. WBPH Resistance Score Analysis after Treatment with C7 and cq-9
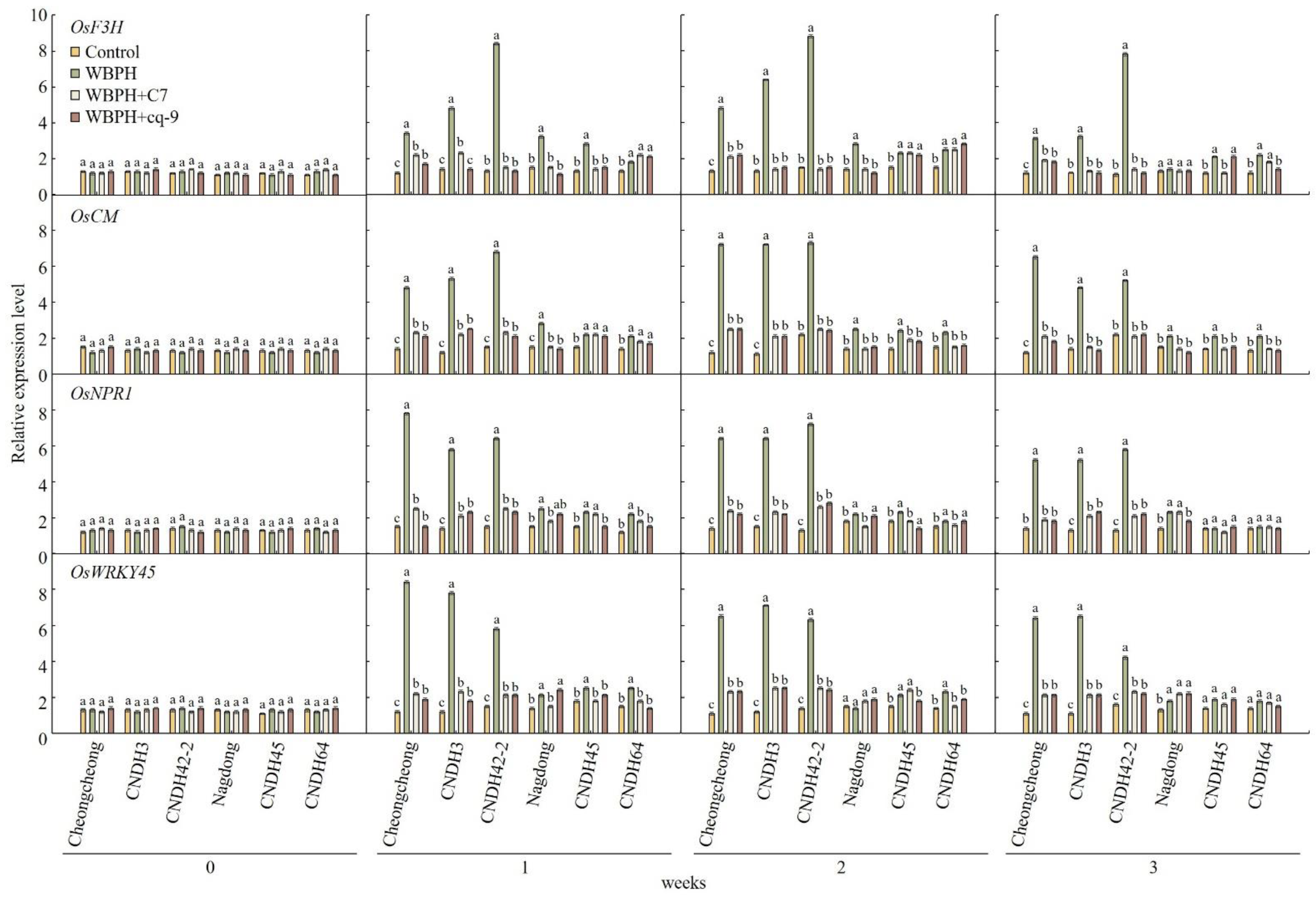
2.6. Expression Levels of Genes Related to Stress Resistance after Treatment with C7 and cq-9
3. Discussion
4. Materials and Methods
4.1. Rearing of WBPH for Repellent Test
4.2. Extracting C7 and cq-9 from Rice
4.3. Evaluation of WBPH Behavior against Rice Extracts
4.4. Evaluation of Plant Height and Bio-Scoring
4.5. Leaf Sampling and RNA Extraction
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nishad, R.; Ahmed, T.; Rahman, V.J.; Kareem, A. Modulation of plant defense system in response to microbial interactions. Front. Microbiol. 2020, 11, 1298. [Google Scholar] [CrossRef] [PubMed]
- Delaux, P.-M.; Schornack, S. Plant evolution driven by interactions with symbiotic and pathogenic microbes. Science 2021, 371, eaba6605. [Google Scholar] [CrossRef]
- Marrone, P.G. Pesticidal natural products–status and future potential. Pest Manag. Sci. 2019, 75, 2325–2340. [Google Scholar] [CrossRef]
- Wani, S.H.; Anand, S.; Singh, B.; Bohra, A.; Joshi, R. WRKY transcription factors and plant defense responses: Latest discoveries and future prospects. Plant Cell Rep. 2021, 40, 1071–1085. [Google Scholar] [CrossRef]
- Tiwari, R.K.; Lal, M.K.; Kumar, R.; Mangal, V.; Altaf, M.A.; Sharma, S.; Singh, B.; Kumar, M. Insight into melatonin-mediated response and signaling in the regulation of plant defense under biotic stress. Plant Mol.Biol. 2022, 105, 385–399. [Google Scholar] [CrossRef] [PubMed]
- Tuladhar, P.; Sasidharan, S.; Saudagar, P. Role of phenols and polyphenols in plant defense response to biotic and abiotic stresses. In Biocontrol Agents and Secondary Metabolites; Elsevier: Amsterdam, The Netherlands, 2021; pp. 419–441. [Google Scholar]
- Reichel, T.; de Resende, M.L.V.; Monteiro, A.C.A.; Freitas, N.C.; dos Santos Botelho, D.M. Constitutive defense strategy of coffee under field conditions: A comparative assessment of resistant and susceptible cultivars to rust. Mol. Biotechnol. 2022, 64, 263–277. [Google Scholar] [CrossRef]
- Sun, T.; Zhang, Y. Short-and long-distance signaling in plant defense. Plant J. 2021, 105, 505–517. [Google Scholar] [CrossRef] [PubMed]
- Yuan, P.; Tanaka, K.; Poovaiah, B. Calcium/calmodulin-mediated defense signaling: What is looming on the horizon for AtSR1/CAMTA3-mediated signaling in plant immunity. Front. Plant Sci. 2022, 12, 3244. [Google Scholar] [CrossRef]
- Aguirre-Becerra, H.; Vazquez-Hernandez, M.C.; Saenz de la O, D.; Alvarado-Mariana, A.; Guevara-Gonzalez, R.G.; Garcia-Trejo, J.F.; Feregrino-Perez, A.A. Role of stress and defense in plant secondary metabolites production. In Bioactive Natural Products for Pharmaceutical Applications; Springer: Cham, Switzerland, 2021; pp. 151–195. [Google Scholar]
- Khan, A.L.; Hussain, J.; Hamayun, M.; Gilani, S.A.; Ahmad, S.; Rehman, G.; Kim, Y.-H.; Kang, S.-M.; Lee, I.-J. Secondary metabolites from Inula britannica L. and their biological activities. Molecules 2010, 15, 1562–1577. [Google Scholar] [CrossRef] [Green Version]
