Top and Side Lighting Induce Morphophysiological Improvements in Korean Ginseng Sprouts (Panax ginseng C.A. Meyer) Grown from One-Year-Old Roots
Abstract
:1. Introduction
2. Results
2.1. Morphology and Growth Parameters
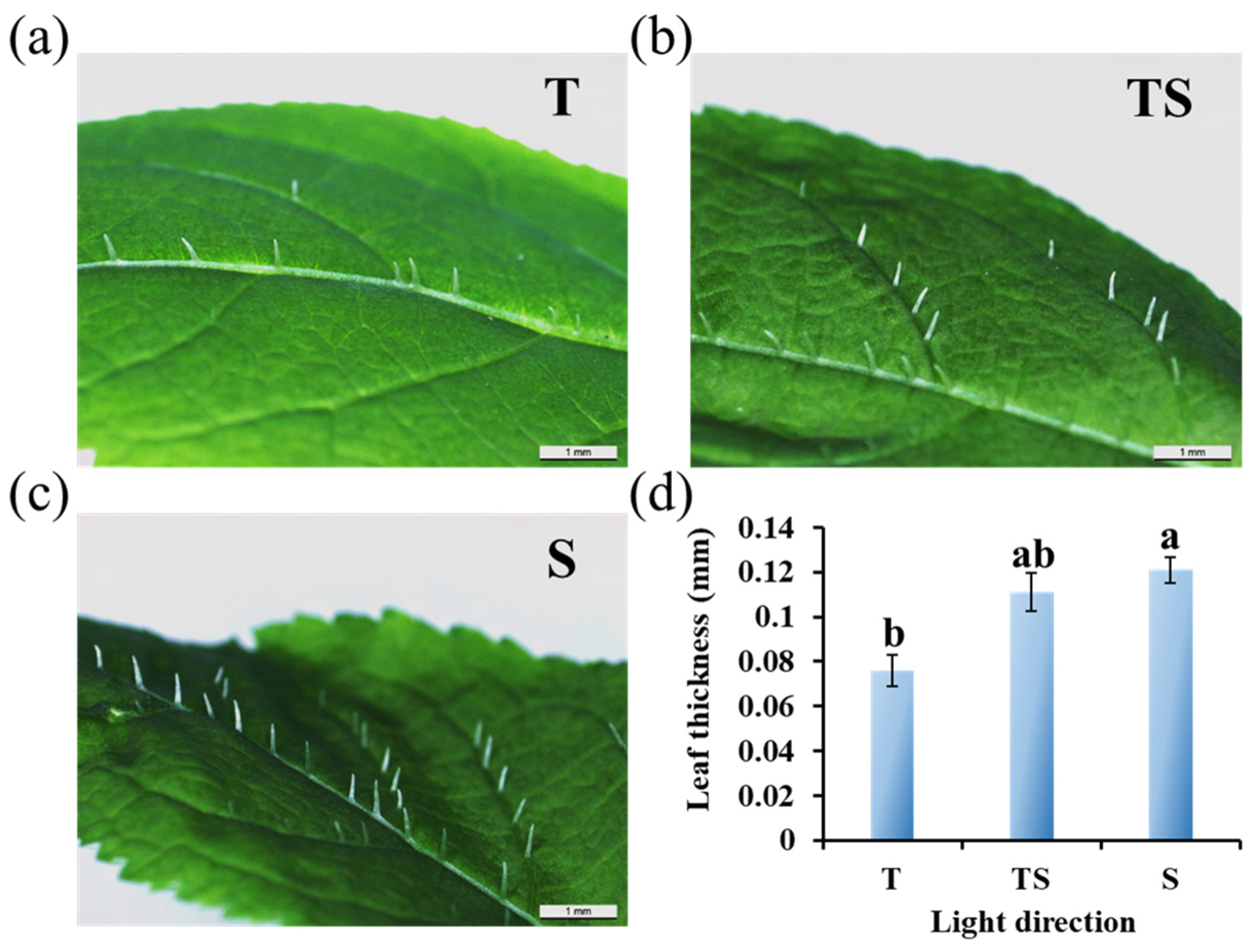
2.2. Micro-Observation of Trichomes and Leaf Thickness
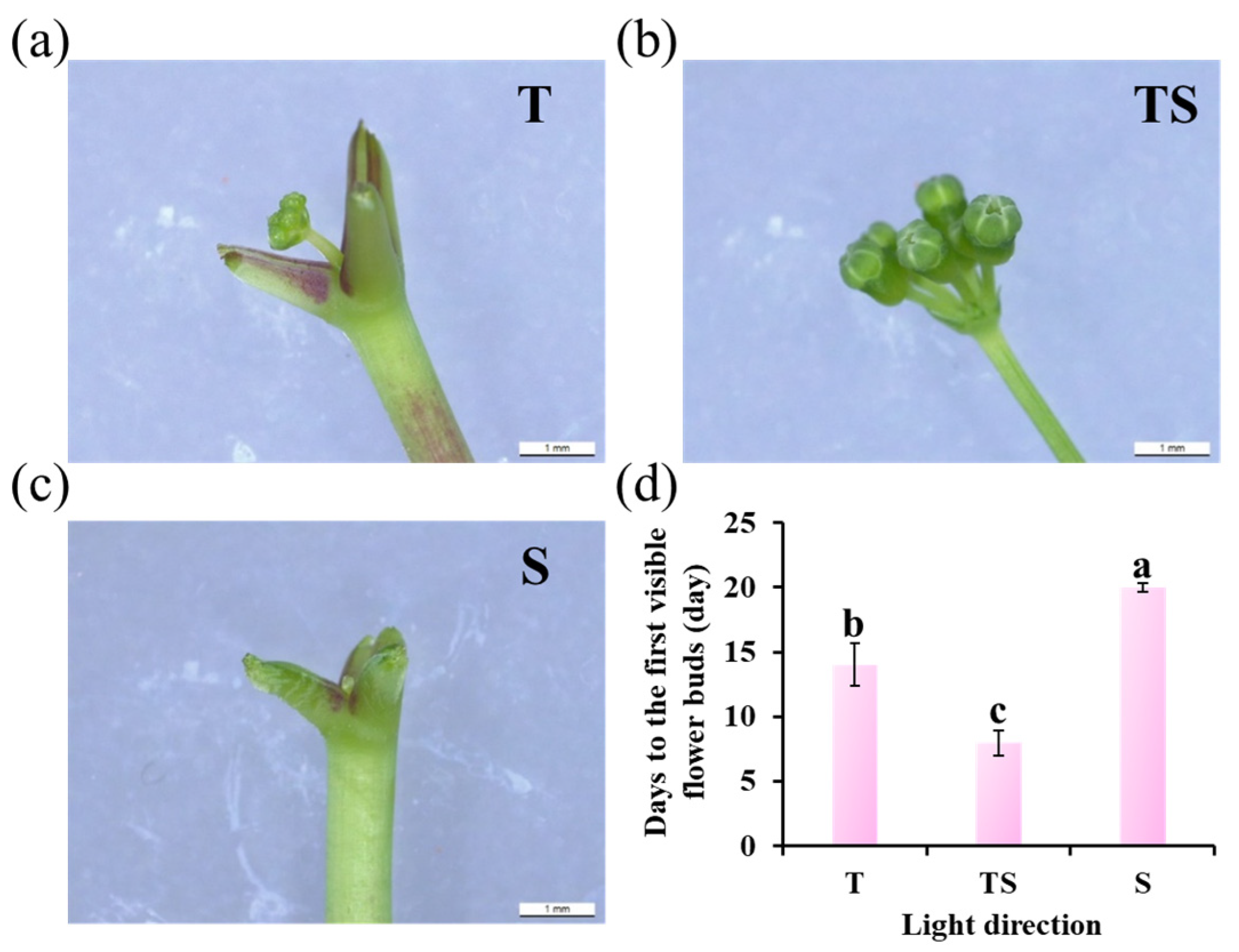
2.3. Flower Bud Formation
2.4. Photosynthesis-Related Pigment Contents
2.5. Photosynthetic and Chlorophyll Fluorescence Characteristics
3. Discussion
3.1. Shortened Shoots, Enlarged Leaves, and Strong Roots
3.2. Early Flower Bud Formation
3.3. Photosynthetic and Chlorophyll Fluorescence Characteristics
3.4. Further Research
4. Conclusions
5. Materials and Methods
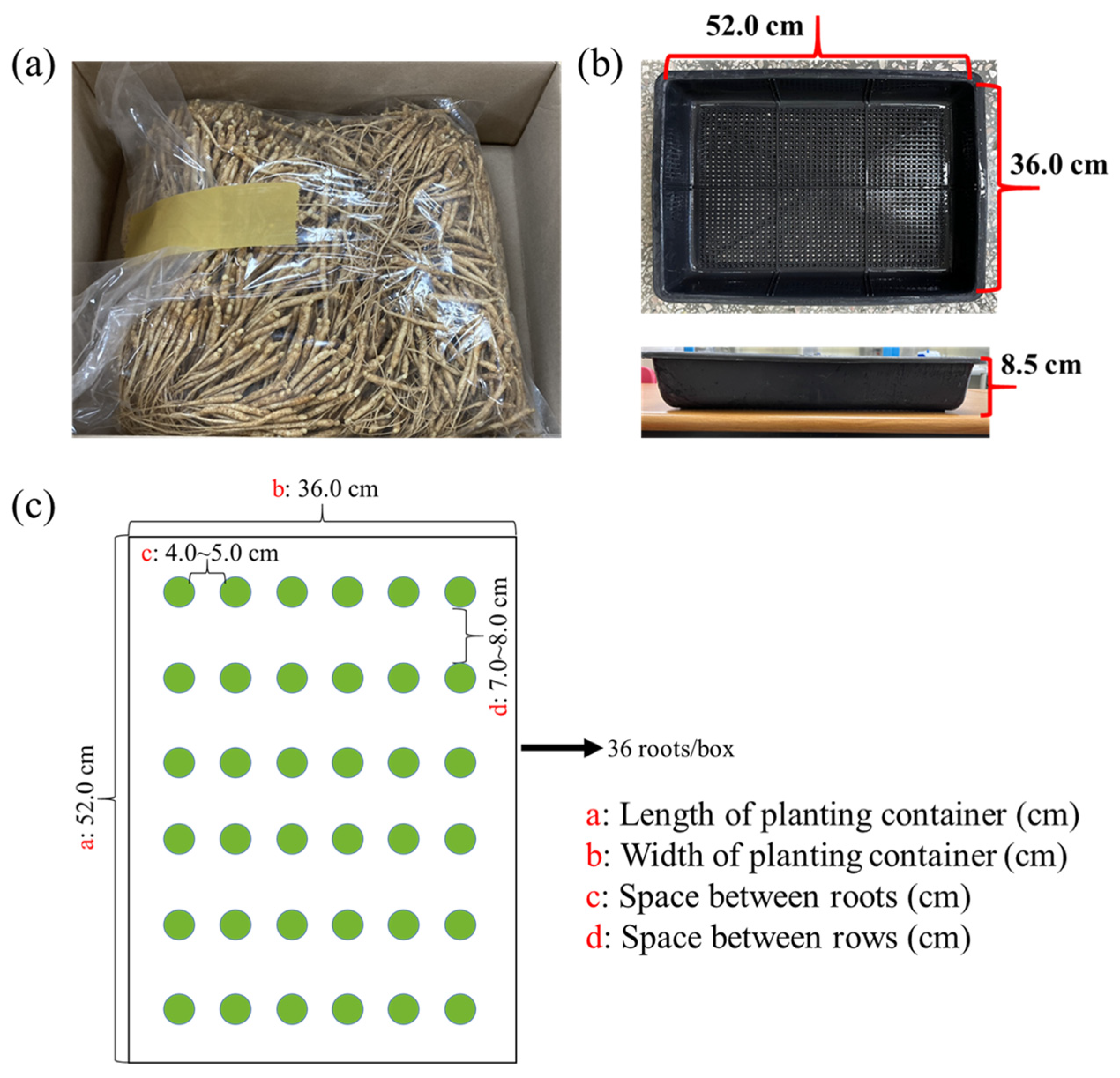
5.1. Plant Materials and Growth Conditions
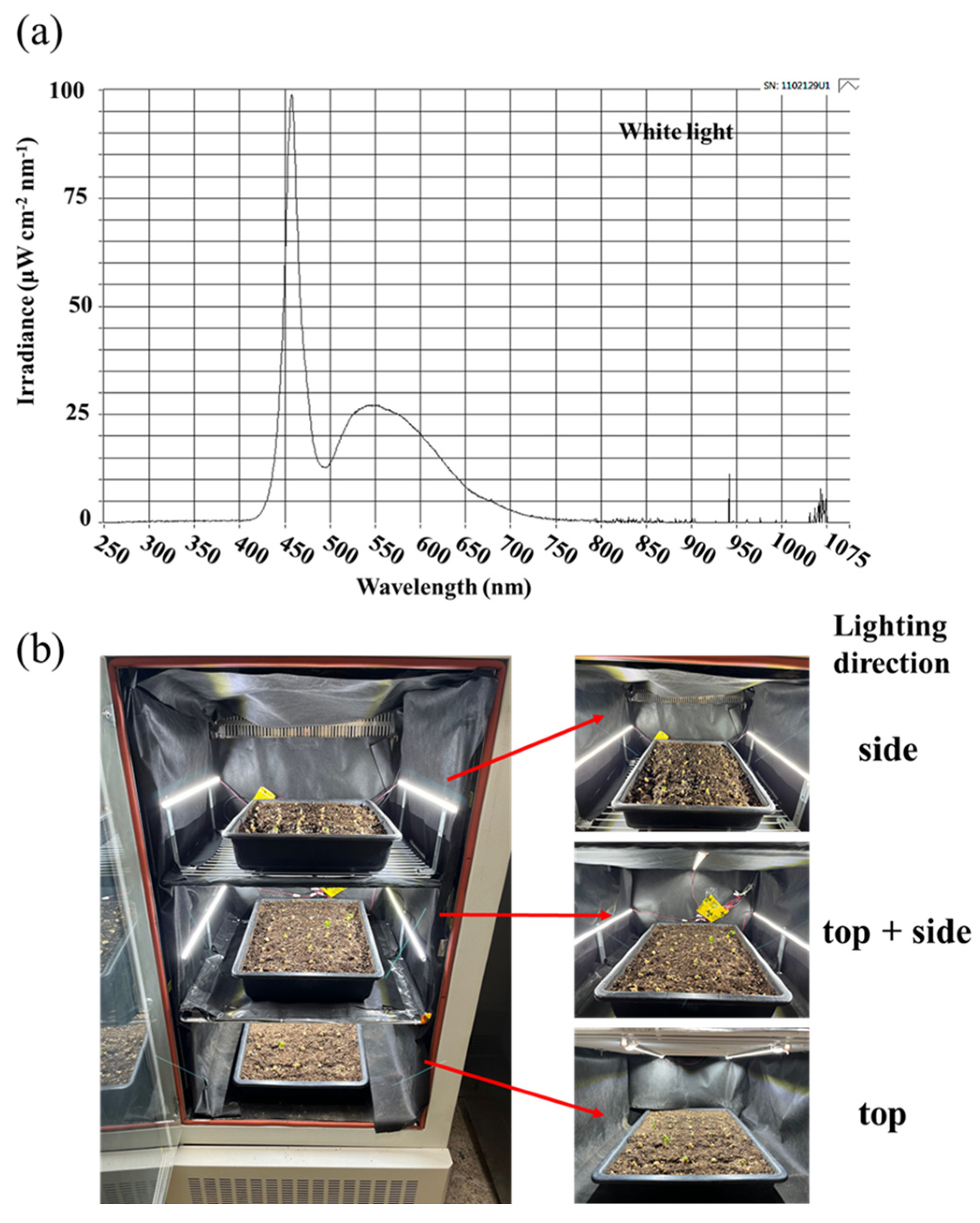
5.2. Lighting Treatments
5.3. Measurement of the Growth Parameters, Calculation of the Relative Growth Rate, and Observation of the Leaf Trichomes and Flower Buds
5.4. Measurement of the Photosynthetic Pigment Contents
5.5. Measurement of Photosynthesis and Chlorophyll Fluorescence
5.6. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- He, M.; Huang, X.; Liu, S.; Guo, C.; Xie, Y.; Meijer, A.H.; Wang, M. The difference between white and red ginseng: Variations in ginsenosides and immunomodulation. Planta Med. 2018, 84, 845–854. [Google Scholar] [CrossRef] [Green Version]
- Thompson Coon, J.; Ernst, E. Panax ginseng: A systematic review of adverse effects and drug interactions. Drug Safety 2002, 25, 323–344. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, S.A.; Gadhvi, K.V.; Chaudhary, A.B. Comprehensive review on world herb trade and most utilized medicinal plant. Int. J. Appl. Biol. Pharma. Technol. 2010, 1, 517. [Google Scholar]
- Baeg, I.H.; So, S.H. The world ginseng market and the ginseng (Korea). J. Ginseng Res. 2013, 37, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chung, S.I.; Kang, M.Y.; Lee, S.C. In vitro and in vivo antioxidant activity of aged ginseng (Panax ginseng). Prev. Nutr. Food Sci. 2016, 21, 24. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.H. Pharmacological and medical applications of Panax ginseng and ginsenosides: A review for use in cardiovascular diseases. J. Ginseng Res. 2018, 42, 264–269. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.D.; Kim, J.T.; Kim, S.H.; Chung, S.H. Ginseng and diabetes: The evidences from in vitro, animal and human studies. J. Ginseng Res. 2012, 36, 27. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Ren, C.; Zhang, Y.; Wu, X. Ginseng: An nonnegligible natural remedy for healthy aging. Aging Dis. 2017, 8, 708. [Google Scholar] [CrossRef] [Green Version]
- Kaneko, H.; Nakanishi, K. Proof of the mysterious efficacy of ginseng: Basic and clinical trials: Clinical effects of medical ginseng, Korean red ginseng: Specifically, its anti-stress action for prevention of disease. J. Pharmacol. Sci. 2004, 95, 158–162. [Google Scholar] [CrossRef] [Green Version]