- Nabavi, S.M.; Šamec, D.; Tomczyk, M.; Milella, L.; Russo, D.; Habtemariam, S.; Suntar, I.; Rastrelli, L.; Daglia, M.; Xiao, J. Flavonoid biosynthetic pathways in plants: Versatile targets for metabolic engineering. Biotechnol. Adv. 2020, 38, 107316. [Google Scholar] [CrossRef]
- Goławska, S.; Sprawka, I.; Łukasik, I.; Goławski, A. Are naringenin and quercetin useful chemicals in pest-management strategies? J. Pest Sci. 2014, 87, 173–180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Riveros, A.J.; Gronenberg, W. The flavonoid rutin protects the bumble bee Bombus impatiens against cognitive impairment by imidacloprid and fipronil. J. Exp. Biol. 2022, 225, jeb244526. [Google Scholar] [CrossRef]
- Anderson, R.; Bayer, P.E.; Edwards, D. Climate change and the need for agricultural adaptation. Curr. Opin. Plant Biol. 2020, 56, 197–202. [Google Scholar] [CrossRef] [PubMed]
- Hussain, S.; Huang, J.; Huang, J.; Ahmad, S.; Nanda, S.; Anwar, S.; Shakoor, A.; Zhu, C.; Zhu, L.; Cao, X. Rice production under climate change: Adaptations and mitigating strategies. In Environment, Climate, Plant and Vegetation Growth; Springer: Cham, Switzerland, 2020; pp. 659–686. [Google Scholar]
- Ali, E.; Mao, K.; Liao, X.; Jin, R.; Li, J. Cross-resistance and biochemical characterization of buprofezin resistance in the white-backed planthopper, Sogatella furcifera (Horvath). Pest. Biochem. Physiol. 2019, 158, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Pu, L.; Xie, G.; Ji, C.; Ling, B.; Zhang, M.; Xu, D.; Zhou, G. Transmission characteristics of Southern rice black-streaked dwarf virus by rice planthoppers. Crop Prot. 2012, 41, 71–76. [Google Scholar] [CrossRef]
- Zheng, Y.L.; Xu, L.; Wu, J.C.; Liu, J.L.; DuanMu, H.L. Time of occurrence of hopperburn symptom on rice following root and leaf cutting and fertilizer application with brown planthopper, Nilaparvata lugens (stål) infestation. Crop Prot. 2007, 26, 66–72. [Google Scholar] [CrossRef]
- Nuruzzaman, M.; Sharoni, A.M.; Satoh, K.; Karim, M.R.; Harikrishna, J.A.; Shimizu, T.; Kikuchi, S. NAC transcription factor family genes are differentially expressed in rice during infections with Rice dwarf virus, Rice black-streaked dwarf virus, Rice grassy stunt virus, Rice ragged stunt virus, and Rice transitory yellowing virus. Front. Plant Sci. 2015, 6, 676. [Google Scholar] [CrossRef] [Green Version]
- Sharma, K.R.; Raju, S.; Jaiswal, D.K. Influence of environmental effect on the population dynamics of brown plant hopper, Nilaparvata lugens (Stal) and white-backed plant hopper, Sogatella furcifera (Hovarth) in Varanasi region. J. Entomol. Res. 2018, 42, 339. [Google Scholar] [CrossRef]
- Dhaka, S.; Rai, M.; Rai, M.; Yadav, A. Field evaluation of novel insecticides against brown planthopper (Nilaparvata lugens) and white backed planthopper (Sogatella furcifera) in rice. Indian J. Agric. Sci. 2020, 90, 1528–1531. [Google Scholar] [CrossRef]
- Sharma, K.; Raju, S. Field efficacy of some combination insecticide formulations against paddy planthoppers. Pestic. Res. J. 2019, 31, 119–125. [Google Scholar] [CrossRef]
- Hladik, M.L.; Main, A.R.; Goulson, D. Environmental risks and challenges associated with neonicotinoid insecticides. Environ. Sci. Technol. 2018, 52, 3329–3335. [Google Scholar] [CrossRef] [Green Version]
- Bolzonella, C.; Lucchetta, M.; Teo, G.; Boatto, V.; Zanella, A. Is there a way to rate insecticides that is less detrimental to human and environmental health? Glob. Ecol. Conserv. 2019, 20, e00699. [Google Scholar] [CrossRef]
- Khare, R.; Das, G.; Kumar, S.; Bendigeri, S.; Sachan, S.; Saiyam, R.; Banerjee, D.; Khare, D. Herbal insecticides and acaricides: Challenges and constraints. Int. J. Chem. Stud. 2019, 7, 118–125. [Google Scholar]
- Verma, C.; Ebenso, E.E.; Bahadur, I.; Quraishi, M. An overview on plant extracts as environmental sustainable and green corrosion inhibitors for metals and alloys in aggressive corrosive media. J. Mol. Liq. 2018, 266, 577–590. [Google Scholar] [CrossRef]
- Fatima, I.; Safdar, N.; Akhtar, W.; Munir, A.; Saqib, S.; Ayaz, A.; Zaman, W. Evaluation of potential inhibitory effects on acetylcholinesterase, pancreatic lipase, and cancer cell lines using raw leaves extracts of three fabaceae species. Heliyon 2023, 9, e15909. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.E.; Hong, S.J.; Hasan, N.; Baek, E.J.; Kim, J.T.; Kim, Y.D.; Park, M.K. Repellent efficacy of essential oils and plant extracts against Tribolium castaneum and Plodia interpunctella. Entomol. Res. 2020, 50, 450–459. [Google Scholar] [CrossRef]
- Wangai, L.N.; Kamau, K.K.; Munyekenye, G.; Nderu, D.; Maina, E.; Gitau, W.; Murigi, M.; Kamau, S.; Njuguna, M.; Gichuki, J. Efficacy of plant-based repellents against Anopheles mosquitoes: A systematic review. Biomed. Sci. 2020, 6, 44. [Google Scholar]
- Blundell, R.; Schmidt, J.E.; Igwe, A.; Cheung, A.L.; Vannette, R.L.; Gaudin, A.; Casteel, C.L. Organic management promotes natural pest control through altered plant resistance to insects. Nat. Plants 2020, 6, 483–491. [Google Scholar] [CrossRef] [PubMed]
- Shinde, B.H.; Inamdar, S.N.; Nalawade, S.A.; Chaudhari, S.B. A systematic review on antifungal and insecticidal applications of biosynthesized metal nanoparticles. Mater. Today 2023, 73, 412–417. [Google Scholar] [CrossRef]
- Wang, L.; Li, X.; Gao, F.; Liu, Y.; Lang, S.; Wang, C.; Zhang, D. Effect of ultrasound combined with exogenous GABA treatment on polyphenolic metabolites and antioxidant activity of mung bean during germination. Ultrason. Sonochem. 2023, 94, 106311. [Google Scholar] [CrossRef]
- Kabera, J.N.; Semana, E.; Mussa, A.R.; He, X. Plant secondary metabolites: Biosynthesis, classification, function and pharmacological properties. J. Pharm. Pharmacol. 2014, 2, 377–392. [Google Scholar]