- Basati, G.; Ghanadi, P.; Abbaszadeh, S. A review of the most important natural antioxidants and effective medicinal plants in traditional medicine on prostate cancer and its disorders. J. Herbmed. Pharmacol. 2020, 9, 112–120. [Google Scholar] [CrossRef]
- Kim, H.G.; Cho, J.H.; Yoo, S.R.; Lee, J.S.; Han, J.M.; Lee, N.H.; Ahn, Y.C.; Son, C.G. Antifatigue effects of Panax ginseng CA Meyer: A randomised, double-blind, placebo-controlled trial. PLoS ONE 2013, 8, e61271. [Google Scholar]
- Chen, W.; Balan, P.; Popovich, D.G. Review of ginseng anti-diabetic studies. Molecules 2019, 24, 4501. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, S.; Wang, Z.; Huang, Y.; O’Barr, S.A.; Wong, R.A.; Yeung, S.; Chow, M.S.S. Ginseng and anticancer drug combination to improve cancer chemotherapy: A critical review. Evid.-Based Complement. Altern. Med. 2014, 2014, 168940. [Google Scholar] [CrossRef] [PubMed]
- Park, T.Y.; Hong, M.; Sung, H.; Kim, S.; Suk, K.T. Effect of Korean Red Ginseng in chronic liver disease. J. Ginseng Res. 2017, 41, 450–455. [Google Scholar] [CrossRef]
- Kang, S.; Min, H. Ginseng, the ‘immunity boost’: The effects of Panax ginseng on immune system. J. Ginseng Res. 2012, 36, 354. [Google Scholar] [CrossRef] [Green Version]
- Choi, K.T. Botanical characteristics, pharmacological effects and medicinal components of Korean Panax ginseng CA Meyer. Acta Pharmacol. Sin. 2008, 29, 1109–1118. [Google Scholar] [CrossRef] [Green Version]
- Chen, W.; Balan, P.; Popovich, D.G. Comparison of the ginsenoside composition of Asian ginseng (Panax ginseng) and American ginseng (Panax quinquefolius L.) and their transformation pathways. Stud. Nat. Prod. Chem. 2019, 63, 161–195. [Google Scholar]
- Kiefer, D.S.; Pantuso, T. Panax ginseng. Am. Fam. Physician 2003, 68, 1539–1542. [Google Scholar]
- Qu, C.; Bai, Y.; Jin, X.; Wang, Y.; Zhang, K.; You, J.; Zhang, H. Study on ginsenosides in different parts and ages of Panax quinquefolius L. Food Chem. 2009, 115, 340–346. [Google Scholar] [CrossRef]
- Xie, J.T.; Mehendale, S.R.; Wang, A.; Han, A.H.; Wu, J.A.; Osinski, J.; Yuan, C.S. American ginseng leaf: Ginsenoside analysis and hypoglycemic activity. Pharmacol. Res. 2004, 49, 113–117. [Google Scholar] [CrossRef]
- Chen, W.; Balan, P.; Popovich, D.G. Ginsenosides analysis of New Zealand–grown forest Panax ginseng by LC-QTOF-MS/MS. J. Ginseng Res. 2020, 44, 552–562. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Peng, D.; Xie, J. Ginseng leaf-stem: Bioactive constituents and pharmacological functions. Chin. Med. 2009, 4, 20. [Google Scholar] [CrossRef] [Green Version]
- Ligor, T.; Ludwiczuk, A.; Wolski, T.; Buszewski, B. Isolation and determination of ginsenosides in American ginseng leaves and root extracts by LC-MS. Anal. Bioanal. Chem. 2005, 383, 1098–1105. [Google Scholar] [CrossRef] [PubMed]
- Jackson, C.J.C.; Dini, J.P.; Lavandier, C.; Faulkner, H.; Rupasinghe, H.; Proctor, J.T. Ginsenoside content of North American ginseng (Panax quinquefolius L. Araliaceae) in relation to plant development and growing locations. J. Ginseng Res. 2003, 27, 135–140. [Google Scholar]
- KFDA. Food Code; Korea Food and Drug Administration: Seoul, Republic of Korea, 2002.