- Biondi, A.; Guedes, R.N.C.; Wan, F.-H.; Desneux, N. Ecology, worldwide spread, and management of the invasive South American tomato pinworm, Tuta absoluta: Past, present, and future. Annu. Rev. Entomol. 2018, 63, 239–258. [Google Scholar] [CrossRef] [PubMed]
- Sogawa, K.; Liu, G.; Qiang, Q.; Heong, K.; Hardy, B. Prevalence of whitebacked planthoppers in Chinese hybrid rice and whitebacked planthopper resistance in Chinese japonica rice. In Planthoppers: New Threats to the Sustainability of Intensive Rice Production Systems in Asia; International Rice Research Institute: Los Banos, Philippines, 2009; pp. 257–280. [Google Scholar]
- Ahmed, N.; Alam, M.; Saeed, M.; Ullah, H.; Iqbal, T.; Al-Mutairi, K.A.; Shahjeer, K.; Ullah, R.; Ahmed, S.; Ahmed, N.A.A.H. Botanical insecticides are a non-toxic alternative to conventional pesticides in the control of insects and pests. In Global Decline of Insects; IntechOpen: London, UK, 2021. [Google Scholar]
- Khan, T.; Shah, S.M.; Khan, S.A.; Hassan, A.; Khan, A.R.; Akhtar, G.; Sajjad, Y. Evaluating the antioxidative defense response of selected indoor plants against benzene and formaldehyde. Environ. Sci. Pollut. Res. 2023, 1–11. [Google Scholar]
- Treesubsuntorn, C.; Lakaew, K.; Autarmat, S.; Thiravetyan, P. Enhancing benzene removal by Chlorophytum comosum under simulation microgravity system: Effect of light-dark conditions and indole-3-acetic acid. Acta Astronaut. 2020, 175, 396–404. [Google Scholar] [CrossRef]
- Kumar, S.; Abedin, M.M.; Singh, A.K.; Das, S. Role of phenolic compounds in plant-defensive mechanisms. In Plant Phenolics in Sustainable Agriculture; Springer: Singapore, 2020; Volume 1, pp. 517–532. [Google Scholar]
- Treutter, D. Significance of flavonoids in plant resistance: A review. Environ. Chem. Lett. 2006, 4, 147–157. [Google Scholar] [CrossRef]
- Naboulsi, I.; Aboulmouhajir, A.; Kouisni, L.; Bekkaoui, F.; Yasri, A. Plants extracts and secondary metabolites, their extraction methods and use in agriculture for controlling crop stresses and improving productivity: A review. J. Med. Plants 2018, 6, 223–240. [Google Scholar]
- Rosenthal, G.A. The biochemical basis for the deleterious effects of L-canavanine. Phytochemistry 1991, 30, 1055–1058. [Google Scholar] [CrossRef]
- Sadasivam, K.; Kumaresan, R. Theoretical investigation on the antioxidant behavior of chrysoeriol and hispidulin flavonoid compounds–A DFT study. Comput. Theor. Chem. 2011, 963, 227–235. [Google Scholar] [CrossRef]
- Ogungbe, I.V.; Erwin, W.R.; Setzer, W.N. Antileishmanial phytochemical phenolics: Molecular docking to potential protein targets. J. Mol. Graph. 2014, 48, 105–117. [Google Scholar] [CrossRef]
- Jan, R.; Asaf, S.; Asif, S.; Kim, E.-G.; Jang, Y.-H.; Kim, N.; Al-Harrasi, A.; Lee, G.-S.; Kim, K.-M. Enhancing the Expression of the OsF3H Gene in Oryza sativa Leads to the Regulation of Multiple Biosynthetic Pathways and Transcriptomic Changes That Influence Insect Resistance. Int. J. Mol. Sci. 2022, 23, 15308. [Google Scholar] [CrossRef]