- Kim, S.D.; Do, J.H.; Oh, H.I. Effects of processing methods on the quality of ginseng leaf tea. Korean J. Food Sci. Technol. 1981, 13, 267–272. [Google Scholar]
- Kwon, J.H.; Byun, M.W.; Choi, K.J.; Kwon, D.W.; Cho, H.O. Effects of decontamination treatments on chemical components of Panax ginseng-leaf tea. Korean J. Food Sci. Technol. 1992, 24, 65–69. [Google Scholar]
- Shin, B.K.; Kwon, S.W.; Park, J.H. Chemical diversity of ginseng saponins from Panax ginseng. J. Ginseng Res. 2015, 39, 287–298. [Google Scholar] [CrossRef] [Green Version]
- Li, K.K.; Li, S.-S.; Xu, F.; Gong, X.J. Six new dammarane-type triterpene saponins from Panax ginseng flower buds and their cytotoxicity. J. Ginseng Res. 2020, 44, 215–221. [Google Scholar] [CrossRef]
- Wang, Y.S.; Jin, Y.P.; Gao, W.; Xiao, S.Y.; Zhang, Y.W.; Zheng, P.H.; Wang, J.; Liu, J.X.; Sun, C.H.; Wang, Y.P. Complete 1H-NMR and 13C-NMR spectral assignment of five malonyl ginsenosides from the fresh flower buds of Panax ginseng. J. Ginseng Res. 2016, 40, 245–250. [Google Scholar] [CrossRef]
- Sun, Y. Structure and biological activities of the polysaccharides from the leaves, roots and fruits of Panax ginseng CA Meyer: An overview. Carbohyd. Polym. 2011, 85, 490–499. [Google Scholar] [CrossRef]
- Zhao, B.; Lv, C.; Lu, J. Natural occurring polysaccharides from Panax ginseng CA Meyer: A review of isolation, structures, and bioactivities. Int. J. Biol. Macromol. 2019, 133, 324–336. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.; Wang, J.; Huang, R.; Tan, Y.; Zhang, F.; Zhou, Y.; Sun, L. Analysis of pectin from Panax ginseng flower buds and their binding activities to galectin-3. Int. J. Biol. Macromol. 2019, 128, 459–467. [Google Scholar] [CrossRef] [PubMed]
- Li, T.S. Asian and American ginseng—A review. HortTechnology 1995, 5, 27–34. [Google Scholar] [CrossRef] [Green Version]
- Cheon, S.G.; Mok, S.G.; Lee, S.S. Effects of light intensity and quality on the growth and quality of Korean ginseng (Panax ginseng CA Meyer) II. Relationship between light intensity and planting density. J. Ginseng Res. 1991, 15, 31–35. [Google Scholar]
- Parmenter, G.; Littlejohn, R. The effect of irradiance during leaf development on photoinhibition in Panax ginseng CA Meyer. J. Ginseng Res. 1998, 22, 102–113. [Google Scholar]
- Yang, D.; Yoo, H.; Yoon, J. Investigation on the photo-oxidation of pigment in leaf-burning disease of Panax ginseng: II. Investigation and analysis of physiological reaction mechanism on the chlorophyll bleaching phenomenon. Korean J. Ginseng Sci. 1987, 11, 101–110. [Google Scholar]
- Lee, S.S.; Proctor, J.T.; Choi, K.T. Influence of monochromatic light on photosynthesis and leaf bleaching in Panax species. J. Ginseng Res. 1999, 23, 1–7. [Google Scholar]
- Fournier, A.R.; TA, J.; Khanizadeh, S.; Gosselin, A.; Dorais, M. Acclimation of maximum quantum yield of PSII and photosynthetic pigments of Panax quinquefolius L. to understory light. J. Ginseng Res. 2008, 32, 347–356. [Google Scholar]
- Hendrick, M.F.; Finseth, F.R.; Mathiasson, M.E.; Palmer, K.A.; Broder, E.M.; Breigenzer, P.; Fishman, L. The genetics of extreme microgeographic adaptation: An integrated approach identifies a major gene underlying leaf trichome divergence in Yellowstone Mimulus Guttatus. Mol. Ecol. 2016, 25, 5647–5662. [Google Scholar] [CrossRef]
- Mazie, A.R.; Baum, D.A. Clade-specific positive selection on a developmental gene: BRANCHLESS TRICHOME and the evolution of stellate trichomes in Physaria (Brassicaceae). Mol. Phylogenet. Evol. 2016, 100, 31–40. [Google Scholar] [CrossRef] [Green Version]
- Ning, P.; Wang, J.; Zhou, Y.; Gao, L.; Wang, J.; Gong, C. Adaptional evolution of trichome in Caragana korshinskii to natural drought stress on the Loess Plateau, China. Ecol. Evol. 2016, 6, 3786–3795. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tian, D.; Peiffer, M.; De Moraes, C.M.; Felton, G.W. Roles of ethylene and jasmonic acid in systemic induced defense in tomato (Solanum lycopersicum) against Helicoverpa zea. Planta 2014, 239, 577–589. [Google Scholar] [CrossRef] [PubMed]
- Kenneth, V. Thimann. Chapter I—Phototropism. Compr. Biochem. 1967, 27, 1–29. [Google Scholar]
- Yang, J.; Jeong, B.R. Side lighting enhances morphophysiology by inducing more branching and flowering in chrysanthemum grown in controlled environment. Int. J. Mol. Sci. 2021, 22, 12019. [Google Scholar] [CrossRef]
- Yang, J.; Song, J.; Jeong, B.R. Side lighting enhances morphophysiology and runner formation by upregulating photosynthesis in strawberry grown in controlled environment. Agronomy 2022, 12, 24. [Google Scholar] [CrossRef]
- Yang, J.; Song, J.; Jeong, B.R. Lighting from top and side enhances photosynthesis and plant performance by improving light usage efficiency. Int. J. Mol. Sci. 2022, 23, 2448. [Google Scholar] [CrossRef] [PubMed]
- Seo, J.; Lee, J.S.; Shim, S.L.; In, J.-G.; Park, C.S.; Lee, Y.J.; Ahn, H.J. Development and authentication of Panax ginseng cv. Sunhong with high yield and multiple tolerance to heat damage, rusty roots and lodging. Hortic. Environ. Biotechnol. 2023, 1–12. [Google Scholar] [CrossRef]
- Liscum, E.; Askinosie, S.K.; Leuchtman, D.L.; Morrow, J.; Willenburg, K.T.; Coats, D.R. Phototropism: Growing towards an understanding of plant movement. Plant Cell 2014, 26, 38–55. [Google Scholar] [CrossRef] [Green Version]
- Mullen, J.L.; Weinig, C.; Hangarter, R.P. Shade avoidance and the regulation of leaf inclination in Arabidopsis. Plant Cell Environ. 2006, 29, 1099–1106. [Google Scholar] [CrossRef] [Green Version]
- Van Zanten, M.; Pons, T.; Janssen, J.; Voesenek, L.; Peeters, A. On the relevance and control of leaf angle. Crit. Rev. Plant Sci. 2010, 29, 300–316. [Google Scholar] [CrossRef]
- Kozai, T.; Kino, S.; Jeong, B.; Kinowaki, M.; Ochiai, M.; Hayashi, M.; Mori, K. A sideward lighting system using diffusive optical fibers for production of vigorous micropropagated plantlets. Acta Hortic. 1992, 319, 237–242. [Google Scholar] [CrossRef]
- Génard, M.; Dauzat, J.; Franck, N.; Lescourret, F.; Moitrier, N.; Vaast, P.; Vercambre, G. Carbon allocation in fruit trees: From theory to modeling. Trees 2008, 22, 269–282. [Google Scholar] [CrossRef]
- Ping, X.; Zhou, G.; Sun, J. Advances in the study of photosynthate allocation and its controls. Chin. J. Plant Ecol. 2010, 34, 100–111. [Google Scholar]
- Poorter, H.; Nagel, O. The role of biomass allocation in the growth response of plants to different levels of light, CO2, nutrients and water: A quantitative review. Aust. J. Plant Physiol. 2000, 27, 595–607. [Google Scholar]
- De Groot, C.C.; Marcelis, L.F.M.; Van Den Boogaard, R.; Lambers, H. Interactive effects of nitrogen and irradiance on growth and partitioning of dry mass and nitrogen in young tomato plants. Funct. Plant Biol. 2002, 29, 1319–1328. [Google Scholar] [CrossRef] [PubMed]
- Kotowski, W.; van Andel, J.; van Diggelen, R.; Hogendorf, J. Responses of ten plant species to groundwater level and light intensity. Plant Ecol. 2001, 155, 147–156. [Google Scholar] [CrossRef]
- Van Gelderen, K.; Kang, C.; Pierik, R. Light signaling, root development, and plasticity. Plant Physiol. 2018, 176, 1049–1060. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vandenbussche, F.; Pierik, R.; Millenaar, F.F.; Voesenek, L.A.; Van Der Straeten, D. Reaching out of the shade. Curr. Opin. Plant Biol. 2005, 8, 462–468. [Google Scholar] [CrossRef] [PubMed]
- Sheerin, D.J.; Hiltbrunner, A. Molecular mechanisms and ecological function of far-red light signalling. Plant Cell Environ. 2017, 40, 2509–2529. [Google Scholar] [CrossRef]
- Weraduwage, S.M.; Chen, J.; Anozie, F.C.; Morales, A.; Weise, S.E.; Sharkey, T.D. The relationship between leaf area growth and biomass accumulation in Arabidopsis thaliana. Front. Plant Sci. 2015, 6, 167. [Google Scholar] [CrossRef] [Green Version]
- Marchi, S.; Tognetti, R.; Minnocci, A.; Borghi, M.; Sebastiani, L. Variation in mesophyll anatomy and photosynthetic capacity during leaf development in a deciduous mesophyte fruit tree (Prunus persica) and an evergreen sclerophyllous Mediterranean shrub (Olea europaea). Trees 2008, 22, 559–571. [Google Scholar] [CrossRef]
- Akhtar, M.Q.; Qamar, N.; Yadav, P.; Kulkarni, P.; Kumar, A.; Shasany, A.K. Comparative glandular trichome transcriptome-based gene characterization reveals reasons for differential (−)-menthol biosynthesis in Mentha species. Physiol. Plant 2017, 160, 128–141. [Google Scholar] [CrossRef] [PubMed]