- Park, J.-R.; Jan, R.; Park, S.-G.; Handoyo, T.; Lee, G.-S.; Yun, S.; Jang, Y.-H.; Du, X.-X.; Lee, T.; Kwon, Y.-S. The Quantitative Trait Loci Mapping of Rice Plant and the Components of Its Extract Confirmed the Anti-Inflammatory and Platelet Aggregation Effects In Vitro and In Vivo. Antioxidants 2021, 10, 1691. [Google Scholar] [CrossRef]
- Jan, R.; Khan, M.A.; Asaf, S.; Lee, I.-J.; Kim, K.-M. Overexpression of OsF3H modulates WBPH stress by alteration of phenylpropanoid pathway at a transcriptomic and metabolomic level in Oryza sativa. Sci. Rep. 2020, 10, 14685. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.-G.; Yun, S.; Park, J.-R.; Kim, K.-M. Identification of F3H, major secondary metabolite-related gene that confers resistance against whitebacked planthopper through qtl mapping in rice. Plants 2021, 10, 81. [Google Scholar] [CrossRef] [PubMed]
- Jan, R.; Khan, M.A.; Asaf, S.; Lee, I.-J.; Bae, J.-S.; Kim, K.-M. Overexpression of OsCM alleviates BLB stress via phytohormonal accumulation and transcriptional modulation of defense-related genes in Oryza sativa. Sci. Rep. 2020, 10, 19520. [Google Scholar] [CrossRef] [PubMed]
- Park, J.-R.; Yun, S.; Jan, R.; Kim, K.-M. Screening and identification of brown planthopper resistance genes OsCM9 in rice. Agronomy 2020, 10, 1865. [Google Scholar] [CrossRef]
- Kim, E.-G.; Yun, S.; Park, J.-R.; Jang, Y.-H.; Farooq, M.; Yun, B.-J.; Kim, K.-M. Bio-Efficacy of Chrysoeriol7, a Natural Chemical and Repellent, against Brown Planthopper in Rice. Int. J. Mol. Sci. 2022, 23, 1540. [Google Scholar] [CrossRef]
- Jang, Y.-H.; Yun, S.; Park, J.-R.; Kim, E.-G.; Yun, B.-J.; Kim, K.-M. Biological Efficacy of Cochlioquinone-9, a Natural Plant Defense Compound for White-Backed Planthopper Control in Rice. Biology 2021, 10, 1273. [Google Scholar] [CrossRef]
- Jang, Y.-H.; Park, J.-R.; Kim, K.-M. Antimicrobial activity of chrysoeriol 7 and chochlioquinone 9, white-backed planthopper-resistant compounds, against rice pathogenic strains. Biology 2020, 9, 382. [Google Scholar] [CrossRef]
- Asadollahi, A.; Khoobdel, M.; Zahraei-Ramazani, A.; Azarmi, S.; Mosawi, S.H. Effectiveness of plant-based repellents against different Anopheles species: A systematic review. Malar. J. 2019, 18, 436. [Google Scholar] [CrossRef]
- da Silva, M.R.M.; Ricci-Júnior, E. An approach to natural insect repellent formulations: From basic research to technological development. Acta Trop. 2020, 212, 105419. [Google Scholar] [CrossRef]
- Ren, J.; Wang, W.; Li, H.; Hou, P.; Ma, Z.; Feng, J.; Wu, H. Efficacy of Ginkgo biloba L. and Derris trifoliata binary extract combinations against aphids (Hemiptera: Aphididae). Crop Prot. 2023, 164, 106144. [Google Scholar] [CrossRef]
- Jiang, T.; Guo, K.; Liu, L.; Tian, W.; Xie, X.; Wen, S.; Wen, C. Integrated transcriptomic and metabolomic data reveal the flavonoid biosynthesis metabolic pathway in Perilla frutescens (L.) leaves. Sci. Rep. 2020, 10, 16207. [Google Scholar] [CrossRef]