- Bryant, L.; Patole, C.; Cramer, R. Proteomic analysis of the medicinal plant Artemisia annua: Data from leaf and trichome extracts. Data Brief 2016, 7, 325–331. [Google Scholar] [CrossRef] [Green Version]
- Champagne, A.; Boutry, M. A comprehensive proteome map of glandular trichomes of hop (Humulus lupulus L.) female cones: Identification of biosynthetic pathways of the major terpenoid-related compounds and possible transport proteins. Proteomics 2017, 17, 1600411. [Google Scholar] [CrossRef]
- Maes, L.; Van Nieuwerburgh, F.C.; Zhang, Y.; Reed, D.W.; Pollier, J.; Vande Casteele, S.R.; Inzé, D.; Covello, P.S.; Deforce, D.L.; Goossens, A. Dissection of the phytohormonal regulation of trichome formation and biosynthesis of the antimalarial compound artemisinin in Artemisia annua plants. New Phytol. 2011, 189, 176–189. [Google Scholar] [CrossRef] [Green Version]
- Schilmiller, A.; Shi, F.; Kim, J.; Charbonneau, A.L.; Holmes, D.; Daniel Jones, A.; Last, R.L. Mass spectrometry screening reveals widespread diversity in trichome specialized metabolites of tomato chromosomal substitution lines. Plant J. 2010, 62, 391–403. [Google Scholar] [CrossRef] [Green Version]
- Tiwari, P. Recent advances and challenges in trichome research and essential oil biosynthesis in Mentha arvensis L. Ind. Crop Prod. 2016, 82, 141–148. [Google Scholar] [CrossRef]
- Li, W.; Chen, T.; Chen, Y.; Lei, M. Role of trichome of Pteris vittata L. in arsenic hyperaccumulation. Sci. China Ser. C 2005, 48, 148–154. [Google Scholar]
- Crook, M.J.; Ennos, A.R. Stem and root characteristics associated with lodging resistance in four winter wheat cultivars. J. Agric. Sci. 1994, 123, 167–174. [Google Scholar] [CrossRef]
- Sirajul Islam, M.; Peng, S.; Visperas, R.M.; Ereful, N.; Sultan Uddin Bhuiya, M.; Julfiquar, A.W. Lodging-related morphological traits of hybrid rice in a tropical irrigated ecosystem. Field Crop Res. 2007, 101, 240–248. [Google Scholar] [CrossRef]
- Ma, C.; Dai, S. Advances in photoreceptor-mediated signaling transduction in flowering time regulation. Chin. Bull. Bot. 2019, 54, 9. [Google Scholar]
- Blümel, M.; Dally, N.; Jung, C. Flowering time regulation in crops—What did we learn from Arabidopsis? Curr. Opin. Biotechnol. 2015, 32, 121–129. [Google Scholar] [CrossRef]
- Samach, A.; Onouchi, H.; Gold, S.E.; Ditta, G.S.; Schwarz-Sommer, Z.; Yanofsky, M.F.; Coupland, G. Distinct roles of CONSTANS target genes in reproductive development of Arabidopsis. Science 2000, 288, 1613–1616. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abe, M.; Kobayashi, Y.; Yamamoto, S.; Daimon, Y.; Yamaguchi, A.; Ikeda, Y.; Ichinoki, H.; Notaguchi, M.; Goto, K.; Araki, T. FD, a bZIP protein mediating signals from the floral pathway integrator FT at the shoot apex. Science 2005, 309, 1052–1056. [Google Scholar] [CrossRef] [PubMed]
- Adeyemo, O.S.; Chavarriaga, P.; Tohme, J.; Fregene, M.; Davis, S.J.; Setter, T.L. Overexpression of Arabidopsis FLOWERING LOCUS T (FT) gene improves floral development in cassava (Manihot esculenta, Crantz). PLoS ONE 2017, 12, e0181460. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kozai, T. Resource use efficiency of closed plant production system with artificial light: Concept, estimation and application to plant factory. Proc. Jpn. Acad. Ser. B 2013, 89, 447–461. [Google Scholar] [CrossRef] [Green Version]
- Liao, J.X.; Ge, Y.; Huang, C.C.; Zhang, J.; Liu, Q.X.; Chang, J. Effects of irradiance on photosynthetic characteristics and growth of Mosla chinensis and M. scabra. Photosynthetica 2005, 43, 111–115. [Google Scholar] [CrossRef]
- Yin, Q.; Tian, T.; Kou, M.; Liu, P.; Wang, L.; Hao, Z.; Yue, M. The relationships between photosynthesis and stomatal traits on the Loess Plateau. Glob. Ecol. Conserv. 2020, 23, e01146. [Google Scholar] [CrossRef]
- Ma, J.; Zhu, Q.S.; Ma, W.B.; Tian, Y.H.; Yang, J.C.; Zhou, K.D. Studies on the photosynthetic characteristics and assimilate’s accumulation and transformation in heavy panicle type of rice. Agric. Sci. China 2003, 2, 602–608. [Google Scholar]
- Yamori, W.; Kusumi, K.; Iba, K.; Terashima, I. Increased stomatal conductance induces rapid changes to photosynthetic rate in response to naturally fluctuating light conditions in rice. Plant Cell Environ. 2020, 43, 1230–1240. [Google Scholar] [CrossRef]