- Bicalho, B.; Gonçalves, R.A.; Zibordi, A.P.M.; Manfio, G.P.; Marsaioli, A.J. Antimicrobial compounds of fungi vectored by Clusia spp. (Clusiaceae) pollinating bees. Z. Naturforsch. C 2003, 58, 746–751. [Google Scholar] [CrossRef] [PubMed]
- Al-Huqail, A.A.; Behiry, S.I.; Salem, M.Z.; Ali, H.M.; Siddiqui, M.H.; Salem, A.Z. Antifungal, antibacterial, and antioxidant activities of Acacia saligna (Labill.) HL Wendl. flower extract: HPLC analysis of phenolic and flavonoid compounds. Molecules 2019, 24, 700. [Google Scholar] [CrossRef] [Green Version]
- Govindarajan, M.; Sivakumar, R. Adulticidal and repellent properties of indigenous plant extracts against Culex quinquefasciatus and Aedes aegypti (Diptera: Culicidae). Parasitol. Res. 2012, 110, 1607–1620. [Google Scholar] [CrossRef] [PubMed]
- Souto, A.L.; Sylvestre, M.; Tölke, E.D.; Tavares, J.F.; Barbosa-Filho, J.M.; Cebrián-Torrejón, G. Plant-derived pesticides as an alternative to pest management and sustainable agricultural production: Prospects, applications and challenges. Molecules 2021, 26, 4835. [Google Scholar] [CrossRef]
- Tomás-Menor, L.; Barrajón-Catalán, E.; Segura-Carretero, A.; Martí, N.; Saura, D.; Menéndez, J.A.; Joven, J.; Micol, V. The promiscuous and synergic molecular interaction of polyphenols in bactericidal activity: An opportunity to improve the performance of antibiotics? Phytother. Res. 2015, 29, 466–473. [Google Scholar] [CrossRef]
- Choudhury, D.; Dobhal, P.; Srivastava, S.; Saha, S.; Kundu, S. Role of botanical plant extracts to control plant pathogens-A review. Indian J. Agric. Res. 2018, 52, 341–346. [Google Scholar]
- Kim, K.-M.; Park, Y.-H. Studies of the life cycle and rearing methods of whitebacked planthopper (Sogatella furcifera Horvath). J. Life Sci. 2018, 28, 357–360. [Google Scholar]
- Tu, Z.; Ling, B.; Xu, D.; Zhang, M.; Zhou, G. Effects of southern rice black-streaked dwarf virus on the development and fecundity of its vector, Sogatella furcifera. Virol. J. 2013, 10, 145. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.; Qin, Y.; Sohn, J. Analysis of QTLs related to resistance to brown planthopper in rice. Korean J. Breed. Sci. 2009, 41, 236–243. [Google Scholar]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, J.-R.; Kim, E.-G.; Jang, Y.-H.; Nam, S.Y.; Kim, K.-M. Investigation of the Relationship between Genetic and Breeding Characteristics of WBPH Behavior according to Resistant Materials in Rice. Plants 2023, 12, 2821. https://doi.org/10.3390/plants12152821
Park J-R, Kim E-G, Jang Y-H, Nam SY, Kim K-M. Investigation of the Relationship between Genetic and Breeding Characteristics of WBPH Behavior according to Resistant Materials in Rice. Plants. 2023; 12(15):2821. https://doi.org/10.3390/plants12152821
Chicago/Turabian StylePark, Jae-Ryoung, Eun-Gyeong Kim, Yoon-Hee Jang, Sang Yong Nam, and Kyung-Min Kim. 2023. "Investigation of the Relationship between Genetic and Breeding Characteristics of WBPH Behavior according to Resistant Materials in Rice" Plants 12, no. 15: 2821. https://doi.org/10.3390/plants12152821