- Park, Y.G.; Jeong, B.R. Both the quality and positioning of the night interruption light are important for flowering and plant extension growth. J. Plant Growth Regul. 2020, 39, 583–593. [Google Scholar] [CrossRef] [Green Version]
- Dai, Y.; Shen, Z.; Liu, Y.; Wang, L.; Hannaway, D.; Lu, H. Effects of shade treatments on the photosynthetic capacity, chlorophyll fluorescence, and chlorophyll content of Tetrastigma hemsleyanum Diels et Gilg. Environ. Exp. Bot. 2009, 65, 177–182. [Google Scholar] [CrossRef]
- Liang, Y.; Feng, L.; Yin, C. Current status and prospect of chlorophyll fluorescence technique in the study of responses of microalgae to environmental stress. Mar. Sci.-Qingdao-Chin. Ed. 2007, 31, 71. [Google Scholar]
- Zhang, Y.; Liu, G.-j. Effects of cesium accumulation on chlorophyll content and fluorescence of Brassica juncea L. J. Environ. Radioact. 2018, 195, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.; Pham, M.D.; Cui, M.; Lee, H.; Hwang, H.; Jang, I.; Chun, C. Growth and physiological responses of Panax ginseng seedlings as affected by light intensity and photoperiod. Hortic. Environ. Biotechnol. 2022, 63, 835–846. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Buschmann, C. Chlorophylls and carotenoids: Measurement and characterization by UV-VIS spectroscopy. Curr. Protoc. Food Anal. Chem. 2001, 1, F4. 3.1–F4. 3.8. [Google Scholar] [CrossRef]
- Genty, B.; Briantais, J.M.; Baker, N.R. The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochim. Biophys. Acta (BBA)—Gen. Subj. 1989, 990, 87–92. [Google Scholar] [CrossRef]
- Roháček, K. Chlorophyll fluorescence parameters: The definitions, photosynthetic meaning, and mutual relationships. Photosynthetica 2002, 40, 13–29. [Google Scholar] [CrossRef]


| Light Direction 1 | Shoot FW 2 (g) | Shoot DW 3 (g) | Pre-Root FW (g) | Post-Root FW (g) | Pre-Root DW (g) |
| T | 0.793 ± 0.011 b 4 | 0.124 ± 0.008 b | 0.714 ± 0.094 | 0.836 ± 0.021 b | 0.121 ± 0.006 |
| TS | 1.075 ± 0.017 a | 0.187 ± 0.010 a | 1.087 ± 0.014 a | ||
| S | 0.372 ± 0.020 c | 0.056 ± 0.007 c | 0.797 ± 0.032 bc | ||
| Light Direction | Post-Root DW (g) | Pre-Root Length (cm) | Post-Root Length (cm) | Pre-Root Diameter (mm) | Post-Root Diameter (mm) |
| T | 0.153 ± 0.012 b | 10.871 ± 1.023 | 12.004 ± 1.147 b | 3.164 ± 1.001 | 4.142 ± 0.987 b |
| TS | 0.198 ± 0.010 a | 15.863 ± 1.263 a | 5.341 ± 1.000 a | ||
| S | 0.124 ± 0.009 c | 12.075 ± 1.536 b | 3.790 ± 1.023 bc |
| Light Direction 1 | Pn 2 (μmol CO2 m−2·s−1) | Tr 3 (mmol H2O m−2·s−1) | Gs 4 (mol H2O m−2·s−1) | Ci 5 (μmol CO2 mol−1) |
| T | 12.137 ± 0.351 b 10 | 1.893 ± 0.084 a | 0.437 ± 0.021 b | 438.337 ± 15.119 b |
| TS | 15.004 ± 0.435 a | 1.542 ± 0.071 b | 0.586 ± 0.037 a | 486.274 ± 13.996 a |
| S | 10.016 ± 0.375 c | 1.165 ± 0.080 c | 0.368 ± 0.023 c | 404.158 ± 14.772 c |
| Light Direction | Fv/Fm 6 | Fv′/Fm′ 7 | qP 8 | NPQ 9 |
| T | 0.860 ± 0.011 a | 0.526 ± 0.010 b | 0.425 ± 0.009 b | 2.625 ± 0.067 ab |
| TS | 0.862 ± 0.012 a | 0.597 ± 0.018 a | 0.473 ± 0.012 a | 2.347 ± 0.074 b |
| S | 0.812 ± 0.007 b | 0.523 ± 0.011 b | 0.353 ± 0.023 c | 2.801 ± 0.083 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, J.; Song, J.; Shilpha, J.; Jeong, B.R. Top and Side Lighting Induce Morphophysiological Improvements in Korean Ginseng Sprouts (Panax ginseng C.A. Meyer) Grown from One-Year-Old Roots. Plants 2023, 12, 2849. https://doi.org/10.3390/plants12152849
Yang J, Song J, Shilpha J, Jeong BR. Top and Side Lighting Induce Morphophysiological Improvements in Korean Ginseng Sprouts (Panax ginseng C.A. Meyer) Grown from One-Year-Old Roots. Plants. 2023; 12(15):2849. https://doi.org/10.3390/plants12152849
Chicago/Turabian StyleYang, Jingli, Jinnan Song, Jayabalan Shilpha, and Byoung Ryong Jeong. 2023. "Top and Side Lighting Induce Morphophysiological Improvements in Korean Ginseng Sprouts (Panax ginseng C.A. Meyer) Grown from One-Year-Old Roots" Plants 12, no. 15: 2849. https://doi.org/10.3390/plants12152849
APA StyleYang, J., Song, J., Shilpha, J., & Jeong, B. R. (2023). Top and Side Lighting Induce Morphophysiological Improvements in Korean Ginseng Sprouts (Panax ginseng C.A. Meyer) Grown from One-Year-Old Roots. Plants, 12(15), 2849. https://doi.org/10.3390/plants12152849







